Abstract
Stroke is one of the world's leading causes of mortality and morbidity. Greater understanding is required of the underlying relationships in ischemic brains in order to prevent stroke or to develop effective treatment. This review highlights new findings about the relationship of blood–brain barrier with astrocytes, pentraxin-3 (PTX3), and other factors expressed during or after ischemic stroke. These are discussed with respect to their ameliorative or deleterious effects. These effects are measured in vivo in animal models as well as in vitro in cell cultures. Evidence was found to suggest that astrocytes play a key role in stroke by expressing PTX3, which, in turn, enhances endothelial tightness, increases tight junction proteins, and inhibits vascular endothelial growth factor. The role of astrocytes and PTX3 is examined in relation to hypoxic stress and conditioning as well as mitochondrial transfer. Astrocytes and PTX3 are placed in the context of brain circulation and related areas.
Keywords: Astrocyte, blood–brain barrier, conditioning, endothelium, hypoxia, mitochondria, pentraxin-3, stroke, tight junction proteins, vascular endothelial growth factor
Overview of Stroke, Blood–Brain Barrier, Astrocytes, and Pentraxin-3
Stroke is one of the most common causes of death worldwide, with recent United States estimates ranging between the third and fifth highest.[1,2] In addition, even for cases that do not directly contribute to mortality statistics, stroke is also the leader in causes of long-term disability.[3] Ischemic stroke is the most common classification of stroke and is defined by a blockage or reduction of blood flow to the brain.[4] Few treatments currently exist for such a prevalent disease, and they are quite time sensitive. Greater understanding of stroke's pathology is needed in order to treat it, or even better, to prevent it from ever happening in the first place.
One of the most significant sources of secondary damage following a stroke is the increased vulnerability and leakage of blood–brain barrier (BBB).[5] Under normal conditions, the BBB functions to protect the central nervous system (CNS) from various harmful agents.[6] The BBB is assisted in this function by cerebral endothelial cells, and these are, in turn, supported by astrocytes, a kind of glial cell.[7,8] Astrocytes structurally and functionally support the BBB and the CNS as a whole.[9] In addition, the glia limitans, part of the BBB, is formed by the processes of the astrocytes.[9] Under ischemic stroke conditions, the usual astrocyte functions may be disturbed, potentially causing them to exert both adverse and ameliorative effects.[10]
Shindo et al. at the Massachusetts General Hospital have published novel findings on this subject. In the reviewed article, the researchers investigated one of these effects by examining the role of pentraxin-3 (PTX3), which is believed to have ameliorative effects in the acute phase of stroke.[10] PTX3 is a member of the long pentraxin family, a group of proteins, and was selected for its representative qualities.[10] The hypothesis advances the notion that reactive astrocytes are responsible for secreting mediators such as PTX3 as a way of aiding cerebral endothelial cells, which, in turn, help maintain BBB homeostasis following an ischemic stroke.[10] In particular, the research interest primarily resides in determining if PTX3 originated from reactive astrocytes in vivo after an ischemic stroke.[10] Once this was determined, an equally interesting research area is assessing how PTX3 may interact with the BBB in vitro.[10]
In Vivo Relationship of Astrocytes, Pentraxin-3, Blood–Brain Barrier, and Vascular Endothelial Growth Factor
Ten 3-month-old spontaneous hypertensive male rats were used for the in vivo portion of this experiment. Half of the rats underwent a sham operation to serve as controls, whereas the remaining half underwent a transient middle cerebral artery occlusion (MCAO) to induce focal cerebral ischemia.[10] Three days after the procedures, the brains were harvested to examine the origin of PTX3.
Compared to the controls, the MCAO brains had statistically significantly higher levels of PTX3 (P < 0.05).[10] After analysis of a variety of marker antibodies, the PTX3-positive cells stained with a type of antibody and in a pattern primarily indicative of astrocytic origin, suggesting an answer to one of the primary research interests.[10] In addition, immunoglobulin G staining provided evidence that BBB leakage in the peri-infarct area was observed primarily in regions with low amounts of PTX3, and that the opposite was true of regions with high amounts of PTX3. This negative correlation (R2 = 0.7123, P < 0.05) suggests that PTX3 derived from astrocytes may play a key role in regulating BBB permeability during acute stroke.[10]
Vascular endothelial growth factor (VEGF) also influences BBB permeability and increases BBB leaks during acute stroke conditions.[10] Prior studies have established that VEGF and PTX3 bind, so this study also investigated whether this binding might be partially responsible for PTX3's protective influence on the BBB.[10] Post-MCAO, brains displayed increased expression of VEGF, and it was confirmed that reactive astrocytes can produce both PTX3 and VEGF, answering one of the authors' original questions.[10] In summary, these in vivo results attest to an important relationship between astrocytes, PTX3, BBB, and VEGF in acute ischemic stroke.
In Vitro Relationship of Astrocytes, Pentraxin-3, Blood–Brain Barrier, Tight Junction Proteins, and Vascular Endothelial Growth Factor
In order to further examine some of the in vivo findings, additional tests were conducted on cultures of primary rat astrocytes.[10] First, PTX3 was confirmed to originate from astrocytes with Western blots of astrocyte conditioned media (ACM) that were prepared from primary cultured rat astrocytes.[10] Next, further tests on PTX3's effect on BBB permeability were conducted using a standard transwell system using either ACM or a specialized ACM from which the PTX3 has been depleted (ACMPTX3−).[10] In concordance with the evidence from the in vivo trials, the ACM increases endothelial tightness, which leads to a less permeable BBB.[10] Meanwhile, the ACMPTX3 − did not affect endothelial tightness.[10]
Continuing the aforementioned line of investigation of the role of VEGF in vivo, additional in vitro tests of VEGF were performed using these cell cultures. Most importantly, these results demonstrated that VEGF, which normally increases cerebral endothelial cell permeability, may be inhibited when PTX3 binds to it.[10] This suggests yet another possible protective and compensatory role of PTX3 in acute ischemic stroke conditions.
In addition, the cultures were subjected to hypoxic conditions for 6 h to simulate pathological conditions.[10] This yielded evidence that only the ACM condition, and not the ACMPTX3 − condition, continued to significantly decrease permeability, even under hypoxic stress.[10] There is speculation on the exact mechanism for these observed differences, but evidence suggests that it may involve tight junction proteins ZO-1 and claudin 5, which were significantly higher in the ACM condition than in the ACMPTX3 − condition.[10]
To further examine the interaction of PTX3, ZO-1, and claudin 5, additional tests were conducted on more cell cultures, using RBE.4 rat endothelial cells this time.[10] PTX3 was added to the brain endothelial cells to investigate its effect on permeability and ZO-1 and claudin 5 levels.[10] Corresponding to its established effect on the transwell system, the added PTX3 had an enhancement effect on endothelial barrier integrity and increased ZO-1 and claudin 5 levels.[10] Notably, assays evinced that this difference was not due to a change in cell viability or number in the culture.[10] For one, a major source of VEGF's deleterious effect on the BBB is its downregulation of claudin 5, so this suggests another way by which PTX3 counteracts VEGF.[10,11] Furthermore, the aforementioned in vivo data also supported a mild correlation between PTX3 and ZO-1 in the peri-infarcted site.[10] The expression of these proteins suggests a possible line of investigation into the mechanism of PTX3's protective effects.
Possible Limitations of Pentraxin-3 and Astrocytes
This study has advanced research into the compensatory and protective properties of astrocyte-derived PTX3 and uncovered several novel results. However, there are some limitations that should be kept in mind. Angiogenesis, the process by which new blood vessels are formed, is correlated with neuroprotection before stroke and is a key component of recovery after stroke.[12,13] Fibroblast growth factor (FGF)-2 (FGF-2) is thought to play a role in several processes including cell proliferation, angiogenesis, and wound healing.[14,15] However, PTX3 may inhibit angiogenesis through its inhibition of FGF-2. Some in vitro trials have found that PTX3 binds to FGF-2 and blocks angiogenesis induced by FGF-2.[16] In contrast, there is evidence that PTX3 has little-to-no effect with other growth factors and cytokines, such as interleukin-1, nerve growth factor, and FGF-1.[16] Together, this demonstrates the possible damaging effects of PTX3.
In addition, while VEGF has deleterious effects during acute ischemic stroke, this is not always true in other situations. In the chronic phase of stroke, VEGF, true to its growth factor name, helps with remodeling the brain by increasing the permeability of the BBB.[10,17] Therefore, it is reasonable to suspect that PTX3's tendency of binding to VEGF may become maladaptive if it continues during the recovery phase.
Furthermore, while some reactive astrocytes showed clear indications of PTX3 expression, others did not, and the reason is unknown as of yet.[10] Thus, more research is needed into the factors determining the heterogeneity of reactive astrocytes' expression of PTX3.[10] Overall, there are certain limitations and unknown variables of both PTX3 and astrocytes that merit further exploration.
Pentraxin-3, Conditioning, and Hypoxia
As mentioned previously, Shindo et al. examined the interaction between oxygen supplies, BBB permeability, and astrocytes with and without PTX3.[10] Tests under hypoxic stress revealed that only astrocytes with PTX3 maintained BBB tightness, whereas PTX3-deprived astrocytes did not.[10] This test is meant to model hypoxic conditions that may be experienced locally or generally during ischemic stroke.
Hypoxia is defined here as a state of inadequate oxygen supply which may be due to low oxygen partial pressure, stroke, injury, or otherwise.[18] Under these conditions, hypoxia is more often experienced in the brain as it normally has some of the highest oxygen demands and is therefore more sensitive, relative to other organs.[19] There is evidence to suggest that conditioning the brain to tolerate semi-hypoxic environments prior to stroke may result in better outcomes. For instance, a recent study in an animal model found that prophylactic cardiovascular exercise may advance the cerebrovascular network and thus provide better neuroprotection.[20] Correspondingly, a lack of physical activity has deleterious effects on neurogenesis in animal models, which may begin to explain the relatively poor outcomes of inactive patients who are diagnosed with neurological disorders.[21] Further evidence suggests that the mediator for these improvements, or lack thereof, is indeed the individual's amount of conditioning to a low level of oxygen, as another study manipulated this with hyperbaric oxygen therapy preconditioning and found significant neuroprotective effects.[22]
Returning to PTX3, Dr. Shindo's findings of its protective effects on BBB tightness under hypoxic conditions represent an important area for future investigation.[10] To start with, one study has found increased glial scarring and edema in PTX3 knockout mice.[23] It seems likely that the protective effects of exercise and hyperbaric oxygen preconditioning would be related to the expression of PTX3, possibly with PTX3 as the mechanism behind some of the findings.
Astrocytes in Context
According to some studies, astrocytes are the most common cell type in the CNS.[24] This makes sense given that they are one of the cell types most responsible for maintaining cerebrovascular homeostasis.[10] It is, therefore, natural that they should play a central role in stroke research. Structurally and functionally, astrocytes act in the gliovascular network as the support system of the brain.[25,26] Structurally, astrocytes' fibrous composition allows them to act as scaffolding for other neuronal elements.[26] This is done in large part by their closely packed, stellate-patterned processes, or end feet, which sometimes wrap all the way around vascular walls.[26] Functionally, their normal responsibilities include regularizing local pH and ion levels, delivering glucose, clearing waste products, and providing metabolic substrates.[26] Importantly, several of these functions directly and indirectly support the BBB. For example, directly, astrocytes physically support the BBB by using tight junction proteins to glue it together, as indicated previously.[27] Indirectly, glial-derived neurotrophic factor, which originates from astrocytes, helps maintain the BBB.[28] As astrocytes play such a vital role, disturbances caused by ischemic stroke can have far-reaching effects. The aforementioned expression of both VEGF and PTX3 is an example of this. It is possible that the expression of PTX3 develops as an adaptive response to the, at times, maladaptive expression of VEGF.
In addition, under stroke conditions, neurons may deplete their stores of adenosine triphosphate (ATP) faster than the surrounding glial cells.[29] In response, astrocytes have recently been observed sending their own healthy mitochondria to neurons in need, in order to replenish the neurons' ATP stores.[29,30,31] This exciting discovery demonstrates yet another way that astrocytes support the BBB and the CNS as a whole, and how observations of natural processes can lead to the innovation of new therapies. For example, recent studies explore the potential of having stem cells perform this same function of transferring healthy mitochondria, after being inspired by the natural actions of astrocytes.[32] Finding ways of supporting or simulating astrocytes in their responsibilities opens a new area of therapeutic options for before, during, and after stroke, as well as for other neurological conditions.
Conclusion
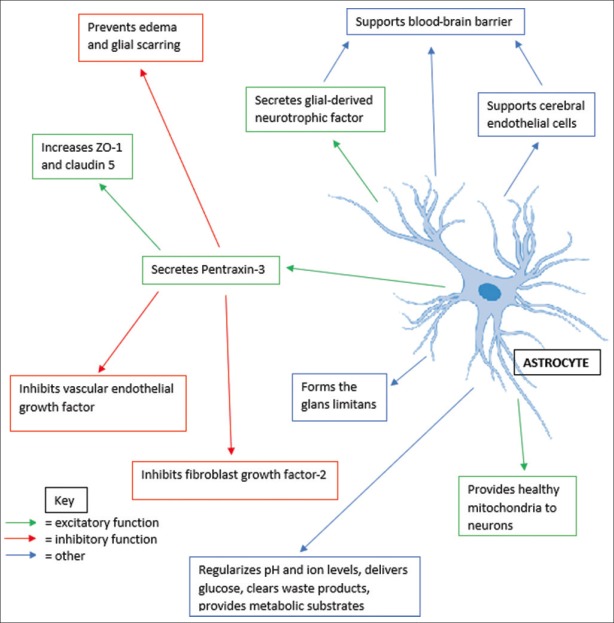
This review article is focused on the findings of Shindo et al. from their study “Astrocyte-derived pentraxin 3 supports blood–brain barrier integrity under acute phase of stroke” published in 2016 in Stroke.[10] This study examined the role of astrocytes, PTX3, VEGF, BBB, and tight junction proteins.[10] These were investigated in vivo in animal models and in vitro with cell cultures.[10] Highlights from this study include discovering that astrocyte is a major source of PTX3 in brain, as well as identifying numerous factors through which astrocytes may interact with the BBB[10] [Figure 1]. Further study is needed, but this study also provides preliminary data on tight junction proteins, ZO-1, and claudin 5 and PTX3's role during hypoxic conditions.[10] This review also aims to place key agents from this study, including PTX3 and astrocytes, in their proper context. It does so by acknowledging the limitations of each as well as additional research that shed light on the background relationships between these agents in the brain. To the end, this article intends to bring attention to new discoveries related to stroke, as well as possible areas of interest for therapeutic study.
Figure 1.
Multipronged functions and interactions of astrocytes and pentraxin-3
Financial support and sponsorship
NIH R01NS065089.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A Report from the American heart association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: A Report from the American heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Cosky EE, Ding Y. Exercise conditioning in acute ischemic stroke. Cond Med. 2018;1:204–11. [Google Scholar]
- 4.Heiss WD. The concept of the penumbra: Can it be translated to stroke management? Int J Stroke. 2010;5:290–5. doi: 10.1111/j.1747-4949.2010.00444.x. [DOI] [PubMed] [Google Scholar]
- 5.Xie L, Yang SH. Interaction of astrocytes and T cells in physiological and pathological conditions. Brain Res. 2015;1623:63–73. doi: 10.1016/j.brainres.2015.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad-Tóvolli R, Dragano NRV, Ramalho AFS, Velloso LA. Development and function of the blood-brain barrier in the context of metabolic control. Front Neurosci. 2017;11:224. doi: 10.3389/fnins.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–49. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 8.Borlongan CV, Rodrigues AA, Jr, Oliveira MC. Breaking the barrier in stroke: What should we know? A mini-review. Curr Pharm Des. 2012;18:3615–23. doi: 10.2174/138161212802002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 10.Shindo A, Maki T, Mandeville ET, Liang AC, Egawa N, Itoh K, et al. Astrocyte-derived pentraxin 3 supports blood-brain barrier integrity under acute phase of stroke. Stroke. 2016;47:1094–100. doi: 10.1161/STROKEAHA.115.012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–82. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Y. Exercise, angiogenesis and neurogenesis: A conditioning medicine in Stroke? 5th International Symposium on Conditioning Medicine. 2018. Nov, Available from: http://www.conditionmed.org/Data/View/4550 . Last accessed on 2019 Sep 23.
- 13.Cosky EEP, Ding Y. The role of vascular endothelial growth factor in angiogenesis and brain circulation after stroke. Brain Circ. 2018;4:73–5. doi: 10.4103/bc.bc_8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–23. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey D, Nandhini G, Rajkumar K. Fibroblast growth factors and their role in disease and therapy. SRM J Res Dent Sci. 2015;6:41. [Google Scholar]
- 16.Rusnati M, Camozzi M, Moroni E, Bottazzi B, Peri G, Indraccolo S, et al. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004;104:92–9. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 17.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 18.Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–76. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 19.Milton SL. Constitutive preconditioning: The anoxia tolerant freshwater turtle as a model organism of the preconditioned phenotype. Cond Med. 2019;2:90–105. [Google Scholar]
- 20.Pianta S, Lee JY, Tuazon JP, Castelli V, Mantohac LM, Tajiri N, et al. A short bout of exercise prior to stroke improves functional outcomes by enhancing angiogenesis. Neuromolecular Med. 2019 doi: 10.1007/s12017-019-08533-x. [In press]. doi: 10.1007/s12017-019-08533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippert T, Watson N, Ji X, Yasuhara T, Date I, Kaneko Y, et al. Detrimental effects of physical inactivity on neurogenesis. Brain Circ. 2016;2:80–5. doi: 10.4103/2394-8108.186278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liska GM, Lippert T, Russo E, Nieves N, Borlongan CV. A dual role for hyperbaric oxygen in stroke neuroprotection: Preconditioning of the brain and stem cells. Cond Med. 2018;1:151–66. [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Grande B, Swana M, Nguyen L, Englezou P, Maysami S, Allan SM, et al. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J Cereb Blood Flow Metab. 2014;34:480–8. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zang YW, Gu XD, Xiang JB, Chen ZY. Brain metastases from colorectal cancer: Microenvironment and molecular mechanisms. Int J Mol Sci. 2012;13:15784–800. doi: 10.3390/ijms131215784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borlongan CV, Glover LE, Sanberg PR, Hess DC. Permeating the blood brain barrier and abrogating the inflammation in stroke: Implications for stroke therapy. Curr Pharm Des. 2012;18:3670–6. doi: 10.2174/138161212802002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–30. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Cabezas R, Avila M, Gonzalez J, El-Bachá RS, Báez E, García-Segura LM, et al. Astrocytic modulation of blood brain barrier: Perspectives on Parkinson's disease. Front Cell Neurosci. 2014;8:211. doi: 10.3389/fncel.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, et al. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophys Res Commun. 1999;261:108–12. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: New insight and implications. Aging Dis. 2018;9:924–37. doi: 10.14336/AD.2017.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25:31. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo E, Napoli E, Borlongan CV. Healthy mitochondria for stroke cells. Brain Circ. 2018;4:95–8. doi: 10.4103/bc.bc_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo E, Nguyen H, Lippert T, Tuazon J, Borlongan CV, Napoli E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018;4:84–94. doi: 10.4103/bc.bc_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]