Abstract
A community-based participatory research was utilized to address the coastal community’s concern regarding Deepwater Horizon oil contamination of seafood. Therefore, we analyzed polycyclic aromatic hydrocarbons (PAHs), major toxic constituents of crude oil, in the seafood collected from gulf coast (Louisiana, Alabama and Mississippi) during December 2011-February 2014. PAHs were extracted from edible part of shrimp, oysters, and crabs by the QuEChERS/dsPE procedure and analyzed by gas chromatography-mass spectrometry. The total PAHs data were further analyzed using the General Linear Mixed Model procedure of the SAS (Version 9.3, SAS Institute, Inc., Cary, NC) statistical software. Brown shrimp showed statistically significant differences in PAHs levels with respect to time and locations while white shrimp showed differences at various time points. PAHs levels in oyster and crab samples were not statistically different at the Type I error of 0.05. Overall, the PAHs levels are far below FDA levels of concern for human consumption.
Keywords: Petrogenic PAHs, Seafood, Deepwater Horizon Oil Spill
1. Introduction
The University of Texas Medical Branch (UTMB) has maintained long-standing relationships with coastal communities during disasters including Hurricanes Katrina, Rita, and Ike [Croisant et al., 2017]. This working relationship facilitated early contact with affected communities following the Deepwater Horizon (DWH) oil spill regarding their concerns about its detrimental effects on the seafood and human health. This resulted in the development of a consortium involving several multi-disciplinary, multi-institutional academic partners and six Gulf Coast communities impacted by the spill [Croisant et al., 2017; Sullivan et al., 2018]. The consortium developed a research proposal, the Gulf Coast Health Alliance: Health Risks related to the Macondo Spill (GC-HARMS) study, utilizing a Community-Based Participatory Research (CBPR) approach that was subsequently funded by the National Institute of Environmental Health Sciences as a U19 grant. We analyzed polycyclic aromatic hydrocarbons (PAHs) levels in shrimp (both brown and white), crabs, and oysters supplied to us by community partners and reported back the data to them on a routine basis during GC-HARMS meetings and their cumulative levels on a yearly basis which are reported in this manuscript.
The Gulf of Mexico fishery produces the majority of domestic shrimp (60%) and oysters for the U.S., and to a lesser extent crab and finfish [Rotkin-Ellman et al., 2012]. Crude oil is composed of ~ 17,000 organic compounds with differing volatility, solubility, and toxicity to marine life [Allan et al., 2012]. DWH crude oil largely contains low molecular weight hydrocarbons followed by saturated alkanes and PAHs (Liu et al., 2012). Petrogenic PAHs primarily consist of 2-3 ring compounds with their alkylated analogues representing the bulk of PAHs in the crude oil [Pampanin, 2017], PAHs are also the byproducts of combustion processes and ubiquitously found in air, food, water, dust and soil. In general, humans are exposed to low-level PAHs via contaminated foods and via inhalation. Aguilera et al. [2010] reviewed the short-term effects of exposure to spilled oils on human health. Pampanin [2017] reported that alkylated PAHs (petrogenic in nature) had higher toxicity than non-alkylated PAHs and are known to cause inflammatory diseases and cancer [Agency for Toxic Substances and Disease Registry (ATSDR), 1995; Lewtas, 2007; Schober, 2007], Among PAHs, benzo[a]pyrene (B[a] P) has been identified as a human carcinogen [Boffetta et al., 1997], In addition to the carcinogenic risk, PAHs also cause increased rates of cardiovascular diseases including fatal ischemic heart disease [Burstyn et al., 2005], Several PAHs are known toxic constituents of the crude oil that can accumulate in marine species; higher molecular weight PAHs are more likely to persist in tissues longer than low molecular weight PAH’s [Meador et al., 1995; Beyer et al., 2010; Gohlke et al., 2011], In aquatic species, PAH toxicity is thought to be mediated through metabolism/oxidation of the parent compound [Dasgupta et al., 2014]. Fish are effective metabolizers, transforming PAHs to their more toxic oxidized forms [Pampanin, 2017]. Hence an investigation into the presence of PAH metabolites, especially alkylated PAHs, would provide a better measure of the toxicity [Huang et al., 2017].
Despite the natural and anthropomorphic processes to rid the environment of the spilled oil from the Gulf, its effect on seafood quality is not known. In the early stages of the oil spill, research focused primarily on its distribution, composition and toxicity [Reddy et al., 2012; Mulabagal et al., 2013; Paul et al., 2013]. Moreover, a substantial fraction of the oil persisted near the coast and underwent weathering [Aeppli et al., 2012; Liu et al., 2012; Sammarco et al., 2013]. It is worth bearing in mind that considerable amounts of oil consistently enter the Gulf water through natural seeps, discharges from transportation activities, and as combustion by-products [Ocean Studies Board and Marine Board, 2003]. However, the amount of oil released during the DWH oil spill was estimated to be ~ 7 times more than those associated with the aforementioned releases [Murawski et al., 2014]. Much of the oil released during the spill formed a plume that existed about 100-1200 meters beneath the surface [Camilli et al., 2010; Diercks et al., 2010; Ryerson et al., 2012], Even though dissolution and biodegradation are suggested for the oil plume’s fate, the final consequence of this plume is unknown and its impact on the marine ecosystem remains undetermined [Camilli et al., 2010; Reddy et al., 2012; Valentine et al., 2014].
Shrimp is America’s most popular seafood, and 60% of Americans regularly eat seafood [Ramalhosa et al., 2009; Norli et al., 2011; Johnson, 2012]. To begin evaluating the risk for adverse human health outcomes and to address community concerns in the Gulf Coast region associated with seafood consumption following the oil spill, we monitored the levels of the petrogenic PAHs in marine species regularly consumed by the subsistence fishing communities. Our project involved various community partners, and the PAH residue data presented in this paper will aid in providing a comprehensive picture of the contamination of the seafood by oil-derived PAHs and the overall health of the affected communities. Seafood samples were collected over a three-year span at selected locations (total 15) through community partners with a focus mainly on brown shrimp (Farfantepenaeus aztecus), and white shrimp (Litopenaeus setiferus), blue crabs (Callinectes sapidus) and oysters (Crassostrea virginica) which are known to accumulate PAHs [Meador et al., 1995; Hylland, 2006].
Seafood samples were collected and transferred to us for the PAHs analysis. We analyzed samples from inshore and near-shore regions in Alabama, Mississippi, and Louisiana from 12/13/2011 to 02/03/2014, collected by community partners (local fisherman). Analytical assessments determined that the total PAHs found in the seafood samples were below the level of concern established by the U.S. Food and Drug Administration (FDA) guidelines for any individual PAH. However, the present guidelines do not include alkylated PAHs which are the dominant PAHs in oil.
2. Materials and Methods
2.1. Sample collection
Seafood (shrimp, oyster, and crab) samples were collected from six community hubs organized across the tristate area directly affected by the oil spill: three in Louisiana, two in Mississippi, and one in Alabama [Sullivan et al., 2018]. The project established a coordinated community field science network with proper training of fishermen responsible for acquiring the seafood samples as previously described [Sullivan et. al, 2018]. These sampling hubs were located in Houma, Louisiana (United Houma Nation; UHN), Gulfport, Mississippi (Mississippi Vietnamese-American Fishing Community; MVC) and Coden / Bayou La Batre, Alabama (Alabama Fisheries Cooperative, AFC). The sample collection sites were at UHN: Atchafalaya (4 League Bay), Lower Terrebonne/Lafourche, Grand Isle, Barataria/Lafitte, Venice/Boothville, Point-a-la-Hache, and St. Bernard); and MVC: Bay St. Louis, Waveland/Pass Christian, Gulfport, Biloxi, and Pascagoula/Moss Point; and AFC: Mobile Bay (east/west), Dauphin Island, and Bayou La Batre [Sullivan et al.,2018]. The samples were collected from December 2011 through February 2014. At the early stages of the oil spill most state waters extending from Louisiana to Florida were closed for fishing [Ylitalo et al., 2012]. Working protocols were developed for sample collection and processing using Environmental Protection Agency (EPA)-approved procedures [Sullivan et. al., 2018; Croisant et al., 2017]. These procedural details ensured the quality of samples, their geographical location (GPS coordinates), and a documented chain-of-custody.
For the seafood-sampling period (December 2011-February 2014), the community partners successfully collected samples of shrimp (brown and white), blue crab, and oysters, all of which were used in the present study. The GC-HARMS detailed sampling map is available (http://gcharms.leanweb.org/seafood-sampling-map/)
2.2. Extraction Method
Five grams of edible meat from seafood was homogenized in a 50 ml Falcon tube with 12 ml of water. Deuterated phenanthrene was added to the homogenate as an internal standard and vortexed for 1 minute. The homogenate was mixed with 15 ml acetonitrile, and the mixture was vortexed for 15 minutes. The QuEChERS pouch content containing MgSCU and NaCI (Agilent p/n 5982-6555) was added and shaken vigorously for 15 minutes. The mixture was centrifuged and 8 ml of the organic layer transferred to Agilent AOAC fatty sample dSPE 15 ml tube (Agilent p/n 5982-5158) and vortexed for 1 minute. Each sample was centrifuged and the acetonitrile layer transferred to a glass tube and reduced under a gentle stream of nitrogen to 500 pi. To each 100 μΙ aliquot of the extract added mixed running standards of deuterated naphthalene, acenaphthene and perylene, and analyzed by GC-MS (adapted from Agilant technical note 5990-6668N and modified for optimal recovery [Smith and Lynam, 2012]). The GC-MS method for the analyses of PAHs in seafood samples are well established [Olson et al., 2004; Gratz et al., 2011; Mastovska et al., 2015; Overton et al., 2016].
2.3. Analysis
2.3.1. Instrumentation:
A GC-MS (Agilent 6890N/5975C) with a DB-5MS column (30m x 0.25 mm x 0.25 μm, Agilant p/n 122-5532) and auto sampler (Gerstel multipurpose sampler MPS2) was used [Smith and Lynam, 2012]. The instrument performance was monitored by running a tune file on a daily basis.
2.3.2. Optimization:
To develop optimal GC conditions (Agilant note 5990-6668N), we used the 16 PAHs stock solution (Agilant p/n 8500-6035), (2H)-deuterated 16 PAH standards (RTC p/n S-4405-100-BD), PAH/NPD stock (RTC p/n 4007-ASS-AC) and a crude oil sample. To validate the limit of quantification and detection, standard PAHs of different concentrations of 16 PAHs were used. For the smaller ring compounds (2-3 rings), concentrations down to 0.001 μg/ml could be detected while for the higher ring compounds (4-6 rings) limit-of-detection was 0.005 μg/ml. Test samples consisted of three deuterated standards representing 2 ring (naphthalene), 3 ring (acenaphthene) and 5 ring (perylene) PAHs (all purchased from Cambridge isotope laboratories, Cambridge, MA, USA). The Selected Ion Monitoring (SIM) groups of non-deuterated naphthalene, acenaphthene and perylene with minimum interfering matrix peaks were used. At the concentrations 0.1 and 0.5 pg/ml the recovery was excellent with ~5 fold change as compared to blank (acetonitrile)
2.4. Statistical Analysis
The Shapiro-Wilk test for normality and homogeneity assessment test for the total PAHs concentration were evaluated for the model assumption of normality and equal variances under parametric analysis of variance (ANOVA) test. The total PAHs concentrations with a highly skewed and unequal distribution obtained from GC-MS analysis were subjected to the Kruskal-Wallis test, a nonparametric analysis of variance (ANOVA) by Ranks test. This test is a non-parametric method that determines if there is a statistical significant difference in location or time groups of independent variables on continuous dependent outcomes. The data were compared across locations (states) and times for total shrimp, white shrimp and brown shrimp separately; oysters, and crab. The results are presented using Box and Whisker plots. The p-values were compared based on the ANOVA F test on ranks statistics to the alpha of 0.05 significance level to assess the hypothesis that the means are all equal. Two-way ANOVA tests on ranks were used to assess differences between locations and times, and the Tukey post hoc test (Multiplicity adjustment) was employed.
3. Results and Discussion
3.1. PAHs
The level of PAHs are frequently measured in the air, water, and food to assess environmental levels and/or predict their adverse effects [Poster et al., 2006; Forsberg et al., 2011; Clark III et al., 2012; Best et al., 2016]. These complex mixtures of PAHs are preferentially analyzed by GC-MS for their greater selectivity, resolution, and sensitivity [Poster et al., 2006; Frosberg et al., 2011; Mastovska et al., 2015]. Health effects associated with chronic exposure to coal tar, a residual material following distillation of oil-containing PAHs, causes several types of cancer [Boffetta et al., 1997; Lewtas, 2007; Schober et al., 2007; ATSDR,1995]. Exposure to ultraviolet light may increase PAH toxicity, probably via oxidative modification [Dasgupta et al., 2014]. Naphthalene, one of the most abundant PAHs of crude oil, is a skin irritant and its vapors can cause headache, nausea, vomiting, and diarrhea [ATSDR,1995]. The PAHs are metabolized/oxidized differently in accordance with the varying numbers of aromatic rings and presence of aliphatic substituents. The resultant metabolites may impart distinct target organ toxicities not necessarily apparent by analyzing the parent PAHs and/or their alkylated analogues. These might be better estimated using bioassays based on defined mechanistic parameters [Wilson et al., 2015; Jackson et al., 2019]. The other noteworthy caveat is that in response to the oil spill in April 2010, a large amount of dispersant was added to the Gulf waters. How dispersants affected the oil-derived PAH bioavailability and degradation is currently not known. Within the spectrum of PAHs that we measured, the low molecular weight species are known to evaporate while higher ring PAHs tend to accumulate as oil undergoes the weathering process [Pampanin, 2017]. Hence, the PAHs profile changes over time due to weathering and this has been monitored after the DWH spill [Yin et al., 2015]. We have measured the parent PAHs together with their alkylated homologs in the seafood samples, namely naphthalene and its alkylated analogues (C1, C2, C3, C4), acenaphthalene, acenaphthylene, florene and their alkylated analogues (C1, C2, C3), phenanthrene/anthracene and their alkylated analogues (C1, C2, C3, C4), fluoranthene /pyrene and their alkylated analogs (C1, C2), benz[a]anthracene, chrysene and its alkylated analogs (C1, C2, C3) and benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-c,d]pyrene, dibenz[a,h]anthracene and bezo[g,h,i]perylene by quantitating qualifying ions used for the identification of PAHs [Olson et al., 2004; Overton et al, 2016]. In one pilot study we compared edible shrimp meat vs. shell-on shrimp and observed that there was no significant difference in the PAHs present between the two samples, demonstrating that the shell is not a significant source of PAHs. Hence, subsequent analyses were performed on edible meat only.
3.2. Seafood sampling
After the DWH oil spill, one of the main concerns voiced by our partner communities was centered on seafood quality and potential health effects. To address their concerns, the GC-HARMS consortium studied PAHs contamination in the seafood and human health of the affected communities [Croisant et al., 2017; Sullivan et al., 2018; Jackson et al., 2019]. Our sample collections began from December 2011; ~18 months after the oil spill was capped, by that time affected areas were reopened for seafood harvesting. The seafood was collected by local fishermen (community partners) who fished for commercial purposes as well as personal consumption. At the same time of seafood collection, a 4-year longitudinal study of >400 individuals was underway to monitor the health of subsistence fishing communities impacted by the spill. Therefore results obtained for PAH levels are being reconciled with the human consumption data in the same population. While relatively higher median plasma PAH levels were found in populations from communities impacted by the oil spill compared to the reference community, no association was observed between overall clinical status and plasma PAH levels for any of the clinical categories that were assessed by investigators (Croisant et al., Manuscript under consideration).
PAH levels in various seafood species depends upon their environmental concentrations, the metabolic capacity of the organism, their lifespan, and their mobility [Meador et al., 1995]. Finfish exhibits the fastest metabolism (not analyzed in the present study), followed by shrimp, crabs, and oysters being the slowest [Seibel and Drazen, 2007; Pampanin, 2017]. In terms of mobility, finfish cover the greatest range followed by shrimp and crabs, with oysters being largely stationary. At the time of the sample analysis, we did not come across any directly oiled samples collected along the Louisiana, Alabama and Mississippi coastline (Seafood Sampling Map, http://gcharms.leanweb.org/seafood-sampling-map/;GC-HARMS; covering Louisiana, Mississippi, and Alabama). The collection period for various hubs was: UHN from 12/13/2011 to 6/30/2013; AFC from 12/16/2011 to 2/03/2014; and MVC from 12/16/2011 to 8/24/2013. Samples were periodically collected concomitant with the harvest seasons. The collection protocol and our association with the fishing community and fishermen have been described previously [Sullivan et al., 2018].
3.3. Total PAHs analysis in Shrimp, Oysters and Crabs
Our studies provide an insight into the general distribution of PAHs in shrimp, oysters, and crabs. Table 1 summarizes the range of total PAHs found in various seafood species analyzed from each state. Within the 94 white shrimp sampled, the highest PAH levels were detected in samples obtained from the AFC and UHN sites. The interquartile range (IQR) is the difference between the upper (Q3) and lower (Q1) quartiles, and describes the middle 50% of values when ordered from lowest to highest. The distribution among the AFC samples was much wider as compared to MVC and UHN. In a similar analysis on brown shrimp, the highest value was obtained in samples found at the UHN site, with a wider interquartile range. For crabs, the highest PAH levels were identified in the UHN samples followed by AFC. The UHN and AFC sites exhibited equivalent high average PAHs burden in the oyster samples. Overall, UHN seafood samples (shrimp, crab) were contaminated with PAHs to a greater extent when compared to MVC.
Table 1.
High and low levels of PAHs found in various sites and species (ng/g wet weight).
| Species | Louisiana | Alabama | Mississippi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Low | High | N | Low | High | N | Low | High | |
| White Shrimp | 35 | 19.8±6.1 | 62.1 ±6.7 | 30 | 14.4±2.0 | 64.4±46.5 | 29 | 13.1±2.2 | 41.6±10.9 |
| Brown Shrimp | 25 | 24.4±3.5 | 107.9±24.3 | 10 | 34.3±27.8 | 37.9±20.0 | 19 | 20.6±23.2 | 85.2±15.8 |
| Blue Crab | 43 | 19.9±4.1 | 77.2±18.5 | 12 | 40.4±6.5 | 68.9 ±6.1 | 13 | 8.3±5.6 | 57.71±9.9 |
| Oyster | 86 | 18.5±17.7 | 71.3±9.62 | 30 | 13.6±4.9 | 71.8±20.0 | 5 | 21.1±9.0 | - |
N= Total samples analyzed and values are mean ± SEM (n = 5). In cases where n were less than 5, at least 3 were analyzed and used for computation of the high and the low values.
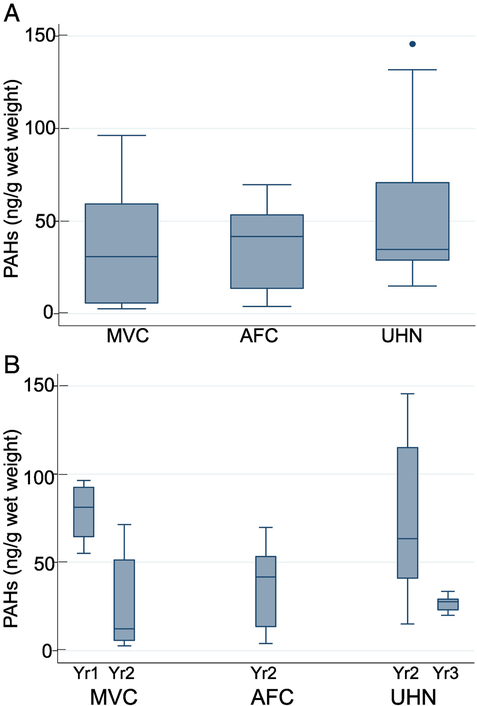
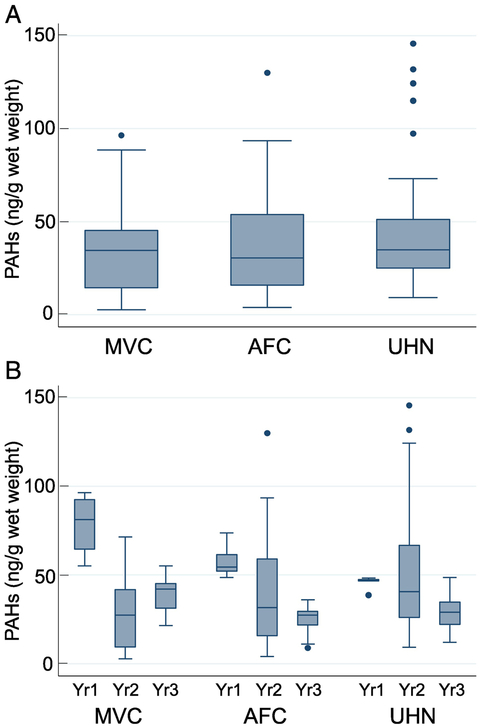
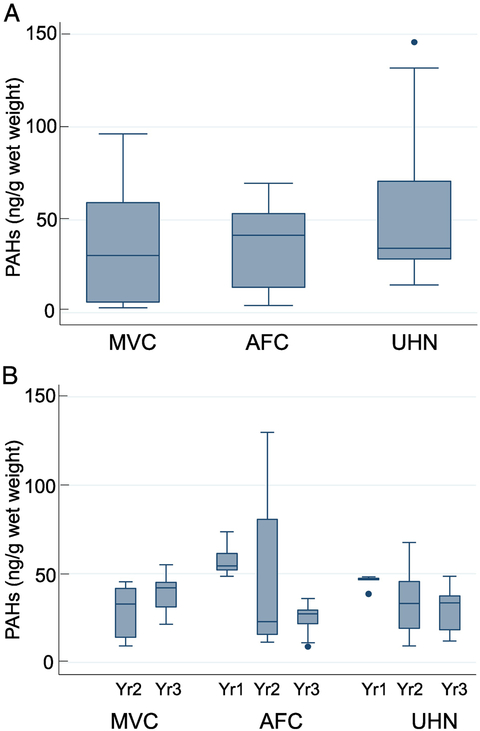
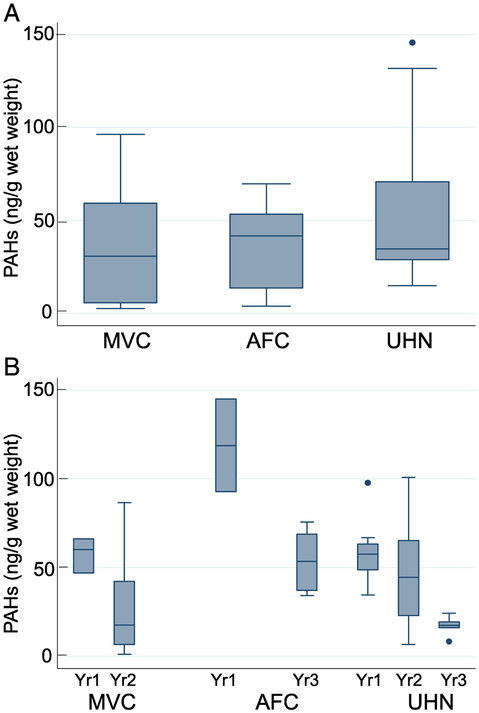
The statistical analysis of the average distribution of total PAHs in brown, white, and total shrimp (brown+white) are shown in Figures 1-3. The rationale for analyzing brown and white shrimp separately was predicated on the knowledge that during their respective life cycles, these species occupy different—albeit overlapping—coastal ecosystems, and hence their exposure to oil may differ accordingly. Since this study relied on wild-caught seafood samples, there were occasional difficulties in receiving samples in a timely fashion. Accordingly, problems related to obtaining brown shrimp samples from each location for all time points unfortunately prevented a comprehensive longitudinal assessment. The data show a decreasing trend in MVC and UHN for the two-year period (Figure 1B). In a similar analysis of white shrimp, data are plotted for three time points for AFC and UHN, while samples were received for only two time points from MVC (Figure 2B). The distribution of the average values for the samples from AFC showed a much broader range and accordingly, a wide interquartile range. However, the median PAH levels decreased with time at each location to a relatively stable level, suggestive of a new steady-state baseline. White shrimp samples from the MVC location were not available for the initial time period (Figure 2B). Because the harvesting periods for the brown and white shrimp are seasonal, Spring and Fall respectively, our collection periods spanned both species. Hence, we opted to combine the data for white and brown shrimp summarized in Figure 3. The results reveal comparable median values from all three states as equivalent (~50 ng total PAHs/g edible meat), despite several outliers obtained in the UHN location. When the data are analyzed on a yearly basis, we observed a gradual decrease from the initial time period, and consider the latter year results indicative of the new steady-state baseline for each region impacted by the oil spill (Figure 3B). The median PAH values for AFC decreased from 54.4 to 27.4 ng/g, while median values for UHN decreased from 46.9 to 27.8 ng/g. The MVC location revealed a drop from 81.3 to 14.7 ng/g during the first two collection periods that subsequently increased to 42.0 ng/g in the final year for reasons that remain unclear. The total number of shrimp analyzed for both species from the MVC, AFC, and UHN locations was 43, 40, and 45 respectively; with final year mean values of 34.7, 39.0, and 42.3 ng/g. Our analysis did not identify a significant difference in PAH levels between locations or collection times for either shrimp species analyzed independently or together. Our sampling protocol endeavored to account for age by analyzing shrimp of similar size independent of sex. We used a statistically significant sample number (five individual shrimp) to calculate the average total PAH level for a given location and sampling event.
Figure 1.
PAH levels in brown shrimp at various sites (Figure 1A) and at different time intervals (Figure 1B). The method used for statistical analysis is described in the methods section. The error bars show the range (lowest to highest value) obtained. The lowest value is still within 25th percentile −1.5XIQR while the highest value is within the 75th percentile +1.5XIQR, and the horizontal line in the box represents the median value.
Figure 3.
PAH levels in all shrimp at various sites (Figure 3A) and at different time intervals (Figure 3B). The method used for statistical analysis is described in the methods section. The error bars show the range (lowest to highest value) obtained. The lowest value is still within 25th percentile −1.5XIQR while the highest value is within the 75th percentile +1.5XIQR, and the horizontal line in the box represents the median value.
Figure 2.
PAH levels in white shrimp at various sites (Figure 2A) at different time intervals (Figure 2B). The method used for statistical analysis is described in the methods section. The error bars show the range (lowest to highest value) obtained. The lowest value is still within 25th percentile −1.5XIQR while the highest value is within the 75th percentile +1.5XIQR, and the horizontal line in the box represents the median value.
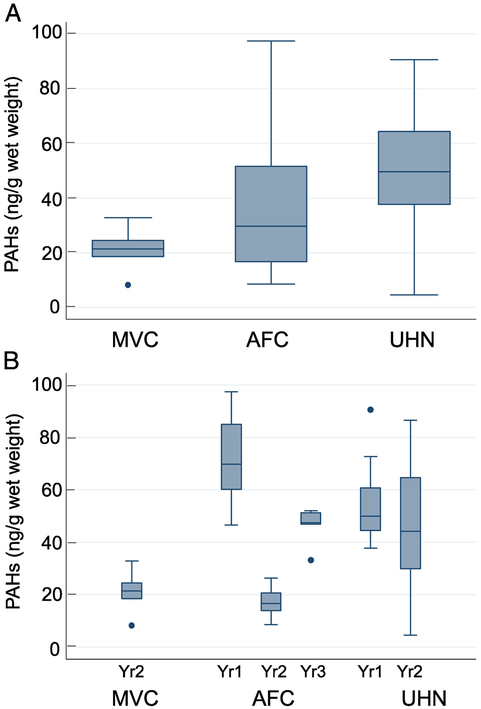
We also performed a two-way ANOVA rank F test analysis for oysters and crabs. The data are shown in Figures 4 and 5. The number of oyster samples received during the three-year period varied significantly from state-to-state with the lowest of 5 from MVC to the highest of 48 from UHN, while AFC had a total of 20. The mean values for PAH levels were 21.1 for MVC, 37.9 for AFC, and 48.2 ng/g for UHN. AFC and UHN had standard deviations > 20. Lack of adequate samples from each site prevented us from year-to-year comparisons. MVC samples were obtained only in the second year while AFC data spanning two years showed a significant decrease with time. The UHN data encompassing each collection time revealed similar median values albeit with considerable variation in the standard deviations. Nevertheless, there was a trend showing a decrease in PAHs levels over time.
Figure 4.
PAH levels in oysters at various sites (Figure 4A) and at different time intervals (Figure 4B). The method used for statistical analysis is described in the methods section. The error bars show the range (lowest to highest value) obtained. The lowest value is within 25th percentile −1.5XIQR while the highest value is still within the 75th percentile +1.5XIQR, and the horizontal line in the box represents the median value.
Figure 5.
PAH levels in crabs at various sites (Figure 5A) and at different time intervals (Figure 5B). The method used for statistical analysis is described in the methods section. The error bars show the range (lowest to highest value) obtained. The lowest value is still within 25th percentile −1.5XIQR while the highest value is within the 75th percentile +1.5XIQR, and the horizontal line in the box represents the median value.
The analysis of 13, 12, and 42 crab samples from MVC, AFC, and UHN showed median values of 42.1, 63.8, and 46.6 ng/g, respectively (Figure 5A). The total PAH distribution among the available samples during the three-year time period is shown in Figure 5B, and brings to light a gradual decrease in each geographical location where samples were collected. We did not observe any statistically significant changes among the various sites or time periods for either shrimp, oyster or crab samples. Kruskal- Wallis tests, a nonparametric analog to ANOVA, provide insignificant results for comparing the medians of locations and times. We are only focusing on the main factor ANOVA rank tests with no interaction effect.
3.4. Limitations:
1. Our collection of samples used in this study began ~18 months after the oil spill ended, by which time fishermen were once again allowed to catch seafood in the designated areas. This may account for the low levels of PAHs reported in this study.
2. PAH content over time in our samples suggests that seafood contamination with PAHs has returned to a baseline. The absence of samples prior to the spill precludes assessment of whether the current baseline is comparable to the pre-spill baseline. However, the observed PAHs levels are below the levels of concern for human consumption set by the FDA. The fact that there are detectable petrogenic PAHs in the seafood also suggests that perennial exposures due to natural seeps and other discharges need to be taken into consideration for assessing seafood contamination due to the DWH oil spill.
3. Sample collection was routinely performed with GPS coordinates. Although efforts were made to collect longitudinal samples from the same locations, it was impossible to do so on every occasion given that our samples were acquired as part of normal commercial fishing operations, and subject to circumstances associated with this practice. Furthermore, certain species are quite mobile, and thus we are unable to account for exposures that occurred in locations remote from the collection sites. We also acknowledge that the age of the organisms can influence the PAH body burden, hence age differences may influence data interpretation despite efforts to account for age by analyzing similarly sized specimens.
4. The PAHs analyses were all performed on raw seafood samples. However, individual consumption habits, including preferential methods of preparation by the different fisherfolk communities involved in our study that affect the fate of the PAHs, ensures that the human health risks associated with PAH exposures will in part be predicated on variables separate from the seafood contamination levels. Hence, our findings need to be communicated with those considerations in mind [Jackson et al., 2019].
5. The sensitivity of PAH detection decreased with the increasing ring number. Therefore, the limits of detection may have underestimated the presence of the larger— and more toxic—PAHs. Additionally, we recognized that the metabolites of naphthalene, fluorine, phenanthrene and pyrene have previously been shown to positively and negatively correlate with human diseases [Clark et al., 2012]. Hence, our focus on analyzing the parent compounds in the seafood samples may be a proxy for the presence of metabolites following consumption of contaminated seafood in lieu of direct measurements of the metabolites.
4. Conclusions
The strength associated with our project includes use of a community-based participatory research approach for this longitudinal study of the analysis of a variety of commercially important seafood over a period of three years. All samples were procured by working (or retired) fisherfolk and their families, recruited through a series of forums facilitated throughout the Gulf states [Sullivan et al., 2018]. These forums served an important purpose for researchers as an opportunity for bi-directional exchange of information and commentary on the project. The “Fishermen’s Citizen Science Network” came to include small-scale commercial and subsistence shrimpers, crabbers and oystermen, as well as recreational fin fishermen, all of whom contributed invaluable expertise to the project related to the sampling schema and interpretation of results.
We analyzed both motile (shrimp, crab) and stationary (oysters) benthic organisms known to accumulate PAHs to varying degrees. Given that results were obtained over time, the PAH levels detected in the latter samples are assumed to reflect movement toward the pre-spill baseline. If so, this indicates that the DWH spill’s effects were transient, and that the persistent detection of PAHs in seafood are likely a consequence of natural oil seeps and other modes of discharge. The data also demonstrate that the amount of PAHs present in seafood do not approach levels that can cause an adverse health effects based upon current FDA levels of concern guide lines, using the 90th percentile of national consumption data from the National Health and Nutrition Examination Survey (NHANES) for fish, shrimp, crab and oysters to calculate risk of PAH exposure in high-level consumers of seafood products. This observation is consistent with the findings reported earlier [Xia et al., 2012; Wilson et al., 2015; Dickey and Huettel, 2016; Wickliffe etal., 2018; Stuchal et al., 2019]. However, little is known about the potential adverse human health effects of petrogenic PAHs, including the alkylated analogs found in seafood samples, necessitating further comprehensive research on their toxicity and improved risk communication [Wickliffe et al. 2014; Jackson et al., 2019]. Moreover, the subsistence fisherfolk represent a population whose seafood consumption patterns often exceeds the 90th percentile and may thus be at risk for adverse health effects due to PAH exposures. Accordingly, these studies should include development of exposure and effect biomarkers. Until such time, the effects of the DWH oil spill on human health may not be fully realized, and ideally continued monitoring of the Gulf seafood and the subsistence communities should be carried out if we are to fully appreciate the impact of the DWH disaster on human health.
Highlights:
Levels of PAHs found in the Gulf coast seafood 21 months after DWH oil spill
Longitudinal analyses indicate low-level contamination of PAHs in the Gulf coast.
PAHs in seafood are well below the level of concern established by FDA
Acknowledgements
This work was supported by 1U19ES020679 and center grant P30ES06676 both from NIH/NIEHS. Thanks to Dr. Edward Overton (Department of Environmental Sciences, Louisiana State University, Baton Rouge, LA) and Dr. John Munson Department of Environmental and Global Health, University of Florida, Gainesville, FL) for helpful discussions.
Footnotes
Competing Interests Statement:
All authors declare no competing interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Aeppli C, Carmichael CA, Nelson RK, Lemkau KL, Graham WM, Redmond MC, Valentine DL, Reddy CM 2012. Oil weathering after the Deepwater Horizon disaster led to the formation of oxygenated residues. Environ. Sci. Technol. 46, 8799–8807. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) 1995. Toxicologial profile for polycyclic aromatic hydrocarbons (http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf). [PubMed]
- 3.Aguilera F, Mendez J, Pasaro E, Laffon BJ 2010. Review on the effects of exposure to spilled oils on human health. Appl. Toxicol. 4, 291–301. [DOI] [PubMed] [Google Scholar]
- 4.Allan SE, Smith BW, Anderson KA 2012. Impact of the deepwater horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ. Sci. Technol. 46, 2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best EA, Juarez-Colunga E, James K, LeBlanc WG, Serdar B 2016. Biomarkers of Exposure to Polycyclic Aromatic Hydrocarbons and Cognitive Function among Elderly in the United States (National Health and Nutrition Examination Survey: 2001–2002). PLoS One. 11, e0147632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer J, Jonsson G, Porte C, Krahn MM, Ariese F 2010. Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile. Environ. Toxicol. Pharmacol. 30, 224–244. [DOI] [PubMed] [Google Scholar]
- 7.Boffetta P, Jourenkova N, Gustavsson P 1997. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 8, 444–472. [DOI] [PubMed] [Google Scholar]
- 8.Burstyn I, Kromhout H, Partanen T, Svane O, Langard S, Ahrens W, Kauppinen T, Sicker I, Shaham J, Heederik D, Ferro G, Heikkila P, Hooiveld M,, Johansen C, Randem BG, Boffetta P. 2005. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology 16, 744–750. [DOI] [PubMed] [Google Scholar]
- 9.Camilli R, Reddy CM, Yoerger DR, Van Mooy BAS, Jakuba MV, Kinsey JC, McIntyre CP, Sylva SP, Maloney JV 2010. Tracking Hydrocarbon Plume Transport and Biodegradation at Deepwater Horizon. Science 330, 201–204. [DOI] [PubMed] [Google Scholar]
- 10.Clark III JD, Serdar B, Lee DJ, Arheart K, Wilkinson JD, Fleming LE 2012. Exposure to polycyclic aromatic hydrocarbons and serum inflammatory markers of cardiovascular disease. Environ. Res. 117, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croisant SA, Lin Y, Shearer JJ, Prochaska J, Phillips-Savoy A, Gee J, Jackson D, Panettieri R, Howarth M, Sullivan J, Black BJ, Tate J, Nguyen D, Anthony A, Khan A, Fernando H, Ansari GAS, Rowe G, Howrey B, Singleton C, and Elferink C 2017. The Gulf Coast Health Alliance: Health Risks Related to the Macondo Spill (GC-HARMS) Study: Self-Reported Health Effects. Inter. J. Environ. Res. Public Health 14 (11) 132 (1–18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta S, Cao A, Mauer B, Yan B, Uno S, McElroy A 2014. Genotoxicity of oxy-PAHs to Japanese medaka (Oryzias latipes) embryos assessed using the comet assay. Environ. Sci. Pollut. Res. 21, 13867–13876. [DOI] [PubMed] [Google Scholar]
- 13.Dickey R, Huettel M 2016. Seafood and beach safety in the aftermath of the Deepwater horizon oil spill. Oceanography 29, 196–203. [Google Scholar]
- 14.Diercks AR, Highsmith RC, Asper VL, Joung D, Zhou Z, Guo L, Shiller AM, Joye SB, Teske AP, Guinasso N, Wade TL, Lohrenz SE 2010. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophys. Res. Lett. 37, 1–6. [Google Scholar]
- 15.Forsberg ND, Wilson GR, Anderson KA 2011. Determination of parent and substituted polycyclic aromatic hydrocarbons in high-fat salmon using a modified QuEChERS extraction, dispersive SPE and GC-MS. J. Agric. Food Chem. 59, 8108–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohlke JM, Doke D, Tipre M, Leader M, Fitzgerald T 2011. A review of seafood safety after the deepwater horizon blowout. Environ Health Perspect. 119, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratz SR, Ciolino LA, Mohrhaus AS, Gamble BM, Gracie JM, Jackson DS, Roetting JP 2nd, McCauley HA, Heitkemper DT, Fricke FL, Krol WJ, Arsenault TL, White JC, Flottmeyer MM, Johnson YSJ 2011. Screening and determination of polycyclic aromatic hydrocarbons in seafoods using QuEChERS-based extraction and high-performance liquid chromatography with fluorescence detection. J. AOAC Int, 94, 1601–1616. [DOI] [PubMed] [Google Scholar]
- 18.Huang M, Mesaros C, Hackfeld LC, Hodge RP, Blair IA, Penning TM 2017. Potential metabolic activation of representative alkylated polycyclic aromatic hydrocarbons 1-methylphenanthrene and 9-ethylphenanthrene associated with the deepwater horizon oil spill in human hepatoma (HepG2) cells. Chem. Res. Toxicol. 30, 2140–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hylland K Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. 2006. J. Toxicol. Environ. Health A. 69, 109–123. [DOI] [PubMed] [Google Scholar]
- 20.Jackson D, Huang M, Fernando H, Ansari G, Howarth M, Mesaros C, Penning T, Elferink C 2019. Using precision environmental health principles in risk evaluation and communication of deepwater horizon oil spill. New Solutions 28, 599–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson YSJ 2012. Determination of polycyclic aromatic hydrocarbons in edible seafood by QuEChERS-based extraction and gas chromatography-tandem mass spectrometry. Food Sci. 77, T131–T137. [DOI] [PubMed] [Google Scholar]
- 22.Lewtas J 2007. Air pollution combustion emissions: Characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects. Mut. Res. Reviews in Mut. Res. 636, 95–133. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Liu J, Zhu Q, Wu W 2012. The weathering of oil after the Deepwater Horizon oil spill: insights from the chemical composition of the oil from the sea surface, salt marshes and sediments. Environ. Res. Lett. 7, 035302. [Google Scholar]
- 24.Mastovska K, Sorenson WR, Hajslova JJ 2015. Determination of polycyclic aromatic hydrocarbons (PAHs) in seafood using gas chromatography-mass spectrometry: collaborative study. J. AOAC Int. 98, 477–505. [DOI] [PubMed] [Google Scholar]
- 25.Meador JP, Stein JE, Reichert WL, Varanasi U 1995. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev. Environ. Contam. Toxicol. 143, 79–165. [DOI] [PubMed] [Google Scholar]
- 26.Mulabagal V, Yin F, John GF, Hayworth JS, Clement TP 2013. Chemical fingerprinting of petroleum biomarkers in Deepwater Horizon oil spill samples collected from Alabama shoreline. Mar. Pollut. Bull. 70, 147–154. [DOI] [PubMed] [Google Scholar]
- 27.Murawski SA, Hogarth WT, Peebles EB, Barbeiri L 2014. Prevalence of External Skin Lesions and Polycyclic Aromatic Hydrocarbon Concentrations in Gulf of Mexico Fishes, Post-Deepwater Horizon. Trans. Am. Fish. Soc. 143, 1084–1097. [Google Scholar]
- 28.Norli HR, Christiansen A, Deribe E 2011. Application of QuEChERS method for extraction of selected persistent organic pollutants in fish tissue and analysis by gas chromatography mass spectrometry. J. Chromatogr A. 1218, 7234–7241. [DOI] [PubMed] [Google Scholar]
- 29.Ocean Studies Board and Marine Board (2003) oil in the sea III Inputs, fates, and effects. National Academic Press, Washington, D. C. [PubMed] [Google Scholar]
- 30.Olson MC, Iverson JL, Furlong ET, Schroeder MP 2004. Methods of analysis by the U. S. Geological Survey National Water Quality Laboratory-Determination of polycyclic aromatic hydrocarbon compounds in sediment by gas chromatography/mass spectrometry. Water-resources investigations report 03–4318. [Google Scholar]
- 31.Overton EB, Wade TL, Radovic JR, Meyer BM, Miles MS, Larter SR 2016. Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 29, 50–63. [Google Scholar]
- 32.Pampanin DM in Petrogenic Polycyclic Aromatic Hydrocarbons in the aquatic environment. Chapter 1.2017. Edited by Pampanin DM, and Sydnes MO pp3–13. [Google Scholar]
- 33.Paul JH, Hollander D, Coble P, Daly KL, Murasko S, English D, Basso J, Delaney J, McDaniel L, Kovach CW 2013. Toxicity and mutagenicity of Gulf of Mexico waters during and after the deepwater horizon oil spill. Environ. Sci. Technol. 47, 9651–9659. [DOI] [PubMed] [Google Scholar]
- 34.Poster DL, Schantz MM, Sander LC, Wise SA 2006. Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: a critical review of gas chromatographic (GC) methods. Anal. Bioanal. Chem. 386, 859–881. [DOI] [PubMed] [Google Scholar]
- 35.Ramalhosa MJ, Paiga P, Morais S, Delerue-Matos C, Oliveira MB 2009. Analysis of polycyclic aromatic hydrocarbons in fish: evaluation of a quick, easy, cheap, effective, rugged, and safe extraction method. J. Sep. Sci. 32, 3529–3538. [DOI] [PubMed] [Google Scholar]
- 36.Reddy CM, Arey JS, Seewald JS, Sylva SP, Lemkau KL, Nelson RK, Carmichael CA, McIntyre CP, Fenwick J, Ventura GT, Van Mooy BA, Camilli R 2012. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. 109, 20229–20234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotkin-Ellman M, Wong KK, Solomon GM 2012. Seafood Contamination after the BP Gulf Oil Spill and Risks to Vulnerable Populations: A Critique of the FDA Risk Assessment. Environ. Health. Perspec. 120, 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryerson TB, Camilli R, Kessler JD, Kujawinski EB, Reddy CM, Valentine DL, Atlas E, Blake DR, de Gouw J, Meinardi S, Parrish DD, Peischl J, Seewald JS, Warneke C 2012. Chemical data quantify Deepwater Horizon hydrocarbon flow rate and environmental distribution. Proc. Natl. Acad. Sci. 109, 20246–20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sammarco PW, Kolian SR, Warby RA, Bouldin JL, Subra WA, Porter SA 2013. Distribution and concentrations of petroleum hydrocarbons associated with the BP/Deepwater Horizon Oil Spill, Gulf of Mexico. Mar. Pollut. Bull. 73, 129–143. [DOI] [PubMed] [Google Scholar]
- 40.Seafood Sampling Map - GC-HARMS: http://gcharms.leanweb.org/seafood-sampling-map/
- 41.Schober W, Lubitz S, Belloni B, Gebauer G, Lintelmann J, Matuschek G, Weichenmeier I, Eberlein-Konig B, Buters J, Behrendt H 2007. Environmental polycyclic aromatic hydrocarbons (PAHs) enhance allergic inflammation by acting on human basophils. Inhal. Toxicol. 19, Suppl 1, 151–156. [DOI] [PubMed] [Google Scholar]
- 42.Seibel BA, Drazen JC 2007. The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc Lond B Biol Sci. 362, 2061–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith D, Lynam K 2012. Polycyclic aromatic hydrocarbons (PAH) Analysis in Fish by GC/MS using agilent bond elut QuEChERS dSPE sample preparation and a high efficiency DB-5ms Ultra Inert GC column. Agilent Technical note5990–6668EN. [Google Scholar]
- 44.Stuchal LD, Charles-Ayinde MKS, Kane AS, Kozuch M, Roberts SM 2019. Probabilistic risk assessment for high-end consumers of seafood on the northeastern Gulf coast. J. Exp. Sci. Eviron. Epidem. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan J, Croisant S, Howarth M, Rowe GT, Fernando H, Phillips-Savoy A, Jackson D, Prochaska J, Ansari GAS, Penning TM, Elferink C, and Community Partners. 2018. Building and Maintaining a Citizen Science Network with Fishermen and Fishing Communities Post Deepwater Horizon Oil Disaster Using a CBPR Approach. New Solutions 28,416–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valentine DL, Fisher GB, Bagby SC, Nelson RK, Reddy CM, Sylva SP, Woo MA 2014. Fallout plume of submerged oil from Deepwater Horizon. Proc. Natl. Acad. Sci. 111, 15906–15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wickliffe JK, Simon-Friedt B, Howard JL, Frahm E, Meyer BM, Wilson MJ, Pangeni D, Overton EB 2018. Consumption of fish and shrimp from southeast Louisiana poses no unacceptable lifetime cancer risks attributable to high-priority polycyclic aromatic hydrocarbons. Risk Analysis 38, 1944–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wickliffe JK, Overton EB, Frickel S, Howard J, Wilson M, Simon B, Echsner S, Nguyen D, Gauthe D, Blake D, Miller C, Elferink C, Ansari S, Fernando H, Trapido E, Kane A 2014. Evaluation of polycyclic aromatic hydrocarbons using analytical methods, toxicology, and risk assessment research: Seafood safety after a petroleum spill as an example. Environ. Health. Perspec. 122, 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson MJ, Frickel S, Nguyen D, Bui T, Echsner S, Simon BR, Howard JL Miller K, Wickliffe JK 2015. A Targeted health risk assessment following the deepwater horizon oil spill: Polycyclic aromatic hydrocarbon exposure in Vietnamese-American Shrimp consumers. Environ. Health. Perspec. 123, 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia K, Hagood G, Childers C, Atkins J, Rogers B, Ware L, Armbrust K, Jewell J, Diaz D, Gatian N, Folmer H 2012. Polycyclic aromatic hydrocarbons (PAHs) in Mississippi seafood from areas affected by the deepwater horizon oil spill. 46, 5310–5318. [DOI] [PubMed] [Google Scholar]
- 51.Yin F, Hayworth JS, Clement TP 2015. A tale of two recent spills-comparison of 2014 Galveston Bay and 2010 Deepwater Horizon oil spill residues. PLoS One. 10, e0118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ylitalo GM, Krahn MM, Dickhoff WW, Stein JE, Walker CC, Lassitter CL, Garrett ES, Desfosse LL, Mitchell KM, Noble BT, Wilson S, Beck NB, Benner RA, Koufopoulos PN, Dickey RW 2012. Federal seafood safety response to the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. 109, 20274–20279. [DOI] [PMC free article] [PubMed] [Google Scholar]