Abstract
Although hyperbaric oxygen therapy (HBOT) is common as a treatment for injuries, this study aimed to research the ability of HBOT in preconditioning to diminish any potential damage. The hypothesis stated that HBOT preconditioning alleviated the death of cells in primary rat neuronal cells (PRNCs) by transferring mitochondria from astrocytes. In this experiment, PRNCs were given an HBOT treatment before a tumor necrosis factor-alpha or lipopolysaccharide injury which resembled cell death associated with stroke and traumatic brain injury (TBI). After being examined, the study found more cell viability in the PRNCs that had received HBOT precondition and a mitochondrial transfer. The mitochondrial transfer was visualized by a series of images showing the transfer after the HBOT treatment. This study demonstrated the ability of HBOT preconditioning as a treatment for inflammation in stroke and TBI, with the transfer of mitochondria from astrocytes to PRNCs reducing cell death. Along with discussion of the study, this review also focuses on different stroke treatments in comparison with HBOT.
Keywords: Hyperbaric oxygen therapy, lipopolysaccharide, mitochondria, preconditioning, primary rat neuronal cells, stroke, traumatic brain injury, tumor necrosis factor-alpha
Introduction: Status of Stroke and Traumatic Brain Injury
Diseases associated with the central nervous system incorporate a diverse set of pathologies.[1] However, the two most dominant adult neurodegenerative diseases in the United States, namely stroke and traumatic brain injury (TBI), affect a large array of people.[1] The American Heart Association found that stroke is responsible for 130,000 deaths each year, making it the fifth-leading cause of death in the United States. Stroke is the number one cause for long-term disability in the United States, making the associated health-care costs surpass $33 billion per year.[2] In 2013 alone, TBI led to 2.8 million emergency room visits, hospitalizations, and deaths, while approximately 3.1 billion people were living with this disease with the health-care cost of $76.5 billion in 2012.[3,4]
Cell Death
Stroke and TBI utilize similar pathologies for the primary and secondary cell death mechanism, which results from recurring neuroinflammation.[5] An important pathological feature includes the creation of a necrotic tissue core, which becomes irreparable after stroke and TBI.[6,7,8] Secondary cell death of stroke and TBI have been linked to the breakdown of the blood–brain barrier (BBB), which allows for inflammatory cytokines to cross the BBB and ultimately increase inflammatory response, thus damaging the outcomes.[9] Additional neurodegeneration following the damaged BBB has been found due to factors such as oxidative stress, apoptosis, and mitochondrial dysfunction.[10,11,12,13]
Hyperbaric Oxygen Therapy: A Novel Therapeutic Approach
Hyperbaric oxygen therapy (HBOT) has been presented as a possible treatment for TBI and stroke.[14,15] This method uses a pressurized chamber of 2–3 absolute atmospheres, which results in hyperoxygenation of tissues, thus inducing angiogenesis and the recruitment of progenitor cells to the damaged regions.[16,17,18] HBOT can be used for patients with open wounds from burns or diabetic ulcers.[19,20] The chronic stages of stroke are a possible window for HBOT to be used, as the acute stage is more difficult and in need of higher technology to successfully accomplish this therapy.[14] This therapy works to alleviate impairments associated with strokes such as memory loss, language, and comprehension deficits.[14,21] The unpredictable occurrence of TBI impedes the usage of HBOT as treatment for brain trauma patients.[22] Secondary cell death has become the main target for HBOT treatment, as reduction in the levels of inflammatory cytokines has been associated with limiting peri-infarct/peri-impact tissue loss.[23,24] There is still more to investigate surrounding HBOT and the sequestration of inflammation.[25]
The Effectiveness of Hyperbaric Oxygen Therapy
The role of the preconditioning paradigm is yet to be explored regarding HBOT. Studies have demonstrated preclinical efficacy of HBOT preconditions for neuronal cell loss, but rarely, any mechanism-based assessments have explained the function of HBOT.[26,27,28,29] Alternative therapies to treat stroke and TBI have been investigated further.[30] The mitochondria is an important point of investigation to further develop the understanding of HBOT preconditioning.[31,32,33] Functioning extracellular mitochondria were transferred from astrocytes to neurons after neuronal cell death due to stroke.[34] The current study looked at HBOT preconditioning in the limitation of neuronal cell death after the inflammatory response, which imitated secondary cell death associated with stroke and TBI. The possibility of transferring mitochondria as the treatment of neuronal cells was also explored in this study.[35,36,37,38,39,40] The hypothesis states that when astrocytic mitochondria were transferred into neurons, after HBOT preconditioning, neuronal cell viability after inflammatory response would be improved.
Transferring Mitochondria
This study illustrated the effects of HBOT preconditioning as a therapy treatment against cell death that is associated with neurodegenerative diseases, specifically stroke and TBI. The study found that when primary neuronal cells were subjected to HBOT preconditioning before an inflammatory insult, there was a reduced number of cell deaths. On further investigation, the study discovered an increase in the number of astrocytic mitochondria found in the primary neuronal cells. These findings indicate that HBOT can reduce the inflammatory response of the neuronal cells through the transfer of mitochondria. The function of mitochondria is important in stroke and TBI since it plays a role in the secondary injury mechanism.[41,42] However, using HBOT preconditioning as a treatment for these diseases has provided mixed results ranging from therapeutic to harmful.[14]
Reducing Cell Death
The use of HBOT as therapeutic treatments has only been discovered recently. Studies have proposed a variety of mechanisms that allow for HBOT's positive effects, such as reducing inflammation and stabilizing the BBB.[43,44,45,46] These mechanisms are associated with the destruction of mitochondria which led the current study to research the role of mitochondria as a target of HBOT.[47,48] After stroke, the transfer of mitochondria was observed lining up with the research.[34] This discovery forms the possibility of an HBOT treatment for individuals at a high risk of the diseases by providing a way to reduce secondary cell death. The current study was able to detect an increase in astrocytic mitochondria in the primary rat neuronal cells of the HBOT treated group using Mitotracker labeling.
Resisting Inflammatory Response
Researchers also found that injured neurons who had been exposed to tumor necrosis factor-alpha (TNF-alpha) or lipopolysaccharide (LPS) increased the astrocytic mitochondrial transfer. It was found that HBOT preconditioning in common conditions aided astrocytic mitochondrial transfer in comparison to the TNF-alpha and LPS groups. HBOT preconditioning combined with the inflammatory response increased the transfer of mitochondria. This indicates that neurons with more astrocytic mitochondria are more likely to survive an inflammatory response than neurons with less astrocytic mitochondria. This information supports the idea that astrocytic mitochondria are more resistant to inflammatory response than neuronal mitochondria.[35,49]
Hyperbaric Oxygen Therapy Induces Neuroprotection
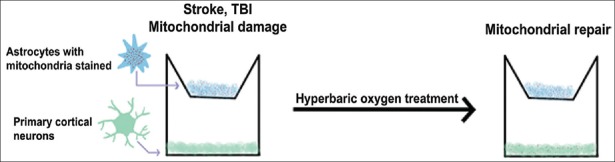
The study demonstrated that the HBOT treatment was tolerated because the cells remained viable marking this a safe and effective procedure [Figure 1]. It was also found that the mitochondrial transfer occurred almost immediately after the HBOT treatment and lasted for around 20 min after the treatment ceased. Using HBOT treatment for a short period induces neuroprotection and protects the cells from the effects of prolonged HBOT.[50,51] This study had some limitations. The images that were collected were taken after the HBOT, which prevented the researchers from accurately detecting the beginning of the mitochondrial transfers.
Figure 1.
Stroke impairs the mitochondria in cultured cells, which is repaired by hyperbaric oxygen therapy
Applying Hyperbaric Oxygen Therapy to Clinics
Although this study demonstrated a single HBOT treatment, multiple short HBOT treatments may also provide a functional result due to the neurological deficits caused by stroke and TBI. Additional studies will continue to occur exploring both post- and pre-injury HBOT. To begin using this treatment at the clinic, a trial will need to take place with a population of individuals who are at a higher risk of these cerebrovascular injuries. HBOT treatments will need to be observed in in vivo disease models. To find the safest and effective treatment of HBOT various trials under different conditions will need to be tested.[52] Recent studies have been able to test HBOT treatments on rodent models allowing them to find successful protocols.[53]
The Potential of Hyperbaric Oxygen Therapy
Following the Food and Drug Administration regulations, HBOT will be implemented in clinics once treatments are revised for human.[19,20] HBOT preconditioning provides a possible treatment for inflammation associated with many cerebrovascular diseases. HBOT can be an alternative method to other treatments for TBI and stroke such as invasive procedures such cell transplants.[54] The astrocytic mitochondrial transfer to neurons acts as a mechanism of HBOT to provide protections against inflammation. This ability to limit damage done by cerebrovascular injuries in high-risk individuals may reduce the burden of these diseases on our economy.
Current Stroke Treatments
Revascularization has been explored as a stroke therapy method; however, new research points to nondrug neuroprotective therapies such as oxygen therapy as a way to prevent brain damage that results from a stroke.[55] An ischemic stroke occurs when the blood supply to the brain is blocked, therefore depriving the brain of sufficient oxygen.[56] Studies have shown that HBOT can minimize neurological impairment caused due to a stroke by increasing oxygen supply, therefore reducing ischemia injury.[56] HBO preconditioning performed on rats has also been found to enhance an enzyme that protects against MCAO.[57] Along with HBO, normobaric oxygen (NBO) therapy has been explored. In contrast to HBO, NBO administers 100% oxygen at one atmosphere. Studies have shown that NBO counteracts hypoxic conditions induced by an ischemic event.[58] NBO protects the BBB from damage by inhibiting an NADPH oxidase enzyme complex that is unregulated during a stroke.[59] HBO therapies have been administered following an ischemic event. Results from a study show that these therapies had neuroprotective effects by facilitating the BBB integrity.[60,61,62,63] In a different study, focused on neuroprotection mediated by hormetic mechanisms, hormetic dose responses were observed as decreasing the amount of damage caused by stroke and TBI.[61] Along with these stroke therapies, one study found that transplanting amniotic fluid stem cells may help reduce the damage caused due to a stroke by promoting neurogenesis.[64] Stem cells are a viable option for stroke treatment since they can initiate regenerative processes in the brain.[65]
Mitochondrial dysfunction has been discovered to play a role in the neural damage that results from an ischemic event. There is evidence that transferring healthy mitochondria from stem cells to replace ischemic-injured cells is a viable method for treating damaged cells.[66]
Financial support and sponsorship
Dr. Borlongan is funded by National Institutes of Health (NIH) R01NS090962, NIH R01NS102395, NIH R21NS109575, and Veterans Affairs Merit Review I01 BX001407.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Borlongan CV, Burns J, Tajiri N, Stahl CE, Weinbren NL, Shojo H, et al. Epidemiological survey-based formulae to approximate incidence and prevalence of neurological disorders in the United States: A meta-analysis. PLoS One. 2013;8:e78490. doi: 10.1371/journal.pone.0078490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths – United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–6. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95:986–950. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, et al. Potential of stem/progenitor cells in treating stroke: The missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–50. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen H, Aum D, Mashkouri S, Rao G, Vega Gonzales-Portillo JD, Reyes S, et al. Growth factor therapy sequesters inflammation in affording neuroprotection in cerebrovascular diseases. Expert Rev Neurother. 2016;16:915–26. doi: 10.1080/14737175.2016.1184086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Giunta B, Obregon D, Velisetty R, Sanberg PR, Borlongan CV, Tan J. The immunology of traumatic brain injury: A prime target for Alzheimer's disease prevention. J Neuroinflammation. 2012;9:185. doi: 10.1186/1742-2094-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash R, Carmichael ST. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol. 2015;28:556–64. doi: 10.1097/WCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, et al. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res. 2010;88:3530–9. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo W, Zhang S, Xia CY, Guo XF, He WB, Chen NH. Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: The role of inhibition of drp1 in ischemic brain damage. Neuropharmacology. 2014;86:103–15. doi: 10.1016/j.neuropharm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Okazawa H, Ikawa M, Tsujikawa T, Kiyono Y, Yoneda M. Brain imaging for oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Q J Nucl Med Mol Imaging. 2014;58:387–97. [PubMed] [Google Scholar]
- 13.Liu B, Zhang YH, Jiang Y, Li LL, Chen Q, He GQ, et al. Gadd45b is a novel mediator of neuronal apoptosis in ischemic stroke. Int J Biol Sci. 2015;11:353–60. doi: 10.7150/ijbs.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai WW, Sun L, Yu ZQ, Chen G. Hyperbaric oxygen therapy in experimental and clinical stroke. Med Gas Res. 2016;6:111–8. doi: 10.4103/2045-9912.184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Q, Manaenko A, Xu T, Guo Z, Tang J, Zhang JH. Hyperbaric oxygen therapy for traumatic brain injury: Bench-to-bedside. Med Gas Res. 2016;6:102–10. doi: 10.4103/2045-9912.184720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–77. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 17.Thom SR. Hyperbaric oxygen: Its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):131S–41S. doi: 10.1097/PRS.0b013e3181fbe2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhutani S, Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45:316–24. doi: 10.4103/0970-0358.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: A systematic review. Eur J Vasc Endovasc Surg. 2014;47:647–55. doi: 10.1016/j.ejvs.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Selçuk CT, Ozalp B, Durgun M, Tekin A, Akkoç MF, Alabalik U, et al. The effect of hyperbaric oxygen treatment on the healing of burn wounds in nicotinized and nonnicotinized rats. J Burn Care Res. 2013;34:e237–43. doi: 10.1097/BCR.0b013e318270092e. [DOI] [PubMed] [Google Scholar]
- 21.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann Transl Med. 2014;2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seule M, Brunner T, Mack A, Hildebrandt G, Fournier JY. Neurosurgical and intensive care management of traumatic brain injury. Facial Plast Surg. 2015;31:325–31. doi: 10.1055/s-0035-1562884. [DOI] [PubMed] [Google Scholar]
- 23.Meng XE, Zhang Y, Li N, Fan DF, Yang C, Li H, et al. Hyperbaric oxygen alleviates secondary brain injury after trauma through inhibition of TLR4/NF-KB signaling pathway. Med Sci Monit. 2016;22:284–8. doi: 10.12659/MSM.894148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shojo H, Kaneko Y, Mabuchi T, Kibayashi K, Adachi N, Borlongan CV. Genetic and histologic evidence implicates role of inflammation in traumatic brain injury-induced apoptosis in the rat cerebral cortex following moderate fluid percussion injury. Neuroscience. 2010;171:1273–82. doi: 10.1016/j.neuroscience.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Gill AL, Bell CN. Hyperbaric oxygen: Its uses, mechanisms of action and outcomes. QJM. 2004;97:385–95. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- 26.Gamdzyk M, Małek M, Bratek E, Koks A, Kaminski K, Ziembowicz A, et al. Hyperbaric oxygen and hyperbaric air preconditioning induces ischemic tolerance to transient forebrain ischemia in the gerbil. Brain Res. 2016;1648:257–65. doi: 10.1016/j.brainres.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Tang J, Chen Q, Jiang B, Zhang B, Tao Y, et al. Hyperbaric oxygen preconditioning attenuates neuroinflammation after intracerebral hemorrhage in rats by regulating microglia characteristics. Brain Res. 2015;1627:21–30. doi: 10.1016/j.brainres.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 28.André-Lévigne D, Modarressi A, Pignel R, Bochaton-Piallat ML, Pittet-Cuénod B. Hyperbaric oxygen therapy promotes wound repair in ischemic and hyperglycemic conditions, increasing tissue perfusion and collagen deposition. Wound Repair Regen. 2016;24:954–65. doi: 10.1111/wrr.12480. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty JE. The role of hyperbaric oxygen therapy in crush injuries. Crit Care Nurs Q. 2013;36:299–309. doi: 10.1097/CNQ.0b013e318294ea41. [DOI] [PubMed] [Google Scholar]
- 30.Newman MB, Misiuta I, Willing AE, Zigova T, Karl RC, Borlongan CV, et al. Tumorigenicity issues of embryonic carcinoma-derived stem cells: Relevance to surgical trials using NT2 and HNT neural cells. Stem Cells Dev. 2005;14:29–43. doi: 10.1089/scd.2005.14.29. [DOI] [PubMed] [Google Scholar]
- 31.Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol. 2012;167:699–719. doi: 10.1111/j.1476-5381.2012.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014:102158. doi: 10.1155/2014/102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: The role of the mitochondria. Oncology (Williston Park) 2011;25:400–10, 413. [PubMed] [Google Scholar]
- 34.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–5. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlongan CV, Yamamoto M, Takei N, Kumazaki M, Ungsuparkorn C, Hida H, et al. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 2000;14:1307–17. doi: 10.1096/fj.14.10.1307. [DOI] [PubMed] [Google Scholar]
- 36.Fahrig T, Gerlach I, Horváth E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson's disease and traumatic brain injury. Mol Pharmacol. 2005;67:1544–55. doi: 10.1124/mol.104.008177. [DOI] [PubMed] [Google Scholar]
- 37.Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, et al. Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: A novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–74. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- 38.Figiel I. Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta Neurobiol Exp (Wars) 2008;68:526–34. doi: 10.55782/ane-2008-1720. [DOI] [PubMed] [Google Scholar]
- 39.Neniskyte U, Vilalta A, Brown GC. Tumour necrosis factor alpha-induced neuronal loss is mediated by microglial phagocytosis. FEBS Lett. 2014;588:2952–6. doi: 10.1016/j.febslet.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gresa-Arribas N, Viéitez C, Dentesano G, Serratosa J, Saura J, Solà C. Modelling neuroinflammation in vitro: A tool to test the potential neuroprotective effect of anti-inflammatory agents. PLoS One. 2012;7:e45227. doi: 10.1371/journal.pone.0045227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiebert JB, Shen Q, Thimmesch AR, Pierce JD. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350:132–8. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 42.Yang L, Hei MY, Dai JJ, Hu N, Xiang XY. Effect of hyperbaric oxygenation on mitochondrial function of neuronal cells in the cortex of neonatal rats after hypoxic-ischemic brain damage. Braz J Med Biol Res. 2016;49:e5187. doi: 10.1590/1414-431X20165187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jadhav V, Zhang JH. Surgical brain injury: Prevention is better than cure. Front Biosci. 2008;13:3793–7. doi: 10.2741/2968. [DOI] [PubMed] [Google Scholar]
- 44.Ostrowski RP, Graupner G, Titova E, Zhang J, Chiu J, Dach N, et al. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 2008;29:1–3. doi: 10.1016/j.nbd.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soejima Y, Ostrowski RP, Manaenko A, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia enhanced hemorrhagic transformation after transient MCAO in rats. Med Gas Res. 2012;2:9. doi: 10.1186/2045-9912-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen SY, Huang E, Wang V, Fan YM, Ho CF, Yip PK. Improvement of clinical outcome and cerebral perfusion in a patient of atherosclerotic cerebral infarction after repetitive hyperbaric oxygen treatment – A case report and literature review. Undersea Hyperb Med. 2011;38:375–9. [PubMed] [Google Scholar]
- 47.Aliev G, Smith MA, de la Torre JC, Perry G. Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer's disease. Mitochondrion. 2004;4:649–63. doi: 10.1016/j.mito.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 48.Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: Novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–9. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Adrenomedullin gene delivery protects against cerebral ischemic injury by promoting astrocyte migration and survival. Hum Gene Ther. 2004;15:1243–54. doi: 10.1089/hum.2004.15.1243. [DOI] [PubMed] [Google Scholar]
- 50.Yin D, Zhou C, Kusaka I, Calvert JW, Parent AD, Nanda A, et al. Inhibition of apoptosis by hyperbaric oxygen in a rat focal cerebral ischemic model. J Cereb Blood Flow Metab. 2003;23:855–64. doi: 10.1097/01.WCB.0000073946.29308.55. [DOI] [PubMed] [Google Scholar]
- 51.Bennett MH, Trytko B, Jonker B. Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev. 2012;12:CD004609. doi: 10.1002/14651858.CD004609.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borlongan CV. Cell therapy for stroke: Remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–8. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing P, Ma K, Li L, Wang D, Hu G, Long W. The protection effect and mechanism of hyperbaric oxygen therapy in rat brain with traumatic injury. Acta Cir Bras. 2018;33:341–53. doi: 10.1590/s0102-865020180040000006. [DOI] [PubMed] [Google Scholar]
- 54.Saporta S, Cameron DF, Borlongan CV, Sanberg PR. Survival of rat and porcine sertoli cell transplants in the rat striatum without cyclosporine – A immunosuppression. Exp Neurol. 1997;146:299–304. doi: 10.1006/exnr.1997.6493. [DOI] [PubMed] [Google Scholar]
- 55.Ji X. Forward thinking in stroke treatment: Advances in cerebrovascular reperfusion and neurorehabilitation. Brain Circ. 2015;1:1–2. [Google Scholar]
- 56.Chandra A, Stone CR, Du X, Li WA, Huber M, Bremer R, et al. The cerebral circulation and cerebrovascular disease III: Stroke. Brain Circ. 2017;3:66–77. doi: 10.4103/bc.bc_12_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson CW, Escobar I, Xu J, Perez-Pinzon MA. Effects of ischemic preconditioning on mitochondrial and metabolic neruoprotection: 5' adenosine monophosphate-activated protein kinase and sirtuins. Brain Circ. 2018;4:54–61. doi: 10.4103/bc.bc_7_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thibodeau A, Geng X, Previch LE, Ding Y. Pyruvate dehydrogenase complex in cerebral ischemia-reperfusion injury. Brain Circ. 2016;2:61–6. doi: 10.4103/2394-8108.186256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li W, Yang S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016;2:153–63. doi: 10.4103/2394-8108.195279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gidday JM. Cerebrovascular ischemic protection by pre-and post-conditioning. Brain Circ. 2015;1:97–103. [Google Scholar]
- 61.Calabrese EJ, Calabrese V, Giordano J. The role of hormesis in the functional performance and protection of neural systems. Brain Circ. 2017;3:1–3. doi: 10.4103/2394-8108.203257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chau M, Zhang J, Wei L, Yu SP. Regeneration after stroke: Stem cell transplantation and trophic factors. Brain Circ. 2016;2:86–94. doi: 10.4103/2394-8108.186279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knecht T, Borlongan C, Dela Peña I. Combination therapy for ischemic stroke: Novel approaches to lengthen therapeutic window of tissue plasminogen activator. Brain Circ. 2018;4:99–108. doi: 10.4103/bc.bc_21_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diaco NS, Diamandis ZM, Borlongan CV. Amniotic fluid-derived stem cells as an effective cell source for transplantation therapy in stroke. Brain Circ. 2015;1:119–24. [Google Scholar]
- 65.Russo E, Lippert T, Tuazon JP, Borlongan CV. Advancing stem cells: New therapeutic strategies for treating central nervous system disorders. Brain Circ. 2018;4:81–3. doi: 10.4103/bc.bc_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo E, Nguyen H, Lippert T, Tuazon J, Borlongan CV, Napoli E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018;4:84–94. doi: 10.4103/bc.bc_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]