Abstract
Mammalian transient receptor potential (TRP) channels mediate Ca2+ flux and voltage changes across membranes in response to environmental and cellular signals. At the plasma membrane, sensory TRPs act as neuronal detectors of physical and chemical environmental signals, and receptor-operated (metabotropic) TRPs decode extracellular neuroendocrine cues to control body homeostasis. In intracellular membranes, such as lysosomes, organellar TRPs respond to compartment-derived signals to control membrane trafficking, signal transduction, and organelle function. Complementing mouse genetics, human genetics, and high-resolution structural studies, physiological studies employing natural agonists and synthetic inhibitors have become critical to resolving the in vivo functions of metabotropic, sensory, and organellar TRPs.
Introduction
Transient receptor potential (TRP) was initially identified as a receptor-operated sensory cation channel required for sustained light responses in Drosophila1–3. Subsequent homology cloning revealed a superfamily of cation channels in mammals4–8. Based on sequence homology, mammalian TRPs can be divided into 6 subfamilies: TRPC1–7 (C for canonical), TRPV1–6 (V for vanilloid), TRPM1–8 (M for melastatin), TRPA1 (A for Ankyrin), TRPML1–3 (ML for mucolipin), and TRPP1–3 (P for polycystin) (see Table 1). A common feature of TRP channels is the homotetrameric assembly of subunits containing six transmembrane segments (S1–6; see Fig. 1)9–14. Most TRPs are Ca2+-permeable cation channels, and virtually all are gated by chemical signals, ranging from environmental sensory signals to extracellular neurotransmitters and intracellular messengers8,15. Hence, TRPs can be considered as a superfamily of ligand-gated cation channels, although some TRPs can also be gated by physical signals, such as temperature, mechanical force, and voltage8.
Table 1.
TRP summary table.
| TRP | Subgroup$ | Activation | Subcellular distribution# | Genetic phenotypes | Human disease/Drug target |
|---|---|---|---|---|---|
| C1 | PM | ↓ Salivation | |||
| C2 | S, M | Pheromones, DAG | PM | ↓ Pheromone detection | |
| C3 | M | GPCR-PLC, DAG | PM, Secretory vesicles, Mitochondria | Ataxia; motor coordination defects | |
| C4 | M | GPCR-PLC, Englerin A | PM, Secretory vesicles | Impaired vascular function | |
| C5 | S, M | GPCR-PLC, Cool25–37 °C, Englerin A | PM, Secretory vesicles | ↓ Anxiety behaviors | |
| C6 | S, M | GPCR-PLC, DAG | PM | ↑ Arterial contractility | FSGS (GOF) |
| C7 | S, M | GPCR-PLC, DAG | PM | ↓ Non-image forming photoreception | |
| V1 | S, M, O | Heat>42 °C, H+, Vanolloids, DkTx | PM, ER, Mitochondria | ↓ nociception; ↓ thermal hyperalgesia; ↓ bladder function | Analgesia (clinical trial) |
| V2 | S, O | Heat>52 °C, 2-APB | PM, EE (?) | Susceptibility to bacterial infection | |
| V3 | S | Warm30–35 °C, 2-APB, Carvacrol, Incensole | LELs (?), PM | Abnormal hair morpho-genesis; compromised skin barrier | Olmsted syndrome (GOF) |
| V4 | S | Hypotonicity, Warm24–38°C, 4α-PDD | PM | ↑ Bone density, altered urinary function | CMT type 2C, skeletal dysplasias (GOF); pulmonary edema (clinical trial) |
| V5 | PM, Secretory vesicles | ↓ Renal Ca2+ absorption; hypercalciuria; kidney stones | |||
| V6 | PM, Secretory vesicles | Defective intestinal Ca2+ absorption; osteopenia; infertility | |||
| A1 | S, M, O | ROS, 4-HNE, AITC | PM, LELs | Defective chemosensation | Familial episodic pain syndrome (GOF); chronic pain (clinical trial) |
| M1 | M, O | Melanosomes | Impaired vision | Congenital stationary night blindness | |
| M2 | S, O | ROS, Warm>35 °C, ADPR | PM, LELs | ↓ Inflammation response | |
| M3 | S | Heat>40 °C, PS, Sphingolipids | PM | Defective thermosensation | |
| M4 | S, M | Tastants, Ca2+ | PM | Defective gustation | Brugada syndrome; familial heart block 1 (GOF) |
| M5 | S, M | Tastants, Heat>35 °C, Ca2+ | PM | Defective gustation | |
| M6 | O (?) | PM, M7-like vesicles (?) | Embryonic lethal | Heritable hypomagnesaemia | |
| M7 | O | ROS | PM, M7-like vesicles | Embryonic lethal | |
| M8 | S, O | Cool<25 °C, Menthol, Icilin | PM, ER | Defective cold sensation | Analgesia (clinical trial) |
| ML1 | O | PI(3,5)P2, ROS, ML-SAs, SF51, Mk-683 | LELs, TVs | Neuro- and retinal degeneration, muscle dystrophy, hypochlorhydria | Mucolipidosis type IV |
| ML2 | O | PI(3,5)P2, ROS, ML-SAs | Recycling endosomes, LELs | Impaired innate immune responses | |
| ML3 | O | PI(3,5)P2, ML-SAs, SFs | Early endosomes, LELs | Varitint-Waddler (GOF) | |
| P1 | O | Cilia, ER | Embryonic lethal | ADPKD | |
| P2 | O | Calmidazolium | Cilia | Renal, retinal, and intestinal defects. | |
| P3 | O | Cilia |
Abbreviations: 4-HNE, 4-hydroxynonenal; 4α-PDD, 4α-phorbol 12–13-didecanoate; ADPKD, autosomal dominant polycystic kidney disease; ADPR, cyclic ADP ribose; AITC, allyl isothiocyanate; DkTx, double-knot toxin; DVT, decavanadate; FSGS, familial focal segmental glomerulosclerosis; GPCR, G-protein coupled receptor; IC, intracellular; ML-SA, mucolipin synthetic agonist; ML-SI, mucolipin synthetic inhibitor; LEL, late endosome and lysosome; PM, plasma membrane; PS, pregnenolone sulfate; ROS, reactive oxygen species; TV, tubulovesicles.
S, sensory TRPs, M, metabotripic TRPs, O, organellar TRPs.
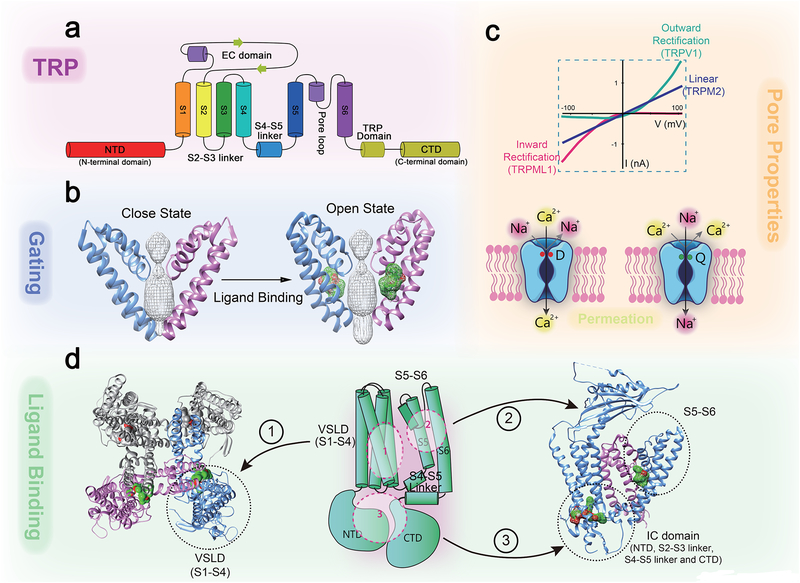
Fig. 1. Architecture and functional elements of a TRP channel.
Structural biology analyses reveal critical domains and amino acid residues for Ca2+ permeation and selectivity, and allow visualization of elements such as selectivity filter, activation gates, agonist binding sites, and explain gating-coupling machinery. a. TRPs are 6 transmembrane (TM, S1–S6) cation channels with N- and C- terminal domains facing the cytosol. b, Ligand binding to the S5–S6 domain leads to opening the lower S6 gate. c. Pore properties of TRP channels. Upper panel: representative TRP current-voltage (I-V) traces. Lower panels: the pore-loop between S5 and S6 forms the selectivity filter and upper gate of the channel. Negatively charged residues (e.g. Asp541 in TRPV6; red, the left panel) form the high-affinity Ca2+-binding sites required for Ca2+ permeation and selectivity. In TRPs with very low PCa, specific neutral and polar amino acid residues (e.g., Gln977 in TRPM4; green, the righ panel) form binding sites that favor monovalent cations over Ca2+. d. Ligand binding sites are localized in S1–4 VSLD (site 1), S5–S6 region (site 2; see agonist-bound cryo-EM structures of TRP in the zoomed-in image), or intracellular (IC) domains (site 3; see agonist-bound cryo-EM structures of TRP in the zoomed-in image). The S4–S5 linker and TRP domain act as the binding-gating coupling machinery that interacts to pull the S6 gate open upon ligand-binding.
TRP channels have been intensively studied for over 20 years, due to their crucial roles in both sensory and signal transduction7. Exploration of the biological functions of TRPs relies heavily on human genetics (channelopathy studies) and reverse mouse genetics. TRP knockout (KO) mouse studies revealed the essential roles of TRPs in temperature and pain sensation (TRPV1), pheromone detection (TRPC2), taste sensation (TRPM5), and innate fear responses (TRPC5)7,16–19. The cellular functions of TRPs are extremely diverse, partly due to their diverse activation mechaniams and different subcellular localizations20: most TRPs are expressed at the plasma membrane, whereas others may function as organellar channels that actively participate in signal transduction and organelle biology (see Table 1).
The biophysical properties of TRP channels are usually established in heterologous overexpression systems using whole-cell and whole-organelle patch clamps8 (Box 1). Ca2+ imaging assays have also been useful to study TRP channel physiology in intact cells and have allowed high-throughput screening of small molecule modulators15,21. High-resolution structural studies and the use of chemical modulators have further improved the reliability of channel characterization and become critical to resolving the in vivo functions of TRPs22,23 (Box 1 & Fig. 1). Individually, each of these approaches have inherent limitations, and it is thus essential to integrate different types of studies (Box 1).
Research in the TRP channel field has benefited enormously from the use of an integrated approach, such that the same channel modulators used in the channel studies and high-resolution structures are expected to produce TRP-specific effects in cellular, tissue and behavioral analyses (see Box Fig. 1). Phenotypes at the animal level may be dampened by compensatory mechanisms in KO mice, or be due to indirect gain-of-function effects in transgenic mice. For example, in TRPC6 KO mice, other TRPCs are upregulated in a compensatory mechanism, resulting in a paradoxical increase in neurotransmitter-induced arterial contractility8,24. Hence, the complementary use of biochemical and genetic approaches provides a safeguard against complications produced by pharmacological off-target effects and genetic compensation issues when each is used alone, respectively. Therefore, the fact that consistent temperature and pain phenotypes are observed across TRPV1 KO and pharmacological inhibition studies, has provided great confidence in the findings19.
In this review, we summarize our current knowledge of TRP channels, focusing in particular on the least-known functional group, the organellar TRPs, to bring together findings from studies on channel modulation, atomic structure, cell biology, animal physiology, and disease.
Physiology and architecture of TRP channels
TRPs are Ca2+-flux channels that can be activated by both physical and chemical signals7. How physical factors, such as temperature and mechanical force, activate TRPs is not yet known, though the domains and residues from TRPV1 involved in the temperature response have been identified25. Liposome reconstitution studies have indicated that some TRPs, e.g., TRPV1 and TRPM8, are activated directly by thermal stimulation26, and mutagenesis analyses suggest that thermosensitivity and chemosensitivity can be segregated in specific TRPs27. Some physical factors, e.g. light and hypotonicity, activate TRPs indirectly, through derived chemical signals28–30. Chemcial signals, either environmental cues or intracellular messengers, may activate TRPs by binding directly to channel proteins10 (Fig. 1d). When activated, TRP channels can permeate at least three cation groups, contributing to their diverse cellular functions. First, Ca2+ permeation results in changes in cytoplasmic Ca2+ levels, either global or juxta-organellar31. Second, Na+ flux reduces transmembrane voltage potential either across the plasma or organellar membrane20. Third, some TRPs (e.g., TRPM7 and TRPML1) are permeable to metal ions such as Mg2+, Zn2+, and Fe2+, whose dehydration energy is too high for non-TRP ion channels32,33 but can be reduced34 or accommodated as partially hydrated ions within the large TRP pore13,14,22,35,36.
TRP channel protomers have 6 transmembrane segments (S1–S6) with N- and C- terminal domains facing the cytosol (Fig. 1a). The S1–S4 form a voltage-sensor-like domain (VSLD; Fig. 1d). However, although many TRP channels are weakly modulated by voltage, the VSLD may not be the primary determinant for voltage sensitivity in most TRPs10,37. Instead, VSLD may serve as the ligand-binding domain for many TRPs22. The S5–S6 domain forms the cationic selectivity filter and channel activation gate (Fig. 1). In some TRPs such as TRPMLs and TRPPs, the large S1–S2 extracellular domain may also contribute to Ca2+ permeation12. Several intracellular domains, including the S2–S3 linker, S4–S5 linker, the TRP domain, intracellular N- and C- terminal doamins, may be involved in ligand binding and coupling of ligand-binding to opening of the channel gate (Fig. 1d).
The TRP selectivity filter is formed by a pore loop between S5 and S635,38,39 (Fig. 1). The pore size at the selectivity filter ranges from 2 to 8 Ǻ, allowing the passage of dehydrated or partially-hydrated Na+ and Ca2+ 10,13,14,22,36,39. The broad range of Ca2+ permeability to Na+ permeability ratios (PCa/PNa) among TRP channels can be attributed to selectivity filter features. For example, in TRPV5 and TRPV6 channels, with high PCa/PNa (>100), four aspartate residues in the selectivity filter region of each subunit form a high-affinity Ca2+-binding site that excludes monovalent permeation (Fig. 1c)40,41. Conversely, TRPM4 and TRPM5 have very low PCa/PNa (< 0.05), due to a ring of glutamine residues in the selectivity filter that bind preferentially monovalent ions (Fig. 1c)37–39. TRPs with PCa/PNa in the 1~10 range have intermediate-affinity Ca2+ binding sites in the selectivity filter, composed of negatively-charged residues10,11,13,14,22,36,42.
There are one or two activation gates in TRPs. The lower activation gate is found in all TRPs, and is formed by the S6 helices (Fig. 1)10,22, similarly to that in voltage-gated K+ channels. Mutations affecting the lower gate can result in constitutively active TRP channels10,43. The binding of agonists to different regions of the channel may cause conformational changes that converge at the S6 gate, leading to channel opening12,14,39. The upper gate is present only in some TRPs (e.g., TRPV1 and TRPC5), and is formed by the pore loop involved in selectivity10,22. Binding of extracellular ligands such as H+ and toxin opens the upper gate of these TRPs10,19,44. The coupling mechanisms between the two gates remain to be established.
Many endogenous and synthetic TRP agonists bind to the VSLD. Comparison of apo- and ligand-bound structures revealed that ligand binding causes conformational changes in the S4–S5 linker region, which in turn can interact directly with the S6 helices (lower gate) in another subunit (a so-called domain swap) or indirectly, through the TRP domain that is present in most TRPs and lies parallel to the membrane10,11,14,45 (Fig. 1d). Binding of intracellular ligands (e.g., Ca2+, PIP2, or ATP) to or phosphorylation events in cytoplasmic regions, such as the N-terminal domains (e.g., ankyrin repeats in TRPC, -V, and -A, TRPM-homology-regions and pre-S1 domains), S2–S3 linkers, or C-terminal domains (e.g. coiled-coil domain)10,11,14,45, control S6 gating through the S4–S5 linker or TRP domain (Fig. 1d). Thus, the S4–S5 linker, similar to that in other tetrameric channels, and the TRP domain are essential for coupling ligand binding and gating in most TRPs. However, recent studies revealed that some other agonists, e.g., ML-SA1 for TRPML1 and TRPML3, bind to the S5–S6 region, where they could exert direct force on the S6 gate, and possibly also the upper gate36,46 (Fig. 1d). Hence, there exist multiple ligand-binding sites and gating mechanisms in TRPs.
Functional classification of TRPs
Ion channels are commonly classified based on their selectivity and/or gating mechanisms. TRPs are generally cation non-selective, and the same activing stimulus may activate a subset of TRPs in different sequence homology-based subfamilies, e.g., temperature activation of TRPV1–4, TRPM2–5, 8, and TRPC58. Therefore, TRPs can be more informatively classified, based on their physiological function and endogenous activation mechanism, into three subgroups: metabotropic, sensory, and organellar TRPs (Table 1). Each functional subgroup contains members from individual TRP subfamilies. Because individual TRPs can be involved in multiple functions, and many TRPs are activated by both environmental and cellular signals, some TRPs belong to more than function groups. For instance, the fly TRP has both sensory (activated by light) and metabotropic (coupled to rhodopsin) functionality3.
Metabotropic TRPs.
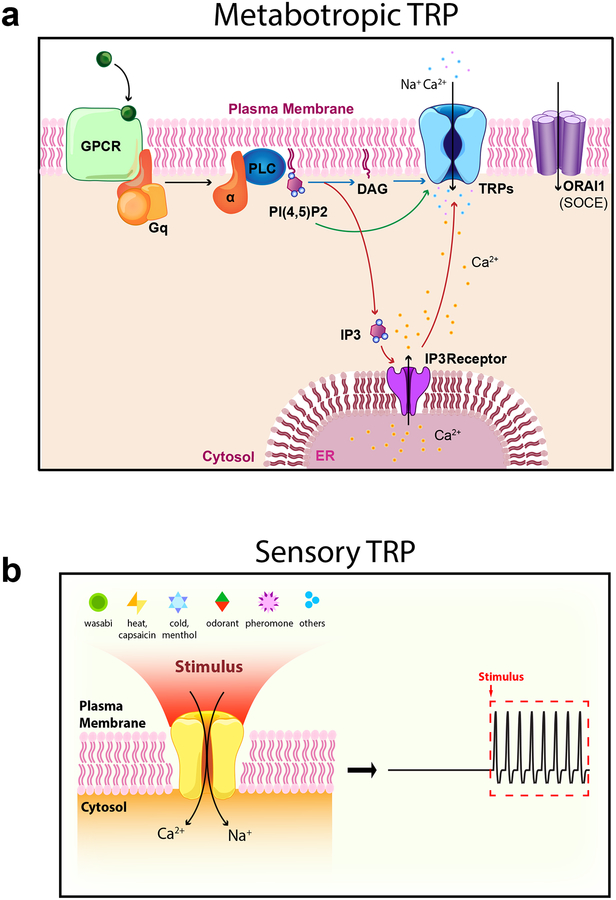
Most animal tissues and organs are regulated by both nervous and endocrine systems, and neuroendocrine signals need to be transduced at the cellular level8. Metabotropic TRPs are signal transducers in cells that express phospholipase (PLC)-dependent G protein-coupled receptors (GPCRs). This group includes TRPCs and members from each of the other subfamilies (Table 1). TRPC channels are expressed in the heart, smooth muscle, and kidney podocytes; they are regulated by sympathetic and parasympathetic transmitters, and mediate agonist-induced Ca2+-entry pathways4–7. Activation of Gq-coupled receptors stimulates PLC activity, resulting in hydrolysis of PI(4,5)P2 into DAG and IP3; IP3 then induces Ca2+ release from the ER through IP3 receptors (Fig. 2a). Such PLC-dependent signal transduction mechanisms then activate metabotrophic TRPs and store-operated Ca2+ entry channels, whose pore are formed by the Orai proteins47,48.
Fig 2. Metabotropic and sensory TRPs.
a. Metabotropic TRPs couple extracellular cues to biology. Excellular neuroendocrine signals, e. g., neurotransmitters, act on G protein-coupled receptors (GPCRs). Metabotropic TRPs are signal transducers in cells that also express phospholipase (PLC)-dependent GPCRs. Activation of Gq-coupled receptors stimulates PLC activity, which hydrolyze PI(4,5)P2 into DAG and IP3. IP3 then induces Ca2+ release from the ER through IP3 receptors. PLC-dependent signal transduction mechanisms activate metabotropic TRPs, as well as store-operated Ca2+ entry channels, whose pore-forming subunits are Orai proteins. Details see Table 1. b. Sensory TRPs couple environmental cues to biology. Sensory TRPs are activated by environmental signals such as light, temperature change, osmo-mechanical force, and plant-derived compounds, pain-/itch-inducing chemicals, tastants, and pheromones. These physical and chemical signals activate sensory TRP-mediated Na+ entry, increasing the membrane excitability of various sensory cells, including DRG neurons, taste receptor cells, hair cells, and retinal ganglion cells through sensory TRPs. Details see Table 1.
Sensory TRPs.
Animals detect environmental cues such as light, temperature change, osmo-mechanical force, and natural compounds, pain/itch-inducing chemicals, tastants, and pheromones19. These physical and chemical signals increase the membrane excitability of various sensory cells (e.g. DRG neurons, taste receptor cells, hair cells, and retinal ganglion cells) through sensory TRPs (Fig. 2b). This group includes the founding member of the TRPV subfamily, TRPV1, discovered as a cation channel activated by somatosensory cues such as heat and capsaicin (an alkaloid in chili peppers that produces a burning sensation)49. Other TRPs involved in sensory functions include TRPM8, a cold and menthol receptor found in DRG neurons50,51, and TRPA1, a temperature receptor for the spice wasabi (allylisothiocyanate, AITC)52,53(Table 1). Other thermosensors include TRPM3 in DRG neurons54, TRPM2 in sympathetic and central neurons55,56, and TRPV3/4 in keratinocytes57.
TRPV1 is also sensitive to itch-inducing substances such as histamine and injury-evoked inflammatory mediators (e.g., bradykinin) through indirect receptor-dependent mechanisms19. In taste receptor cells, tastants activate TRPM4/5 and membrane depolarization through GPCR taste receptors58. TRPC2 in mouse VNO neurons is activated by pheromones through GPCR pheromone receptors16. In these cases, GPCRs, but not TRPs, are the direct targets of sensory signals, these sensory TRPs are also metabotropic: metabotrophic sensory TRPs.
Organellar TRPs.
This is the least-understood group among TRP channels. Most TRPs observed in intracellular locations are thought to be plasma membrane channels going through biosynthetic or secretory processes, en route to their final destination20. The intracellular localization of organellar TRPs is usually demonstrated by overexpression of GFP fusions, tag-knock-in studies, and knockout (KO)-controlled immunohistochemistry20. More importantly, organelle electrophysiology and organelle-targeted Ca2+ imaging provide validation for the functions of organellar TRPs23,59,60. The key members in this group are the TRPMLs, which are activated by compartment-specific intracellular cues59. TRPML1–3 and TRPP1–3 are distantly related TRPs, whose channel functions remained mysterious until the development of organelle-specific patch-clamp techniques33,61. In the remainder of this review, we discuss the roles of organellar TRPs in decoding cellular signals.
Organellar TRPs couple intracellular cues to biology
Sensory TRPs are activated by physical and chemical environmental signals; metabotropic TRPs are activated by extracellular neuroendocrine signals. Similarly, cellular signals generated in the cytoplasm and other cellular compartments are thought to activate organellar TRPs. Notably, the same environmental signals that activate sensory TRPs, such as oxidants, pH, and osmo-mechanical force, may also regulate organellar TRPs62–64.
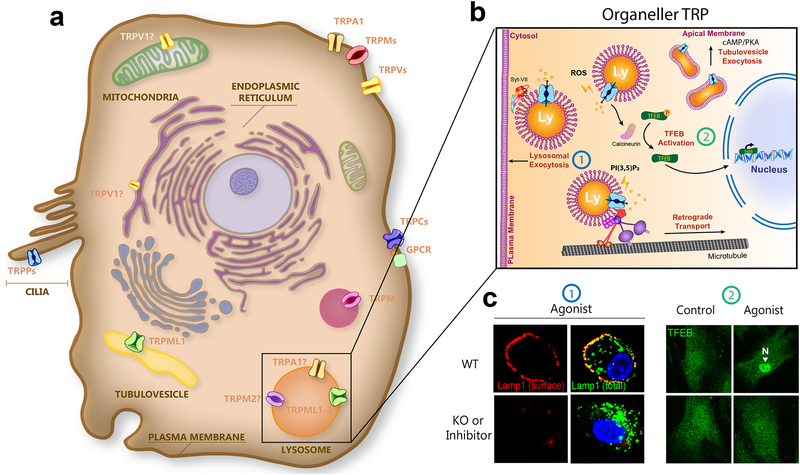
Most organellar TRPs function either in biosynthetic/secretory pathways (in the endoplasmic reticulum (ER) and Golgi), or in the endocytic pathway (e.g. in endosomes and lysosomes20). Candidate (if not functionally validated by organell electrophysiology yet) and confirmed organellar TRPs include TRPV1 and TRPP1, which are known to be localized on the ER membranes; and TRPC3–5, TRPV5–6, TRPM2, TRPM8, TRPA1, and TRPML1–3, which are present in secretory vesicles, early and recycling endosomes, and lysosomes (Table 1). All those organelles are intracellular Ca2+ stores, with luminal Ca2+ concentrations ranging from 0.3 to 0.7 mM65. Their Ca2+ permeability allows organellar TRPs to contribute to cellular signal transduction, by causing global changes in cytoplasmic Ca2+ levels31. More importantly, Ca2+ release by organellar TRP channels may increase juxta-organellar Ca2+ levels, specifically affecting the dynamics anf function of the organelles20. In addition, changes in membrane potential across organellar membranes may also affect organellar functions23.
Organellar TRPs can be divided into two groups: those predominantly localized in the organellar membranes, referred to as “committed” organellar TRPs; and those dually localized at plasma membrane and organellar membranes, referred to as “non-committed” organellar TRPs. We review our current knowledge of the members in each group below.
Committed organellar TRPs
TRPY1 in the yeast vacuole.
TRPY1, the only TRP-like protein in yeast, is localized in the membrane of the vacuole, which is equivalent to the mammalian lysosome62. In response to osmotic shock, changes in cytoplasmic ionic strength and/or mechanical force on the vacuolar membrane, activate TRPY1 to mediate vacuolar Ca2+ release and possibly vacuolar membrane fission62. Hence, TRPY1 is has dual sensory (activated by an environmental signal) and organellar (vacuole) functionalities62.
TRPML1 in the lysosome.
Lysosomes degrade cargo materials delivered via endocytosis or autophagy, converting them into catabolites and building blocks (e.g. amino acids) for reuse by the cell66,67. The degradation products are transported out of the lysosomes via vesicular trafficking and catabolite exporters23,68. All these lysosome trafficking steps, including cargo and enzyme import, lysosomal degradation, and catabolite export, are regulated by various intracellular signals produced according to the status of luminal cargos and products23,68. Both lumen-to-cytosol and cytosol-to-lumen signals need to be decoded23. As lysosomes are highly heterogeneous, compartment-specific intracellular signals such as changes in lysosomal lipid composition, membrane potential, and juxta-lysosomal Ca2+ levels, may differentially regulate trafficking of individual lysosomes60,69.
TRPML1 (a.k.a MCOLN1) channels are the major Ca2+-permeable channels in the lysosomes of all cell types. TRPML1 KO cells exhibit defective lysosomal membrane fusion and fission23,70. Whole-endolysosome patch-clamp studies using artificially-enlarged lysosomes59, suggested that TRPML1 may conduct Ca2+, Fe2+, Zn2+, Na+, and K+ across lysosomal membranes33. Employing lysosome-targeted genetically encoded Ca2+ sensors, it was shown that TRPML1 mediates Ca2+ release from lysosomes in intact cells60,71.
TRPML1 channels are activated by cellular cues that regulate lysosome trafficking and function. PI(3,5)P2, a lysosome-specific phosphoinositide, was the first endogenous signal identified for lysosomal TRPML1, activating it in a physiological, low-nanomolar range12,59. PI(3,5)P2 binds to positively-charged amino acid residues in the N-terminus region of the channel, resulting in opening of the S6 gate through the S2–S3 linker 12. PI(3,5)P2 levels may increase transiently prior to fusion of two lysosomes72, and during phagocytic uptake of large particles73. Hence, it is likely PI(3,5)P2 dynamics serves as the cellular cue that activates lysosomal TRPML1.
Several synthetic small-molecule TRPML agonists and inhibitors have been identified60,74. Although synthetic agonists activate TRPML1 independent of endogenous cues36,60, they provide useful tools to probe the channel’s cellular functions. ML-SA1 is an agonist that binds directly to the S5–S6 region of the channel36, where the bound compound may exert a direct force on the S6 gate36,46. In macrophages, acute ML-SA1 treatment induces the fusion of lysosomes with the plasma membrane, i.e., lysosomal exocytosis, through activation of Ca2+ sensor synaptotagmin VII (Syt-VII)75 (see Fig. 3) in wild-type but not TRPML1 KO cells73. Moreover, retrograde movement of lysosomes to the perinuclear region, which is required for autophagosome-lysosome fusion, is increased with TRPML1 over-expression or synthetic agonism through Ca2+ sensor EF-hand-protein ALG276, but reduced by TRPML1 KO, synthetic inhibition or PI(3,5)P2 deficiency76 (see Fig. 2). Hence, activation of TRPML1 may allow cellular cues such as lysosomal lipids to control/regulate lysosomal trafficking by triggering increase in juxta-lysosomal Ca2+.
Fig. 3. Organellar TRP channels.
a. Though most TRPs are located at the plasma membrane, TRPMLs are localized in intracellular endosomes and lysosomes, and TRPPs are localized in primary cilia. In addition, TRPV1 is localized in the ER and possibly mitochondria, and TRPM7 is localized in M7-like vesicles. In specialized cell types, TRPA1 and TRPM2 are functionally expressed in lysosomes. In parietal cells, TRPML1 is an organellar TRP that functions in both lysosomes and tubulovesicles (TVs). b. Endogenous (e.g. ROS and PI(3,5)P2) or synthetic agonists induce TRPML1-mediated lysosomal Ca2+ release to trigger lysosomal exocytosis (example 1), retrograde transport, and TFEB nuclear translocation (example 2). In the parietal cells, histamine-induced cAMP/protein kinase A signaling activates TV-localized TRPML1, increasing TV trafficking and exocytosis. c. In example 1, activation of TRPML1 triggers lysosomal exocytosis, detected by the surface expression of Lamp1 proteins in WT, but not in TRPML1 KO cells. Images are modified with permission from ref.64; In example 2, agonist activation of TRPML1 leads to nuclear translocation of TFEB (green), a transcription factor for lysosome biogenesis and autophagy in WT, but not TRPML1 KO cells. Images are modified with permission from ref. 102.
TRPML1 is also directly activated by reactive oxygen species (ROS), which are released from mitochondria under stress conditions to activate autophagosome and lysosome biogenesis64. Activation of TRPML1 triggers Ca2+ sensor calcineurin, which in turn promotes the nuclear translocation of transcription factor EB (TFEB), a master regulator of autophagy and lysosome function (Fig. 3)64. Activation of TFEB is sufficient to promote mitophagy, removing damaged mitochondria to reduce excessive ROS in the cell64.
The regulation of TRPML1 by endogenous cellular cues and synthetic modulators can illuminate how organellar TRPs are involved in different cellular processes. Although regulation by lipids and ROS is a general feature of TRPs7, co-localization of the membrane-delimited activating signal and channel within the same compartment defines a specific organelle function for such regulation. Hence, a signal that acts as an environmental cue for a sensory TRP (e.g., ROS) can also act as an intracellular agonist for an organellar TRP such as TRPML164,77. The specific cellular process regulated by TRPML1 is determined by both activating signals and downstream effectors, e.g., Ca2+ sensors (see Fig. 3). Given that synthetic agonists activate TRPML1 independent of PI(3,5)P2 and ROS12,60,64, it is likely that additional endogenous agonists for TRPML1 exist. It has been demonstrated that ROS sensitivity, but not PI(3,5)P2 sensitivity, is required for the TFEB activation upon mitochondria damage64. Finally, although the major lysosomal Δψ regulator is likely two-repeat (2× 6TM) TPC channels78, cellular cues that affect lysosomal membrane potential may also regulate lysosome function in a TRPML1-dependent manner 23,59, given that TRPML1 currents are strongly rectifying (see Fig. 1c).
TRPML2/3 in the endosomes and lysosomes.
Both TRPML2 and TRPML3 are also localized in the lysosomes, but only in certain cell types23,70,79. TRPML1 and TRPML3 play complementary roles in cells expressing both channels, such as intestinal enterocytes and cochlear hair cells80. As PI(3,5)P2 activates all three TRPMLs59, it is possible that PI(3,5)P2 serves as the endogenous cellular cue that activates lysosomal TRPML2/3 channels. In the bladder epithelial cells, TRPML3 is activated by lysosomal alkalization to mediate exosome release and bacterial extrusion63.
TRPML2 and TRPML3 also function in early and recycling endosomes of certain cell types23,70,79. For example, TRPML3 is the primary endosomal channel in some macrophage types79, but the cellular signals that activate early-endosomal TRPML3 are still unknown. Moreover, early endosomal functions of TRPML2/3 are not firmly established; lysosomal defects associated with the lack of TRPML2/3 could indirectly affect the functions of early endosomes, such as endocytosis and endosome-autophagosome fusion23,70.
TRPML1 in tubulovesicles.
In specialized cell types, TRPMLs are also expressed in endosome- or lysosome-related vesicles. For example, TRPML1 is expressed in tubulovesicles (TVs), the specialized organelles of acid-secreting gastric parietal cells81, and is required for TV Ca2+ release that triggers TV exocytosis in response to cAMP signaling downstream of histamine, a neurotransmitter that induces gastric acid secretion82. TV exocytosis is essential for gastric acid secretion in response to neurotransmitter histamine81,82. In this context, the organellar TRPML1 also acts as a receptor-operated channel. Clinically, loss-of-function mutations of TRPML1 underlie type IV mucolipidosis (ML-IV), a genetic disease in which patients experience hyposecretion of gastric acid23,83. In concordance, TRPML1 KO or inhibition suppresses gastric acid secretion, while TRPML1 overexpression or activation augments gastric acid secretion in mice82,84.
TRPPs in cilia.
Primary cilia are isolated cellular organelles in eukaryotic cells85. TRPP1 (a.k.a, PKD2) and TRPP2 (PKD2L1) channels are expressed in primary cilia, and their channel physiology characteristics were established in whole-cilium recordings with and without synthetic channel modulators61. However, cilium-specific TRPP-activating cues are yet to be identified.
Non-committed organellar TRPs
Most intracellularly-localized TRPs are thought to be plasma membrane channels that are temporarily associated with intracellular membranes as they go through their biosynthetic or secretory pathways20. However, several TRPs have been shown to be functional in intracellular membranes, and hence referred to as non-committed organellar TRPs.
TRPM2 and TRPA1 in the lysosomes of specialized cells.
In Dorsal Root Ganglion (DRG) neurons, TRPA1 is localized in the lysosomes that mediate lysosomal Ca2+ release in response to AITC86. However, TRPA1 is not present in the lysosomes of TRPA1-transfected HEK293 cells, suggesting that TRPA1 is targeted to lysosomes via a DRG neuron-specific mechanism86. TRPA1-mediated lysosomal Ca2+ release promotes the exocytosis of neuropeptide-containing, dense-core vesicles86. Likewise, in pancreatic β cells, ROS may generate another intracellular signal, ADP-ribose, which then activates lysosome-localized TRPM2, triggering lysosomal Ca2+ release to evoke insulin secretion87. In both cases, lysosomal Ca2+ release regulates the same functions as their plasma membrane counterparts. To separate organellar from plasma membrane functions of dually-localized TRPs, it is necessary to genetically modify the organelle-targeting motif and/or to develop membrane-permeable (organelle targeting) and impermeable channel inhibitors. It remains unknown whether lysosomal TRPA1 and TRPM2 are activated by organelle-specific luminal or cytosolic factors to regulate specific lysosome functions.
TRPM7 in vesicles.
TRPM7, ubiquitously expressed at the plasma membrane of most cell types, is permeable to both Mg2+ and Zn2+, in addition to Ca2+ and Na+ 8,88,89. However, TRPM7 is also localized in the so-called M7-like vesicles32 and in the synaptic vesicles of sympathetic neurons90. Consistent with the intracellular localization, TRPM7 mediates Zn2+ release from intracellular vesicles in response to ROS elevation32. Other organellar TRPs, e.g., TRPML1, may also contribute to heavy metal release from intracellular vesicles such as lysosomes33. As organellar TRPs may also mediate vesicular Ca2+/Na+ release, selective metal chelators (e.g., Ca2+ chelator BAPTA) may enable the actions of permeant ions to be distinguished20. Additionally, knock-in mutations with altered Ca2+/Fe2+/Zn2+ selectivity produced by genome editing tools may help to separate these functions.
TRPs in the ER.
ER-localized TRPs in the biosynthetic pathway are expected to be inactive since they lack glycosylation modifications that take place in the Golgi after ER exit 20. However, several TRPs, including TRPV1, TRPM8, and TRPP1, may be functionally expressed in the ER 20,91. For example, activation of TRPV1 using exogenous native agonists increased cytosolic Ca2+, but decreased ER luminal Ca2+ 20,91. The latter result suggests that the high surface/volume ratio in intracellular organelles may confer a regulation of luminal ionic homeostasis by organellar TRPs.
Organellar TRPs in disease and therapeutics
More than a dozen inheritable diseases are associated with gain-of-function (GOF) or loss-of-function TRP mutations (see Table 1). Among them, loss-of-function mutations in TRPML1 cause ML-IV, a neurodegenerative lysosome storage disease23,83. Given their diverse biological functions and the technical feasibility of developing high-throughput screening of TRP modulators based on Ca2+ imaging, pharmaceutical companies are pursuing TRPs, including TRPV1 and TRPA1 as potential drug targets15,21.
The demonstrated roles of organellar TRPs in regulating organelle function suggest that small molecule TRP modulators may boost organelle function. For instance, TRPML agonists can potentially enhance lysosome function in ML-IV patients with partial loss of TRPML1 activity92. There are more than 50 lysosome storage diseases (LSDs), many of which at the cellular level exhibit lysosomal trafficking defects similar to those seen in ML-IV23,93; thus, TRPML agonists may be used to up-regulate organelle function in LSDs characterized by lysosome impairment23,60. For instance, in Niemann-Pick type C disease, TRPML1-mediated lysosomal Ca2+ release and lysosomal trafficking are partially blocked60. Likewise, in PI(3,5)P2-deficient cells, TRPML1 activity is also reduced, which may cause lysosomal trafficking defects and storage94. Furthermore, lysosomal trafficking is also defective in many common neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases93. In some lysosomal diseases, mutations in hydrolases or exporters disrupt lysosomal storage, which in turn affects lysosomal degradation and trafficking, leading to accumulation of secondary materials in that organelle (“secondary lysosome storage”) and, ultimately, a vicious cycle23,60. Hence, TRPML1 channel dysregulation in the lysosome may be a primary cause of secondary storage in many lysosomal diseases. If so, then increasing TRPML1 activity could break the vicious cycle and facilitate lysosomal trafficking to clear lysosomal storage23,60. Indeed, TRPML1 overexpression or TRPML1 agonism increases cholesterol clearance in Niemann-Pick type C patient-derived cells23,60. Similarly to TRPML1, TFEB overexpression also induces cellular clearance in most LSDs, except ML-IV95. Hence, TRPML1 and TFEB may constitute a cellular clearance program for lysosomal storage23,96. However, the in vivo efficacy of TRPML1 agonists has not been reported.
Future directions
After 20 years of intensive research, we have reached a nearly complete characterization of basic TRP channel physiology. With the advent of cryo-EM development, more atomic-resolution TRP structures are available to provide mechanistic insights into the selectivity and activation of TRPs, which may lead to the development of potent, selective agents, both for research and therapeutic purposes. The list of identified extracellular and intracellular, physical and chemical signals that activate TRPs continues to grow, specific channel modulators have been developed, and various genetically-engineered mice (with global or local/conditional knockout and knockin of TRPs) are available for research applications. Despite such progress, the mechanisms by which TRPs are activated by cellular cues in vivo are not well understood. Furthermore, how organellar TRPs regulate organelle function remain largely unknown. In the future, we expect to see more KO-controlled in vitro studies and an expansion of in vivo animal studies using TRP modulators.
TRPs are activated by polymodal signals in vitro, and there are often multiple phenotypes associated with TRP KO and inhibition. Thus, an open question in the TRP channel field is which activation mechanisms are physiologically relevant in vivo. For example, although TRPV1 inhibitors increased core body temperature in clinical trials21, such effect might not be related to the thermosensitivity of TRPV1 per se, but more likely it involves TRPV1 activation by visceral non-thermal cues97. The availability of genome-editing technologies, such as CRISPR/Cas998, has made it possible to study the biological functions of specific modes of TRP modulation. In Conclusion, TRPs are activated by both environmental and cellular signals, but the in vivo relevance of such activation awaits further studies taking advantage of mouse genetics in combination with biochemical manipulations.
Supplementary Material
Box Fig. 1. An integrated approach to study TRPs.
An integrated approach to establish functions of TRPs with respect to channel physiology, cell and tissue physiology, and organismal biology, is illustrated below, in anticlockwise direction. Top right, agonist-evoked inward currents (measured at negative voltages) are abolished in TRP KO cells or by synthetic inhibitors, suggestive of the specificity of the response. Bottom right, structural biology analyses reveal critical domains and amino acid residues as agonist binding sites. Middle right, corresponding mutations in the binding sites abolished agonist-induced Ca2+ imaging responses. Bottom left, cell biological functions of TRPs (e.g. agonist activation of TRPML1 leads to nuclear translocation of transcription factor TFEB in WT, but not KO cells). Top left, animal biology and physiology of TRPs. For example, agonist (e.g., capsaicin for TRPV1) application increases firing frequency in WT, but not TRP KO, DRG neurons.
Acknowledgements
We apologize to researchers whose works are not cited due to space limitations. The TRP channel field has become so large that it is difficult to cover all the important findings. Research programs in the authors’ laboratory are supported by NIH grants (NS062792, AR060837, and DK115474). We thank Drs. L. Yue, D. Clapham, Y. Jiang, and R. Hume for reading the manuscript and appreciate the encouragement and helpful comments provided by other members of the Xu laboratory.
References
- 1.Minke B, Wu C & Pak WL Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 258, 84–87 (1975). [DOI] [PubMed] [Google Scholar]
- 2.Montell C & Rubin GM Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323 (1989). [DOI] [PubMed] [Google Scholar]
- 3.Hardie RC & Minke B The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Wes PD et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci U S A 92, 9652–9656 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Chu PB, Peyton M & Birnbaumer L Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett 373, 193–198 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Harteneck C, Plant TD & Schultz G From worm to man: three subfamilies of TRP channels. Trends Neurosci 23, 159–166 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Clapham DE TRP channels as cellular sensors. Nature 426, 517–524, doi: 10.1038/nature02196 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Wu LJ, Sweet TB & Clapham DE International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev 62, 381–404, doi: 10.1124/pr.110.002725 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoenderop JG et al. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22, 776–785, doi: 10.1093/emboj/cdg080 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao E, Liao M, Cheng Y & Julius D TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118, doi: 10.1038/nature12823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulsen CE, Armache JP, Gao Y, Cheng Y & Julius D Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517, doi: 10.1038/nature14367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q et al. Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature 550, 415–418, doi: 10.1038/nature24035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q et al. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res, doi: 10.1038/s41422-018-0038-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin Y et al. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359, 237–241, doi: 10.1126/science.aan4325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilius B & Szallasi A Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 66, 676–814, doi: 10.1124/pr.113.008268 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Stowers L, Holy TE, Meister M, Dulac C & Koentges G Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295, 1493–1500, doi: 10.1126/science.1069259 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Riccio A et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137, 761–772, doi: 10.1016/j.cell.2009.03.039 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julius D TRP channels and pain. Annu Rev Cell Dev Biol 29, 355–384, doi: 10.1146/annurev-cellbio-101011-155833 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Dong XP, Wang X & Xu H TRP channels of intracellular membranes. J Neurochem 113, 313–328, doi: 10.1111/j.1471-4159.2010.06626.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran MM TRP Channels as Potential Drug Targets. Annu Rev Pharmacol Toxicol 58, 309–330, doi: 10.1146/annurev-pharmtox-010617-052832 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Madej MG & Ziegler CM Dawning of a new era in TRP channel structural biology by cryo-electron microscopy. Pflugers Arch 470, 213–225, doi: 10.1007/s00424-018-2107-2 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Xu H & Ren D Lysosomal physiology. Annual review of physiology 77, 57–80, doi: 10.1146/annurev-physiol-021014-071649 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso-Carbajo L et al. Muscling in on TRP channels in vascular smooth muscle cells and cardiomyocytes. Cell Calcium 66, 48–61, doi: 10.1016/j.ceca.2017.06.004 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Franulic I, Caceres-Molina J, Sepulveda RV, Gonzalez-Nilo F & Latorre R Structure-Driven Pharmacology of Transient Receptor Potential Channel Vanilloid 1. Mol Pharmacol 90, 300–308, doi: 10.1124/mol.116.104430 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Cao E, Cordero-Morales JF, Liu B, Qin F & Julius D TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77, 667–679, doi: 10.1016/j.neuron.2012.12.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bandell M et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci 9, 493–500, doi: 10.1038/nn1665 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Vriens J et al. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A 101, 396–401, doi: 10.1073/pnas.0303329101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue T et al. Melanopsin signalling in mammalian iris and retina. Nature 479, 67–73, doi: 10.1038/nature10567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardie RC & Franze K Photomechanical responses in Drosophila photoreceptors. Science 338, 260–263, doi: 10.1126/science.1222376 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Clapham DE Calcium signaling. Cell 131, 1047–1058, doi:S0092–8674(07)01531–0 [pii] 10.1016/j.cell.2007.11.028 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Abiria SA et al. TRPM7 senses oxidative stress to release Zn(2+) from unique intracellular vesicles. Proc Natl Acad Sci U S A 114, E6079–E6088, doi: 10.1073/pnas.1707380114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong XP et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996, doi: 10.1038/nature07311 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan J et al. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1810719115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao M, Cao E, Julius D & Cheng Y Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112, doi: 10.1038/nature12822 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmiege P, Fine M, Blobel G & Li X Human TRPML1 channel structures in open and closed conformations. Nature 550, 366–370, doi: 10.1038/nature24036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J et al. Structures of the calcium-activated, non-selective cation channel TRPM4. Nature 552, 205–209, doi: 10.1038/nature24997 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilius B et al. The selectivity filter of the cation channel TRPM4. The Journal of biological chemistry 280, 22899–22906, doi: 10.1074/jbc.M501686200 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Winkler PA, Huang Y, Sun W, Du J & Lu W Electron cryo-microscopy structure of a human TRPM4 channel. Nature 552, 200–204, doi: 10.1038/nature24674 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Voets T, Janssens A, Droogmans G & Nilius B Outer pore architecture of a Ca2+-selective TRP channel. The Journal of biological chemistry 279, 15223–15230, doi: 10.1074/jbc.M312076200 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Saotome K, Singh AK, Yelshanskaya MV & Sobolevsky AI Crystal structure of the epithelial calcium channel TRPV6. Nature 534, 506–511, doi: 10.1038/nature17975 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owsianik G, Talavera K, Voets T & Nilius B Permeation and selectivity of TRP channels. Annual review of physiology 68, 685–717, doi: 10.1146/annurev.physiol.68.040204.101406 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Hofmann L et al. The S4---S5 linker - gearbox of TRP channel gating. Cell Calcium 67, 156–165, doi: 10.1016/j.ceca.2017.04.002 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Xu SZ et al. TRPC channel activation by extracellular thioredoxin. Nature 451, 69–72, doi: 10.1038/nature06414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y, Cao E, Julius D & Cheng Y TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351, doi: 10.1038/nature17964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou X et al. Cryo-EM structures of the human endolysosomal TRPML3 channel in three distinct states. Nat Struct Mol Biol 24, 1146–1154, doi: 10.1038/nsmb.3502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prakriya M & Lewis RS Store-Operated Calcium Channels. Physiol Rev 95, 1383–1436, doi: 10.1152/physrev.00020.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feske S et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185, doi: 10.1038/nature04702 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Caterina MJ et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824, doi: 10.1038/39807 (1997). [DOI] [PubMed] [Google Scholar]
- 50.McKemy DD, Neuhausser WM & Julius D Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58, doi: 10.1038/nature719 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Peier AM et al. A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Story GM et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Jordt SE et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265, doi: 10.1038/nature02282 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Vriens J et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494, doi: 10.1016/j.neuron.2011.02.051 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Tan CH & McNaughton PA The TRPM2 ion channel is required for sensitivity to warmth. Nature 536, 460–463, doi: 10.1038/nature19074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song K et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353, 1393–1398, doi: 10.1126/science.aaf7537 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186, doi: 10.1038/nature00882 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Dutta Banik D, Martin LE, Freichel M, Torregrossa AM & Medler KF TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc Natl Acad Sci U S A 115, E772–E781, doi: 10.1073/pnas.1718802115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong XP et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1, 38, doi: 10.1038/ncomms1037 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen D et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun 3, 731, doi: 10.1038/ncomms1735 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeCaen PG, Delling M, Vien TN & Clapham DE Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318, doi: 10.1038/nature12832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer CP et al. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci U S A 98, 7801–7805, doi: 10.1073/pnas.141036198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao Y, Li G, Zhang X, Xu H & Abraham SN A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell 161, 1306–1319, doi: 10.1016/j.cell.2015.05.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7, 12109, doi: 10.1038/ncomms12109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Zhao Z, Gu M, Feng X & Xu H Release and uptake mechanisms of vesicular Ca(2+) stores. Protein Cell, doi: 10.1007/s13238-018-0523-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huotari J & Helenius A Endosome maturation. EMBO J 30, 3481–3500, doi: 10.1038/emboj.2011.286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luzio JP, Pryor PR & Bright NA Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8, 622–632, doi: 10.1038/nrm2217 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Xiong J & Zhu MX Regulation of lysosomal ion homeostasis by channels and transporters. Sci China Life Sci 59, 777–791, doi: 10.1007/s11427-016-5090-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383, doi: 10.1016/j.cell.2012.08.036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Venkatachalam K, Wong CO & Zhu MX The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 58, 48–56, doi: 10.1016/j.ceca.2014.10.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garrity AG et al. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife 5, doi: 10.7554/eLife.15887 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li X et al. Genetically encoded fluorescent probe to visualize intracellular phosphatidylinositol 3,5-bisphosphate localization and dynamics. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1311864110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samie M et al. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev Cell 26, 511–524, doi: 10.1016/j.devcel.2013.08.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grimm C et al. Small molecule activators of TRPML3. Chem Biol 17, 135–148, doi: 10.1016/j.chembiol.2009.12.016 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czibener C et al. Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J Cell Biol 174, 997–1007, doi: 10.1083/jcb.200605004 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X et al. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 18, 404–417, doi: 10.1038/ncb3324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bessac BF et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118, 1899–1910, doi: 10.1172/JCI34192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calcraft PJ et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600, doi: 10.1038/nature08030 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen CC et al. Small Molecules for Early Endosome-Specific Patch Clamping. Cell Chem Biol 24, 907–916 e904, doi: 10.1016/j.chembiol.2017.05.025 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Remis NN et al. Mucolipin co-deficiency causes accelerated endolysosomal vacuolation of enterocytes and failure-to-thrive from birth to weaning. PLoS Genet 10, e1004833, doi: 10.1371/journal.pgen.1004833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao X & Forte JG Cell biology of acid secretion by the parietal cell. Annual review of physiology 65, 103–131, doi: 10.1146/annurev.physiol.65.072302.114200 (2003). [DOI] [PubMed] [Google Scholar]
- 82.Sahoo N et al. Gastric Acid Secretion from Parietal Cells Is Mediated by a Ca(2+) Efflux Channel in the Tubulovesicle. Dev Cell 41, 262–273 e266, doi: 10.1016/j.devcel.2017.04.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bargal R et al. Identification of the gene causing mucolipidosis type IV. Nat Genet 26, 118–123, doi: 10.1038/79095 (2000). [DOI] [PubMed] [Google Scholar]
- 84.Chandra M et al. A role for the Ca2+ channel TRPML1 in gastric acid secretion, based on analysis of knockout mice. Gastroenterology 140, 857–867, doi: 10.1053/j.gastro.2010.11.040 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delling M, DeCaen PG, Doerner JF, Febvay S & Clapham DE Primary cilia are specialized calcium signalling organelles. Nature 504, 311–314, doi: 10.1038/nature12833 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shang S et al. Intracellular TRPA1 mediates Ca2+ release from lysosomes in dorsal root ganglion neurons. J Cell Biol 215, 369–381, doi: 10.1083/jcb.201603081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lange I et al. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal 2, ra23, doi: 10.1126/scisignal.2000278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nadler MJ et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595, doi: 10.1038/35079092 (2001). [DOI] [PubMed] [Google Scholar]
- 89.Runnels LW, Yue L & Clapham DE TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047, doi: 10.1126/science.1058519 (2001). [DOI] [PubMed] [Google Scholar]
- 90.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM & Clapham DE The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron 52, 485–496, doi: 10.1016/j.neuron.2006.09.033 (2006). [DOI] [PubMed] [Google Scholar]
- 91.Zhao R & Tsang SY Versatile Roles of Intracellularly Located TRPV1 Channel. J Cell Physiol 232, 1957–1965, doi: 10.1002/jcp.25704 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Chen CC et al. A small molecule restores function to TRPML1 mutant isoforms responsible for mucolipidosis type IV. Nat Commun 5, 4681, doi: 10.1038/ncomms5681 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Fraldi A, Klein AD, Medina DL & Settembre C Brain Disorders Due to Lysosomal Dysfunction. Annu Rev Neurosci 39, 277–295, doi: 10.1146/annurev-neuro-070815-014031 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Dong XP et al. PI(3,5)P(2) Controls Membrane Traffic by Direct Activation of Mucolipin Ca Release Channels in the Endolysosome. Nat Commun 1, doi: 10.1038/ncomms1037 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medina DL et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell 21, 421–430, doi: 10.1016/j.devcel.2011.07.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Medina DL et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17, 288–299, doi: 10.1038/ncb3114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gavva NR et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136, 202–210, doi: 10.1016/j.pain.2008.01.024 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Komor AC, Badran AH & Liu DR CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 168, 20–36, doi: 10.1016/j.cell.2016.10.044 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.