Abstract
Background:
The health care industry is witnessing an increasing trend in the use of generic medicines because of their presumed low cost compared with innovator medicines. The aim of this study was to determine and compare the performance of the copy drug Osveral® and its innovator drug deferasirox (Exjade®).
Methods:
A prospective observational study including 223 patients receiving the branded medicine Exjade® and 101 patients receiving the copy Osveral® was carried out. Data were assessed for a 1-year period and included clinical symptoms, serum ferritin (SF), serum creatinine (SC), and alanine aminotransferase (ALT). Data were analyzed with SPSS version 22 software (SPSS, Chicago, IL, USA).
Results:
The median age of the sample was 8 years. There was no significant difference in gender distribution between the two groups (p = 0.625). Nausea was the most frequently reported adverse effect followed by diarrhea and abdominal pain in both groups. Patients receiving Exjade® had a higher relative reduction of SF at the end of the study compared with the Osveral® group (19.9% versus 9.93%, p = 0.028). SC was found to be significantly higher in the Osveral® group than in the Exjade® group throughout the study period. The mean platelet count was higher in the Exjade® group. ALT was significantly higher among patients receiving Osveral® over the last three months of the study.
Conclusions:
Exjade® showed a better ability to reduce SF, with less liver toxicity, and better hemostasis profile. No congenital anomalies associated with short-term use of both drugs during pregnancy were observed or reported.
Keywords: copy, deferasirox, Iraq, thalassemia
Introduction
B-thalassemia, an inherited autosomal recessive blood disorder, is prevalent across the Italian, Greek, Middle Eastern, South Asian, and African populations.1 Although frequent blood transfusions are vital to survive,2–4 an effective iron-chelating therapy is warranted coherently to prevent complications of chronic iron overload, such as organ failure.4–6
Generic medicines are copies of the innovator (branded) drug which are usually marketed after the patent expiry of the innovator drug. The health care industry is witnessing an increasing trend in the use of generic medicines because of their presumed low cost compared with innovator medicines. Generic and innovator medicines might be different with regards to coloring and bulking agents. Nonetheless, both medicines should demonstrate bioequivalence.7
The Food and Drug Agency (FDA) uses a limit of 80–125% of the bioavailability and a variance of 25% when assessing a new generic medicine against the innovator.8 A study investigating 12 years bioequivalence of 2070 single-dose clinical studies showed that the average difference in absorption into the body between the generic and the innovator was 3.5% indicating good bioequivalence.9
Studies comparing the efficacy of innovator and generic medicines have shown contradictory results.
A quantitative systematic review of 52 reports revealed that laypeople were more likely to perceive generic medicines as less effective, while doctors believed they cause more side effects, and pharmacists think they are of inferior quality. Both doctors and pharmacists reported concerns about the safety of generic medicines.10 Bioequivalent studies of psychoactive drugs showed that seizures had recurred among epileptic patients who switched from branded to generic carbamazepine. A significant difference was found in the pharmacokinetic profile between brand-name versus generic diazepam.11
In contrast, a review of regulations, policy, and safety issues for oncology generic drugs from the USA, Canada, Japan, India, and the EU concluded that there were no safety concerns where regulation and enforcement were strong, and no major problems have been reported from developed countries. However, safety issues are still encountered in developing countries owing to less intensive oversight.12
Deferasirox (ICL670), also known as Exjade,® is a member of a new class of tridentate iron chelators. It is readily metabolized in the liver and causes fewer side effects compared with earlier agents such as desferrithiocin.13 It is orally bioavailable and its terminal elimination half-life (t1/2) is between 8 and 16 h, allowing for once-daily administration.14 No significant drug–drug interactions have been identified to date. So far, there is no licensed generic drug that is equivalent to Exjade®. Nonetheless, a drug with a trading name of Osveral® has been produced by Iranian pharmaceutical company and circulated in a few Middle Eastern countries as equivalent to Exjade®. In the sole study of the efficacy of Osveral® among a sample of Iranian patients, serum ferritin (SF) level was reported to be reduced significantly with the use of Osveral®. More than one-third (36.6%) of the participants experienced at least one adverse effect.15 A study of the physicochemical properties of Osveral® compared with the original medicine Ejaxade® showed that the properties of Osveral® were similar to those of Exjade®, and the similarity factor was more than 50% for all doses.16
In a period of rising financial instability, the Ministry of Health (MOH) of Iraq has limited resources to provide patients with branded medications due to their high costs. To date, there is no generic medicine equivalent to Exjade® that is licensed by the FDA, and the European Medicines Agency. In Iraq, only patients with high iron overload were given priority to receive Exjade® due to resource constraints. As a result, Osveral® has been used by a number of patients against their physician’s advice. The objective of this study is to determine and compare the performance of the copy drug Osveral® with its branded drug version deferasirox (Exjade®) in relation to selected blood markers and clinical outcomes.
Materials and methods
Patients/sample
A prospective observational study design was used. The study included patients with β-thalassemia syndrome (either thalassemia major or intermedia), who were attending Baghdad Hereditary Anemia Center at Ibn Al-Baladi hospital, aged 2 years and above, with SF levels of ⩾1000 ng/ml and a negative serum β-hCG pregnancy test at the start of a study period. Patients with comorbidities or complications, such as cardiac, liver, renal, hematologic, auditory, and ocular, were excluded from this study.
Patients were divided into two groups. The first group of 223 patients received the branded form of deferasirox (Exjade®) manufactured by NOVARTIS, Basel, Switzerland. which was supplied by the government. The second group of 101 patients received the copy form of deferasirox (Exjade®) manufactured by an Iranian Pharmaceutical Company known as Osveral® which is produced by Osvah Pharmaceuitcal Company (OSVE), Tehran, Iran. Patients of the latter group obtained the drug from the black market without the approval of their managing specialist. Data collected included the clinical symptoms: nausea, vomiting, dizziness, diarrhea, abdominal pain, skin rash, hair loss, tachypnea, or chest pain; the blood markers: SF, platelet count, SC, and ALT; and the dose of medication given at each visit. Both groups underwent a baseline assessment and then were followed up every 3 months for 12 months.
Informed consent was obtained from all participants and the study was approved by the institutional ethics committee of Al-Kindy Medical College, Bagdad University.
Statistical analyses
Numerical variables were described by mean and standard deviation while categorical variables were described by frequency and percentage. Independent samples t test was used to test the significance of the mean difference between the two groups. A paired t test was used to test the significance of the mean difference within each group. Repeated measures analysis of variance (ANOVA) was used to ascertain the mean difference within and between the groups. The percentage of relative change was calculated from baseline measurement to the intended follow-up measurement. The significance of the difference in relative change was assessed with Z statistics. The significance level was set at 0.05. All data were entered and analyzed using SPSS V.22 software.
Results
The sample median age was 8 years. Overall, there were more men (57%) than women (43%) in this study, however, there was no significant difference in gender distribution between the two groups (p = 0.625).
Out of the 324 recruited patients, three women (two from the Exjade® group and one from the Osveral® group) became pregnant after initiating the treatment and were excluded from the study, thus 321 patients completed the follow-up period. The pregnancies were detected at 6–8 weeks of gestation, there were no pregnancy complications, newborns were delivered via Cesarean section and no congenital anomalies were reported upon assessment by an attending pediatrician.
Adverse drug reaction
In terms of adverse drug effects, it was found that nausea was reported the most frequent followed by diarrhea, and abdominal pain. There was no statistically significant difference between the two groups (Table 1).
Table 1.
Distribution of adverse drug effects.
| Exjade® n (%) | Osveral® n (%) | p value* | ||
|---|---|---|---|---|
| Gender | Men | 128 (57.9) | 55 (55) | 0.625 |
| Women | 93 (42.1) | 45 (45) | ||
| Dizziness | No | 221 (100) | 99 (99) | 0.137 |
| Yes | 0 (0) | 1 (1) | ||
| Nausea | No | 165 (74.7) | 71 (71) | 0.491 |
| Yes | 56 (25.3) | 29 (29) | ||
| Diarrhea | No | 192 (86.9) | 83 (83) | 0.358 |
| Yes | 29 (13.1) | 17 (17) | ||
| Abdominal pain | No | 203 (91.9) | 87 (87) | 0.173 |
| Yes | 18 (8.1) | 13 (13) | ||
| Skin rash | No | 196 (88.7) | 90 (90) | 0.727 |
| Yes | 25 (11.3) | 10 (10) | ||
| Hair loss | No | 221 (100) | 98 (98) | 0.035 |
| Yes | 0 (0) | 2 (2) | ||
| Tachypnea | No | 221 (100) | 99 (99) | 0.137 |
| Yes | 0 (0) | 1 (1) |
p value for the chi-squared test.
SF and dosage of the drug
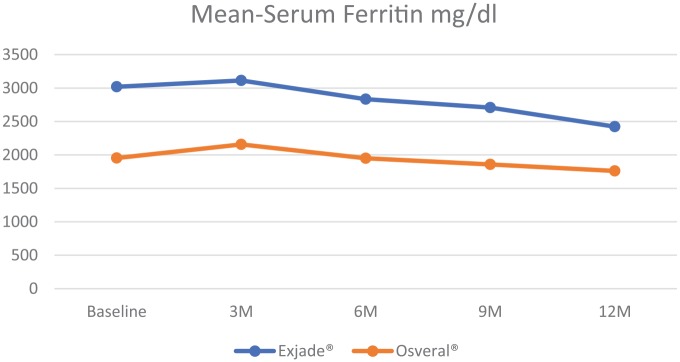
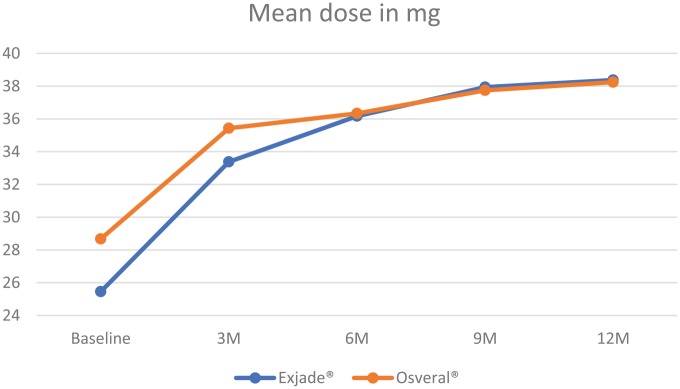
The mean SF was found to be higher among patients who received Exjade® at the commencement of the study. Nonetheless, the Exjade® group had a significantly higher reduction in the SF level compared with the Osveral® group. The dose was increased significantly over the study period and was almost equal between the study groups (Table 2, Figures 1 and 2).
Table 2.
Comparison of mean serum ferritin and dose between the two groups.
| Timeline | Group | Mean serum ferritin (±SD) mg/dl | p value* | % Relative change from baseline | p value** | Mean dose (±SD) mg/kg | p value* |
|---|---|---|---|---|---|---|---|
| Baseline | Exjade® | 3019.72 (±292.79) | <0.001 | 25.46 (±1.42) | <0.001 | ||
| Osveral® | 1953.61 (±319.51) | 28.67 (±2.77) | |||||
| 3 months | Exjade® | 3113.34 (±294.85) | <0.001 | 3.10 | 0.006 | 33.38 (±1.43) | <0.001 |
| Osveral® | 2157.5 (±321.24) | 10.44 | 35.43 (±1.48) | ||||
| 6 months | Exjade® | 2833.5 (±295.56) | <0.001 | 6.17 | 0.014 | 36.17 (±1.69) | 0.396 |
| Osveral® | 1949.31 (±319.98) | 0.22 | 36.33 (±1.16) | ||||
| 9 months | Exjade® | 2708.62 (±303.61) | <0.001 | 10.30 | 0.101 | 37.94 (±1.16) | 0.097 |
| Osveral® | 1858.44 (±327.69) | 4.87 | 37.74 (±0.47) | ||||
| 12 months | Exjade® | 2422.96 (±296.73) | <0.001 | 19.76 | 0.028 | 38.37 (±1.23) | 0.317 |
| Osveral® | 1759.6 (±328.96) | 9.93 | 38.24 (±0.47) |
p value for independent samples t test.
p value for Z test.
Figure 1.
Mean serum ferritin by study group.
Figure 2.
Mean dose by study group.
Effect of drug on SC
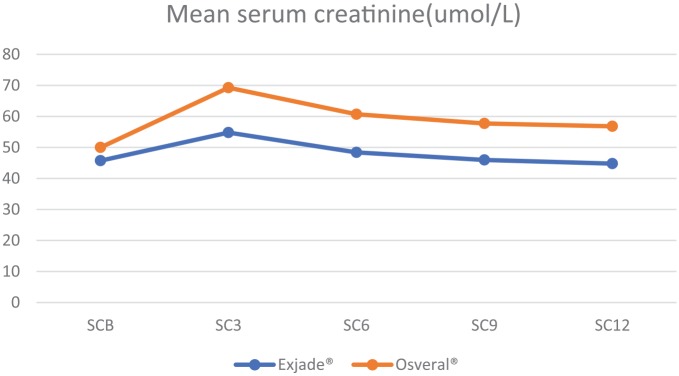
SC was found to be significantly higher in the Osveral® group compared with the Exjade® group throughout the follow-up period. Repeated measures ANOVA showed the results hold significance for within-group difference (p < 0.001) and between-group difference (p < 0.001) (Table 3, Figure 3).
Table 3.
Mean serum creatinine by study group.
| Group | n | Mean (SD) | p value* | |
|---|---|---|---|---|
| SCB | Exjade® | 221 | 45.71 (3.12) | <0.001 |
| Osveral® | 100 | 49.98 (3.69) | ||
| SC3 | Exjade® | 221 | 54.78 (3.51) | <0.001 |
| Osveral® | 100 | 69.24 (4.02) | ||
| SC6 | Exjade® | 221 | 48.4 (3.71) | <0.001 |
| Osveral® | 100 | 60.7 (4.11) | ||
| SC9 | Exjade® | 221 | 45.96 (4.03) | <0.001 |
| Osveral® | 100 | 57.71 (4.31) | ||
| SC12 | Exjade® | 221 | 44.77 (4.29) | <0.001 |
| Osveral® | 100 | 56.82 (4.34) |
p value for independent samples t test.
SCB, Serum creatinine baseline; SC3 serum creatinine 3 months; SC6, serum creatinine 6 months; SC9 serum creatinine 9 months; SC12, serum creatinine 12 months.
Figure 3.
Mean serum creatinine by study groups.
Effect of drug on ALT
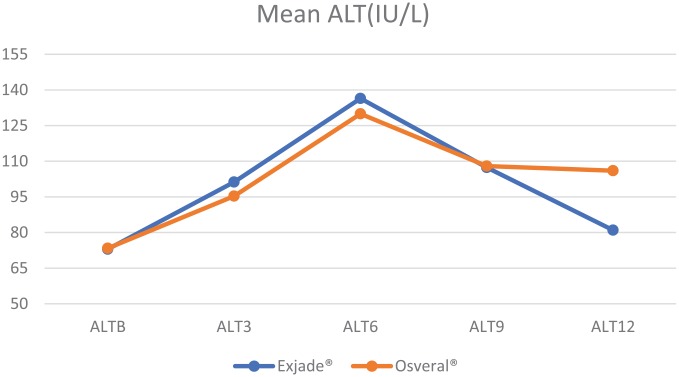
Mean ALT distribution is shown in Table 4 and Figure 4. It was observed that until the sixth month of observation, ALT was significantly higher in the Exjade® group while over the last 3 months of the study it was significantly higher among patients receiving Osveral®. Repeated measures ANOVA showed the results hold significance for within-group difference (p < 0.001), and no significance for between-groups difference (p = 0.211).
Table 4.
Mean ALT by group.
| Group | n | Mean (SD) | p value* | |
|---|---|---|---|---|
| ALTB | Exjade® | 221 | 72.95 (17.47) | 0.853 |
| Osveral® | 100 | 73.34 (17.82) | ||
| ALT3 | Exjade® | 221 | 101.22 (17.75) | 0.007 |
| Osveral® | 100 | 95.33 (18.16) | ||
| ALT6 | Exjade® | 221 | 136.44 (18.07) | 0.003 |
| Osveral® | 100 | 129.94 (18.21) | ||
| ALT9 | Exjade® | 221 | 107.36 (18.40) | 0.786 |
| Osveral® | 100 | 107.96 (18.50) | ||
| ALT12 | Exjade® | 221 | 81 (19.03) | 0.000 |
| Osveral® | 100 | 105.99 (18.67) |
p value for independent samples t test.
ALT, alanine aminotransferase; ALTB, alanine aminotransferase baseline; ALT3, alanine aminotransferase 3 month; ALT6, alanine aminotransferase 6 month; ATL9, alanine aminotransferase 9 month; ALT12, alanine aminotransferase 12 month.
Figure 4.
Mean ALT by study groups.
Effect of drug on hemostasis profile
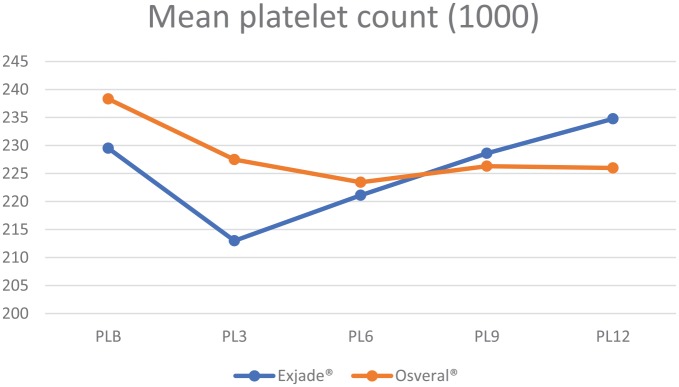
The mean platelet count distribution is shown in Table 5 and Figure 5. It was observed that the mean platelet count was higher in the Exjade® group, however, the difference was not statistically significant. Repeated measures ANOVA showed the results were significant for within-group difference (p < 0.001) and otherwise for between-groups difference (p = 0.659).
Table 5.
Mean platelet count by group.
| Group | n | Mean (SD) | p value* | |
|---|---|---|---|---|
| PLB | Exjade® | 221 | 229.52 (62.99) | 0.092 |
| Osveral® | 100 | 238.32 (30.26) | ||
| PL3 | Exjade® | 221 | 212.99 (62.82) | 0.005 |
| Osveral® | 100 | 227.48 (29.53) | ||
| PL6 | Exjade® | 221 | 221.12 (62.77) | 0.654 |
| Osveral® | 100 | 223.44 (30.02) | ||
| PL9 | Exjade® | 221 | 228.6 (62.57) | 0.658 |
| Osveral® | 100 | 226.31 (30.19) | ||
| PL12 | Exjade® | 221 | 234.76 (62.25) | 0.099 |
| Osveral® | 100 | 225.99 (32.37) |
p value for independent samples t test.
PLB, platelet baseline; PL3, platelet 3 month; PL6, platelet 6 month; PL9, platelet 9 month; PL12 platelet 12 month.
Figure 5.
Mean platelet count by study groups.
Discussion
The advantages and disadvantages of using generic medicines have been acknowledged in the literature. However, fewer studies have provided objective evidence about the consequences of using generic medicines compared with innovator drugs. This study reported the use of a copy drug which has not been licensed as a generic one. The results of this study are expected to highlight and inform health professionals on the use of a copy drug among thalassemia patients.
Similar to other studies, there was a higher proportion of men in this study, however, the difference was not statistically significant.17,18 The findings of the reported adverse drug effects are not surprising because it is well known that an iron-chelating agent is an oral agent, well absorbed from the gastrointestinal tract, which induces some inevitable adverse effects.19 The finding that nausea and diarrhea were the most frequent adverse drug effects is in line with other reports.14,20,21
The greater reduction in SF level after the use of innovator drugs may reflect its higher efficacy compared with the copy drug. This fact was supported toward the end of the study where the dosage of both drugs was almost equal. The reason that patients receiving Exjade® in this study had higher SF level is attributed to the fact that administration of Exjade® was guarded by a ’priority policy’ of the MOH Iraq. Patients who had high iron overload (high SF) were considered as a priority to receive Exjade® while those with lower SF were put on a waiting list. A number of patients on the waiting list with lower SF opted to obtain Osveral® from the black market despite being notified not to do so. Therefore, this should serve as a mandate that the Iraqi MOH has to scrutinize its drug marketing and distribution policies. In contrast to our results, the poorly designed and reported Iranian study concluded adequate efficacy of Osveral® in reducing SF level without an obvious comparison with any counterpart drug.15
With regard to the safety of the drug, it is noticeable that the SC was higher in the Osveral® group in our study. However, SC was within the normal level. It is well known that increased SC reflects renal toxicity and is considered as one of the drug withdrawal indications, either temporarily or permanently.22 The longer the period of temporary drug withdrawal, the higher the chance of iron accumulation within the body tissues, and the increased failure of iron-chelating therapy.23 Our results are in line with other studies in relation to the safety of Exjade®.14,20
Among the frequently reported adverse drug effects of iron-chelating agents is liver toxicity. The iron-chelating agent is mainly metabolized in the liver and prolonged use is found to cause permanent liver damage.
In our study, there was no clear pattern in favor of either Exjade® or Osveral® as a drug with a better hepatic profile. ALT more than five times the upper limit of normal is a warning sign to discontinue the drug either temporarily or permanently to avoid liver damage.14,24 The ALT level did not reach abnormal figures in this study.
Despite the higher level of ALT among the Osveral® group, it might be too early to determine the possibility of progression to organ damage and failure without a longer period of observation.
One of the main indicators of chelating drug safety is the effect on hemostasis.25,26 There was no clear pattern in favor of either drug in this study. However, the innovator drug Exjade® exhibited better performance in maintaining higher platelets count toward the end of the observation period, hence less risk of bleeding. Deferasirox is associated with agranulocytosis including thrombocytopenia27 or only a slight gradual decrease in platelet count for first 2 months of usage.17 Although some studies revealed no agranulocytosis events14,28 other reports have documented the correlation between acquired platelet defect and iron overload.29 Nonetheless, no hemorrhagic episodes were reported among the patients in our study. Our results are in line with other studies which reported no significant impact of chelating agent on platelets count14,30 but contradict other reports in which chelating agents caused thrombocytopenia.31,32
The exclusion of three female study participants who became pregnant (detected at 6–8 weeks of gestation), was warranted because there is limited safety data on the use of deferasirox during pregnancy.33 In general, iron-chelating drugs, including deferasirox, are strongly contraindicated during pregnancy. However, pregnant women who received chelation treatment unintentionally delivered healthy children. To the best of our knowledge, there is very limited data on the unintentional use of deferasirox during the first trimester of pregnancy in a single patient34 and in nine cases.35 All reports ensured the delivery of babies without evident teratogenicity. Our data, albeit limited, might contribute to previous evidence and either on the safety of Exjade® or its copy drug use during early pregnancy.
Giving the permissible limit of 80–125% of the bioavailability and a variance of 25% used by the FDA, the higher efficacy assumed for originator drug might be attributed to the effect of either the excipients or a nocebo effect.36 In fact, bioequivalence studies provided solid evidence about the utility of generic medicines but perception is still not favoring generic medicine.10
As far as the price is concerned, the difference is one of the major indications for using generic medicines, in our case, it might be argued that the cheaper price with a small margin of superiority of Ejaxade® is a reason to use the Osveral®. The major ethical implication is that Osveral® is not listed under approved drugs by the FDA, otherwise it might be a proper substitute for Ejaxade®. To date, bioequivalence studies would be the best evidence to guide managing specialists on the use of Osveral® in thalassemia patients.
Despite all of our efforts to provide valid and reliable data, there are some limitations that need to be considered when making inferences from the results of this study. First, the use of observational study design implies a lack of randomization which would have helped to control for unknown confounding factors. Second, some sociodemographic data were not collected that might have had an effect on the results.
In conclusion, Exjade® showed a better ability to reduce SF level, less liver toxicity, and a better hemostasis profile. No congenital anomalies associated with short-term use of both drugs during pregnancy were reported. Longer observation periods and bioequivalence studies are needed to provide reliable evidence on efficacy and safety of the generic drug.
Acknowledgments
The authors would like to thank all supporting staff at Ibn Al-Baladi hospital for their efforts in data collection. Authors would also like to thanks Associate Professor Dr Halina Lugova for her contribution to review the manuscript and make necessary language editing.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
ORCID iD: Aqil M. Daher  https://orcid.org/0000-0002-3504-4340
https://orcid.org/0000-0002-3504-4340
Contributor Information
Aqil M. Daher, Department of Community Medicine, Faculty of Medicine and Defense Health, National Defense University of Malaysia, Kuala Lumpur, 57000, Malaysia.
Hayder Al-Momen, Department of Pediatrics, Al-Kindy College of Medicine, University of Baghdad, Baghdad, Iraq.
Shaymaa Kadhim Jasim, Department of Obstetrics and Gynecology, College of Medicine, University of Baghdad, Baghdad, Iraq.
References
- 1. Weatherall DJ, Clegg JB. The thalassaemia syndromes. Fourth ed Oxford: Wiley-Blackwell, 2001. [Google Scholar]
- 2. Porter JB, El-Alfy M, Viprakasit V, et al. Utility of labile plasma iron and transferrin saturation in addition to serum ferritin as iron overload markers in different underlying anemias before and after deferasirox treatment. Eur J Haematol 2016; 96: 19–26. [DOI] [PubMed] [Google Scholar]
- 3. Saliba AN, Musallam KM, Cappellini MD, et al. Serum ferritin values between 300 and 800 ng/mL in nontransfusion-dependent thalassemia: a probability curve to guide clinical decision making when MRI is unavailable. Am J Hematol 2017; 92: E35–E37. [DOI] [PubMed] [Google Scholar]
- 4. Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous β-thalassemia. N Engl J Med 1994; 331: 574–578. [DOI] [PubMed] [Google Scholar]
- 5. Rund D, Rachmilewitz E. β-Thalassemia. N Engl J Med 2005; 353: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 6. Harmatz P, Butensky E, Quirolo K, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 2000; 96: 76–79. [PubMed] [Google Scholar]
- 7. Dunne S, Shannon B, Dunne C, et al. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol 2013; 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simoens S. International comparison of generic medicine prices. Curr Med Res Opin 2007; 23: 2647–2654. [DOI] [PubMed] [Google Scholar]
- 9. Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother 2009; 43: 1583–1597. [DOI] [PubMed] [Google Scholar]
- 10. Colgan S, Faasse K, Martin LR, et al. Perceptions of generic medication in the general population, doctors and pharmacists: a systematic review. BMJ Open 2015; 5: e008915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoative drugs. Clin Ther 2003; 25: 1578–1592. [DOI] [PubMed] [Google Scholar]
- 12. Yang YT, Nagai S, Chen BK, et al. Generic oncology drugs: are they all safe? Lancet Oncol 2016; 17: e493–e501. [DOI] [PubMed] [Google Scholar]
- 13. Nick H, Acklin P, Lattmann R, et al. Development of tridentate iron chelators: from desferrithiocin to ICL670. Curr Med Chem 2003; 10: 1065–1076. [DOI] [PubMed] [Google Scholar]
- 14. Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with β-thalassemia. Blood 2006; 107: 3455–3462. [DOI] [PubMed] [Google Scholar]
- 15. Eshghi P, Farahmandinia Z, Molavi M, et al. Efficacy and safety of Iranian made deferasirox (Osveral®) in Iranian major thalassemic patients with transfusional iron overload: a one year prospective multicentric open-label non-comparative study. Daru 2011; 19: 240. [PMC free article] [PubMed] [Google Scholar]
- 16. Ebrahimnejad P, Salehifar E, Kowsaryan M. Post-market surveillance study of osveral, a branded generic formulation of deferasirox, and the original brand, Exjade. J Mazandaran Univ Med Sci 2016; 26: 238–244. [Google Scholar]
- 17. Al-Momen H. Iron chelation therapy in sickle cell/beta thalassemia syndrome, a 2 years’ extension study. Al-Kindy Col Med J 2017; 13: 76–81. [Google Scholar]
- 18. Hayder HAM, Ali AO, Zena KM, et al. Is deferasirox as effective as desferrioxamine in treatment of iron overload in patients with thalassemia major. IOSR JDMS 2017; 16: 29–34. [Google Scholar]
- 19. Tanaka C. Clinical pharmacology of deferasirox. Clin Pharmacokinet 2014; 53: 679–694. [DOI] [PubMed] [Google Scholar]
- 20. Cappellini MD, Bejaoui M, Agaoglu L, et al. Iron chelation with deferasirox in adult and pediatric patients with thalassemia major: efficacy and safety during 5 years’ follow-up. Blood 2011; 118: 884–893. [DOI] [PubMed] [Google Scholar]
- 21. Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol 2007; 136: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamed EA, ElMelegy NT. Renal functions in pediatric patients with beta-thalassemia major: relation to chelation therapy: original prospective study. Ital J Pediatr 2010; 36: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aldudak B, Bayazit AK, Noyan A, et al. Renal function in pediatric patients with β-thalassemia major. Pediatr Nephrol 2000; 15: 109–112. [DOI] [PubMed] [Google Scholar]
- 24. Deugnier Y, Turlin B, Ropert M, et al. Improvement in liver pathology of patients with β-thalassemia treated with deferasirox for at least 3 years. Gastroenterology 2011; 141: 1202–1211. e3. [DOI] [PubMed] [Google Scholar]
- 25. Mobarra N, Shanaki M, Ehteram H, et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res 2016; 10: 239–247. [PMC free article] [PubMed] [Google Scholar]
- 26. Barradas MA, Jeremy JY, Kontoghiorghes GJ, et al. Iron chelators inhibit human platelet aggregation, thromboxane A2 synthesis and lipoxygenase activity. FEBS Lett 1989; 245: 105–109. [DOI] [PubMed] [Google Scholar]
- 27. Kontoghiorghes GJ. Deferasirox: uncertain future following renal failure fatalities, agranulocytosis and other toxicities. Expert Opin Drug Saf 2007; 6: 235–239. [DOI] [PubMed] [Google Scholar]
- 28. Lee JW, Yoon SS, Shen ZX, et al. Hematologic responses in patients with aplastic anemia treated with deferasirox: a post hoc analysis from the EPIC study. Haematologica 2013; 98: 1045–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dahi AA, Hanafy E, Al Pakra M. Iron overload and platelet function defects: possible correlation. J Investig Med High Impact Case Rep 2016; 4: 2324709616675645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomber S, Saxena R, Madan N. Comparative efficacy of desferrioxamine, deferiprone and in combination on iron chelation in thalassemic children. Indian Pediatr 2004; 41: 21–28. [PubMed] [Google Scholar]
- 31. Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood 2012; 120: 3657–3669. [DOI] [PubMed] [Google Scholar]
- 32. Yang LP, Keam SJ, Keating GM. Deferasirox. Drugs 2007; 67: 2211–2230. [DOI] [PubMed] [Google Scholar]
- 33. Shah F, Prescott E, Kyei-Mensah A. Management of thalassemias in Pregnancy. In: Pavord S, Hunt B. (eds) The obstetric hematology manual. Cambridge: Cambridge University Press, 2018, pp. 66–76. [Google Scholar]
- 34. Vini D, Servos P, Drosou M. Normal pregnancy in a patient with β-thalassaemia major receiving iron chelation therapy with deferasirox (Exjade®). Eur J Haematol 2011; 86: 274–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diamantidis MD, Neokleous N, Agapidou A, et al. Iron chelation therapy of transfusion-dependent β-thalassemia during pregnancy in the era of novel drugs: is deferasirox toxic? Int J Hematol 2016; 103: 537–544. [DOI] [PubMed] [Google Scholar]
- 36. Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron 2008; 59: 195–206. [DOI] [PubMed] [Google Scholar]