Abstract
Disruption in redox signaling and control of cellular processes has emerged as a key player in many pathologies including neurodegeneration. As protein aggregations are a common hallmark of several neuronal pathologies, a firm understanding of the interplay between redox signaling, oxidative and free radical stress, and proteinopathies is required to sort out the complex mechanisms in these diseases. Fortunately, models of toxicant-induced neurodegeneration can be utilized to evaluate and report mechanistic alterations in the proteostasis network (PN). The epidemiological links between environmental toxicants and neurological disease gives further credence into characterizing the toxicant-mediated PN disruptions observed in these conditions. Reviewed here are examples of mechanistic interaction between oxidative or free radical stress and PN alterations. Additionally, investigations into toxicant-mediated PN disruptions, specifically focusing on environmental metals and pesticides, are discussed. Finally, we emphasize the need to distinguish whether the presence of protein aggregations are contributory to phenotypes related to neurodegeneration, or if they are a byproduct of PN deficiencies.
Keywords: Neurodegeneration, Proteostasis Network, Protein Aggregation, Environmental Toxicants, Redox Proteome, Oxidative Stress
1. Introduction
Preservation of a healthy proteome is crucial for cellular and organismal physiology, which is why organisms have developed a sophisticated system responsible for protein quality control called the proteostasis network (PN). The major goals of the PN are proper protein synthesis, correct protein folding into functional structures, and degradation of misfolded and damaged peptides1-3. Dysfunction within the PN has the ability to propagate protein misfolding and aggregate formation. Interestingly, PN collapse is associated with the molecular events involved in the pathology of several disorders such as diabetes4-10, aging2,11-13 and is a major feature of neurodegenerative diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS) (Table 1).
Table 1.
Neurodegenerative diseases and genes associated with proteostasis collapse.
| Table 1 | ||||
|---|---|---|---|---|
| Disease | Protein aggregate | Responsible protein | Disease genes | References |
| Alzheimer’s disease | Aβ Plaques | Aβ peptide | APP | 91,187–189 |
| Alzheimer’s diseasetauopathies | Neurofibrillary tangles | Tau | MAPT | 190 |
| Parkinson’s disease | Lewy bodies | α-synuclein | SNCA | 132,191 |
| Huntington’s disease | Polyglutamine inclusion bodies | Huntingtin | HTT | 90,192,193 |
| ALS | Superoxide dismutase 1 aggregate | Superoxide dismutase 1 | SOD1 | 194,195 |
| ALS | Stress granules | TDP-43/FUS | TARDBP/FUS | 196 |
| Creutzfeld-Jacob disease-Prion diseases | Prion aggregates | PrPSC | PRNP | 197,198 |
Similar to protein aggregation, cellular redox imbalance and free radical damage are also hallmarks of neurodegeneration14-19. Several environmental toxicants are associated with neurodegeneration, with converging mechanisms including mitochondrial dysfunction, ROS production and disruptions in compartmental redox signaling and control19-24. Of great importance to this review, protein folding, autophagy and proteasomal activity can all be modulated through thiol redox signaling and control mechanisms25-31. These observations highlight the significance of the interplay between the proteome and redox homeostasis.
Although redox regulation of the PN is an emerging topic that has not been fully explored, recent studies have yielded significant information. The cell displays several examples of PN tuning or disruption through redox reactions25,29,31-34. This review aims to evaluate mechanisms participating in the cross-talk between these networks of pathways, as well as the relationship between PN disruption and redox imbalance from a toxicological perspective.
2. Redox Regulation of the Proteostasis Network
2.1. The Proteostasis Network
Preserving proper production, function and integrity of the cellular proteome is absolutely necessary for cell survival, since proteins participate in nearly every cellular process35. For this reason, organisms have developed a highly dynamic set of pathways called the PN, which promotes vigilant protein quality control and favors proteome homeostasis13,36. The PN is primarily composed of molecular pathways that regulate translation, folding and degradation of proteins2. Several other secondary but essential molecular circuitries, like the unfolded protein response (UPR)37, participate in the PN and provide its necessary dynamic nature and its ability to respond during stresses. Protein synthesis requires precise translation by the ribosome38, while molecular chaperones aid in co-translational folding35. Accurate control of unstable folding intermediates and misfolded proteins is necessary for a healthy proteome and is also regulated by chaperones systems1. Degradation of misfolded or defective individual peptides happens through proteasomal degradation39, while autophagy is responsible for bulk protein and aggregate clearance40. This section of the review aims to provide an overview of the PN and present examples of redox regulation of PN components.
2.2. Radical and Non-radical Damage to Proteins
In the cellular milieu, the proteome is constantly under an overwhelming quantity of stresses41 and oxidative stress is a well-characterized example of a condition which can facilitate protein damage42–44. Oxidative stress is a process characterized by disruption of cellular redox homeostasis and production of radical and non-radical molecules45. Free radical species (e.g. superoxide, hydroxyl radical) occur naturally in the cell as metabolic by-products46. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) can attack the protein backbone to inhibit function and promote fragmentation of the polypeptide chain, which can result in protein unfolding 42,47,48. Amino acid residues, like histidine, leucine, methionine and aromatic amino-acids phenylalanine, tyrosine, and tryptophan, can undergo oxidative modifications that can lead to protein crosslinking, and aggregation49. Perhaps the most vital signaling disruption due to oxidative stress is within the cysteine-based thiol redox proteome50,51. These critical residues are involved in redox-regulated control of several cellular functions52,53, and their oxidation/reduction states are regulated through activity of glutathione (GSH) and thioredoxin (Trx) systems. Non-radical (NR) oxidant molecules (e.g. peroxides, aldehydes, epoxides) are also produced in the cell and have the ability to oxidize thiols independently of free radical presence54. Oxidation and modification of critical thiol entities can produce cellular redox imbalance to disrupt thiol redox signaling. Pathological conditions, such as neurodegenerative diseases (AD, PD, HD, ALS), are characterized by increased generation of free radicals and non-radicals55,56. Also, general oxidative injury can induce lipid peroxidation and reactive aldehyde production, which can also promote protein damage through adduct formation and favor protein misfolding57-59.
Oxidative damage to components of the PN, such as chaperones, is of great importance to this review. Proper protein folding is regulated by molecular chaperones1,13,36 and their function is an important cell defense to prevent aggregation and abrogate pathogenesis. Direct oxidation or adduct formation of sensitive chaperone thiols can result in inhibition of chaperone function and diminish cellular protein quality control60-62. Ethanol toxicity is a great example of how oxidative damage to members of the chaperone family can impede protein folding63,64. Also, incubation of PC12 neuronal cells with the highly reactive peroxynitrite can promote tyrosine nitration of the chaperone heat shock protein 90 (Hsp90) and promote motor neuron death65. Another important component of chaperone function is sufficient levels of adenosine triphosphate (ATP)66, as many chaperones employ ATP hydrolysis to facilitate folding35. Therefore, energy deficits as a result of xenobiotic-mediated mitochondrial dysfunction can also affect protein folding.
2.3. Redox Control of Proteasomal Degradation
Protein degradation is a cornerstone of protein quality control, since removal of misfolded proteins prone to aggregation is critical to prevent disease pathogenesis67-69. To remove damaged and misfolded proteins, the cell employs the ubiquitin proteasome system (UPS) (for review:70,71). Briefly, misfolded or damaged proteins are labeled with ubiquitin by E1, E2 and E3 ubiquitin ligase enzymes72, which ‘flags’ these misfolded proteins for degradation through the proteasome39. The proteasome is a unique protein complex consisting of two regulatory subunits (19S) and one catalytic subunit (20S). As ubiquitin-tagged proteins are introduced to the proteasome complex, ubiquitin is removed from tagged peptides by the regulatory subunits and then single polypeptides enter the 20S core where they are processed and cleaved by proteolytic subunits. In the presence of mild oxidative stress the activity of the proteasome is increased, since it is responsible for removal of proteins suffering from oxidative damage43,44. Increased intensity of redox imbalance can induce separation of the proteasomal subunits (20s, 19s), switching the mode of proteolytic degradation from Ub-dependent (ATP dependent) to Ub-independent (ATP independent), resulting in degradation of oxidized proteins73. Once again, the importance of energy balance and ATP production in PN preservation is highlighted. However, oxidative stress after major oxidative insults can result in inhibition of proteasomal activity. As reviewed extensively by Pajares et al25, proteasomal subunits can be modified by post-translational modifications (PTMs) (S-glutathionylation, carbonylation, HNE-adduction) that are closely related to redox imbalance as they form as byproducts of oxidative damage30,74-76. S-nitrosylation and S-glutathionylation can also modify critical thiols in ubiquitin-related enzymes responsible for protein ubiquitination, resulting in damaged proteins escaping protein quality mechanisms and disruption of cellular physiology77,78. The proteasome is also responsible for regulation and degradation of several transcription factors (e.g. Nrf2, NF-kB), which are extensively redox regulated. This is an important point as decreased proteolytic activity disturbs the regulatory capacity of these critical transcription factors, potentially leading to system dysregulation and promotion of pathology79-81. Finally, oxidative modification of the 26s proteasome is a common observation in aging70,82 and other neurological disorders83-86; therefore, from a toxicological perspective, the involvement of redox regulation of the proteasome can be of great mechanistic importance.
2.4. Redox Signaling in Autophagy
Autophagy is an essential molecular pathway involved in major cellular processes87-91, like immune function, aggregate clearance, and energy metabolism. There are three different forms of autophagy: 1) chaperone-mediated autophagy, in which the heat shock cognate (Hsc70) chaperone shuttles individual misfolded peptides to the lysosome, where they are degraded (reviewed here92); 2) microautophagy, in which the lysosomal membrane forms invaginations that sequester cytosolic material for degradation (reviewed here 93), and 3) macroautophagy (hereby referred as autophagy), which is the bulk protein degradation pathway of the cell40. The process of autophagy is mainly regulated by the mTOR complex, a central regulator of cell metabolism that functions as a sensor for cellular nutrient and energy levels11,94. At basal conditions, mTOR is activated by several metabolic signals and inhibits autophagy. Under stresses like amino acid depletion or protein aggregate formation, mTOR is inhibited and autophagy is activated. During autophagy, cytosolic material, e.g. protein aggregates, organelles, lipids, is engulfed by a double membrane vesicle called the autophagosome and is transported to the lysosome to undergo degradation (for review:28,89,95). Autophagy is vital for preserving cellular physiology and its importance is highlighted by the fact that autophagic clearance of mitochondria (mitophagy) is the only known procedure that promotes mitochondrial turnover28. Additionally, dysfunction of autophagy is a common observation in neurodegenerative diseases96 and impaired autophagic clearance promotes protein aggregation of pathological proteins (Table 1). Also, autophagy is involved in the removal of oxidized macromolecules33,97-99 and dysfunctional autophagy can result in ROS/RNS production100-102. The interplay between autophagy and thiol redox signaling has not been investigated thoroughly, but it has been reported that ROS and RNS can induce autophagy by inhibiting mTOR103-105. For example, a validated redox switch critical for autophagosome formation includes oxidation of an important Cys residue near the catalytic site of Atg4 family members29. Atg4 proteins possess cysteine protease activity that aids in lipidation of LC3-I and delipidation of LC3-II106. Oxidation or mutation of Cys81 inhibits Atg4 activity, blocks autophagosome formation and restricts the cell from using autophagy. Another convergence point of autophagy and thiol redox signaling involves the p62-Nrf2-Keap1 axis. The autophagy receptor p62 binds ubiquitinated molecules to form the autophagosome cargo107, and reports show that p62 can modulate antioxidant responses by binding Keap1108-111, which is a major regulator of antioxidant defense79,112-114. Dysfunctional p62 clearance results in p62 accumulation, possibly leading to increased Keap1 sequestration and subsequent Nrf2 over-activation, which is associated with cancer pathology115-117. These few examples indicate that exploration of mechanisms governing cross-talk between autophagy and thiol redox signaling can be of great interest and can be used in toxicology to decipher xenobiotic-mediated mechanisms of pathogenesis.
2.5. Endoplasmic Reticulum Stress and Disulfide Bond Formation
The endoplasmic reticulum (ER) serves as a hub for nascent peptide folding, since to-be-secreted proteins enter the ER co-translationally to fold into their proper three-dimensional form118. Disturbance of ER physiology can inhibit protein folding, propagate aggregation and activate the UPR 119,120. This event results in activation of three ER-transmembrane proteins (IRE-1a, PERK, ATF6a) that inhibit translation and transcriptionally activate protein degradation pathways as a defense mechanism. UPR also induces expression of folding facilitators, e.g. chaperones, to help the cell cope with the increased load of misfolded proteins121. Toxicologically, the inability of the cell to defend against prolonged ER stress can eventually result in cell death122. Many xenobiotics that exert toxicity through ER stress have been identified123-125 and examples of thiol redox regulation of the UPR are common 32,105,126,127. This is because a major process in protein folding is the disulfide bond formation that takes place solely in the ER128. Additionally, the formation of intermolecular or intramolecular disulfide bonds between cysteine residues is important for protein stability62. Protein disulfide isomerase (PDI) oxidoreductases work as a disulfide donor by promoting cysteine oxidation of candidate peptides123,129. PDI is also responsible for disulfide bond isomerization in proteins, a rather important process regarding protein folding and its disruption can instigate misfolding. Due to the importance of structural disulfide bonds, reducing factors like dithiothreitol (DTT) can cause ER stress through breaking disulfide bonds and modulation of protein folding124,130. Also, PTMs of cysteines in the active site of PDI can inhibit its function123,131 and might be involved in neurodegeneration, since PDI levels are increased in brains of patients suffering from neurological disorders128,132-134. In general, UPR dysfunction or over-activation is involved in several neurodegenerative disorders and exploration of ER stress induction through several toxicants can provide valuable information regarding development of pathology.
3. Toxicants That Impact the PN via Redox Interactions
With the emergence of PN disruption as a hallmark for multiple pathologies, characterization of toxicants, either from epidemiological studies or research models, has led to better understanding of disease mechanisms. Table 2 represents a snapshot of toxicants that impact the PN and a brief description of pathways/protein targets that are disrupted. It should become apparent that common themes exist between toxicants and across the classic modes of PN dysfunction, such as the profound effect of environmental toxicants (heavy metals, pesticides) in all defined categories. Also, it is important to note that there are many converging mechanisms and crosstalk (signaling, ROS) among defined PN disruptions. For the focus of this review, we will discuss metals and pesticides, and their impact on the redox control of the PN as it relates to neurotoxicology.
Table 2.
Toxicants known to disrupt cellular proteostasis and mechanisms impacted.
| Table 2 | |||
|---|---|---|---|
| Toxicant | Mechanism | References | |
| ER Stress | Acetaminophen | ATF6, CHOP, Caspase-12 | 199–202 |
| HIV drugs (i.e. Efavirenz, Lopinavir) | CHOP, GRP78, eIF2α, XBP1S, ATF4 | 203–206 | |
| Type II diabetes drugs (i.e. troglitazone, ciglitazone) | ERK, PPARγ, eIF2α, MAPK | 207,208 | |
| Ethanol | ATF4, CHOP, GRP78 | 209–212 | |
| Environmental Toxicants (acrolein) | eIF2α, ATF3/4, CHOP | 167 | |
| Chemical Toxicants (iodoacetamide, TBHP, menadione) | Caspase-12, GRP94, GRP78 | 213,214 | |
| Metals (Cd, Cu, Fe, Zn, As, Mn) | CHOP, GADD34, ATF4 | 160,215 | |
| Pesticides (i.e. deltamethrin, PQ, MB) | CHOP, Caspase-12, GRP78 | 162,216–219 | |
| Protein Misfolding and Chaperones | Pesticides (Rotenone, PQ, MB, Chlorpyrifos) | BiP, PDI, CHOP, ATF4, HSPs | 84,168–170,174,175,220 |
| Metals (Cd) | HSPs, Metalloproteins | 156,171 | |
| Proteasome Inhibition | PD Related Pesticides (Rotenone, PQ, MB) | Mitochondrial Dysfunction, 20S inhibition | 85,174,181,221 |
| Metals (Cu, Pb) | Selenium inactivation, 20S inhibition | 179,180,222–224 | |
| Pesticides (i.e. TPT) | Direct Inhibition of Proteasome | 225 | |
| Autophagy | Pesticides (Rotenone, PQ, MB, Chlorpyrifos) | acetylated α-tubulin, Atg7/12, MAPK, Parkin | 174,184,186 |
| Metals (Cd, Mn, Cu, Pb) | mTOR/p70S6K, ERK, GSK-3β | 157,182,183,226,227 | |
| Rapamycin, 3-MA, Chloroquine | mTOR, PI3K, Ca++, Lysosome pH | 228–230 | |
| ROS Generation | PD Related Pesticides (Rotenone, PQ, MB) | Mitochondrial Dysfunction, Redox
Signaling |
151,231 |
| Formaldehyde | SOD1 | 232 | |
| Metals (Cd, Hg, As) | Trx, GSH, NOX, Fenton Reaction, Mitochondrial Dysfunction | 135,136,145,156,233–237 | |
A review by Farina et al does well to describe the vital role of metals in biochemical reactions, as well as the implications of environmental exposure to certain metals associated with oxidative stress and neurodegeneration135. Mechanisms of toxicity including Fenton chemistry, selenium inactivation, direct oxidation of cellular components (lipids, DNA, and proteins), and vital metal replacement impact all aspects of the PN, with redox disruption as a key player in neurodegeneration136,137. Specifically, several metals have been shown to impact the PN at multiple points or compartments (i.e. mitochondria/cytosol): Cadmium (Cd), Copper (Cu), Manganese (Mn), Arsenic (Ar), Mercury (Hg), and Lead (Pb)136,137.
Although evaluation of pesticide safety has led to regulation and control of human exposure, understanding of the toxicological impacts of chronic exposure to low levels of these compounds is still widely unknown. A recent review by Sabarwal et al describes pesticide exposure as well as the many toxic outcomes including cancer, neurodegenerative diseases (i.e. PD and AD), respiratory and reproductive disorders, and endocrine disruptions138. Similar to metals, certain pesticides have been found to be related to PN disruption in neurodegeneration, either through epidemiological studies or mechanistic research, such as those related to PD: rotenone, paraquat (PQ), and maneb (MB)139-141.
3.1. ROS Generation and Cellular Anti-oxidant Defense
As previously mentioned, oxidative and free radical damage of proteins has a widespread impact on the PN as well as the cellular defenses designed to maintain both protein function and redox state of the proteome. Mechanisms of toxicity throughout the PN disruptions listed below may be independent or resultant of toxicant-induced ROS generation. For example, Cu and iron (Fe) can undergo Fenton chemistry to directly produce hydroxyl radicals from hydrogen peroxide resulting in oxidative damage to lipids, DNA, and proteins142. While Cd does not participate in Fenton reactions, it does substitute itself in membrane and cytosolic metalloproteins (i.e. ferritin) leading to a higher abundance of unbound Cu and Fe to impart oxidative stress142-144. Cd exposure does cause ROS generation directly through other ROS species, however there are several ROS-independent mechanisms that contribute to overall oxidative and free radical damage and PN disruption. Additionally, exposure to Cd results in cysteine oxidation, thioredoxin oxidation, and significantly impacts the mitochondrial compartment far more than the cytoplasmic20,145. Another metal of particular interest is Mn and its relation to neurodegeneration involving ROS generation via increased mitochondrial respiration146. It has been proposed that Mn2+ exposure disrupts Ca2+ dynamics as well as directly impacts the electron transport chain (ETC) of the mitochondria146-148. Mn is also the metal component of the dithiocarbamate pesticide MB with similar associations to neurodegeneration through similar, but not identical pathways139. Regarding MB, it has been shown to directly inhibit complex III of the electron transport chain as well as impact mitochondrial membrane dynamics149,150. However, direct ROS production has not been consistently observed with MB exposure, which may be explained by Nrf2 activation and increase in cellular GSH151. In contrast, PQ, used in a co-exposure model of PD with MB, causes ROS production without activation of the Nrf2 response, contributing to the complex interplay of oxidative mechanisms seen in PD151. For the remainder of this review, we will present both ROS-mediated and ROS independent mechanisms of PN disruption.
Another impact of environmental exposures involves the thiol-containing proteins involved in the cellular antioxidant response. Cd, Hg, and As have been shown to significantly impact of the redox states of Trx proteins without impacting the GSH/GSSG redox status145. The disruption of the Trx pathway can have a significant impact on not only the resolution of oxidative damage to proteins through the thiol redox proteome, but through aberrant signaling and control of many cellular functions, such as mitochondrial function, ATP production, and apoptosis20,152,153. As these metals do not undergo Fenton-type chemistry, this impact is proposed to be directly on free thiols, leading to apoptosis pathway induction and/or accumulation of damaged proteins. Furthermore, similar observations are observed in pesticide exposures that mimic neurodegenerative pathology151. MB and PQ have been shown to differentially carbonylate proteins within the cortex and striatum of mice154. While the direct reactivity of MB to protein thiols has been reported, the association between oxidation of thiols and neurodegenerative endpoints such as protein aggregation, ATP depletion, and mitochondrial function are still being investigated19,20,155.
3.2. ER Stress
Metal-induced ER stress is characterized by ROS generation, oxidation of protein thiols, oxidative damage, and the substitution of catalytic metals in enzymes (i.e. Cu/Zn SOD)156. Manganese (Mn), an essential nutrient and trace element, has also been shown to induce activation of ER stress-related proteins, like CHOP and eIF2α, as result of oxidative damage to proteins and induction of the UPR136. Furthermore, Mn has been linked to neurodegeneration via Mn-induced apoptosis of dopaminergic neurons in PD and manganism via ER stress and disrupted autophagy157. One such mechanism includes the abundance and activity of MnSOD, which has been shown to be altered by exogenous Mn exposure158,159. In addition, Zn has also shown induction of ER stress in hypothalamic neurons, with enhancement of toxicity with co-exposure to Cu160. Lead (Pb), a metal that is widely accepted to negatively impact IQ in children, has also been reported to cause ER stress leading to protein aggregation161.
In regards to pesticide-induced ER stress, a recent study published by Hossain et al reports the detrimental impact of deltamethrin, a pyrethroid pesticide, on SK-N-AS human neuroblastoma cells through induction of apoptosis via the UPR pathway 162. Their investigation lead to a description of deltamethrin mechanism involving calpain activation leading to CHOP/GADD153 induction as well as caspase-12 cleavage with following caspase cascade. Although pyrethroid compounds have been shown to induce ROS and oxidative damage, the unique calpain apoptosis pathway activated with deltamethrin presents the possibility of a non ROS-mediated ER stress mechanism163. Combined with epidemiological links of pyrethroid exposure to neurodegeneration, similar induction of calpain-mediated apoptosis via caspase-12 has been observed in neurodegenerative pathologies such as AD, ALS, and PD 164,165. There are also reported associations between PQ and ER stress outcomes, however determination of direct interaction or indirect oxidative damage to proteins has yet to be made125. MB has also been shown to induce the ER stress pathways, potentially due to its ability to modify critical protein thiols19,86,166. Further, the environmental pollutant acrolein found in cigarette smoke has also shown induction of ER pathways as a result of damaged and misfolded proteins via oxidative adducts167.
3.3. Chaperones
As previously mentioned, molecular chaperones, such as the family of heat shock proteins (HSPs), are vital for not only the proper folding of native proteins, but as well as the UPR maintenance of misfolded and damaged proteins leading to recycling or disposal via chaperone-mediated autophagy. Induction of HSPs is not only a marker of pathological ER stress, but can be independently inhibited or altered by toxicant exposure as reported by several investigations168-170. Specifically, HSP70 and HSP40 have been observed to play a key role in PD pathology. While metals have been highly studied due to their association with proteinopathies of the brain, their direct effect on molecular chaperones are still widely unknown. Cd has been reported to induce protein aggregation through multiple mechanisms, one being direct binding and inhibition of unfoldases (DnaK, DnaJ, Hsp70, Hsp60, Hsp104) and ATP-driven proteases (Lon, ClpAB)171. Furthermore, silencing of Hsp70 ameliorated Cd-mediated apoptosis in SN56 neuroblastoma cell culture, possibly due to the modification of an allosteric redox switch on Hsp70172,173. With alterations in molecular chaperones presenting in multiple neurodegenerative diseases, it is no surprise that pesticides have shown similar alterations in HSP abundance and activity168. For instance, co-exposure of MB and PQ causes increased abundance of Hsp70 and Hsp90 in mice174. Investigations of other pesticides and human HSP modulation are rare, but chlorpyrifos and esfenvalerat have been shown to induce HSP expression in salmon175. Combination of toxicant-mediated alterations in native protein folding and UPR described above and disruptions in proper protein degradation and exocytosis creates this complex network of PN deficiencies observed in neurodegeneration.
It is important to note here the impact of oxidative stress and redox modifications on the signaling transduction pathways associated with Heat Shock Factor 1 (HSF-1), the transcriptional regulator of chaperone expression and heat shock response HSR. HSF-1 is heavily regulated through phosphorylation via protein kinase and phosphatase activity, enzymes shown to be modulated by ROS presence176-178. Increased cellular ROS can potentially dampen the HSR, allowing yet another indirect impact of general ROS on PN maintenance.
3.4. Proteasome and Autophagy
Proper function of the ubiquitin-proteasome pathway and removal of defective proteins are imperative to cellular defense against protein aggregation and maintenance of the proteome. Cu has been reported to directly inhibit proteasome activity and induce apoptosis in jurkat T cells and human breast cancer cells179. Additionally, As, Cd, and Pb showed inhibition of proteasomal activity in blood samples of a case-control investigation180. Similarly, the PD-related pesticides rotenone and PQ also show direct inhibition of the catalytic 20S subunit of the proteasome125,181. However, direct mechanistic links between thiol oxidation and proteasome inhibition by environmental toxicants have yet to be reported.
Metal-mediated alterations in autophagy have been highly reviewed in neurodegenerative diseases such as PD, AD, and HD182,183. For instance, Mn exposure in rats revealed dysfunctional lysosomes as well as quenched signaling for autophagy induction through mTOR/p70S6K pathway157,183. PQ and rotenone also have the ability to directly impact the autophagy machinery through alterations of chaperones involved in transport to the lysosome, mTOR signaling, and fusion of the lysosome with the autophagosome174,184. Furthermore, PQ has been shown to disrupt ubiquitin-dependent autophagy by reducing ubiquitin abundance with no reduction in mRNA185. Chlorpyrifos has also been reported to enhance LC3-II expression in a dose-dependent manner, with associations to mitochondrial dysfunction and apoptosis186. Again, a direct mechanistic link to protein thiol oxidation and toxicant-induced deficiencies in autophagy has yet to be made within neurotoxicology.
4. Toxicological Impact of Redox Stress and PN Dysfunction in Neurodegeneration: Separating Disrupted Signaling and Protein Aggregation
Two main pathways describe the major impacts of thiol redox homeostasis disruption on protein aggregation. First, alterations in protein thiols vital for the resolution and maintenance of oxidative damage to proteins will sensitize cells to ER stress and will exacerbate deficiencies in proper autophagy. Because of this, toxicants impacting these redox sensitive systems should display altered protein degradation and aggregation, as seen in rotenone-mediated alteration of α-synuclein metabolism181. However, it is vital to separate the impact of environmental exposures on redox signaling and the end result of protein aggregation, as many interventions target protein aggregations to alleviate pathology. The detrimental effect of protein aggregation on neuronal functions, such as synaptic transmission and autophagy, cannot be discounted, but may also represent a byproduct of upstream disruptions in PN control.
As mechanistic evaluation of toxicant models of neurodegeneration uncover more pathways altered in disease, focus must be made on the wide range of PN disruptions that can occur through modifications of the redox proteome via oxidative and free radical stress (Figure 1). Research performed with this focus will have the potential to find therapeutics that target protein aggregation in the earliest phases of its neurodegenerative phenotype and stop errant protein agglomerations whether as the cause or byproduct of pathology.
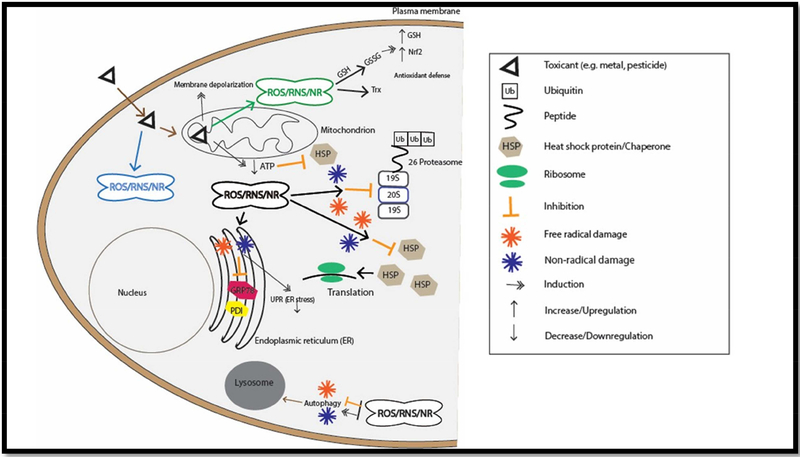
Figure 1: Schematic overview of the possible PN disruptions through toxicant-mediated oxidative adduction, free radical damage, and non-radical modifications.
Briefly, toxicants can impact the PN via direct mechanisms, like redox cycling and direct oxidation of critical proteins involved in proteasomal degradation, autophagy, and heat shock protein chaperones. Additionally, the PN can be negatively impacted by toxicant exposure via toxicant-mediated mitochondrial dysfunction, which can impair ATP production and exacerbate ROS production.
Acknowledgements
This work was supported by funds from the National Institutes of Health (R01 ES027593).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353(6294):aac4354.A thorough review of the core tenents of protein folding and chaperone function to achieve proteostasis.
- 2.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464.Labbadia and Morimoto analyze the components and properties of the proteostasis network in multiple organisms and describe the mechanisms associated with PN dysfunction and pathogenesis of disease.
- 3.Brandvold KR, Morimoto RI. The Chemical Biology of Molecular Chaperones--Implications for Modulation of Proteostasis. J Mol Biol. 2015;427(18):2931–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez CD, Lee MS, Marchetti P, et al. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy. 2011;7(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaisson S, Gillery P. Impaired proteostasis: role in the pathogenesis of diabetes mellitus. Diabetologia. 2014;57(8):1517–1527. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Cui J, He Q, Chen Z, Arvan P, Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol Aspects Med. 2015;42:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marré ML, Profozich JL, Coneybeer JT, et al. Inherent ER stress in pancreatic islet β cells causes self-recognition by autoreactive T cells in type 1 diabetes. J Autoimmun. 2016;72:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan JY, Luzuriaga J, Maxwell EL, West PK, Bensellam M, Laybutt DR. The balance between adaptive and apoptotic unfolded protein responses regulates β-cell death under ER stress conditions through XBP1, CHOP and JNK. Mol Cell Endocrinol. 2015;413:189–201. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZF, Li YB, Han JY, et al. The double-edged effect of autophagy in pancreatic beta cells and diabetes. Autophagy. 2011;7(1):12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costes S, Huang CJ, Gurlo T, et al. β-cell dysfunctional ERAD/ubiquitin/proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes. 2011;60(1):227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garza-Lombó C, Gonsebatt ME. Mammalian Target of Rapamycin: Its Role in Early Neural Development and in Adult and Aged Brain Function. Front Cell Neurosci. 2016;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirstein J, Morito D, Kakihana T, et al. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. EMBO J. 2015;34(18):2334–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hipp MS, Park SH, Hartl FU. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014;24(9):506–514. [DOI] [PubMed] [Google Scholar]
- 14.Kamat PK, Kalani A, Rai S, et al. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol Neurobiol. 2016;53(1):648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBean GJ, López MG, Wallner FK. Redox-based therapeutics in neurodegenerative disease. Br J Pharmacol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel M Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol Sci. 2016;37(9):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valenti D, de Bari L, De Filippis B, Henrion-Caude A, Vacca RA. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: an overview of Down syndrome, autism, Fragile X and Rett syndrome. Neurosci Biobehav Rev 2014;46 Pt 2:202–217. [DOI] [PubMed] [Google Scholar]
- 18.Muchova J, Zitnanova I, Durackova Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol Res. 2014;63(5):535–542. [DOI] [PubMed] [Google Scholar]
- 19.Roede JR, Jones DP. Thiol-reactivity of the fungicide maneb. Redox Biol 2014;2:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Go YM, Roede JR, Orr M, Liang Y, Jones DP. Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of cd toxicity. Toxicol Sci. 2014;139(1):59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzatkowski DM, Trombetta LD. Maneb causes pro-oxidant effects in the hippocampus of Nrf2 knockout mice. Environ Toxicol Pharmacol. 2013;36(2):427–436. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Wei G, Huang Z, et al. Mitochondrial proteomic alterations caused by long-term low-dose copper exposure in mouse cortex. Toxicol Lett. 2016;263:16–25. [DOI] [PubMed] [Google Scholar]
- 23.Bové J, Perier C. Neurotoxin-based models of Parkinson’s disease. Neuroscience. 2012;211:51–76. [DOI] [PubMed] [Google Scholar]
- 24.Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol Sci. 2011;124(2):225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pajares M, Jiménez-Moreno N, Dias IH, et al. Redox control of protein degradation. Redox Biol 2015;6:409–420.This review offers a detailed description of how oxidative stress and redox imbalance can regulate/affect protein degradation (autophagy, 26s proteasome)
- 26.Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Pérez ME, Zaffagnini M, Marchand CH, Crespo JL, Lemaire SD. The yeast autophagy protease Atg4 is regulated by thioredoxin. Autophagy 2014;10(11):1953–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441(2):523–540.This review analyzes studies focused on redox regulation of autophagy with a particular specialization in basic mechanisms of mitophagy. The impact of autophagy on mitochondrial function formation of reactive species is allso discussed.
- 29.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii T, Sakurai T, Usami H, Uchida K. Oxidative modification of proteasome: identification of an oxidation-sensitive subunit in 26 S proteasome. Biochemistry. 2005;44(42):13893–13901. [DOI] [PubMed] [Google Scholar]
- 31.Niforou K, Cheimonidou C, Trougakos IP. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014;2:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eletto D, Chevet E, Argon Y, Appenzeller-Herzog C. Redox controls UPR to control redox. J Cell Sci. 2014;127(Pt 17):3649–3658.This paper details redox mechanisms of activation of the unfolded protein response (UPR) and focuses on endoplasmic reticulum (ER) stress and protein disulfide isomerases (PDI) function.
- 33.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretz-Remy C, Arrigo AP. Modulation of the chymotrypsin-like activity of the 20S proteasome by intracellular redox status: effects of glutathione peroxidase-1 overexpression and antioxidant drugs. Biol Chem. 2003;384(4):589–595. [DOI] [PubMed] [Google Scholar]
- 35.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. [DOI] [PubMed] [Google Scholar]
- 36.Díaz-Villanueva JF, Díaz-Molina R, García-González V. Protein Folding and Mechanisms of Proteostasis. Int J Mol Sci 2015;16(8):17193–17230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matus S, Glimcher LH, Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23(2):239–252. [DOI] [PubMed] [Google Scholar]
- 38.Brar GA, Weissman JS. Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat Rev Mol Cell Biol. 2015;16(11):651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleiger G, Mayor T. Perilous journey: a tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014;24(6):352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bento CF, Renna M, Ghislat G, et al. Mammalian Autophagy: How Does It Work? Annu Rev Biochem. 2016;85:685–713.This extensive review describes the molecular biology of mammalian autophagy by elaborating on roles and regulation of key protein involved in the autophagic process.
- 41.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. [DOI] [PubMed] [Google Scholar]
- 42.Grimm S, Höhn A, Grune T. Oxidative protein damage and the proteasome. Amino Acids. 2012;42(1):23–38.This review offers extensive analysis of free radical protein damage mechanisms and 26s proteasome behavior under oxidative stress and redox imbalance.
- 43.Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305(3):709–718. [DOI] [PubMed] [Google Scholar]
- 44.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11(7):526–534. [PubMed] [Google Scholar]
- 45.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9–10):1865–1879. [DOI] [PubMed] [Google Scholar]
- 46.Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715–748.A current review on methods for evaluating oxidative stress and redox interactions in biomedical research
- 47.Hawkins CL, Davies MJ. Generation and propagation of radical reactions on proteins. Biochim Biophys Acta. 2001;1504(2–3):196–219. [DOI] [PubMed] [Google Scholar]
- 48.Baraibar MA, Liu L, Ahmed EK, Friguet B. Protein oxidative damage at the crossroads of cellular senescence, aging, and age-related diseases. Oxid Med Cell Longev. 2012;2012:919832 This review explores mechanisms of protein damage through direct oxidation and protein adduct formation through interaction with lipid peroxidation and advanced glycated end products. Furthermore, implication of oxidative protein damage in aging and age-related diseases is discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–218. [DOI] [PubMed] [Google Scholar]
- 50.Go YM, Chandler JD, Jones DP. The cysteine proteome. Free Radic Biol Med. 2015;84:227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones DP, Go YM. Mapping the cysteine proteome: analysis of redox-sensing thiols. Curr Opin Chem Biol. 2011;15(1):103–112. [DOI] [PubMed] [Google Scholar]
- 52.Go YM, Jones DP. Redox theory of aging: implications for health and disease. Clin Sci (Lond). 2017;131(14):1669–1688.A current and comprehensive review of oxidative stress, redox signaling and control, and disease.
- 53.Jones DP. Redox sensing: orthogonal control in cell cycle and apoptosis signalling. J Intern Med. 2010;268(5):432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sbodio JI, Snyder SH, Paul BD. Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities. Antioxid Redox Signal. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McBean GJ, Aslan M, Griffiths HR, Torrão RC. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;5:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fritz KS, Galligan JJ, Smathers RL, et al. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol. 2011;24(5):651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen. 2010;51(5):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrera G, Pizzimenti S, Daga M, et al. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants (Basel). 2018;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winter J, Linke K, Jatzek A, Jakob U. Severe oxidative stress causes inactivation of DnaK and activation of the redox-regulated chaperone Hsp33. Mol Cell. 2005;17(3):381–392. [DOI] [PubMed] [Google Scholar]
- 61.Conway ME, Lee C. The redox switch that regulates molecular chaperones. Biomol Concepts. 2015;6(4):269–284. [DOI] [PubMed] [Google Scholar]
- 62.Galligan JJ, Petersen DR. The human protein disulfide isomerase gene family. Hum Genomics. 2012;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galligan JJ, Fritz KS, Backos DS, et al. Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse model of alcoholic liver disease: functional independence of ATPase activity and chaperone function. Free Radic Biol Med. 2014;73:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carbone DL, Doorn JA, Kiebler Z, Sampey BP, Petersen DR. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem Res Toxicol. 2004;17(11):1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franco MC, Ricart KC, Gonzalez AS, et al. Nitration of Hsp90 on Tyrosine 33 Regulates Mitochondrial Metabolism. J Biol Chem. 2015;290(31):19055–19066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magnoni R, Palmfeldt J, Christensen JH, et al. Late onset motoneuron disorder caused by mitochondrial Hsp60 chaperone deficiency in mice. Neurobiol Dis. 2013;54:12–23. [DOI] [PubMed] [Google Scholar]
- 67.Chien V, Aitken JF, Zhang S, et al. The chaperone proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and formation of β-sheet-containing aggregates by human amylin: a potential role for defective chaperone biology in Type 2 diabetes. Biochem J. 2010;432(1):113–121. [DOI] [PubMed] [Google Scholar]
- 68.Eisele YS, Monteiro C, Fearns C, et al. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov. 2015;14(11):759–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aguzzi A, O’Connor T. Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9(3):237–248. [DOI] [PubMed] [Google Scholar]
- 70.Chondrogianni N, Sakellari M, Lefaki M, Papaevgeniou N, Gonos ES. Proteasome activation delays aging in vitro and in vivo. Free Radic Biol Med. 2014;71:303–320. [DOI] [PubMed] [Google Scholar]
- 71.Amm I, Sommer T, Wolf DH. Protein quality control and elimination of protein waste: the role of the ubiquitin-proteasome system. Biochim Biophys Acta. 2014;1843(1):182–196. [DOI] [PubMed] [Google Scholar]
- 72.Ruggiano A, Foresti O, Carvalho P. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol. 2014;204(6):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci. 2001;58(10):1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zmijewski JW, Banerjee S, Abraham E. S-glutathionylation of the Rpn2 regulatory subunit inhibits 26 S proteasomal function. J Biol Chem. 2009;284(33):22213–22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulteau AL, Moreau M, Nizard C, Friguet B. Impairment of proteasome function upon UVA- and UVB-irradiation of human keratinocytes. Free Radic Biol Med. 2002;32(11):1157–1170. [DOI] [PubMed] [Google Scholar]
- 76.Reinheckel T, Ullrich O, Sitte N, Grune T. Differential impairment of 20S and 26S proteasome activities in human hematopoietic K562 cells during oxidative stress. Arch Biochem Biophys. 2000;377(1):65–68. [DOI] [PubMed] [Google Scholar]
- 77.Yao D, Gu Z, Nakamura T, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101(29):10810–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jahngen-Hodge J, Obin MS, Gong X, et al. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272(45):28218–28226. [DOI] [PubMed] [Google Scholar]
- 79.Harder B, Jiang T, Wu T, et al. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem Soc Trans. 2015;43(4):680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vriend J, Reiter RJ. The Keap1-Nrf2-antioxidant response element pathway: a review of its regulation by melatonin and the proteasome. Mol Cell Endocrinol. 2015;401:213–220. [DOI] [PubMed] [Google Scholar]
- 81.Wertz IE. TNFR1-activated NF-κB signal transduction: regulation by the ubiquitin/proteasome system. Curr Opin Chem Biol. 2014;23:71–77. [DOI] [PubMed] [Google Scholar]
- 82.Shringarpure R, Davies KJ. Protein turnover by the proteasome in aging and disease. Free Radic Biol Med. 2002;32(11):1084–1089. [DOI] [PubMed] [Google Scholar]
- 83.Sulistio YA, Heese K. The Ubiquitin-Proteasome System and Molecular Chaperone Deregulation in Alzheimer’s Disease. Mol Neurobiol. 2016;53(2):905–931. [DOI] [PubMed] [Google Scholar]
- 84.Cook C, Stetler C, Petrucelli L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(5):a009423.A thorough review of several aspects of PN disruption (autophagy, chaperones, UPS) in PD and associations of oxidative mechanisms of neurodegeneration
- 85.Fornai F, Schluter OM, Lenzi P, et al. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102(9):3413–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aivazidis S, Coughlan CM, Rauniyar AK, et al. The burden of trisomy 21 disrupts the proteostasis network in Down syndrome. PLoS One. 2017;12(4):e0176307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shibutani ST, Saitoh T, Nowag H, Münz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16(10):1014–1024. [DOI] [PubMed] [Google Scholar]
- 88.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16(8):461–472. [DOI] [PubMed] [Google Scholar]
- 89.Redmann M, Benavides GA, Berryhill TF, et al. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol 2017;11:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sarkar S, Rubinsztein DC. Huntington’s disease: degradation of mutant huntingtin by autophagy. FEBS J. 2008;275(17):4263–4270. [DOI] [PubMed] [Google Scholar]
- 91.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–4091. [DOI] [PubMed] [Google Scholar]
- 92.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19(6):365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7(7):673–682. [DOI] [PubMed] [Google Scholar]
- 94.Crino PB. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol. 2016;12(7):379–392. [DOI] [PubMed] [Google Scholar]
- 95.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11(9):709–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41(5):1103–1130.This review provides valuable mechanistic insight to mTOR-dependent and mTOR-independent pathways of autophagic regulation.
- 97.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8(1–2):152–162. [DOI] [PubMed] [Google Scholar]
- 98.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15(11):4829–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182(7):4046–4055. [DOI] [PubMed] [Google Scholar]
- 100.Wang HL, Chou AH, Wu AS, et al. PARK6 PINK1 mutants are defective in maintaining mitochondrial membrane potential and inhibiting ROS formation of substantia nigra dopaminergic neurons. Biochim Biophys Acta. 2011;1812(6):674–684. [DOI] [PubMed] [Google Scholar]
- 101.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12(3):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu JJ, Quijano C, Chen E, et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY). 2009;1(4):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, Wang K, Lei Y, Li Q, Nice EC, Huang C. Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med. 2015;89:452–465. [DOI] [PubMed] [Google Scholar]
- 104.Huang YC, Yu HS, Chai CY. Roles of oxidative stress and the ERK1/2, PTEN and p70S6K signaling pathways in arsenite-induced autophagy. Toxicol Lett. 2015;239(3):172–181. [DOI] [PubMed] [Google Scholar]
- 105.Wang J, Yang X, Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal. 2016;28(8):1099–1104. [DOI] [PubMed] [Google Scholar]
- 106.Nakatogawa H, Ishii J, Asai E, Ohsumi Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy. 2012;8(2):177–186. [DOI] [PubMed] [Google Scholar]
- 107.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J. 2015;282(24):4672–4678. [DOI] [PubMed] [Google Scholar]
- 109.Dodson M, Redmann M, Rajasekaran NS, Darley-Usmar V, Zhang J. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem J. 2015;469(3):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27(20):2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gañán-Gómez I, Wei Y, Yang H, Boyano-Adánez MC, García-Manero G. Oncogenic functions of the transcription factor Nrf2. Free Radic Biol Med. 2013;65:750–764. [DOI] [PubMed] [Google Scholar]
- 112.Lacher SE, Slattery M. Gene regulatory effects of disease-associated variation in the NRF2 network. Curr Opin Toxicol 2016;1:71–79.A detailed review regarding the regulation of the NRF2 major antioxidant pathway and its association with disease.
- 113.Kubben N, Zhang W, Wang L, et al. Repression of the Antioxidant NRF2 Pathway in Premature Aging. Cell. 2016;165(6):1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gan L, Johnson JA. Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta. 2014;1842(8):1208–1218. [DOI] [PubMed] [Google Scholar]
- 115.Ichimura Y, Komatsu M. Activation of p62/SQSTM1-Keap1-Nuclear Factor Erythroid 2-Related Factor 2 Pathway in Cancer. Front Oncol. 2018;8:210.The p62--Keap1-Nrf2 axis is a significant converging point of autophagy and antioxidant defense pathways and in this publication provides an overview for its association with tumorigenesis.
- 116.Ichimura Y, Waguri S, Sou YS, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51(5):618–631. [DOI] [PubMed] [Google Scholar]
- 117.Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. [DOI] [PubMed] [Google Scholar]
- 118.Dufey E, Sepúlveda D, Rojas-Rivera D, Hetz C. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 1. An overview. Am J Physiol Cell Physiol. 2014;307(7):C582–594. [DOI] [PubMed] [Google Scholar]
- 119.Liu XD, Ko S, Xu Y, et al. Transient aggregation of ubiquitinated proteins is a cytosolic unfolded protein response to inflammation and endoplasmic reticulum stress. J Biol Chem. 2012;287(23):19687–19698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Plate L, Cooley CB, Chen JJ, et al. Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hetz C The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. [DOI] [PubMed] [Google Scholar]
- 122.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Okumura M, Kadokura H, Hashimoto S, et al. Inhibition of the functional interplay between endoplasmic reticulum (ER) oxidoreduclin-1α (Ero1α) and protein-disulfide isomerase (PDI) by the endocrine disruptor bisphenol A. J Biol Chem. 2014;289(39):27004–27018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carpio MA, Michaud M, Zhou W, Fisher JK, Walensky LD, Katz SG. BCL-2 family member BOK promotes apoptosis in response to endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2015;112(23):7201–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chinta SJ, Rane A, Poksay KS, Bredesen DE, Andersen JK, Rao RV. Coupling endoplasmic reticulum stress to the cell death program in dopaminergic cells: effect of paraquat. Neuromolecular Med. 2008;10(4):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaplan A, Gaschler MM, Dunn DE, et al. Small molecule-induced oxidation of protein disulfide isomerase is neuroprotective. Proc Natl Acad Sci U S A. 2015;112(17):E2245–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamura T, Prikhodko OA, Pirie E, et al. Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol Dis. 2015;84:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perri ER, Thomas CJ, Parakh S, Spencer DM, Atkin JD. The Unfolded Protein Response and the Role of Protein Disulfide Isomerase in Neurodegeneration. Front Cell Dev Biol. 2015;3:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Araki K, Iemura S, Kamiya Y, et al. Ero1-α and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J Cell Biol. 2013;202(6):861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kannan M, Sivaprakasam C, Prinz WA, Nachiappan V. Endoplasmic reticulum stress affects the transport of phosphatidylethanolamine from mitochondria to the endoplasmic reticulum in S.cerevisiae. Biochim Biophys Acta. 2016;1861(12 Pt A):1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol. 2010;41(2–3):55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Colla E, Coune P, Liu Y, et al. Endoplasmic reticulum stress is important for the manifestations of α-synucleinopathy in vivo. J Neurosci. 2012;32(10):3306–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoo BC, Krapfenbauer K, Cairns N, Belay G, Bajo M, Lubec G. Overexpressed protein disulfide isomerase in brains of patients with sporadic Creutzfeldt-Jakob disease. Neurosci Lett. 2002;334(3):196–200. [DOI] [PubMed] [Google Scholar]
- 134.Atkin JD, Farg MA, Walker AK, McLean C, Tomas D, Horne MK. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30(3):400–407. [DOI] [PubMed] [Google Scholar]
- 135.Farina M, Avila DS, da Rocha JB, Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int. 2013;62(5):575–594.This very comprehensive review describes the oxidative and free radical stress associated with a variety of metals and their associations with neurodegenerative disease
- 136.Chen P, Miah MR, Aschner M. Metals and Neurodegeneration. F1000Res. 2016;5.A current review covering a broad range of metals and their connections to neurodegenerative disease including metals presented in this review.
- 137.Hartwig A Metal interaction with redox regulation: an integrating concept in metal carcinogenesis? Free Radic Biol Med. 2013;55:63–72. [DOI] [PubMed] [Google Scholar]
- 138.Sabarwal A, Kumar K, Singh RP. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ Toxicol Pharmacol. 2018;63:103–114.A current review on pesticides and outcomes such as cancer, neurodegenerative disease, and oxidative stress
- 139.Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases--a mechanistic approach. Toxicol Lett. 2014;230(2):85–103. [DOI] [PubMed] [Google Scholar]
- 140.Bastias-Candia S, Zolezzi JM, Inestrosa NC. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol Neurobiol. 2018.A current review of rotenone and paraquat associations with Parkinson’s disease
- 141.Jakaria M, Park SY, Haque ME, et al. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front Mol Neurosci 2018;11:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2–3):65–87. [DOI] [PubMed] [Google Scholar]
- 143.Branca JJV, Morucci G, Pacini A. Cadmium-induced neurotoxicity: still much ado. Neural Regen Res. 2018;13(11):1879–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mohajeri M, Rezaee M, Sahebkar A. Cadmium-induced toxicity is rescued by curcumin: A review. Biofactors. 2017;43(5):645–661. [DOI] [PubMed] [Google Scholar]
- 145.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40(1):138–145. [DOI] [PubMed] [Google Scholar]
- 146.Martinez-Finley EJ, Gavin CE, Aschner M, Gunter TE. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med. 2013;62:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20(2–3):445–453. [PubMed] [Google Scholar]
- 148.Smith MR, Fernandes J, Go YM, Jones DP. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem Biophys Res Commun. 2017;482(3):388–398.A detailed review of manganese and its involvement in redox disruption in Parkinson’s disease
- 149.Zhang J, Fitsanakis VA, Gu G, et al. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neurochem. 2003;84(2):336–346. [DOI] [PubMed] [Google Scholar]
- 150.Domico LM, Zeevalk GD, Bernard LP, Cooper KR. Acute neurotoxic effects of mancozeb and maneb in mesencephalic neuronal cultures are associated with mitochondrial dysfunction. Neurotoxicology. 2006;27(5):816–825. [DOI] [PubMed] [Google Scholar]
- 151.Roede JR, Hansen JM, Go YM, Jones DP. Maneb and paraquat-mediated neurotoxicity: involvement of peroxiredoxin/thioredoxin system. Toxicol Sci. 2011;121(2):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Briston T, Hicks AR. Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention. Biochem Soc Trans. 2018;46(4):829–842.Briston and Hicks describe how protein misfolding can promote mitochondrial dysfunction in neurodegenerative disorders and suggest new therapeutic approaches to tackle pathogenesis.
- 153.Roede JR, Go YM, Jones DP. Redox equivalents and mitochondrial bioenergetics. Methods Mol Biol. 2012;810:249–280. [DOI] [PubMed] [Google Scholar]
- 154.Coughlan C, Walker DI, Lohr KM, et al. Comparative Proteomic Analysis of Carbonylated Proteins from the Striatum and Cortex of Pesticide-Treated Mice. Parkinsons Dis. 2015;2015:812532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anderson CC, Aivazidis S, Kuzyk CL, Jain A, Roede JR. Acute Maneb Exposure Significantly Alters Both Glycolysis and Mitochondrial Function in Neuroblastoma Cells. Toxicol Sci. 2018;165(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trojsi F, Monsurro MR, Tedeschi G. Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: state of the art and research perspectives. Int J Mol Sci 2013;14(8):15286–15311.This review covers both metals and pesticides as they relate to ALS both through mechanistic evalutaion and epidemiological comparison
- 157.Zhang J, Cao R, Cai T, et al. The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotox Res 2013;24(4):478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bresciani G, Cruz IB, de Paz JA, Cuevas MJ, Gonzalez-Gallego J. The MnSOD Ala16Val SNP: relevance to human diseases and interaction with environmental factors. Free Radic Res. 2013;47(10):781–792. [DOI] [PubMed] [Google Scholar]
- 159.Li L, Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev. 2018;2018:7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tanaka KI, Kawahara M. Copper Enhances Zinc-Induced Neurotoxicity and the Endoplasmic Reticulum Stress Response in a Neuronal Model of Vascular Dementia. Front Neurosci 2017;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang J, Cai T, Zhao F, et al. The role of alpha-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci 2012;8(7):935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and the ER stress pathway. Toxicol Sci. 2011;122(2):512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang Y, Liu W, Wang J, Zhang Y, Xu W, Tao L. The different effects of natural pyrethrins and beta-cypermethrin on human hepatocyte QSG7701 cells by ROS-mediated oxidative damage. Environ Sci Pollut Res Int. 2018;25(24):24230–24240. [DOI] [PubMed] [Google Scholar]
- 164.Doi H, Kikuchi H, Murai H, et al. Motor neuron disorder simulating ALS induced by chronic inhalation of pyrethroid insecticides. Neurology. 2006;67(10):1894–1895. [DOI] [PubMed] [Google Scholar]
- 165.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38(1):78–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roede JR, Uppal K, Park Y, Tran V, Jones DP. Transcriptome-metabolome wide association study (TMWAS) of maneb and paraquat reveals network level interactions in toxicologic mechanism. Toxicology Reports. 2014;1:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohammad MK, Avila D, Zhang J, et al. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265(1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dimant H, Ebrahimi-Fakhari D, McLean PJ. Molecular chaperones and co-chaperones in Parkinson disease. Neuroscientist 2012;18(6):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Holtz WA, O’Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278(21):19367–19377. [DOI] [PubMed] [Google Scholar]
- 170.Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16(6):653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jacobson T, Priya S, Sharma SK, et al. Cadmium Causes Misfolding and Aggregation of Cytosolic Proteins in Yeast. Mol Cell Biol. 2017;37(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moyano P, Garcia JM, Lobo M, et al. Cadmium alters heat shock protein pathways in SN56 cholinergic neurons, leading to Abeta and phosphorylated Tau protein generation and cell death. Food Chem Toxicol. 2018;121:297–308. [DOI] [PubMed] [Google Scholar]
- 173.Vignols F, Mouaheb N, Thomas D, Meyer Y. Redox control of Hsp70-Co-chaperone interaction revealed by expression of a thioredoxin-like Arabidopsis protein. J Biol Chem. 2003;278(7):4516–4523. [DOI] [PubMed] [Google Scholar]
- 174.Wills J, Credle J, Oaks AW, et al. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS One 2012;7(1):e30745.A thorough investigation into PQ-mediated alterations on the proteasome, leading to protein accumulation and aggregation in mice
- 175.Eder KJ, Leutenegger CM, Kohler HR, Werner I. Effects of neurotoxic insecticides on heat-shock proteins and cytokine transcription in Chinook salmon (Oncorhynchus tshawytscha). Ecotoxicol Environ Saf. 2009;72(1):182–190. [DOI] [PubMed] [Google Scholar]
- 176.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. [DOI] [PubMed] [Google Scholar]
- 177.Calderwood SK, Xie Y, Wang X, et al. Signal Transduction Pathways Leading to Heat Shock Transcription. Sign Transduct Insights. 2010;2:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Natarajan V, Scribner WM, al-Hassani M, Vepa S. Reactive oxygen species signaling through regulation of protein tyrosine phosphorylation in endothelial cells. Environ Health Perspect. 1998;106 Suppl 5:1205–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xiao Y, Chen DI, Zhang X, et al. Molecular study on copper-mediated tumor proteasome inhibition and cell death. Int J Oncol. 2010;37(1):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Neslund-Dudas C, Mitra B, Kandegedara A, et al. Association of metals and proteasome activity in erythrocytes of prostate cancer patients and controls. Biol Trace Elem Res. 2012;149(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Betarbet R, Canet-Aviles RM, Sherer TB, et al. Intersecting pathways to neurodegeneration in Parkinson’s disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22(2):404–420. [DOI] [PubMed] [Google Scholar]
- 182.Chatterjee S, Sarkar S, Bhattacharya S. Toxic metals and autophagy. Chem Res Toxicol. 2014;27(11):1887–1900. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Z, Miah M, Culbreth M, Aschner M. Autophagy in Neurodegenerative Diseases and Metal Neurotoxicity. Neurochem Res. 2016;41(1–2):409–422.A detailed review of common themes among metal-induced disruptions in autophagy and deficiencies in neurodegenerative disease and protein aggregation
- 184.Dagda RK, Das Banerjee T, Janda E. How Parkinsonian toxins dysregulate the autophagy machinery. Int J Mol Sci. 2013;14(11):22163–22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Navarro-Yepes J, Anandhan A, Bradley E, et al. Inhibition of Protein Ubiquitination by Paraquat and 1-Methyl-4-Phenylpyridinium Impairs Ubiquitin-Dependent Protein Degradation Pathways. Mol Neurobiol. 2016;53(8):5229–5251.Investigation into the mechanistic link between PD toxins (PQ) and disruption in proper protein degradation via the proteasome leading to protein aggregation
- 186.Park JH, Lee JE, Shin IC, Koh HC. Autophagy regulates chlorpyrifos-induced apoptosis in SH-SY5Y cells. Toxicol Appl Pharmacol. 2013;268(1):55–67. [DOI] [PubMed] [Google Scholar]
- 187.Yun HM, Jin P, Han JY, et al. Acceleration of the development of Alzheimer’s disease in amyloid beta-infused peroxiredoxin 6 overexpression transgenic mice. Mol Neurobiol. 2013;48(3):941–951. [DOI] [PubMed] [Google Scholar]
- 188.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. [DOI] [PubMed] [Google Scholar]
- 189.Jang H, Arce FT, Ramachandran S, et al. Truncated beta-amyloid peptide channels provide an alternative mechanism for Alzheimer’s Disease and Down syndrome. Proc Natl Acad Sci U S A. 2010;107(14):6538–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279(17):17957–17962. [DOI] [PubMed] [Google Scholar]
- 191.Deas E, Cremades N, Angelova PR, et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid Redox Signal. 2016;24(7):376–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Finkbeiner S Huntington’s Disease. Cold Spring Harb Perspect Biol. 2011;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arrasate M, Finkbeiner S. Protein aggregates in Huntington’s disease. Exp Neurol. 2012;238(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yerbury JJ, Gower D, Vanags L, Roberts K, Lee JA, Ecroyd H. The small heat shock proteins αB-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones. 2013;18(2):251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Silverman JM, Fernando SM, Grad LI, et al. Disease Mechanisms in ALS: Misfolded SOD1 Transferred Through Exosome-Dependent and Exosome-Independent Pathways. Cell Mol Neurobiol. 2016;36(3):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li YR, King OD, Shorter J, Gitler AD. Stress granules as crucibles of ALS pathogenesis. J Cell Biol. 2013;201(3):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aguzzi A, Nuvolone M, Zhu C. The immunobiology of prion diseases. Nat Rev Immunol. 2013;13(12):888–902. [DOI] [PubMed] [Google Scholar]
- 198.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. [DOI] [PubMed] [Google Scholar]
- 199.Nagy G, Kardon T, Wunderlich L, et al. Acetaminophen induces ER dependent signaling in mouse liver. Arch Biochem Biophys. 2007;459(2):273–279. [DOI] [PubMed] [Google Scholar]
- 200.Nagy G, Szarka A, Lotz G, et al. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2010;243(1):96–103. [DOI] [PubMed] [Google Scholar]
- 201.Uzi D, Barda L, Scaiewicz V, et al. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59(3):495–503. [DOI] [PubMed] [Google Scholar]
- 202.Wang X, Thomas B, Sachdeva R, et al. Mechanism of arylating quinone toxicity involving Michael adduct formation and induction of endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2006;103(10):3604–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Apostolova N, Gomez-Sucerquia LJ, Alegre F, et al. ER stress in human hepatic cells treated with Efavirenz: mitochondria again. J Hepatol. 2013;59(4):780–789. [DOI] [PubMed] [Google Scholar]
- 204.Gomez-Sucerquia LJ, Blas-Garcia A, Marti-Cabrera M, Esplugues JV, Apostolova N. Profile of stress and toxicity gene expression in human hepatic cells treated with Efavirenz. Antiviral Res. 2012;94(3):232–241. [DOI] [PubMed] [Google Scholar]
- 205.Kao E, Shinohara M, Feng M, Lau MY, Ji C. Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology. 2012;56(2):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Parker RA, Flint OP, Mulvey R, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol Pharmacol. 2005;67(6):1909–1919. [DOI] [PubMed] [Google Scholar]
- 207.Gardner OS, Dewar BJ, Earp HS, Samet JM, Graves LM. Dependence of peroxisome proliferator-activated receptor ligand-induced mitogen-activated protein kinase signaling on epidermal growth factor receptor transactivation. J Biol Chem. 2003;278(47):46261–46269. [DOI] [PubMed] [Google Scholar]
- 208.Gardner OS, Shiau CW, Chen CS, Graves LM. Peroxisome proliferator-activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: correlation with endoplasmic reticulum stress. J Biol Chem. 2005;280(11):10109–10118. [DOI] [PubMed] [Google Scholar]
- 209.Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G54–63. [DOI] [PubMed] [Google Scholar]
- 210.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–1499. [DOI] [PubMed] [Google Scholar]
- 211.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29(8):1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Magne L, Blanc E, Legrand B, et al. ATF4 and the integrated stress response are induced by ethanol and cytochrome P450 2E1 in human hepatocytes. J Hepatol. 2011;54(4):729–737. [DOI] [PubMed] [Google Scholar]
- 213.Muruganandan S, Cribb AE. Calpain-induced endoplasmic reticulum stress and cell death following cytotoxic damage to renal cells. Toxicol Sci. 2006;94(1):118–128. [DOI] [PubMed] [Google Scholar]
- 214.Ryan PM, Bedard K, Breining T, Cribb AE. Disruption of the endoplasmic reticulum by cytotoxins in LLC-PK1 cells. Toxicol Lett. 2005;159(2):154–163. [DOI] [PubMed] [Google Scholar]
- 215.Naranmandura H, Xu S, Koike S, et al. The endoplasmic reticulum is a target organelle for trivalent dimethylarsinic acid (DMAIII)-induced cytotoxicity. Toxicol Appl Pharmacol. 2012;260(3):241–249. [DOI] [PubMed] [Google Scholar]
- 216.Hossain MM, DiCicco-Bloom E, Richardson JR. Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol Sci. 2015;143(1):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J Neuroinflammation. 2009;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sama DM, Norris CM. Calcium dysregulation and neuroinflammation: discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res Rev. 2013;12(4):982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22(24):10690–10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang XF, Li S, Chou AP, Bronstein JM. Inhibitory effects of pesticides on proteasome activity: implication in Parkinson’s disease. Neurobiol Dis. 2006;23(1):198–205. [DOI] [PubMed] [Google Scholar]
- 222.Chen D, Cui QC, Yang H, Dou QP. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66(21):10425–10433. [DOI] [PubMed] [Google Scholar]
- 223.Schrauzer GN. Selenium and selenium-antagonistic elements in nutritional cancer prevention. Crit Rev Biotechnol. 2009;29(1):10–17. [DOI] [PubMed] [Google Scholar]
- 224.Wang F, Zhai S, Liu X, et al. A novel dithiocarbamate analogue with potentially decreased ALDH inhibition has copper-dependent proteasome-inhibitory and apoptosis-inducing activity in human breast cancer cells. Cancer Lett. 2011;300(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shi G, Chen D, Zhai G, et al. The proteasome is a molecular target of environmental toxic organotins. Environ Health Perspect. 2009;117(3):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Di Gioacchino M, Petrarca C, Perrone A, et al. Autophagy as an ultrastructural marker of heavy metal toxicity in human cord blood hematopoietic stem cells. Sci Total Environ. 2008;392(1):50–58. [DOI] [PubMed] [Google Scholar]
- 227.Scheiber IF, Mercer JF, Dringen R. Metabolism and functions of copper in brain. Prog Neurobiol. 2014;116:33–57. [DOI] [PubMed] [Google Scholar]
- 228.Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. [DOI] [PubMed] [Google Scholar]
- 229.Bolt AM, Klimecki WT. Autophagy in toxicology: self-consumption in times of stress and plenty. J Appl Toxicol. 2012;32(7):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Autophagy Zhang J. and Mitophagy in Cellular Damage Control. Redox Biol 2013;1(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pan-Montojo F, Reichmann H. Considerations on the role of environmental toxins in idiopathic Parkinson’s disease pathophysiology. Transl Neurodegener 2014;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yu GY, Song XF, Liu Y, Sun ZW. Inhaled formaldehyde induces bone marrow toxicity via oxidative stress in exposed mice. Asian Pac J Cancer Prev. 2014;15(13):5253–5257. [DOI] [PubMed] [Google Scholar]
- 233.Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27(11–12):1405–1412. [DOI] [PubMed] [Google Scholar]
- 234.Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L442–449. [DOI] [PubMed] [Google Scholar]
- 235.Singh AP, Goel RK, Kaur T. Mechanisms pertaining to arsenic toxicity. Toxicol Int. 2011;18(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Argos M, Ahsan H, Graziano JH. Arsenic and human health: epidemiologic progress and public health implications. Rev Environ Health. 2012;27(4):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Watanabe T, Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 2013;87(6):969–979. [DOI] [PubMed] [Google Scholar]