Abstract
Progress in stem cell research demonstrates stem cells' potential for treating neurodegenerative diseases. Stem cells have proliferative/differentiative properties and produce a variety of paracrine factors that can potentially be used to regenerate nervous tissue. Previous studies have shown the positive regenerative effects of endothelial progenitor cells (EPCs), and thus, they may be used as a tool for regeneration. A study by Di Santo et al. explored whether EPC-derived conditioned medium (EPC-CM) promotes the survival of cultured striatal progenitor cells and attempted to find the paracrine factors and signaling pathways involved with EPC-CM's effects. The neuronal progenitor cells that were cultured with EPC-CM had much higher densities of GABA-immunoreactive (GABA-ir) neurons. It was shown that phosphatidylinositol-3-kinase/AKT and mitogen-activated protein kinase/ERK signaling pathways are involved in the proliferation of GABAergic neurons, as inhibition of these pathways decreased GABAergic densities. In addition, the results suggest that paracrine factors from EPC, both proteinaceous and lipidic, significantly elevated the viability and/or differentiation in the cultures. Importantly, it was found that EPC-CM provided neuroprotection against toxins from 3-nitropropionic acid. In sum, EPC-CM engendered proliferation and regeneration of the cultured striatal cells through paracrine factors and imparted neuroprotection. Furthermore, the effects of EPC-CM may generate a cell-free therapeutic strategy to address neurodegeneration.
Keywords: 3-nitropropionic acid, endothelial progenitor cells, GABAergic neurons, neuroprotection, paracrine factors, regeneration, signaling pathways, striatal progenitor cells, therapeutic strategy against neurodegeneration
Novel Discoveries about the Potential of Endothelial Progenitor Cells as a Therapeutic Tool
Effective treatments for neurodegenerative diseases are still lacking. The advancements in stem cell research have revealed the proliferative and differentiation properties of these cells; therefore, these cells can hypothetically be used to regenerate nervous tissues. Stem cells are not only capable of replacing injured cells but also have the ability to produce a variety of factors. The humoral actions of stem cells, rather than transdifferentiation/engraftment, are now thought to have the most influence on tissue regeneration. Evidence demonstrates that bone marrow-derived endothelial precursor cells release paracrine factors that increase the viability of various tissues. Recent studies have demonstrated the potential of endothelial progenitor cell (EPC) “secretome” within its conditioned medium form (EPC-CM).[1,2,3] EPCs and their soluble factors have also been shown to be successful in treating traumatic brain injury, ischemic stroke, and white matter damage models. However, the potential of EPCs has often been exploited for neurovascular repair.[4,5,6,7]
Effects of Endothelial Progenitor Cell-Derived Conditioned Medium on GABA-immunoreactive Neurons and the Cellular Mechanisms Involved
Substantial evidence suggests that stem cells and progenitor cells utilize paracrine factors to carry out regeneration.[1,2,3,4] Recent studies have demonstrated that the viability of brain microvascular cells is significantly increased by incubation with EPC-CM.[3] The recent study of Di Santo et al. provided evidence that EPC-CM might support neuronal progenitors from ganglionic eminence (GE).[8] To investigate this, primary cultures from fetal rat embryonic (E14) GE were utilized, and these were grown for 7 days in vitro (DIV). The striatal cultures incubated with the EPC-CM in the DIV5–7 period were found to have significantly higher densities of GABA-immunoreactive (GABA-ir) neurons. When the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) pathways were inhibited, the effects of EPC-CM were reduced, as the inhibition of the pathways significantly impaired EPC-CM's ability to increase the density of GABA-ir cells. Similar results occurred when EPC-CM endured proteolytic digestion and lipid extraction, which impeded translation. Overall, these results suggest that paracrine factors from EPC significantly enhanced the survival and/or differentiation in the cultured striatal progenitor cells through proteinaceous and lipidic factors.
Endothelial Progenitor Cells Produce Paracrine Factors that Promote Regeneration
Several studies have documented the regenerative potential of EPCs, and their capacity to sustain a functional vascular system, which is vital to transporting nutrients, signaling molecules, and cells to the site of tissue injury.[9,10,11,12] Research from the last decade suggests that EPC's regenerative ability may be effective in more than just vascular tissue.[13,14,15,16]
Preclinical trials have shown that EPC paracrine factors can be employed as a therapeutic option.[17] Similarly, mesenchymal stem cells were found to be a plentiful source of paracrine factors and, similar to EPC, can potentially be utilized for a wide variety of regenerative therapies, such as for myocardial infarction and stroke.[18] Furthermore, it has been shown that EPC-CM alleviates ischemic injury in skeletal and myocardial muscles.[1,19,20,21] In recent studies, EPC-CM showed encouraging neuroprotective capabilities for treating ischemic stroke,[22,23] increased the frequency of the dopaminergic phenotype in neuronal stem cells,[24] and enhanced the number of doublecortin-positive neuronal precursors in the subventricular zone of adult rats.[25]
Endothelial Progenitor Cell-Derived Conditioned Medium Increases GABAergic Densities through Cellular Factors and Signaling Pathways
The study of Di Santo et al.[8] is the first of its kind to specifically examine how EPC-CM affects progenitor striatal cells and aims to identify the cellular components and pathways involved. The results of this study demonstrated that EPC-CM increases the densities of GABA-ir neurons through various methods, either through greater rates of proliferation and/or increased cell viability and differentiation of progenitor neurons. The outcome of the Western blot analysis for proliferating cell nuclear antigen (a cell proliferation marker) suggests that EPC-CM increased neuronal cell proliferation in the cultures. Nevertheless, it is improbable that this observation is applicable for the increase in GABAergic cell densities observed after day 2 of the treatment. The total amount of protein was much higher in the cultures treated with EPC-CM, indicating that EPC-CM enhanced proliferation in other neurons and in other cell types. This is also demonstrated by the substantial increase of cells expressing the neuronal marker NeuN in the EPC-CM-treated cultures. In addition, the increase of glial fibrillary acidic protein in treated cultures demonstrated in the Western blot analysis may also imply EPC-CM's effects on glial cells.
Equally compelling evidence is provided on characterizing the type of cellular factors (proteinaceous and lipidic), and signaling pathways are involved with EPC-CM[8] [Figure 1]. Activation of PI3K/AKT and MAPK/ERK signaling cascades promotes the neuroprotective effects of EPC-CM. Through these pathways, creatine also stimulated the GABAergic phenotype in the striatal cultures.[26]
Figure 1.
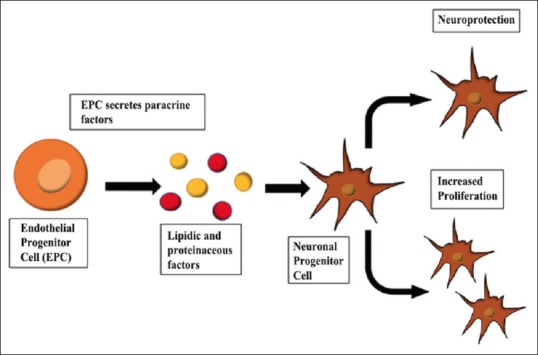
Endothelial progenitor cell and neuroprotection. Endothelial progenitor cells secrete therapeutic factors, including lipidic and proteinaceous factors. These paracrine factors exert neuroprotective effects and exhibit proliferative capacity
PI3K/AKT and MAPK/ERK's role in EPC-CM-induced effects is not unexpected due to the presence of several growth factors: brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, neuritin, and vascular endothelial growth factor. These growth factors are strong inducers of the signaling pathways.[27,28] AKT/PI3K signaling plays an important role in the central nervous system, as it imparts neuroprotection against differing stresses. For example, it provides protection against oxidative stress in the adult brain by hindering pro-apoptotic mechanisms.[29] In the in vitro and in vivo models of HD, ERK activation imparts neuroprotection.[30] Nonetheless, to depict the role of the neurotrophic factors mentioned above in EPC-CM-induced effects, direct evidence needs to be gathered through experiments blocking each of the neurotrophic factors. Interestingly, the blockage of the ROCK pathway did not reduce EPC-CM's impact on GABA-ir cell densities. ROCK is thought to influence neurite outgrowth, dendritic branching, and spine formation, which are hindered by the expression of constitutively activated RhoA.[31,32,33] The protein translation inhibitor cycloheximide abolished the actions of EPC-CM by blocking translation, suggesting that some of EPC-CM's effects were carried out indirectly, such as through growth factors in autocrine signaling.
The advantages of stem cell secretomes among varying clinical conditions have spurred investigations aiming to identify the wide range of bioactive molecules involved. Although a multitude of peptides present in EPC-CM have been discovered through proteomics,[19,34] a thorough examination of the lipidic factors released by secretomes has yet to occur. Even though EPC releases prostaglandins,[35] it was excluded from this study because it cannot be extracted by chloroform.[36] Sphingosine-1-phosphate (S1P) is an important lipid mediator released by bone marrow progenitor cells, which include EPC, and mature endothelial cells.[37] Importantly, S1P influences neuronal differentiation both directly[38] and indirectly through processes involving astrocytes.[39] It is thus reasonable to speculate that S1P might be involved in the effects of EPC-CM; however, its actual role in EPC-CM needs to be investigated.
Recent studies reveal that EPC-CM contains exosomes and microvesicles which can transport microRNA.[14,40] MicroRNA also acts as a regulator for neurogenesis.[41,42] Possibly, the identification of some exosomal cargoes[43] might be an easier task compared to the identification of the soluble proteins and lipids present in the EPC-CM.
Endothelial Progenitor Cells Exert Neuroprotection
The effects of EPC-CM are not limited to the enhancement of neuronal viability and maturation. Indeed, EPC-CM provided neuroprotection against 3-NP (an irreversible succinate dehydrogenase inhibitor). It can be hypothesized that EPC-CM may be pivotal in treating neurodegenerative diseases where energy metabolism is lessened on account of an impaired mitochondrial complex II and complex III.[44,45,46] Mitochondrial dysfunction is thought to be a major cause of neuronal deterioration in age-related stroke. Negative conditioning of mitochondrial dysfunction can potentially be a therapeutic method to ameliorate the quality and function of mitochondria.[47] Surprisingly, in the current study, EPC-CM increased the cytoprotective effect of viable cells before the tissue injury; however, a change in GABA-ir neuron densities was not observed. This suggests that EPC-CM also bestows cytoprotective effects to other cell subpopulations in the neuronal cultures. Moreover, 3-NP also displays toxicity for cultured hippocampal, septal, and hypothalamic neurons.[48] In addition, EPC-CM significantly protected dopaminergic neurons against 1-methyl-4-phenylpyridinium (MPP+). MPP+ toxicity involves the generation of excessive oxidative stress and provides an effective tool for assessment of neuroprotection on dopaminergic neurons.[49] In conclusion, the study suggests that EPC-CM, in conjunction with other methods, may pave the way to overcoming neuronal degeneration.
Other Mechanisms that Impart Neuroprotection in the Treatment of Traumatic Brain Injury and Stroke-Induced Damage
Stroke continues to be the leading cause of death, and those who survive the incident endure long-term disabilities. A major type of pharmacological treatment is the thrombolytic agent tissue plasminogen activator. However, this treatment is not ideal because it has a limited therapeutic time window and could potentially lead to intracranial hemorrhage. In addition, the tissue plasminogen activator fails to provide neuroprotection.[50] The development of neuroprotectants is of great importance in treating poststroke injuries. Recent evidence demonstrates that simple medicinal cannabis formulations can function as effective neuroprotectants.[51] Hyperbaric oxygen therapy (HBOT) is another potential neuroprotective strategy for treating ischemic stroke. HBOT preconditioning of the brain demonstrates promising results as priming the brain with mild oxidative stress prepares the brain for the full-fledged oxidative stress induced by a stroke.[52] Moreover, EPC-CM may act as an essential tool in treating poststroke injuries because EPC-CM can impart neuroprotection as well.
Additional Examples of Trophic Factors and Signaling Pathways That May Play a Role in Neuronal Regeneration
Stem cell transplantations are becoming a pivotal area of research in finding treatments for poststroke injuries. Several beneficial effects have been ascribed to stem cells in treating ischemic stroke, including secreting therapeutic factors around the injured area, initiating regeneration, and promoting recovery.[53] An example of a trophic factor involved in neural activity after stroke is bone morphogenetic protein 7 (BMP7), which can promote DNA synthesis. In a rodent distal middle cerebral artery occlusion (MCAO) model, treatment with BMP7 showed positive results in that sensorimotor function improved, body asymmetry decreased, and locomotor activity escalated.[54] Thus, proteinaceous factors such as BMP7 and those found in EPC-CM may be valuable for treating stroke-induced injuries.
The study by Di Santo et al.[8] demonstrates that EPC-CM's effects stem from the activation of signaling pathways such as PI3K/AKT and MAPK/ERK. The cascade of events, involving both cytotoxic and cytotropic effects, that occur in the inflammatory response of the central nervous system may be altered to impart regenerative properties. In traumatic brain injury or stroke, chronic brain inflammation inhibits neuronal regeneration. Although the inflammatory response acts as a growth inhibitor, it can be altered so that it becomes protective and reparative.[55]
The Importance and Efficacy of Conditional Medicine for Treating Stroke-Induced Brain Injury and Neurodegenerative Diseases
An array of conditioning stimuli, such as ischemia or hypoxia, may provide protection against poststroke brain injury.[56] Evidence also suggests that conditional stimuli can provide protection to the cerebral vasculature, which includes the blood–brain barrier.[56] In addition, Parkinson's disease is associated with a loss in dopaminergic neurons. EPC-CM promoted dopaminergic phenotype in neuronal stem cells, and thus, EPC-CM may act as another tool for treating Parkinson's disease and other neurodegenerative disorders. Likewise, a study conducted by Leak suggests the efficiency of preconditioning dopaminergic neurons in treating Parkinson's disease. In early stages of Parkinson's disease, cells are exposed to mild stress and natural defenses escalate. However, as the patient ages, stress levels substantially increase to a point where toxic cellular responses can no longer be prevented. These results suggest that if dopaminergic systems are preconditioned with mild stress, the stress would strengthen the cells and reduce the toxic effects of stresses that follow.[57] Overall, the current neurological research focuses on utilizing stem cells such as EPC-CM, due to their regenerative and neuroprotective properties, to treat stroke-induced injuries and other neurodegenerative diseases.
Conclusion
The study by Di Santo et al.[8] investigated whether EPCs were capable of spurring proliferation and differentiation in striatal progenitor cells. It was found that through paracrine factors and signaling pathways, EPCs increased GABAergic densities in the cultures. In addition, EPCs imparted neuroprotection in the cultures, which indicates the potential of EPCs in treating brain injury. Therefore, EPCs may become a novel tool for conquering neurodegenerative diseases and traumatic brain injury.
Financial support and sponsorship
This research was supported by the HANELA Foundation (HRW) and the Swiss National Science Foundation NRP63 No. 406340_128124 (SDS).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, et al. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Santo S, Seiler S, Fuchs AL, Staudigl J, Widmer HR. The secretome of endothelial progenitor cells promotes brain endothelial cell activity through PI3-kinase and MAP-kinase. PLoS One. 2014;9:e95731. doi: 10.1371/journal.pone.0095731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Santo S, Fuchs AL, Periasamy R, Seiler S, Widmer HR. The cytoprotective effects of human endothelial progenitor cell-conditioned medium against an ischemic insult are not dependent on VEGF and IL-8. Cell Transplant. 2016;25:735–47. doi: 10.3727/096368916X690458. [DOI] [PubMed] [Google Scholar]
- 4.Rosell A, Morancho A, Navarro-Sobrino M, Martínez-Saez E, Hernández-Guillamon M, Lope-Piedrafita S, et al. Factors secreted by endothelial progenitor cells enhance neurorepair responses after cerebral ischemia in mice. PLoS One. 2013;8:e73244. doi: 10.1371/journal.pone.0073244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Li Y, Wang S, Han Z, Huang X, Li S, et al. Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J Surg Res. 2013;185:441–9. doi: 10.1016/j.jss.2013.05.073. [DOI] [PubMed] [Google Scholar]
- 6.Park KJ, Park E, Liu E, Baker AJ. Bone marrow-derived endothelial progenitor cells protect postischemic axons after traumatic brain injury. J Cereb Blood Flow Metab. 2014;34:357–66. doi: 10.1038/jcbfm.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki T, Morancho A, Martinez-San Segundo P, Hayakawa K, Takase H, Liang AC, et al. Endothelial progenitor cell secretome and oligovascular repair in a mouse model of prolonged cerebral hypoperfusion. Stroke. 2018;49:1003–10. doi: 10.1161/STROKEAHA.117.019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santo SD, Seiler S, Andres R, Widmer HR. Endothelial progenitor cells conditioned medium supports number of GABAergic neurons and exerts neuroprotection in cultured striatal neuronal progenitor cells. Cell Transplant. 2019;28:367–78. doi: 10.1177/0963689719835192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–8. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 11.Hecht N, Schneider UC, Czabanka M, Vinci M, Hatzopoulos AK, Vajkoczy P, et al. Endothelial progenitor cells augment collateralization and hemodynamic rescue in a model of chronic cerebral ischemia. J Cereb Blood Flow Metab. 2014;34:1297–305. doi: 10.1038/jcbfm.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong Z, Hong Y, Zhu J, Cheng X, Liu Y. Endothelial progenitor cells improve functional recovery in focal cerebral ischemia of rat by promoting angiogenesis via VEGF. J Clin Neurosci. 2018;55:116–21. doi: 10.1016/j.jocn.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Cao JP, He XY, Xu HT, Zou Z, Shi XY. Autologous transplantation of peripheral blood-derived circulating endothelial progenitor cells attenuates endotoxin-induced acute lung injury in rabbits by direct endothelial repair and indirect immunomodulation. Anesthesiology. 2012;116:1278–87. doi: 10.1097/ALN.0b013e3182567f84. [DOI] [PubMed] [Google Scholar]
- 14.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–27. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 15.Kado M, Tanaka R, Arita K, Okada K, Ito-Hirano R, Fujimura S, et al. Human peripheral blood mononuclear cells enriched in endothelial progenitor cells via quality and quantity controlled culture accelerate vascularization and wound healing in a porcine wound model. Cell Transplant. 2018;27:1068–79. doi: 10.1177/0963689718780307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–69. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keighron C, Lyons CJ, Creane M, O'Brien T, Liew A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med (Lausanne) 2018;5:354. doi: 10.3389/fmed.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunawardena TN, Rahman MT, Abdullah BJ, Abu Kasim NH. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J Tissue Eng Regen Med. 2019;13:569–86. doi: 10.1002/term.2806. [DOI] [PubMed] [Google Scholar]
- 19.Felice F, Piras AM, Rocchiccioli S, Barsotti MC, Santoni T, Pucci A, et al. Endothelial progenitor cell secretome delivered by novel polymeric nanoparticles in ischemic hindlimb. Int J Pharm. 2018;542:82–9. doi: 10.1016/j.ijpharm.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Doyle B, Sorajja P, Hynes B, Kumar AH, Araoz PA, Stalboerger PG, et al. Progenitor cell therapy in a porcine acute myocardial infarction model induces cardiac hypertrophy, mediated by paracrine secretion of cardiotrophic factors including TGFbeta1. Stem Cells Dev. 2008;17:941–51. doi: 10.1089/scd.2007.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes B, Kumar AH, O'Sullivan J, Klein Buneker C, Leblond AL, Weiss S, et al. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34:782–9. doi: 10.1093/eurheartj/ehr435. [DOI] [PubMed] [Google Scholar]
- 22.Morancho A, Hernández-Guillamon M, Boada C, Barceló V, Giralt D, Ortega L, et al. Cerebral ischaemia and matrix metalloproteinase-9 modulate the angiogenic function of early and late outgrowth endothelial progenitor cells. J Cell Mol Med. 2013;17:1543–53. doi: 10.1111/jcmm.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev Rep. 2011;7:208–20. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- 24.Di Santo S, Widmer HR. Paracrine factors for neurodegenerative disorders: Special emphasis on Parkinson's disease. Neural Regen Res. 2016;11:570–1. doi: 10.4103/1673-5374.180739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andereggen L, Reitmeir R, Di Santo S, Guzman R, Widmer HR, Marbacher S, et al. Modulation of post-stroke plasticity and regeneration by stem cell therapy and exogenic factors. Springer International Publishing: Cham; 2018. pp. 129–52. [Google Scholar]
- 26.Andres RH, Ducray AD, Huber AW, Pérez-Bouza A, Krebs SH, Schlattner U, et al. Effects of creatine treatment on survival and differentiation of GABA-ergic neurons in cultured striatal tissue. J Neurochem. 2005;95:33–45. doi: 10.1111/j.1471-4159.2005.03337.x. [DOI] [PubMed] [Google Scholar]
- 27.Yao JJ, Gao XF, Chow CW, Zhan XQ, Hu CL, Mei YA. Neuritin activates insulin receptor pathway to up-regulate Kv4.2-mediated transient outward K+current in rat cerebellar granule neurons. J Biol Chem. 2012;287:41534–45. doi: 10.1074/jbc.M112.390260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azuchi Y, Namekata K, Shimada T, Guo X, Kimura A, Harada C, et al. Role of neuritin in retinal ganglion cell death in adult mice following optic nerve injury. Sci Rep. 2018;8:10132. doi: 10.1038/s41598-018-28425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong J. RAS and downstream RAF-MEK and PI3K-AKT signaling in neuronal development, function and dysfunction. Biol Chem. 2016;397:215–22. doi: 10.1515/hsz-2015-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarantos MR, Papanikolaou T, Ellerby LM, Hughes RE. Pizotifen activates ERK and provides neuroprotection in vitro and in vivo in models of Huntington's disease. J Huntingtons Dis. 2012;1:195–210. doi: 10.3233/JHD-120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bramham CR, Wells DG. Dendritic mRNA: Transport, translation and function. Nat Rev Neurosci. 2007;8:776–89. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- 33.Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76(Pt C):639–56. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, Ackerman R, Saleh M, Gotlinger KH, Kessler M, Mendelowitz LG, et al. 20-HETE regulates the angiogenic functions of human endothelial progenitor cells and contributes to angiogenesis in vivo. J Pharmacol Exp Ther. 2014;348:442–51. doi: 10.1124/jpet.113.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauber K, Bohn E, Kröber SM, Xiao YJ, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, et al. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated rac1 and cdc42 signaling. Circ Res. 2009;105:696–704. doi: 10.1161/CIRCRESAHA.109.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan B, Luo Z, Yue Y, Liu Y, Pan L, Yu L, et al. Effects of FTY720 (Fingolimod) on proliferation, differentiation, and migration of brain-derived neural stem cells. Stem Cells Int. 2016:9671732. doi: 10.1155/2016/9671732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spohr TC, Dezonne RS, Nones J, Dos Santos Souza C, Einicker-Lamas M, Gomes FC, et al. Sphingosine 1-phosphate-primed astrocytes enhance differentiation of neuronal progenitor cells. J Neurosci Res. 2012;90:1892–902. doi: 10.1002/jnr.23076. [DOI] [PubMed] [Google Scholar]
- 40.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Yuan Y, Zhang Z, Ding K. Inhibition of miRNA-27b enhances neurogenesis via AMPK activation in a mouse ischemic stroke model. FEBS Open Bio. 2019;9:859–69. doi: 10.1002/2211-5463.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabelström H, Petri R, Shchors K, Jandial R, Schmidt C, Sacheva R, et al. Driving neuronal differentiation through reversal of an ERK1/2-miR-124-SOX9 axis abrogates glioblastoma aggressiveness. Cell Rep. 2019;28:2064–79.e11. doi: 10.1016/j.celrep.2019.07.071. [DOI] [PubMed] [Google Scholar]
- 43.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: A web-based compendium of exosomal cargo. J Mol Biol. 2016;428:688–92. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tracey TJ, Steyn FJ, Wolvetang EJ, Ngo ST. Neuronal lipid metabolism: Multiple pathways driving functional outcomes in health and disease. Front Mol Neurosci. 2018;11:10. doi: 10.3389/fnmol.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington's disease caudate nucleus. Ann Neurol. 1996;39:385–9. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 46.Calabresi P, Gubellini P, Picconi B, Centonze D, Pisani A, Bonsi P, et al. Inhibition of mitochondrial complex II induces a long-term potentiation of NMDA-mediated synaptic excitation in the striatum requiring endogenous dopamine. J Neurosci. 2001;21:5110–20. doi: 10.1523/JNEUROSCI.21-14-05110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvaraji S, Poh L, Natarajan V, Mallilankaraman K, Arumugam TV. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond Med. 2019;2:30–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Fink SL, Ho DY, Sapolsky RM. Energy and glutamate dependency of 3-nitropropionic acid neurotoxicity in culture. Exp Neurol. 1996;138:298–304. doi: 10.1006/exnr.1996.0068. [DOI] [PubMed] [Google Scholar]
- 49.Di Santo S, Seiler S, Ducray AD, Widmer HR. Conditioned medium from endothelial progenitor cells promotes number of dopaminergic neurons and exerts neuroprotection in cultured ventral mesencephalic neuronal progenitor cells. Brain Res. 2019;1720:146330. doi: 10.1016/j.brainres.2019.146330. [DOI] [PubMed] [Google Scholar]
- 50.Leng T, Xiong ZG. Treatment for ischemic stroke: From thrombolysis to thrombectomy and remaining challenges. Brain Circ. 2019;5:8–11. doi: 10.4103/bc.bc_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolb B, Saber H, Fadel H, Rajah G. The endocannabinoid system and stroke: A focused review. Brain Circ. 2019;5:1–7. doi: 10.4103/bc.bc_29_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liska GM, Lippert T, Russo E, Nieves N, Borlongan CV. A dual role for hyperbaric oxygen in stroke neuroprotection: Preconditioning of the brain and stem cells. Cond Med. 2018;1:151–66. [PMC free article] [PubMed] [Google Scholar]
- 53.Chau M, Zhang J, Wei L, Yu SP. Regeneration after stroke: Stem cell transplantation and trophic factors. Brain Circ. 2016;2:86–94. doi: 10.4103/2394-8108.186279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dewan SN, Wang Y, Yu S. Drug treatments that optimize endogenous neurogenesis as a therapeutic option for stroke. Brain Circ. 2017;3:152–5. doi: 10.4103/bc.bc_20_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maclean FL, Horne MK, Williams RJ, Nisbet DR. Review: Biomaterial systems to resolve brain inflammation after traumatic injury. APL Bioeng. 2018;2:021502. doi: 10.1063/1.5023709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang J, Andjelkovic AV, Zhou N, Hua Y, Xi G, Wang MM, et al. Is there a central role for the cerebral endothelium and the vasculature in the brain response to conditioning stimuli? Cond Med. 2018;1:220–32. [PMC free article] [PubMed] [Google Scholar]
- 57.Leak RK. Conditioning against the pathology of Parkinson's disease. Cond Med. 2018;1:143–62. [PMC free article] [PubMed] [Google Scholar]


