Abstract
The process of aging underlies many degenerative disorders that arise in the living body, including gradual neuronal loss of the hippocampus that often leads to decline in both memory and cognition. Recent evidence has shown a significant connection between gut microbiota and brain function, as butyrate production by microorganisms is believed to activate the secretion of brain-derived neurotrophic factor (BDNF). To investigate whether modification of intestinal microbiota could impact cognitive decline in the aging brain, Romo-Araiza et al. conducted a study to test how probiotic and prebiotic supplementation impacted spatial and associative memory in middle-aged rats. Their results showed that rats supplemented with the symbiotic (both probiotic and prebiotic) treatment performed significantly better than other groups in the spatial memory test, though not in that of associative memory. Their data also reported that this improvement correlated with increased levels of BDNF, decreased levels of pro-inflammatory cytokines, and better electrophysiological outcomes in the hippocampi of the symbiotic group. Thus, the results indicated that the progression of cognitive impairment is indeed affected by changes in microbiota induced by probiotics and prebiotics. Potential future applications of these findings center around combatting neurodegeneration and inflammation associated not only with aging but also with the damaging posttraumatic effects of ischemic stroke.
Keywords: Associative memory, brain-derived neurotrophic factor, butyrate, cytokines, hippocampus, microbiota, prebiotics, probiotics, spatial memory, symbiotic
Introduction: Hippocampal Neurodegeneration is Linked with Aging
Degenerative changes within the central nervous system are often associated with aging and lead to decline in memory and learning capacity, both of which depend on the hippocampus.[1,2] Mild cognitive impairment (MCI) is regarded as the intermediate stage between healthy aging and dementia, and the present estimates indicate that 30%–100% of patients with MCI will progress to the latter.[3] Moreover, the rate of evolution from MCI to Alzheimer's disease has been reported to be 15% per year.[4]
MCI is linked to neurodegeneration in the hippocampus, and this loss of neurons has been related to mitochondrial defects, as well as oxidative stress that increases levels of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α.[5,6,7,8] The result of such alterations is often neuroinflammation and consequently increased activity of microglia.[1,9,10]
Neuroinflammation Reduces Synaptic Plasticity and Levels of Brain-derived Neurotrophic Factor
Neuroinflammation is correlated with a decline in cognitive function and memory, primarily because inflammation of the hippocampus tends to cause deleterious changes in synaptic transmission and plasticity.[11] Decreased plasticity can lead to the deficient synthesis of glutamate with N-methyl-D-aspartate receptor (NMDAR) within the dentate gyrus and CA1 and CA3 regions of the hippocampus.[12] A lower number of these receptors seem to alter long-term potentiation (LTP) generation, which represents experimental evidence of synaptic plasticity and memory consolidation.[8,11,13] Neuroinflammation also reduces hippocampal gene expression of brain-derived neurotrophic factor (BDNF) a protein neurotrophic factor that is linked closely to synaptic plasticity and thus to memory consolidation.[14,15,16]
Because BDNF helps to sustain and enhance LTP induction, it serves an essential role in cognitive function.[16] Aging is associated with decreased levels of BDNF, suggesting that the maintenance of adequate BDNF concentrations could potentially help to preclude or delay the onset of cognitive impairment.[17]
Enhancing Short-Chain Fatty Acid Production through Modification of Gut Microbiota
One convenient way to raise BDNF levels is supplementation of butyrate, a short-chain fatty acid (SCFA) that functions as a histone deacetylase inhibitor.[18,19] Butyrate maintains the relaxation of chromatin and thereby enhances BDNF expression in the hippocampus.[18,19,20,21,22] Secretion of pro-inflammatory cytokines may also be inhibited by BDNF, as the latter molecule interferes with activation of nuclear factor-kappa beta (NF-κβ).[23] In addition, the expression of enzymes involved in the production of glutathione (GSH) may also be triggered by butyrate secretion.[24,25,26] GSH is an antioxidant enzyme that relieves oxidative stress – another neurodegenerative risk factor – by reducing hydrogen peroxide and lipid hydroperoxide.[1,24,25]
The intestinal microbiota is responsible for a significant proportion of SCFA production.[1] However, levels of SCFA decline with age due to dysbiosis, a microbial imbalance that often results in a considerable increase in pathological bacteria (Proteobacterium) at the expense of mutualistic ones (Bifidobacterium).[1] Progression of gut dysbiosis has been linked to chronic systemic inflammation, including inflammation of the brain.[27]
One recent study concluded that microbiota likely influences different MCI pathophysiological sequences within the brain.[28] For this reason, supplementation with probiotics and prebiotics may counteract the damaging effects that aging has on the brain by not only lessening inflammation and oxidative stress but also by increasing neurotrophic factors and neuronal plasticity.[28]
Prebiotics are nondigestible food materials that are fermented by gut microbiota, thereby selectively enhancing the growth and activity of these microbes, as well as promoting the production of SCFA.[23] On the other hand, probiotics are living microorganisms that, when consumed in adequate quantities, provide health benefits to the host.[29]
The most commonly used prebiotics are fructo- oligosaccharides (FOS), such as agave inulin.[23] Agave inulin selectively stimulates the growth and activity of Enterococcus faecium, a probiotic that has been observed to reduce concentrations of pro-inflammatory cytokines in the gut and to indirectly promote butyrate synthesis by cross-feeding butyrate-producing bacteria.[30]
Testing the Effects of Microbiota Supplementation on the Memory of Middle-aged Rats
With past evidence supporting the idea that elevated butyrate production by healthy gut microbiota can both reduce neuroinflammation and increase BDNF levels and synaptic plasticity,[17,21,22] Romo-Araiza et al. were prompted to test whether altering gut microbiota would significantly impact memory and cognition.[1] To assess the effects of probiotic and prebiotic supplementation on spatial and associative memory, they utilized a model of middle-aged rats.[1]
Their test paradigm consisted of Sprague-Dawley male rats that were assigned randomly to four conditions (n = 13 per condition), henceforward referred to as the control (water), probiotic (E. faecium), prebiotic (agave inulin), and symbiotic (E. faecium + agave inulin) groups.[1] Treatments were administered daily by oral gavage over a 5-week period.[1] Performance in the Morris water maze (MWM) and Pavlovian autoshaping tests evaluated spatial and associative memory, respectively.[1] Following euthanasia of the rats, hippocampi were extracted, and application of the enzyme-linked immunosorbent assay analyzed tissue concentrations of cytokines (IL-1β and TNF-α), BDNF, and γ-aminobutyric acid.[1] Butyrate levels were also analyzed in feces.[1] Finally, physiological responses were compared in hippocampal tissue samples from the control and symbiotic groups (n = 8 per group).[1]
Symbiotic Supplementation Leads to Improvement in Spatial Memory
Although no significant improvement in the Pavlovian autoshaping tests was observed for any condition, the symbiotic group performed significantly better in the MWM (P < 0.01).[1] Significantly higher quantities of BDNF and butyrate (P < 0.0001) and significantly lower quantities of pro-inflammatory cytokines (P < 0.01) were found in the symbiotic group.[1] The decline in IL-1β, in particular, correlated with superior performance in the MWM (P < 0.05).[1] Thus, these results corroborated the prevailing outlook that inflammation of the brain and a deficiency of BDNF are associated with age-related cognitive impairment.[1]
Multiple Pathways for Anti-inflammation
Neuroinflammation occurs as a consequence of chronic system inflammation, which is known to be linked to aging; neuroinflammation is further related to the incidence of MCI.[1,31,32] When pro-inflammatory cytokines, particularly IL-1β, are overexpressed, a significant impact on spatial memory tasks, as well as a correlation with dementia and delirium, has been observed.[31] Elevated levels of IL-1β also negatively influence late-phase LTP generation and may thereby impair memory consolidation.[32]
Cytokine concentrations in peripheral regions of the brain also apparently affect working memory, as changes in neural activity have been linked to systemic inflammation with high levels of IL-1β, which can irreversibly depolarize the membrane and cause proconvulsive activity and neuronal death.[32] The common conclusion between the aforementioned findings and the study by Romo-Araiza et al. is that perpetual and progressive inflammation within the brain exerts deleterious effects upon cognitive function.[1,31,32] The latter demonstrated that reducing neuroinflammation can be an effective therapeutic strategy for preventing cognitive decline, and it also provided evidence that symbiotic therapy leads to decreased concentrations of pro-inflammatory molecules IL-1β and TNF-α in the hippocampus.[1] In addition, the principally investigated study showed a marked correlation between reduced cytokine levels and superior performance in spatial memory and learning tests.[1] Various pathways may have been affected to induce the overall anti-inflammatory effect, although the data indicated that the symbiotic treatment enhanced the production of butyrate, a SCFA, which, along with stimulating BDNF secretion, exert anti-inflammatory effects by binding to the G protein-coupled receptor 43 (GPR43) on mononuclear cells and by modulating the synthesis of inflammatory cytokines.[1,26,33]
Even in the absence of the probiotic, agave inulin may have also acted as an anti-inflammatory agent by inhibiting the expression of GPR43.[1,34] Inulin can likely function as a signal in immunomodulation by mimicking pathogen-associated molecular patterns and ligand binding to toll-like receptors (TLRs).[34] The result is increased concentrations of anti-inflammatory cytokines, such as IL-10, in human peripheral blood monocytes.[34] Activation of AMP-activated kinase (AMPK) may also be triggered by inulin; AMPK further regulates TLR4 and inflammatory processes by inhibiting the transcription factor NF-κβ.[35] Thus, in the experiment currently investigated, any of the above anti-inflammatory mechanisms may have contributed to the enhancement of cognitive function in the symbiotic group.[1] However, the observed increase in hippocampal levels of BDNF was likely also responsible for improvements in memory and learning.[1]
The Importance of Brain-derived Neurotrophic Factor and the Multipurpose Role of Butyrate
Expression of the gene bdnf IV is apparently essential for long-term memory formation, as BDNF must be present to stimulate hippocampal–neocortical interactions before and during ( first 24 h) the consolidation of memories.[36] BDNF levels have been demonstrated to respond to changes in microbiota, as one study found that mice treated with antibiotics developed dysbiosis and had diminished hippocampal concentrations of BDNF.[37] Both probiotics and prebiotics have been observed to increase BDNF levels in the hippocampus, and the probiotic Bifidobacterium longum, in particular, has been linked to elevated levels of this neurotrophic factor.[1,38] Inulin, a prebiotic FOS, also plays a significant role by stimulating both the transcription of bdnf IV and the synthesis of N-methyl-D-aspartate receptor subunit (a subunit of the NMDAR) in the dentate gyrus.[39]
Regarding the previously mentioned studies, higher levels of BDNF induced by probiotic and prebiotic supplementation were primarily associated with the secretion of butyrate (or other SCFA) by butyric acid-producing bacteria, such as Clostridium butyricum.[40] C. butyricum has been observed to counteract cognitive decline and histopathological alterations in the CA1 region of the hippocampus in vascular dementia mice, which reported increases in both hippocampal BDNF and fecal butyrate concentrations.[40] Butyrate can also act as an anti-inflammatory agent by inhibiting NF-κβ, which thereby regulates inflammatory cytokines including but not limited to IL-1β, TNF-α, and IL-6.[22] IL-1β, specifically, has been observed to disrupt BDNF signaling, suggesting that the decline in cytokine concentrations allows BDNF levels to rise, a relationship consistent with results for the symbiotic group in the principal study under review.[1,41] Although only fecal butyrate concentrations were analyzed in the study by Romo-Araiza et al., the introduction of butyric acid-producing bacteria into microbiota has been linked to higher quantities of butyrate in the brain as well.[1,33,40]
Electrophysiological Testing of Hippocampal Tissue
Finally, the symbiotic treatment evidently altered the passive properties of CA1 pyramidal cells and increased the N-methyl-D-aspartate/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ratio.[1] The symbiotic group also reported more vigorous LTP (P < 0.01).[1]
It seems that the enhanced cognitive performance and electrophysiological responses of the symbiotic-treated rats was the combined effect of both anti-inflammatory mechanisms and BDNF secretion.[1] In the study, improvements in spatial memory were observed in both the prebiotic and probiotic groups in comparison to the control, although only the relative progress made by the symbiotic group was deemed statistically significant.[1] On the other hand, while the symbiotic group also reported the best performance in associative memory, no significant increase was achieved by any of the treatment groups, perhaps because associative memory is a function of the cerebellum as well as the hippocampus.[1,42] Moreover, the hippocampus has been identified as considerably more susceptible to age-related deterioration than most other structures of the brain, potentially explaining why spatial memory may be more noticeably responsive to treatment than is associative memory.[1]
Testing of hippocampal tissue also obtained better electrophysiological outcomes for the symbiotic group.[1] The activation of NMDA receptors mediates the induction of hippocampal LTP, which, in turn, is required to compile and store long-term spatial memories.[1,14,16] Because expression of the functional subunits of NMDA receptors declines with aging,[43] deficient induction of LTP in the hippocampus can result and negatively impact spatial learning capacity.[44] Thus, maintaining adequate production and function of NMDA receptors represents a viable approach to reducing the onset of MCI.[1]
As expected, upregulation of the NMDA receptor in hippocampal tissue of the symbiotic group correlated with enhanced LTP induction relative to that of the control.[1] In regard to the relationship between BDNF and NMDA receptors, there has been previous evidence to suggest that activation of the latter molecule triggers the production and secretion of the neurotrophic factor, which, in turn, interacts with those same NMDA receptors to increase excitatory synaptic transmission in the cortex and hippocampus.[45,46] BDNF has also been associated with generating and preserving LTP in the CA1 region of the hippocampus.[47,48] In the study of primary interest, substantially elevated hippocampal levels of BDNF in rats of the symbiotic group were concomitant with their significantly better performance in the spatial memory test.[1] Accordingly, their superior electrophysiological response results may have been an observed effect of heightened BDNF concentrations.[1]
Conclusions
The overall results of the above examined study by Romo-Araiza et al. indicated that symbiotic supplementation could help counteract age-related memory loss, and it provided evidence to corroborate the prevailing outlook that inflammation of the brain and a deficiency of BDNF, a neurotrophic factor important to memory and learning, are associated with age-related cognitive impairment.[1] Their findings showed that, consumed together at least, probiotics and prebiotics have the capacity to promote BDNF secretion and downregulate the production of pro-inflammatory cytokines within the hippocampus.[1] This study elucidates a significant connection between gut microbiota and brain function, as treatment with symbiotics resulted in an observed improvement in neural plasticity and thus memory and learning in middle-aged rats.[1] Furthermore, these conclusions highlight the potential of symbiotic supplementation as a clinical therapy to treat MCI by counteracting the decline in cognition and memory associated with aging.[1]
The aforementioned benefits to anti-inflammation and neurogenesis suggest that symbiotic supplementation may have far-reaching applications in regard to the treatment of neurological diseases [Figure 1]. Along with helping to combat MCI and other neurodegenerative disorders linked to aging, other SCFAs may positively impact the brain–gut axis in cases of ischemic stroke.[19] Secondary neuronal death following ischemic stroke has been associated with heightened inflammatory responses in the brain,[49,50,51] and activation of microglia by the latter can lead to the release of pro-inflammatory mediators that promote the permeabilization of the blood–brain barrier (BBB) and the physical and biochemical barrier that separates circulating blood from the brain and thereby regulates cerebral homeostasis.[52,53] Neuroinflammation following incidence of ischemic stroke likely contributes to progressive dysfunction of the BBB that can further increase the likelihood of future brain injury and cognitive impairment.[52,53] However, neuroinflammation can be evidently counteracted by the action of SCFAs (butyrate in the study by Romo-Araiza et al. and sodium butyrate in the study by Park and Sohrabji), which can be metabolized by the action of probiotics and prebiotics.[1,19]
Figure 1.
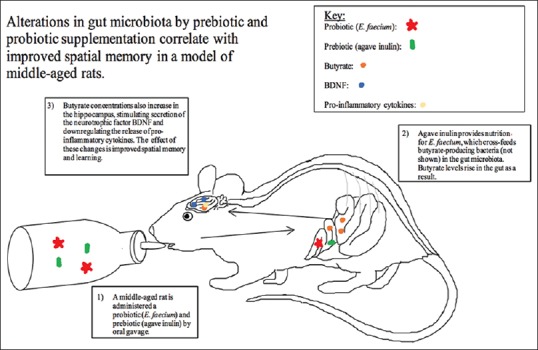
Prebiotics, probiotics, microbiota, and cognitive function. Treatment with prebiotics and probiotics may alter the gut microbiota leading to enhance cognitive function in middle-aged rats
Increasing the levels of the neurotrophic factor BDNF through symbiotic treatment could also be affected in conjunction with regenerative medicine – stem cell therapy – in future studies to test if neurogenesis and thus poststroke cognitive function are enhanced as a result.[54,55,56]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Romo-Araiza A, Gutiérrez-Salmeán G, Galván EJ, Hernández-Frausto M, Herrera-López G, Romo-Parra H, et al. Probiotics and prebiotics as a therapeutic strategy to improve memory in a model of middle-aged rats. Front Aging Neurosci. 2018;10:416. doi: 10.3389/fnagi.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tajiri N, Quach DM, Kaneko Y, Wu S, Lee D, Lam T, et al. NSI-189, a small molecule with neurogenic properties, exerts behavioral, and neurostructural benefits in stroke rats. J Cell Physiol. 2017;232:2731–40. doi: 10.1002/jcp.25847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer's disease patients. Cell Stem Cell. 2019;24:974–82. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan WL, Chopp M, Fan B, Zhang R, Wang X, Hu J, et al. Ablation of the microRNA-17-92 cluster in neural stem cells diminishes adult hippocampal neurogenesis and cognitive function. FASEB J. 2019;33:5257–67. doi: 10.1096/fj.201801019R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan TF, Ferreira Rocha NB, Paes F, Arias-Carrión O, Machado S, de Sá Filho AS. Neural mechanism of exercise: Neurovascular responses to exercise. CNS Neurol Disord Drug Targets. 2015;14:1304–6. doi: 10.2174/1871527315666151111124543. [DOI] [PubMed] [Google Scholar]
- 6.Selvaraji S, Poh L, Natarajan V, Mallilankaraman K, Arumugam TV. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond Med. 2019;2:30–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield DA. The 2013 SFRBM discovery award: Selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic Biol Med. 2014;74:157–74. doi: 10.1016/j.freeradbiomed.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baierle M, Nascimento SN, Moro AM, Brucker N, Freitas F, Gauer B, et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxid Med Cell Longev. 2015;2015:804198. doi: 10.1155/2015/804198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cevenini E, Monti D, Franceschi C. Inflamm-ageing. Curr Opin Clin Nutr Metab Care. 2013;16:14–20. doi: 10.1097/MCO.0b013e32835ada13. [DOI] [PubMed] [Google Scholar]
- 10.Ibarra A, Elisa G. Role of inflammatory signaling pathways in the efficacy of immunization with neural-derived peptides in spinal cord injury. Cond Med. 2018;1:346–9. [Google Scholar]
- 11.Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampà C, et al. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis. 2013;52:229–36. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Kumar A. Long-term potentiation at CA3-CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front Aging Neurosci. 2011;3:7. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL, et al. Neuroinflammation alters the hippocampal pattern of behaviorally induced arc expression. J Neurosci. 2005;25:723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan SM, Nolan YM. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neurosci Biobehav Rev. 2016;61:121–31. doi: 10.1016/j.neubiorev.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Mora F. Successful brain aging: Plasticity, environmental enrichment, and lifestyle. Dialogues Clin Neurosci. 2013;15:45–52. doi: 10.31887/DCNS.2013.15.1/fmora. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: Role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–30. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Pineda-Rodriguez B, Toscano-Tejeida D, García-Vences E, Rodriguez-Barrera R, Flores-Romero A, Castellanos-Canales D, et al. Anterior chamber associated immune deviation used as a neuroprotective strategy in rats with spinal cord injury. PLoS One. 2017;12:e0188506. doi: 10.1371/journal.pone.0188506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009;110:1226–40. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park MJ, Sohrabji F. The histone deacetylase inhibitor, sodium butyrate, exhibits neuroprotective effects for ischemic stroke in middle-aged female rats. J Neuroinflammation. 2016;13:300. doi: 10.1186/s12974-016-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76:677–83. doi: 10.1016/j.neuropharm.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 21.Berni Canani R, Di Costanzo M, Leone L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin Epigenetics. 2012;4:4. doi: 10.1186/1868-7083-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–32. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Franco-Robles E, López MG. Agavins increase neurotrophic factors and decrease oxidative stress in the brains of high-fat diet-induced obese mice. Molecules. 2016;21 doi: 10.3390/molecules21080998. pii: E998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28:88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar EC, Santos LC, Leonel AJ, de Oliveira JS, Santos EA, Navia-Pelaez JM, et al. Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase down-regulation in endothelial cells. J Nutr Biochem. 2016;34:99–105. doi: 10.1016/j.jnutbio.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev. 2014;136-137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Brüssow H. Microbiota and healthy ageing: Observational and nutritional intervention studies. Microb Biotechnol. 2013;6:326–34. doi: 10.1111/1751-7915.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FAO/WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk With Live Lactic Acid Bacteria (Amerian Córdoba Park Hotel, Córdoba. Argentina: FAO/WHO; 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria; pp. 1–34. [Google Scholar]
- 30.Huang Y, Li YL, Huang Q, Cui ZW, Yu DY, Rajput IR, et al. Effect of orally administered Enterococcus faecium EF1 on intestinal cytokines and chemokines production of suckling piglets. Pak Vet J. 2012;32:81–4. [Google Scholar]
- 31.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis. 2011;2:175–95. doi: 10.1177/2040622311399145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skelly DT, Griffin ÉW, Murray CL, Harney S, O'Boyle C, Hennessy E, et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0075-8. doi: 10.1038/s41380-018-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Wang F, Li H, Zhang H, Jin J, Chen W, et al. Neuroprotective effect of sodium butyrate against cerebral ischemia/Reperfusion injury in mice. Biomed Res Int. 2015;2015:395895. doi: 10.1155/2015/395895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capitán-Cañadas F, Ortega-González M, Guadix E, Zarzuelo A, Suárez MD, de Medina FS, et al. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol Nutr Food Res. 2014;58:1098–110. doi: 10.1002/mnfr.201300497. [DOI] [PubMed] [Google Scholar]
- 35.Peshev D, Van den Ende W. Fructans: Prebiotics and immunomodulators. J Funct Foods. 2014;8:348–57. [Google Scholar]
- 36.Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM. A positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J Neurosci. 2014;34:12547–59. doi: 10.1523/JNEUROSCI.0324-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. 609.e1-3. [DOI] [PubMed] [Google Scholar]
- 38.Leung K, Thuret S. Gut microbiota: A Modulator of brain plasticity and cognitive function in ageing. Healthcare (Basel) 2015;3:898–916. doi: 10.3390/healthcare3040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savignac HM, Corona G, Mills H, Chen L, Spencer JP, Tzortzis G, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int. 2013;63:756–64. doi: 10.1016/j.neuint.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlos AJ, Tong L, Prieto GA, Cotman CW. IL-1β impairs retrograde flow of BDNF signaling by attenuating endosome trafficking. J Neuroinflammation. 2017;14:29. doi: 10.1186/s12974-017-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulos AM, Thompson RF. Localization and characterization of an essential associative memory trace in the mammalian brain. Brain Res. 2015;1621:252–9. doi: 10.1016/j.brainres.2014.10.068. [DOI] [PubMed] [Google Scholar]
- 43.Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–52. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 44.Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–92. doi: 10.1038/nrn3677. [DOI] [PubMed] [Google Scholar]
- 45.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 46.Maqsood R, Stone TW. The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem Res. 2016;41:2819–35. doi: 10.1007/s11064-016-2039-1. [DOI] [PubMed] [Google Scholar]
- 47.Diógenes MJ, Costenla AR, Lopes LV, Jerónimo-Santos A, Sousa VC, Fontinha BM, et al. Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology. 2011;36:1823–36. doi: 10.1038/npp.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katche C, Cammarota M, Medina JH. Molecular signatures and mechanisms of long-lasting memory consolidation and storage. Neurobiol Learn Mem. 2013;106:40–7. doi: 10.1016/j.nlm.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 49.Arya AK, Hu B. Brain-gut axis after stroke. Brain Circ. 2018;4:165–73. doi: 10.4103/bc.bc_32_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Islam MR, Young MF, Wrann CD. Neuroprotective potential of exercise preconditioning in stroke. Cond Med. 2017;1:27–34. [PMC free article] [PubMed] [Google Scholar]
- 51.Liska GM, Lippert T, Russo E, Nieves N, Borlongan CV. A dual role for hyperbaric oxygen in stroke neuroprotection: Preconditioning of the brain and stem cells. Cond Med. 2018;1:151–66. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases (Review) Mol Med Rep. 2016;13:3391–6. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdullahi W, Tripathi D, Ronaldson PT. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. 2018;315:C343–56. doi: 10.1152/ajpcell.00095.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russo E, Lippert T, Tuazon JP, Borlongan CV. Advancing stem cells: New therapeutic strategies for treating central nervous system disorders. Brain Circ. 2018;4:81–3. doi: 10.4103/bc.bc_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anthony S, Borlongan CV. Recent progress in regenerative medicine for brain disorders. Brain Circ. 2017;3:121–3. doi: 10.4103/bc.bc_26_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghanekar S, Corey S, Stonesifer C, Lippert T, Diamandis Z, Sokol J, et al. Current challenges in regenerative medicine for central nervous system disorders. Brain Circ. 2016;2:105–7. doi: 10.4103/2394-8108.192516. [DOI] [PMC free article] [PubMed] [Google Scholar]


