Abstract
Background:
Matrix metalloproteinase-9 (MMP-9) is a novel marker of intestinal inflammation. The aim of this study was to assess if serum MMP-9 levels predict clinical flare in patients with quiescent Crohn’s disease (CD).
Methods:
This study was a post hoc analysis of a prospective observational study in which quiescent CD patients were included and followed until clinical relapse or the end of a 2-year follow-up period. Serial C-reactive protein (CRP) and fecal calprotectin (FC) levels were measured, and the patients underwent repeated capsule endoscopies (CEs) every 6 months. Small bowel inflammation was quantified by Lewis score (LS) for CE. A baseline magnetic resonance enterography was also performed, and MaRIA score was calculated. Serum MMP-9 levels in baseline blood samples were quantified by ELISA.
Results:
Out of 58 eligible enrolled patients, 16 had a flare. Higher levels of baseline MMP-9 were found in patients who developed subsequent symptomatic flare compared with patients who did not [median 661 ng/ml, 25–75 interquartile range (IQR; 478.2–1441.3) versus 525.5 ng/ ml (339–662.7), respectively, p = 0.01]. Patients with serum MMP-9 levels of 945 ng/ ml or higher were at increased risk for relapse within 24 months [area under the curve (AUC) of 0.72 [95% confidence interval (CI): 0.56–0.88]; hazard ratio 8.1 (95% CI 3.0–21.9, p < 0.001)]. Serum MMP-9 concentrations showed weak and moderate correlation to baseline LS and FC, respectively (r = 0.31, p = 0.02; r = 0.46, p < 0.001). No correlation was found between serum MMP-9 to CRP and MaRIA score.
Conclusions:
Serum MMP-9 may be a promising biomarker for prediction of clinical flare in CD patients with quiescent disease.
Keywords: Crohn’s disease, biomarker, extracellular matrix, matrix metalloproteinase-9
Introduction
Crohn’s disease (CD) is a chronic and progressive inflammatory disease, frequently associated with accumulation of structural bowel damage.1 Assessing intestinal inflammation remains a difficult challenge. The current reliable method is invasive, costly, and uncomfortable for the patient as it requires frequent endoscopies with biopsy sampling. Moreover, in CD patients, the site of the lesion is not always accessible to endoscopy.2
Clinical remission is poorly correlated with mucosal healing, and subclinical inflammation may persist with a major contribution to the risk of relapse.3,4 The prediction of clinical deterioration in CD patients may lead to optimization of therapeutic strategies and decrease subsequent complications. Therefore, a dire need exists for novel, accurate, and noninvasive markers for this purpose.5,6
Matrix metalloproteinases (MMPs) are a family of 24 zinc-dependent extracellular matrix-degrading endopeptidases. MMPs are key players in extracellular matrix (ECM) turnover by degrading collagens type I, II, III, or IV, in their native form. MMPs also degrade nonmatrix substrates, including cytokines, growth factors, chemokines, and junctional proteins.7 MMPs are involved in multiple pathological processes such as tumor spread and metastasis, cardiovascular disease, rheumatoid arthritis, and initiation and maintenance of chronic inflammatory processes.8–11
Within the MMPs family, MMP-9 (also known as gelatinase B) has been suggested as a possible marker of intestinal inflammation.10,12 MMP-9 is an inducible protease that was shown to be upregulated in several inflammatory conditions. It has been found to be the most abundant MMP in inflamed intestinal tissue of patients with inflammatory bowel disease (IBD). MMP-9 is secreted mainly by neutrophils and other cell types such as mesenchymal cells, fibroblasts, and several inflammatory cells like monocytes or lymphocytes.13–15
The increase in MMP-9 proteolytic activity correlates with histological and endoscopic scores, and with the extent of tissue damage.16–20 Moreover, MMP-9 was suggested as potential marker of mucosal inflammation in several studies.12,21–28 However, in all these studies, MMP-9 was evaluated cross-sectionally with concurrent inflammatory activity, but, to our knowledge, there are hitherto no reports of its diagnostic utility to predict the future course of IBD.
Therefore, present study evaluated serum MMP-9 as a predictor of future exacerbations in CD patients with quiescent disease.
Methods
Patient population
The study was a post hoc analysis of a prospective observational study aimed at identifying predictors of clinical relapse in CD patients with quiescent disease. The patients were followed until clinical flare or the end of the 2-year study.29 The study population included adult CD patients (>18 years) with known small bowel (SB) disease in remission, or mild disease symptoms, as evaluated by a CD activity index (CDAI) of <220. All patients were in corticosteroid-free remission for 3–24 months, and were treated with a stable medication dose [30 days for adalimumab and 5-aminosalicylic acid (5-ASA) agents, 60 days for methotrexate, thiopurines, and infliximab]. CD treatment was unchanged during follow up. The patients were followed prospectively by clinical evaluation and biomarker [C-reactive protein (CRP)/fecal calprotectin (FC) levels] once every 3 months, video capsule endoscopy (VCE) at baseline and every 6 months thereafter, and by magnetic resonance enterography (MRE) examinations at baseline and upon study conclusion. Clinical relapse was defined as an increase of >70 points on CDAI from baseline, and a CDAI > 150, or the need for rescue medication for CD necessitated by disease worsening as determined by physician global assessment (PGA). Patients were excluded from the study if they were unable to provide informed consent; suffered from severe unstable comorbidities such as kidney, liver, metabolic, neurologic, or cardiorespiratory disorders at enrollment; current or history of aspirations or dysphagia; implanted metal objects or cardiac pacemaker, claustrophobia, preventing performance of magnetic resonance imaging; or known or suspected severe stricture or intestinal obstruction. All patients signed an informed consent, and the study was approved by the institutional ethics review board (SMC 13-0218).
Inflammatory biomarkers and disease activity measures
Serum MMP-9 concentrations at baseline were determined using human MMP-9 enzyme-linked immunosorbent assay kit (ELISA; R&D systems, Minneapolis, MN, USA) in accordance with manufacturer’s instructions. The test procedure was standardized using standards provided with the kit. All standards and samples were analyzed in duplicate. The results were measured in units of ng/ml.
Complete blood count (CBC), CRP, and FC were measured every 3 months. FC levels were evaluated using the Quantum Blue calprotectin kit (Bühlmann Laboratories AG, Basel, Switzerland). The reported value range is 30 (detection level) to 300 μg/g (no further quantification was possible above 300 μg/g). Levels >100 μg/g were considered positive. CRP levels were regarded as elevated if >5 mg/l. Patients underwent physician’s assessment and CDAI estimation for disease activity every 3 months.
Imaging and capsule endoscopy studies
Upon enrollment, all patients underwent an MRE. MR image acquisition was performed using a protocol as previously described.4 All patients with active SB disease detected on MRE went through a patency capsule (PC) test. If the PC was not expelled from the SB within 30 h, the patient was withdrawn from the study. In patients with isolated SBCD, a PillCam SB3 capsule (Given Imaging, Yoqneam, Israel) was used. In patients with known ileo-colonic CD, a colonic capsule procedure (PillCam colon2 capsule, Given Imaging, Yoqneam, Israel) was conducted. The SB data retrieved from a colonic capsule was reviewed and analyzed in a process similar to that for the SB capsule. All images were examined using the RAPID 8 software (Given Imaging, Yoqneam, Israel). To ensure visualization of the entire SB, the adaptive frame rate mode was activated. Mucosal inflammation was quantified using the Lewis score (LS). The definition of mucosal healing was LS < 135, mild-to-moderate inflammation as LS of 135–790, and moderate-to-severe inflammation as LS > 790.30 LS was calculated manually when using colonic capsule. The capsule endoscopy videos were read by a board-certified gastroenterologist with over 10 years of experience in the procedure.
Statistics
Categorical variables were described as frequency and percentage. Continuous variables were described as median and interquartile range (IQR). Associations between MMP-9 levels and continuous variables were assessed using Spearman’s correlation coefficient. Associations between MMP-9 levels and categorical variables were assessed using Mann–Whitney U test. In additional analysis, FC was categorized by cut-off level of 250 μg/g, as p proposed in previous studies.31,32 Receiver operating characteristic (ROC) analysis and the Youden index were used to find an optimal cut-off value. Categorical variables were compared between those above the cutoff value and those below using Chi-square test or Fisher’s exact test, and continuous variables were compared using Mann–Whitney U test. Survival without disease relapse was analyzed by Kaplan–Meier curve and log rank test. Logistic regression was used to calculate the propensity score. Age, gender, CRP, FC, medication, and smoking status at presentation were included in the propensity score. Univariate Cox regressions were used to evaluate the association between MMP-9, patient characteristics, medication, and inflammatory markers at presentation with disease relapse. Multivariate Cox regression was used to describe the association between MMP-9 and disease relapse using propensity score. All statistical tests were two sided. p < 0.05 was considered as statistically significant. All statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, version 25, IBM Corp, Armonk, NY, USA). Area under the curve (AUC) was evaluated using survival-ROC package version 1.0.3 in R: a language and environment for statistical computing (The R foundation for statistical computing version 3.3.3, 2017).
Results
A total of 90 patients were screened for eligibility and underwent PC study to verify small bowel patency, of whom 29 failed screening (due mostly to retained PC, n = 17); 61 patients were thus enrolled and underwent capsule endoscopy. After exclusion of 3 patients due to technical causes, there were 58 patients with analyzable samples. The clinical and demographic characteristics of the patients included in the study are described in Table 1.
Table 1.
Patient demographics and baseline characteristics.
| n = 58 | ||
|---|---|---|
| Median age at enrollment, years (IQR) | 29.5 (24–37.5) | |
| Gender | Female | 25 (43%) |
| Male | 33 (57%) | |
| Smoking | 11(19%) | |
| Median disease duration, years (IQR) | 4 (2–9.5) | |
| Median CDAI (IQR) | 42 (24–110) | |
| Disease location | L1 | 36 (62%) |
| L2 | 1 (2%) | |
| L3 | 21 (36%) | |
| Disease behavior | B1 | 39 (67%) |
| B2 | 11 (19%) | |
| B3 | 8 (14%) | |
| Perianal disease | 10 (17%) | |
| Mild symptoms at enrollment (CDAI 150–220) | 6 (10%) | |
| Previous intestinal resection | 10 (17%) | |
| Medication at enrollment | None | 9 (16%) |
| Oral 5-ASA | 9 (16%) | |
| Immunomodulators | 27 (46%) | |
| Anti-TNF | 22 (38%) | |
| Median CRP (mg/l), (IQR) | 2.2 (0.9–5.9) | |
| Median FC (µg/g), (IQR) | 93 (35–204) |
CDAI, Crohn’s disease activity index; CRP, C-reactive protein; FC, Fecal calprotectin; IQR, Interquartile range 25–75; TNF, tumor necrosis factor.
Associations between baseline characteristics, treatment at presentation, and serum MMP-9 are presented in Table 2. Serum MMP-9 concentrations displayed moderate (r = 0.46, p < 0.001) and weak (r = 0.31, p = 0.02) correlation with baseline FC and with capsule endoscopy LS, respectively. Serum MMP-9 was not significantly correlated with age, CRP, MaRIA score of MRE, and CDAI. Smoking status, gender, oral treatment with 5-ASA, and treatment with anti-TNF agents were not associated with MMP-9 levels. Patients receiving immunomodulators medication had significantly higher serum MMP-9 levels at presentation. In categorical analyses, patients who presented with FC ⩾250 µg/g had significantly higher serum MMP-9 levels compared with patients with lower FC (p = 0.012, Figure 1a). Patients with LS ⩾ 790 (i.e. moderate-to-severe mucosal inflammation by conventional LS) had higher median level of serum MMP-9 compared with patients with LS of 135–789, who, in turn, had higher median levels compared with patients with LS < 135 (i.e. no mucosal inflammation). However, this trend did not reach statistical significance (p = 0.09, Figure 1b).
Table 2.
Association between serum MMP-9 levels, patients characteristics and inflammatory indices.
| MMP-9 (ng/ml) | p | |
|---|---|---|
| Age | –0.1 | 0.453 |
| CRP | 0.198 | 0.136 |
| FC (µg/g) | 0.012* | |
| <250 | 524 (352–679) | |
| ⩾250 | 660 (569–1245) | |
| LS | 0.09 | |
| <135 | 323 (211–878) | |
| 135–789 | 528 (373–725) | |
| ⩾790 | 623 (565–818) | |
| MaRIA score | 0.05 | 0.722 |
| CDAI | 0.135 | 0.314 |
| Smoker | 0.519 | |
| No | 565 (344–736) | |
| Yes | 563 (408–878) | |
| Sex | 0.556 | |
| Male | 563 (416–732) | |
| Female | 615 (278–756) | |
| Oral 5-ASA | 0.147 | |
| No | 575 (394–761) | |
| Yes | 429 (238–699) | |
| Immunomodulators | 0.035* | |
| No | 524 (344–636) | |
| Yes | 645 (381–876) | |
| Anti-TNF | 0.95 | |
| No | 549 (379–779) | |
| Yes | 563 (339–705) |
Data are presented as Spearman’s rank correlation coefficient (rs) or median and IQR.
Statistically significant p-values.
5-ASA, 5-aminosalicylic acid; CDAI, Crohn’s disease activity index; CRP, C-reactive protein; FC, fecal calprotectin; IQR, interquartile range 25–75; LS, Lewis score; MMP-9, matrix metalloproteinase-9; TNF, tumor necrosis factor.
Figure 1.
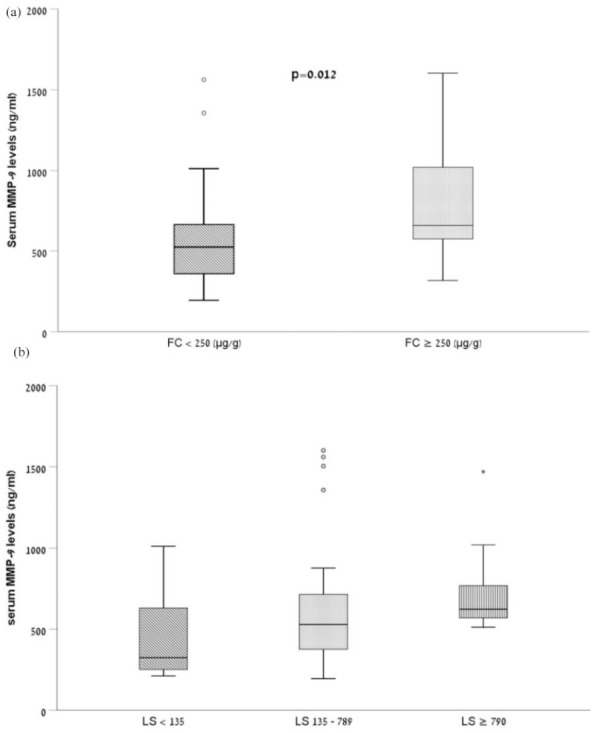
Box-plot representation of serum MMP-9 concentrations in patients with quiescent CD with respect to (a) FC levels and (b) LS. Baseline serum MMP-9 concentration in patients with FC ⩾250 µg/g differed significantly from patients with FC < 250 µg/g (p = 0.012). Baseline serum MMP-9 concentration in patients with LS > 790 tend to be higher than patients with LS 135–789 and patients with LS < 135 but did not reach statistical significance (p = 0.09). The limits of the box represent the first and third quartiles; the black crossbar line represents the median.
CD, Crohn’s disease; CFC, fecal calprotectin; LS, Lewis score; MMP-9, matrix metalloproteinase-9.
Baseline serum MMP-9 levels for prediction of 2-year risk of flare
Overall, 16/58 (28%) of patients relapsed over the 24-month study period, with a median time to relapse of 4.5 months (IQR 3–18). No difference was found between baseline clinical and demographic variables between relapsers and nonrelapsers.
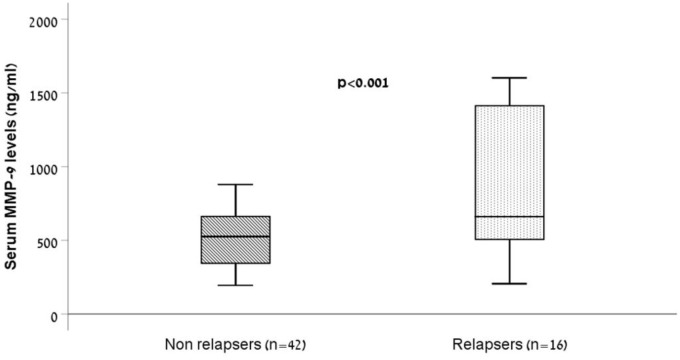
Median baseline levels of serum MMP-9 in the relapse group were significantly higher than MMP-9 levels in the nonrelapse group (661 ng/ml, IQR 478.2–1441.3 versus 525.5 ng/ml, IQR 339–662.7, p < 0.001, Figure 2). Serum MMP-9 had a fair ability to discriminate between patients who subsequently experienced a relapse and those who did not [AUC 0.72, 95% confidence interval (CI) 0.56–0.88, p = 0.012, Figure 3]. A cutoff level of 945 ng/ml of serum MMP-9 showed sensitivity of 44% and specificity of 100% for detecting relapse within 24 months. The positive predictive value (PPV) and negative predictive value (NPV) were 100% and 78%, respectively.
Figure 2.
Box-plot representation of baseline serum MMP-9 measurements in patients with quiescent CD who subsequently flared during follow-up period of 24 months versus those who did not. Baseline serum MMP-9 levels in relapsers differed significantly from nonrelapsers (p < 0.001). The limits of the box represent the first and third quartiles; the black crossbar line represents the median.
CD, Crohn’s disease; MMP-9, matrix metalloproteinase-9.
Figure 3.
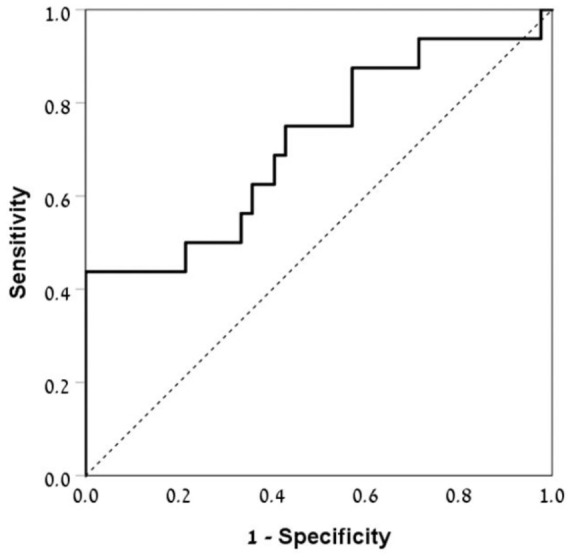
ROC curve illustrating the performance of baseline serum MMP-9 levels for the prediction of relapse in patients with quiescent CD over 24 months follow up. The AUC was 0.72 (95% CI 0.56–0.88, p = 0.012).
AUC, area under the curve; CD, Crohn’s disease; CI, confidence interval; MMP-9, matrix metalloproteinase-9; ROC, receiver operating characteristic.
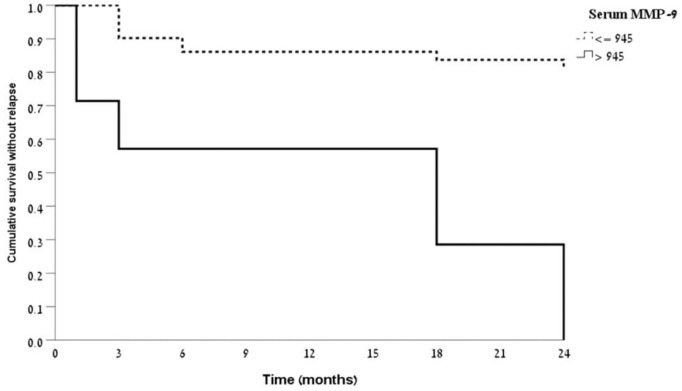
Kaplan–Meyer analysis of survival without relapse during the follow-up period showed that patients with baseline serum MMP-9 > 945 ng/ml had much shorter duration to relapse compared with patients with baseline MMP-9 ⩽ 945 (p < 0.001, Figure 4). The risk of relapse was significantly increased in patients with MMP-9 > 945 ng/ml compared with those below this level (hazard ratio 8.1, 95% CI 3.0–21.9). A formal multivariate analysis was statistically precluded by the relatively limited number of patients who flared (n = 16). Therefore, controlling for possible confounders (age, gender, CRP, FC, medications, and smoking status at presentation) was employed using propensity score. This analysis still yielded significant HR of 6.22 for flare in patients with MMP-9 > 945 ng/ml (95% CI 2.1–17.9, p = 0.001). We did not include the MaRIA MRE score in this propensity scoring as it was not correlated with MMP-9 levels or with future risk of flare, and also because it is not readily available for clinicians in routine practice. Further sensitivity-analysis of the 13/16 relapsed patients defined exclusively by an increase in CDAI after exclusion of patients relapsing by PGA criteria, demonstrated a higher discrimination ability with AUC of 0.77 (95% CI 0.58–0.93, p = 0.006). The same threshold value (945 ng/ml) resulted in 24 months sensitivity of 54% and specificity of 100% for detecting relapse, and PPV and NPV of 100% and 84.2%, respectively.
Figure 4.
Kaplan–Meier analysis of survival without a flare for patients with quiescent CD and baseline serum MMP-9 levels below or above 945 ng/ml. Log rank test for equality of survivor functions, p value <0.001.
CD, Crohn’s disease; MMP-9, matrix metalloproteinase-9.
Discussion
This post hoc analysis of a prospective study demonstrates, for the first time, that serum MMP-9 can identify adult patients with quiescent CD who are at risk of future disease exacerbation. Baseline MMP-9 levels were significantly elevated in patients who relapsed within 24 months compared with nonrelapsers, and a cut-off value of MMP-9 above 945 ng/ml identified patients with high risk of flare during a follow-up period of 24 months. Furthermore, an association was found between serum MMP-9, FC, and LS, which are well validated and widely used tools for assessing bowel inflammation in patients with CD.
Discovery of novel biomarkers that also have a defined pathophysiological role in the disease would improve clinical evaluation and therapeutic strategy. Aberrant tissue remodeling with excessive degradation or accumulation of ECM components is a key event in IBD.10 ECM remodeling processes are executed by MMPs, which are considered predominant proteases involved in IBD.11,33 MMPs possess a conserved catalytic domain with a Zn2+ at the active site and a prodomain that confers latency and requires cleavage during secretion to obtain activity.34–36 Although MMP-9 is not a canonical inflammatory molecule, accumulating evidence suggests that MMP-9 is not only an outcome of inflammatory pathways, but also a propagator of inflammation.10,14 Serum MMP-9 may be derived from increased expression and release from the inflamed intestinal tissues, or from activated neutrophils as they migrate from blood vessels into inflamed tissues.37 The neutrophil is the most important source of MMP-9 during the active inflammatory phase. Upon cleavage by MMP-9, the potent neutrophil activating chemokine IL-8 increases its potency tenfold. This promotes further neutrophil infiltration and activation, leading to enhanced MMP-9 secretion, thus creating a vicious cycle.38 MMP-9 is also involved in generating the collagen-derived fragment proline-glycine-proline (PGP) in the gut, which is a chemo-attractant for neutrophils. PGP, as well as MMP-9 levels are elevated in the intestine of CD patients, and neutrophils from CD patients are capable of producing higher amounts of PGP via MMP-9 secretion than healthy controls.16,17 Thus, our observation of significant correlation between serum MMP-9 and FC is not surprising considering that MMP-9 is normally stored, poised for rapid release, in neutrophil granules, which are also the predominant source of calprotectin.37
Our results are somewhat in line with recent results demonstrating a correlation between fecal MMP-9 and FC, and higher precision for fecal MMP-9 compared with FC in detecting endoscopic ulcerations in CD patients.24 Moreover, fecal MMP-9 was a better biomarker than FC in detecting endoscopic activity in a cohort of patients with ulcerative colitis (UC).23 These findings were in accordance with data from Annahazi and colleagues, who found fecal MMP-9 as an accurate predictor for mucosal healing.22 To our knowledge, except for the EMBARK study, which demonstrated the combination of FC, serum MMP-9, and serum interleukin-22 in CD patients to strongly correlate with endoscopy and imaging-defined inflammation by CTE,26 this is the first report of significant association between serum MMP-9 and FC.
An important finding in our research is the association of baseline serum MMP-9 values with mucosal inflammation also when evaluated by VCE. To date, this study is the first to demonstrate a significant, albeit weak, correlation between serum MMP-9 and LS, which reflects the inflammatory burden in CD patients with SB involvement. This is of particular significance considering that VCE provides accurate assessment of SB inflammation, and that the persistence of subclinical mucosal inflammation may be associated with adverse outcomes in CD. As demonstrated earlier, such residual inflammation may occur in the absence of clinical activity.4 These preliminary results are in accordance with a study showing that a reduction in the cellular expression of MMP-9 was associated with endoscopic and histologic mucosal healing.39
In this study, serum MMP-9 levels at presentation were significantly higher in patients receiving immunomodulators compared with those who did not. Previous report demonstrated that immunosuppressive drugs downregulate MMP-9 expression.39 However, we do not have sera of these patients before starting immunomodulators treatment, and we cannot estimate the effect of the treatment on serum MMP-9 levels in these patients. One may postulate that this finding could be secondary to immunomodulator-treated patients having a more severe underlying disease, reflected in a trend for higher MMP-9 levels, even when symptomatically in remission. However, direct comparative studies are needed to corroborate or refute this association.
MMP-9 has been broadly explored in relation to IBD tissue damage, and, as mentioned above, was suggested as a factor in number of pathological pathways. However, although previous studies claimed that inhibition or genetic deletion of MMP-9 improved experimental colitis, the causality of MMP-9 could not be confirmed in a more recent study in murine models.40 Moreover, the therapeutic potential of anti-MMP-9 antibodies failed to show efficacy in UC and CD.41,42
The close correlation between MMP-9 with endoscopic and histological scores hints at the potential to predict clinical deterioration.19,20,27,28 Several studies associated MMP-9 activity with the disruption of epithelial barrier integrity observed in patients with CD.43,44 Impaired intestinal barrier function has been proposed to precede the onset of inflammation and exacerbation in patients with CD.45 As such, it could be assumed that the increased baseline serum MMP-9 levels in patients with a future flare within the follow-up period may reflect molecular events within the ECM that precede tissue damage associated with CD and lead to clinical exacerbation. However, the molecular events were not explored in the present study, and will need to be further defined by future studies. The predictive ability of serum MMP-9 was comparable to the results of FC to predict relapse in several previously published prospective studies, although some of these studies showed conflicting results and most of them assessed only short-term relapse.46,47 This points to the need for further efforts in the search for noninvasive markers for disease activity. In this context, the advantages of a blood marker over a fecal marker, with respect to patient and caregiver convenience, have to be acknowledged. Collecting stools may be an obstacle for the patient, and a blood sample may be preferable for routine practice.48
Limitations of this study include the relatively modest cohort size, which is nonetheless among the largest CD cohorts ever to undergo comprehensive prospective follow up including serial MREs and VCEs. Only patients who had stable disease for 3–24 months prior to inclusion entered the study. Thus, whether the results can be extended to CD patients with longer remission period remains to be proven. Another limitation of the current post hoc analysis is the lack of zymography analysis of the serum samples. Zymography may reveal, individually, the activated forms and degradation products of MMP-9, whereas with ELISA, these molecules are measured in totality.
Finally, serum MMP-9 was measured only at baseline and not at different time points, and analysis of its possible correlation with intestinal fibrosis, was not assessed, given that intestinal strictures were exclusion criteria for this cohort. Thus, further studies are needed to assess if the change in MMP-9 levels over time could better predict future relapse, and to investigate the correlation of MMP-9 with intestinal wall fibrosis, given its role in ECM remodeling.
In conclusion, elevation of serum MMP-9 precedes a clinical relapse, and may be a useful marker for predicting flare in patients with quiescent CD. These current findings extend previous observations and highlight that serum MMP-9 constitutes an important inflammatory marker for CD. Further studies are warranted to confirm these findings and to determine the role of serum MMP-9 as a prognostic and surrogate biomarker in CD.
Supplemental Material
Supplemental material, TAG for Serum MMP-9: a novel biomarker for prediction of clinical relapse in patients with quiescent Crohn’s disease, a post hoc analysis by Doron Yablecovitch, Uri Kopylov, Adi Lahat, Michal M. Amitai, Eyal Klang, Dana Ben-Ami Shor, Sandra Neuman, Nina Levhar, Ella Fudim, Benjamin Avidan, Ido Laish, Limor Selinger, Noam Zingboim-Orbach, Orit Picard, Miri Yavzori, Rami Eliakim and Shomron Ben-Horin in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Guarantor of the article: Shomron Ben Horin
DY, study design, data collection and analysis, manuscript drafting; UK, AL, DBS, SN, NL, EF, BA, IL, LS, NZO, OP, MY, - data collection and analysis, reviewed the manuscript for important scientific content; EY, MMA, reading of imaging data, reviewed the manuscript for important scientific content; SBH, study conception, initiation and design, reviewed the manuscript for important scientific content; RE, study conception, initiation and design, reading of the capsule studies, reviewed the manuscript for important scientific content. All authors reviewed and approved the final version of the manuscript
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The study was supported by a generous grant from the Leona M. and Harry B. Helmsley Charitable Trust.
Conflict of interest statement: Doron Yablecovitch, none to declare; Uri Kopylov, Speaker fees/advisory Janssen Medtronic Abvvie Takeda MSD; Research support, Janssen Takeda Medtronic; Adi Lahat, none to declare; Michal M. Amitai, none to declare; Eyal Klang, none to declare; Dana Ben-Ami Shor, none to declare; Sandra Neuman, none to declare; Nina Levhar, none to declare; Ella Fudim, none to declare; Benjamin Avidan, none to declare; Limor Selinger, none to declare; Noam Zingboim-Orbach, none to declare; Orit Picard, none to declare; Miri Yavzori, none to declare; Rami Eliakim, Consultation/lecture fee Medtronic/Given Imaging, consultant fee from Abbvie and Takeda; Shomron Ben Horin, Research support or consultancy/Advisory board fees from MSD, AbbVie, Janssen, CellTrion, Pfizer, GSK, and Takeda
ORCID iDs: Doron Yablecovitch  https://orcid.org/0000-0001-6191-9270
https://orcid.org/0000-0001-6191-9270
Uri Kopylov  https://orcid.org/0000-0002-7156-0588
https://orcid.org/0000-0002-7156-0588
Contributor Information
Doron Yablecovitch, Department of Gastroenterology, Sheba Medical Center, Tel Hashomer, Israel, Sackler School of Medicine, Tel- Aviv University, Tel-Aviv, Israel.
Uri Kopylov, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Adi Lahat, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Michal M. Amitai, Department of Diagnostic Imaging, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel
Eyal Klang, Department of Diagnostic Imaging, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Dana Ben-Ami Shor, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Sandra Neuman, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Nina Levhar, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Ella Fudim, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Benjamin Avidan, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Ido Laish, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Limor Selinger, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Noam Zingboim-Orbach, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Orit Picard, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Miri Yavzori, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Rami Eliakim, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
Shomron Ben-Horin, Department of Gastroenterology, Sheba Medical Center and Sackler School of Medicine Tel-Aviv University, Tel-Aviv, Israel.
References
- 1. Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002; 8: 244–250. [DOI] [PubMed] [Google Scholar]
- 2. Samuel S, Bruining DH, Loftus EV, Jr, et al. Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol 2012; 10: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014; 63: 88–95. [DOI] [PubMed] [Google Scholar]
- 4. Kopylov U, Yablecovitch D, Lahat A, et al. Detection of small bowel mucosal healing and deep remission in patients with known small bowel Crohn’s disease using biomarkers, capsule endoscopy, and imaging. Am J Gastroenterol 2015; 110: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 5. Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res 2012; 159: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liverani E, Scaioli E, Digby RJ, et al. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol 2016; 22: 1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003; 92: 827–839. [DOI] [PubMed] [Google Scholar]
- 8. Dollery CM, McEwan JR, Hanney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res 1995; 77: 863–868. [DOI] [PubMed] [Google Scholar]
- 9. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 161–174. [DOI] [PubMed] [Google Scholar]
- 10. Shimshoni E, Yablecovitch D, Baram L, et al. ECM remodeling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 2015; 64: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Bruyn M, Vandooren J, Ugarte-Berzal E, et al. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit Rev Biochem Mol Biol 2016; 51: 295–358. [DOI] [PubMed] [Google Scholar]
- 12. Kofla-Dłubacz A, Matusiewicz M, Krzesiek E, et al. Metalloproteinase- 3 and -9 as novel markers in the evaluation of ulcerative colitis activity in children. Adv Clin Exp Med 2014; 23: 103–110. [DOI] [PubMed] [Google Scholar]
- 13. Baugh MD, Perry MJ, Hollander AP, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 1999; 117: 814–822. [DOI] [PubMed] [Google Scholar]
- 14. Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol 2001; 22: 571–579. [DOI] [PubMed] [Google Scholar]
- 15. Opdenakker G, Van den Steen PE, Dubois B, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol 2001; 69: 851–859. [PubMed] [Google Scholar]
- 16. Xu X, Jackson PL, Tanner S, et al. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One 2011; 6: e15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koelink PJ, Overbeek SA, Braber S, et al. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut 2014; 63: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O’Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm 2015; 2015: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meijer MJW, Mieremet-Ooms MAC, van der Zon AM, et al. Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with Crohn’s disease phenotype. Dig Liver Dis 2007; 39: 733–739. [DOI] [PubMed] [Google Scholar]
- 20. Gao Q, Meijer MJW, Kubben FJGM, et al. Expression of matrix metalloproteinases -2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis 2005; 37: 584–592. [DOI] [PubMed] [Google Scholar]
- 21. Manfredi MA, Zurakowski D, Rufo PA, et al. Increased incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 22. Annahazi A, Molnar T, Farkas K, et al. Fecal MMP-9: a new noninvasive differential diagnostic and activity marker in ulcerative colitis. Inflamm Bowel Dis 2013; 19: 316–320. [DOI] [PubMed] [Google Scholar]
- 23. Farkas K, Saródi Z, Bálint A, et al. The diagnostic value of a new fecal marker, matrix metalloprotease-9, in different types of inflammatory bowel diseases. J Crohns Colitis 2015; 9: 231–237. [DOI] [PubMed] [Google Scholar]
- 24. Buisson A, Vazeille E, Minet-Quinard R, et al. Fecal matrix metalloprotease-9 and lipocalin-2 as biomarkers in detecting endoscopic activity in patients with inflammatory bowel diseases. J Clin Gastroenterol 2018; 52: e53–e62. [DOI] [PubMed] [Google Scholar]
- 25. Kofla-Dlubacz A, Matusiewicz M, Krzystek-Korpacka M, et al. Correlation of MMP-3 and MMP-9 with Crohn’s disease activity in children. Dig Dis Sci 2012; 57: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faubion WA, Fletcher JG, O’Byrne S, et al. EMerging BiomARKers in inflammatory bowel disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol 2013; 108: 1891–900. [DOI] [PubMed] [Google Scholar]
- 27. de Bruyn M, Arijs I, Wollants WJ, et al. Neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate serum marker of mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2014; 20: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 28. de Bruyn M, Arijs I, De Hertogh G, et al. Serum neutrophil gelatinase b-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate marker for mucosal healing in patients with Crohn’s disease. J Crohns Colitis 2015; 9: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 29. Ben-Horin S, Lahat A, Amitai MM, et al. Assessment of small bowel mucosal healing by video capsule endoscopy predicts short and long-term risk of Crohn’s disease flare: a prospective cohort study. Lancet Gastroenterol Hepatol 2019; 4: 519–528. [DOI] [PubMed] [Google Scholar]
- 30. Gralnek IM, Defranchis R, Seidman E, et al. Development of a capsule endoscopy-scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther 2008; 27: 146–154. [DOI] [PubMed] [Google Scholar]
- 31. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224. [DOI] [PubMed] [Google Scholar]
- 32. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018; 390: 2779–2789. [DOI] [PubMed] [Google Scholar]
- 33. Ravi A, Garg P, Sitaraman SV. Matrix metalloproteinases in inflammatory bowel disease: boon or a bane? Inflamm Bowel Dis 2007; 13: 97–107. [DOI] [PubMed] [Google Scholar]
- 34. Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004; 4: 617–629. [DOI] [PubMed] [Google Scholar]
- 35. Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 2002; 3: 207–214. [DOI] [PubMed] [Google Scholar]
- 36. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior? Annu Rev Cell Dev Biol 2001; 17: 464–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 [MMP-9]: the next decade. Crit Rev Biochem Mol Biol 2013; 48: 222–272. [DOI] [PubMed] [Google Scholar]
- 38. Van den Steen PE, Proost P, Wuyts A, et al. Neutrophil gelatinase B potentiates interleukin-8 tenfold by amino terminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 2000; 96: 2673–2681. [PubMed] [Google Scholar]
- 39. Gao Q, Meijer MJW, Schluter UG, et al. Infliximab treatment influences the serological expression of matrix metalloproteinase (MMP)-2 and -9 in Crohn’s disease. Inflamm Bowel Dis 2007; 13: 693–702. [DOI] [PubMed] [Google Scholar]
- 40. de Bruyn M, Breynaert C, Arijs I, et al. Inhibition of gelatinase B/MMP-9 does not attenuate colitis in murine models of inflammatory bowel disease. Nat Commun 2017; 8: 15384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandborn WJ, Bhandari BR, Randall C, et al. Andecaliximab [anti-matrix metalloproteinase-9] induction therapy for ulcerative colitis: a randomized, double-blind, placebo-controlled, phase 2/3 study in patients with moderate to severe disease. J Crohns Colitis 2018; 12: 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schreiber S, Siegel CA, Friedenberg KA, et al. A phase 2, randomized, placebo-controlled study evaluating matrix metalloproteinase-9 inhibitor, andecaliximab, in patients with moderately to severely active Crohns disease. J Crohns Colitis 2018; 12: 1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garg P, Ravi A, Patel NR, et al. Matrix metalloproteinase-9 regulates MUC-2 expression through its effect on goblet cell differentiation. Gastroenterology 2007; 132: 1877–1889. [DOI] [PubMed] [Google Scholar]
- 44. Vermeer PD1, Denker J, Estin M, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 2009; 296: L751–L762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–429. [DOI] [PubMed] [Google Scholar]
- 46. D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol 2008; 103: 2007–2014. [DOI] [PubMed] [Google Scholar]
- 47. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005; 54: 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meuwis MA, Vernier-Massouille G, Grimaud JC, et al. Serum calprotectin as a biomarker for Crohn’s disease. J Crohns Colitis 2013; 7: e678–e683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAG for Serum MMP-9: a novel biomarker for prediction of clinical relapse in patients with quiescent Crohn’s disease, a post hoc analysis by Doron Yablecovitch, Uri Kopylov, Adi Lahat, Michal M. Amitai, Eyal Klang, Dana Ben-Ami Shor, Sandra Neuman, Nina Levhar, Ella Fudim, Benjamin Avidan, Ido Laish, Limor Selinger, Noam Zingboim-Orbach, Orit Picard, Miri Yavzori, Rami Eliakim and Shomron Ben-Horin in Therapeutic Advances in Gastroenterology