Abstract
Background:
There are limited real-world data on the comparative cardiovascular (CV) safety of linagliptin, a dipeptidyl peptidase-4 inhibitor (DPP-4i) available since May 2011.
Objectives:
We aimed to evaluate the safety of linagliptin vs. other glucose-lowering medications in a multi-year monitoring program using insurance claims data.
Methods:
In two commercial U.S. claims databases, we identified three pairwise 1:1 propensity score (PS) matched cohorts of type 2 diabetes (T2D) patients ≥18 years initiating linagliptin or a comparator [other DPP-4i (n=31,492 pairs), pioglitazone (n=23,316 pairs), or 2nd generation sulfonylureas (n=19,731 pairs)] between May 2011 and December 2015. The primary endpoint was the risk of a composite CV outcome (hospitalization for myocardial infarction, stroke, unstable angina, or coronary revascularization). We estimated pooled hazard ratios (HR) and 95% confidence intervals (CI) controlling for >100 baseline characteristics.
Results:
Patient characteristics were well balanced after PS-matching. Mean age was 55 years and mean follow-up was 0.8 years. Linagliptin had a similar risk of the composite CV outcome compared to other DPP-4is (HR = 0.91; 95% CI = 0.79–1.05) and pioglitazone (0.98; 0.84–1.15), and showed a reduced risk of CV outcomes compared to 2nd generation sulfonylureas (0.76; 0.64–0.92). Key findings signaled at the first interim analysis in June 2013 and solidified during ongoing monitoring through 2015.
Conclusion:
Analyses from a large monitoring program in routine care of patients with T2D, showed that linagliptin had similar cardiovascular safety compared to other DPP-4i and pioglitazone, and a reduced cardiovascular risk compared to sulfonylureas.
Keywords: linagliptin, type 2 diabetes, comparative cardiovascular safety, healthcare administrative data, propensity score
Background
Linagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor approved in the United States in May 2011 to improve glycemic control in adults with type 2 diabetes (T2D). It increases insulin secretion and reduces glucagon secretion, thus decreasing blood glucose levels.[1] Randomized controlled trials (RCTs) showed efficacy and safety against placebo.[2–4] Linagliptin does not require dose adjustment in T2D patients at any stage of renal impairment,[5] which may lead to increased use in this vulnerable population.
As cardiovascular disease is the leading cause of morbidity and mortality in patients with T2D,[6] the understanding of the effects of glucose-lowering medications on cardiovascular events is of utmost importance in clinical practice. Available results from large cardiovascular outcome trials among patients with T2D and increased cardiovascular risk, have demonstrated that other DPP-4 inhibitors (saxagliptin, alogliptin, and sitagliptin) neither increased nor decreased major acute cardiovascular events (MACE) compared to placebo.[7–9] With regard to linagliptin, two large randomized cardiovascular outcome trials comparing this agent to either placebo (CARMELINA)[10] or glimepiride (CAROLINA),[11] will provide large-scale information regarding its effects on cardiovascular endpoints. While results for CAROLINA are not available yet, CARMELINA has recently shown that linagliptin added to usual care was non-inferior to placebo with regard to the risk of a composite cardiovascular outcome in approximately 7,000 adults T2D adults with high cardiovascular and renal risk.[12]
However, use of linagliptin in routine care settings outside a highly controlled research environment may differ with respect to patient characteristics and adherence patterns. Large healthcare database studies containing longitudinal recordings of routine clinical practice of tens of thousands of patients, represent an important complementary tool to compare and contrast effectiveness and safety findings with results from clinical trials.[13–16] For newly marketed medications, in particular, database studies can play a crucial role insofar as they allow ongoing monitoring of the safety and effectiveness of these agents in routine care, as real-world data accumulates over time.[17, 18] As time passes, more patients start the new agent and take it for longer durations; longitudinal patient-level study data can be amended with refreshed data in regular time intervals and reanalyzed with an increasing source population, provided the existence of an established real-world data generation mechanism.[17, 19, 20]
Based on data from a pre-specified sequential monitoring program, we aimed to assess the comparative cardiovascular safety of linagliptin versus three alternative agents or classes of glucose-lowering drugs as used in routine care in patients with T2D in two U.S. commercial claims datasets from May 2011 through December 2015.
Methods
Data sources
Data for this study were collected from two U.S. commercial claims datasets (Optum Clinformatics and IBM MarketScan) with nationwide coverage. The Clinformatics database includes data for individuals who are commercially insured or who have insurance through a Medicare Advantage plan. The MarketScan database includes data for individuals who are commercially insured or who have primary traditional (part A & B but no D) Medicare insurance plus a supplemental health plan with a pharmacy benefit. For each insured individual, the two data sources contain demographic information, health plan enrollment status, longitudinal patient-level information on all reimbursed medical services, both inpatient and outpatient diagnoses and procedures along with pharmacy dispensing records, including information on medication start and refill, strength, quantity, and days’ supply. These data sources are used extensively in pharmacoepidemiologic research.[21]
The first data cut for this monitoring program became available for analysis of data through June 2013 and was subsequently updated every six months, at each time undergoing new analyses. The individual-level data were de-identified and the study was approved by the Brigham and Women’s Hospital Institutional Review Board. Signed data license agreements were in place and the study was registered at ENCePP (EUPAS5790) and ClinicalTrials.gov (NCT02197078).
Study design
The monitoring program spans from May 2011 through December 2016, and is comprised of seven pre-specified analyses, each performed based on six-month data refreshes and a final analysis, Here, we report the first 4 years of data, through December 2015. This monitoring system is designed as three sequentially built new-user active-comparator cohorts with 1:1 propensity score (PS) matched patients of either linagliptin or a comparator medication. This study design reduces confounding arising from differences between patients prescribed these two alternative medications.[14, 22] The comparators for linagliptin, i.e., other DPP-4 inhibitors (DPP-4i) (alogliptin, saxagliptin, or sitagliptin), pioglitazone, or 2nd generation sulfonylureas (glimepiride, glipizide, or glyburide), were chosen as they represent common therapeutic strategies used at comparable stages of diabetes progression. This approach is expected to improve clinical equipoise across treatment groups and to reduce confounding.[23]
Cohort entry was the day of the start of any of the drugs listed above. Initiation of drug use was defined for each pair-wise cohort as no prior use of either linagliptin or the specific comparator during the previous six months before cohort entry. Patients who met inclusion criteria could contribute to multiple cohorts, though for a given comparison they were only allowed to contribute once. Individuals eligible for our study were aged 18 years or older, had to have a diagnosis of T2D, defined as an inpatient or outpatient diagnosis (ICD-9 code 250.x0 or 250.x2 or ICD-10 code E11) at any point before cohort entry. We excluded patients with a diagnosis of diabetes type 1 (ICD-9 diagnosis code 250.x1 or 250.x3 or ICD-10 diagnosis code E10), history of secondary or gestational diabetes, malignancy, end-stage renal disease, human immunodeficiency virus, organ transplant, or a nursing home admission in the previous six months.
Follow-up for study outcomes started on the day after treatment initiation and continued in an “as-treated” approach until treatment discontinuation, switch to a comparator, the occurrence of an outcome, a nursing home admission, death, plan disenrollment, or end of the study period, whichever came first. In case of treatment discontinuation, we extended the exposure effect window until 90 days after the end of the last prescription’s supply.
Outcome definition and identification
The primary outcome was defined as a composite cardiovascular outcome comprised of hospitalization for acute myocardial infarction, ischemic or hemorrhagic stroke, unstable angina, or coronary revascularization (eTable-1). Secondary cardiovascular outcomes included the individual components of the primary composite cardiovascular outcome. In previous studies, the positive predictive values of these claims-based algorithms for cardiovascular events were 84% or higher.[24–27] For the last quarter of 2015, outcome definitions included ICD-10 codes. Other secondary outcomes were defined by the protocol but will be reported elsewhere.
Patient Characteristics
Patient characteristics were captured at baseline, i.e., during the six months preceding and including the date of cohort entry. Covariates of interest included demographics, comorbidities, use of medications, and indicators of health care utilization as proxy for overall disease state and care intensity (Table-1 and eTable-2). As previously reported, emphasis was placed on the identification of claims-measured indicators of diabetes severity, e.g., number of glucose-lowering medications at index date and specific past or concurrent diabetes therapy, diabetic nephropathy, neuropathy, retinopathy, diabetic foot, number of HbA1c or glucose tests ordered.[23] Comorbidities were defined using ICD-9 or ICD-10 diagnosis codes and CPT-4 procedure codes.
Table 1.
Selected baseline characteristics of study participants after propensity-score-matching in first and sixth predefined sequential analysis
| May 2011 - June 2013 | May 2011 - December 2015 | May 2011 - June 2013 | May 2011 - December 2015 | May 2011 - June 2013 | May 2011 - December 2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linagliptin | Other DPP-4i | Linagliptin | Other DPP-4i | Linagliptin | Pioglitazone | Linagliptin | Pioglitazone | Linagliptin | Sulfonylureas | Linagliptin | Sulfonylureas | |
| (N=14379) | (N=14379) | (N=31492) | (N=31492) | (N=9966) | (N=9966) | (N=23316) | (N=23316) | (N=8895) | (N=8895) | (N=19731) | (N=19731) | |
| Claims-based patient characteristics | N % or Mean (SD) | N % or Mean (SD) | N % or Mean (SD) | |||||||||
| Demographics | ||||||||||||
| Age (mean, SD) | 54.78 (11.05) | 54.80 (11.03) | 55.61 (11.70) | 55.59 (11.69) | 54.20 (11.05) | 54.23 (11.00) | 55.27 (11.74) | 55.23 (11.64) | 53.84 (11.06) | 53.83 (10.98) | 54.65 (11.65) | 54.63 (11.67) |
| Female | 5793 (40.29) | 5742 (39.93) | 12812 (40.68) | 12840 (40.77) | 4051 (40.65) | 3980 (39.94) | 9504 (40.76) | 9414 (40.38) | 3668 (41.24) | 3600 (40.47) | 8162 (41.37) | 8123 (41.17) |
| Features of medication initiation | ||||||||||||
| Monotherapy | 3790 (26.36) | 3731 (25.95) | 7469 (23.72) | 7468 (23.71) | 2867 (28.77) | 2872 (28.82) | 5961 (25.57) | 6003 (25.75) | 3188 (35.84) | 3163 (35.56) | 6340 (32.13) | 6328 (32.07) |
| Dual therapy | 6428 (44.70) | 6503 (45.23) | 14523 (46.12) | 14575 (46.28) | 4598 (46.14) | 4626 (46.42) | 11030 (47.31) | 10981 (47.10) | 4750 (53.40) | 4763 (53.55) | 10930 (55.40) | 10978 (55.64) |
| Therapy with > 2 agents | 4161 (28.94) | 4145 (28.83) | 9500 (30.17) | 9449 (30.00) | 2501 (25.10) | 2468 (24.76) | 6325 (27.13) | 6332 (27.16) | 957 (10.76) | 969 (10.89) | 2461 (12.47) | 2425 (12.29) |
| Dual therapy with metformin | 4535 (31.54) | 4558 (31.70) | 10360 (32.90) | 10357 (32.89) | 3398 (34.10) | 3396 (34.08) | 8150 (34.95) | 8018 (34.39) | 4073 (45.79) | 4100 (46.09) | 9351 (47.39) | 9478 (48.04) |
| Concomitant initiation of other antidiabetic agents | 1580 (10.99) | 1630 (11.34) | 4744 (15.06) | 4801 (15.25) | 1318 (13.22) | 1328 (13.33) | 3677 (15.77) | 3540 (15.18) | 1142 (12.84) | 1141 (12.83) | 3336 (16.91) | 3255 (16.50) |
| Concomitant initiation of metformin | 1221 (8.49) | 1250 (8.69) | 3499 (11.11) | 3539 (11.24) | 1068 (10.72) | 1076 (10.80) | 2934 (12.58) | 2805 (12.03) | 1028 (11.56) | 1020 (11.47) | 2845 (14.42) | 2805 (14.22) |
| Concomitant initiation of insulin | 133 (0.92) | 127 (0.88) | 336 (1.07) | 342 (1.09) | 104 (1.04) | 97 (0.97) | 251 (1.08) | 261 (1.12) | 81 (0.91) | 81 (0.91) | 222 (1.13) | 226 (1.15) |
| Current use of other antidiabetic agents | 9391 (65.31) | 9450 (65.72) | 20681 (65.67) | 20701 (65.73) | 14540 (62.36) | 14599 (62.61) | 4694 (52.77) | 4744 (53.33) | 10606 (53.75) | 10687 (54.16) | ||
| Current use of metformin | 7093 (49.33) | 7049 (49.02) | 15445 (49.04) | 15387 (48.86) | 4707 (47.23) | 4708 (47.24) | 11097 (47.59) | 11216 (48.10) | 3946 (44.36) | 3988 (44.83) | 8795 (44.57) | 8901 (45.11) |
| Current use of insulin | 1026 (7.14) | 1043 (7.25) | 2577 (8.18) | 2606 (8.28) | 704 (7.06) | 714 (7.16) | 1822 (7.81) | 1844 (7.91) | 656 (7.37) | 629 (7.07) | 1700 (8.62) | 1639 (8.31) |
| Comorbidities at Baseline | ||||||||||||
| Charlson comorbidity score (mean, SD) | 1.36 (0.90) | 1.37 (0.90) | 1.47 (1.01) | 1.47 (1.01) | 1.29 (0.79) | 1.28 (0.79) | 1.39 (0.91) | 1.38 (0.89) | 1.31 (0.84) | 1.32 (0.85) | 1.42 (0.96) | 1.42 (0.96) |
| Diabetic nephropathy | 594 (4.13) | 619 (4.30) | 1741 (5.53) | 1788 (5.68) | 329 (3.30) | 316 (3.17) | 1147 (4.92) | 1171 (5.02) | 290 (3.26) | 289 (3.25) | 889 (4.51) | 871 (4.41) |
| Diabetic retinopathy | 424 (2.95) | 449 (3.12) | 1081 (3.43) | 1106 (3.51) | 253 (2.54) | 252 (2.53) | 694 (2.98) | 711 (3.05) | 207 (2.33) | 212 (2.38) | 560 (2.84) | 578 (2.93) |
| Diabetic neuropathy | 832 (5.79) | 835 (5.81) | 2279 (7.24) | 2252 (7.15) | 538 (5.40) | 534 (5.36) | 1612 (6.91) | 1575 (6.76) | 417 (4.69) | 419 (4.71) | 1231 (6.24) | 1272 (6.45) |
| Peripheral vascular disease | 206 (1.43) | 224 (1.56) | 502 (1.59) | 526 (1.67) | 125 (1.25) | 124 (1.24) | 305 (1.31) | 313 (1.34) | 113 (1.27) | 101 (1.14) | 285 (1.44) | 269 (1.36) |
| Erectile dysfunction | 343 (2.39) | 330 (2.30) | 721 (2.29) | 713 (2.26) | 216 (2.17) | 212 (2.13) | 504 (2.16) | 516 (2.21) | 203 (2.28) | 193 (2.17) | 410 (2.08) | 409 (2.07) |
| Diabetic foot | 128 (0.89) | 118 (0.82) | 327 (1.04) | 305 (0.97) | 75 (0.75) | 73 (0.73) | 206 (0.88) | 203 (0.87) | 61 (0.69) | 56 (0.63) | 186 (0.94) | 187 (0.95) |
| Skin infections | 636 (4.42) | 640 (4.45) | 1406 (4.46) | 1368 (4.34) | 429 (4.30) | 419 (4.20) | 1004 (4.31) | 969 (4.16) | 390 (4.38) | 413 (4.64) | 886 (4.49) | 940 (4.76) |
| Hypoglycemia | 910 (6.33) | 893 (6.21) | 2352 (7.47) | 2270 (7.21) | 587 (5.89) | 577 (5.79) | 1684 (7.22) | 1628 (6.98) | 526 (5.91) | 544 (6.12) | 1360 (6.89) | 1421 (7.20) |
| Hypertension | 5434 (37.79) | 5421 (37.70) | 12441 (39.51) | 12438 (39.50) | 3536 (35.48) | 3532 (35.44) | 8627 (37.00) | 8669 (37.18) | 3214 (36.13) | 3245 (36.48) | 7484 (37.93) | 7457 (37.79) |
| Hyperlipidemia | 4731 (32.90) | 4723 (32.85) | 10830 (34.39) | 10802 (34.30) | 3115 (31.26) | 3082 (30.93) | 7671 (32.90) | 7714 (33.08) | 2809 (31.58) | 2801 (31.49) | 6479 (32.84) | 6502 (32.95) |
| Coronary atherosclerosis | 1201 (8.35) | 1198 (8.33) | 2841 (9.02) | 2842 (9.02) | 717 (7.19) | 731 (7.33) | 1743 (7.48) | 1746 (7.49) | 675 (7.59) | 693 (7.79) | 1662 (8.42) | 1657 (8.40) |
| Acute myocardial infarction | 116 (0.81) | 111 (0.77) | 314 (1.00) | 322 (1.02) | 51 (0.51) | 55 (0.55) | 152 (0.65) | 145 (0.62) | 75 (0.84) | 71 (0.80) | 214 (1.08) | 226 (1.15) |
| Old myocardial infarction | 103 (0.72) | 100 (0.70) | 256 (0.81) | 231 (0.73) | 57 (0.57) | 59 (0.59) | 147 (0.63) | 160 (0.69) | 54 (0.61) | 63 (0.71) | 157 (0.80) | 156 (0.79) |
| Unstable angina | 128 (0.89) | 135 (0.94) | 295 (0.94) | 293 (0.93) | 71 (0.71) | 75 (0.75) | 150 (0.64) | 156 (0.67) | 69 (0.78) | 69 (0.78) | 175 (0.89) | 162 (0.82) |
| Stable angina | 168 (1.17) | 158 (1.10) | 401 (1.27) | 401 (1.27) | 93 (0.93) | 106 (1.06) | 236 (1.01) | 247 (1.06) | 96 (1.08) | 98 (1.10) | 240 (1.22) | 250 (1.27) |
| Other chronic ischemic heart disease | 220 (1.53) | 210 (1.46) | 608 (1.93) | 568 (1.80) | 112 (1.12) | 117 (1.17) | 318 (1.36) | 318 (1.36) | 131 (1.47) | 150 (1.69) | 378 (1.92) | 388 (1.97) |
| Coronary procedure (CABG or PTCA) | 265 (1.84) | 259 (1.80) | 603 (1.91) | 617 (1.96) | 153 (1.54) | 176 (1.77) | 351 (1.51) | 376 (1.61) | 181 (2.03) | 165 (1.85) | 407 (2.06) | 405 (2.05) |
| History of PTCA or CABG | 189 (1.31) | 215 (1.50) | 461 (1.46) | 471 (1.50) | 90 (0.90) | 90 (0.90) | 230 (0.99) | 237 (1.02) | 102 (1.15) | 99 (1.11) | 281 (1.42) | 279 (1.41) |
| Ischemic stroke | 164 (1.14) | 173 (1.20) | 451 (1.43) | 442 (1.40) | 99 (0.99) | 92 (0.92) | 272 (1.17) | 259 (1.11) | 95 (1.07) | 91 (1.02) | 274 (1.39) | 262 (1.33) |
| Congestive heart failure | 265 (1.84) | 268 (1.86) | 727 (2.31) | 741 (2.35) | 117 (1.17) | 124 (1.24) | 284 (1.22) | 289 (1.24) | 135 (1.52) | 146 (1.64) | 425 (2.15) | 449 (2.28) |
| Renal Dysfunction | 1362 (9.47) | 1363 (9.48) | 3723 (11.82) | 3719 (11.81) | 745 (7.48) | 719 (7.21) | 2305 (9.89) | 2239 (9.60) | 737 (8.29) | 730 (8.21) | 2088 (10.58) | 2071 (10.50) |
| Edema | 447 (3.11) | 445 (3.09) | 1064 (3.38) | 1059 (3.36) | 238 (2.39) | 236 (2.37) | 618 (2.65) | 608 (2.61) | 262 (2.95) | 251 (2.82) | 659 (3.34) | 628 (3.18) |
| Use of medications | ||||||||||||
| Past use of other antidiabetic agents | 4694 (32.64) | 4747 (33.01) | 9243 (29.35) | 9289 (29.50) | 2502 (25.11) | 2467 (24.75) | 5811 (24.92) | 5703 (24.46) | 2302 (25.88) | 2245 (25.24) | 4434 (22.47) | 4297 (21.78) |
| Past use of metformin | 2173 (15.11) | 2209 (15.36) | 4302 (13.66) | 4326 (13.74) | 1422 (14.27) | 1434 (14.39) | 3111 (13.34) | 3152 (13.52) | 1280 (14.39) | 1307 (14.69) | 2508 (12.71) | 2492 (12.63) |
| Past use of insulin | 486 (3.38) | 489 (3.40) | 1168 (3.71) | 1204 (3.82) | 329 (3.30) | 332 (3.33) | 827 (3.55) | 805 (3.45) | 300 (3.37) | 288 (3.24) | 768 (3.89) | 751 (3.81) |
| ACE inhibitor | 6029 (41.93) | 6037 (41.98) | 13323 (42.31) | 13376 (42.47) | 4163 (41.77) | 4199 (42.13) | 9865 (42.31) | 9903 (42.47) | 3276 (36.83) | 3245 (36.48) | 7483 (37.93) | 7521 (38.12) |
| ARBs | 3468 (24.12) | 3459 (24.06) | 7647 (24.28) | 7608 (24.16) | 2196 (22.03) | 2155 (21.62) | 5289 (22.68) | 5261 (22.56) | 2155 (24.23) | 2124 (23.88) | 4731 (23.98) | 4677 (23.70) |
| Beta blocker | 3546 (24.66) | 3579 (24.89) | 8194 (26.02) | 8197 (26.03) | 2269 (22.77) | 2255 (22.63) | 5548 (23.79) | 5564 (23.86) | 1982 (22.28) | 2001 (22.50) | 4695 (23.80) | 4608 (23.35) |
| Thiazides | 4218 (29.33) | 4124 (28.68) | 9077 (28.82) | 8971 (28.49) | 2775 (27.84) | 2803 (28.13) | 6579 (28.22) | 6592 (28.27) | 2460 (27.66) | 2415 (27.15) | 5382 (27.28) | 5281 (26.76) |
| Loop diuretics | 1066 (7.41) | 1058 (7.36) | 2450 (7.78) | 2423 (7.69) | 587 (5.89) | 594 (5.96) | 1394 (5.98) | 1372 (5.88) | 579 (6.51) | 557 (6.26) | 1382 (7.00) | 1340 (6.79) |
| Calcium channel blockers | 2938 (20.43) | 2880 (20.03) | 6587 (20.92) | 6495 (20.62) | 1952 (19.59) | 1929 (19.36) | 4615 (19.79) | 4660 (19.99) | 1674 (18.82) | 1662 (18.68) | 3762 (19.07) | 3726 (18.88) |
| Statins | 8002 (55.65) | 7982 (55.51) | 17915 (56.89) | 17811 (56.56) | 5286 (53.04) | 5278 (52.96) | 12849 (55.11) | 12909 (55.37) | 4592 (51.62) | 4573 (51.41) | 10478 (53.10) | 10481 (53.12) |
| Other lipid-lowering drugs | 2095 (14.57) | 2122 (14.76) | 4118 (13.08) | 4020 (12.77) | 1349 (13.54) | 1360 (13.65) | 2869 (12.30) | 2847 (12.21) | 1214 (13.65) | 1217 (13.68) | 2370 (12.01) | 2443 (12.38) |
| Oral anticoagulants | 396 (2.75) | 401 (2.79) | 4 (0.01) | 6 (0.02) | 219 (2.20) | 234 (2.35) | 4 (0.02) | 3 (0.01) | 214 (2.41) | 205 (2.30) | 4 (0.02) | 4 (0.02) |
| Antiplatelet | 785 (5.46) | 778 (5.41) | 1853 (5.88) | 1822 (5.79) | 475 (4.77) | 480 (4.82) | 1168 (5.01) | 1163 (4.99) | 450 (5.06) | 446 (5.01) | 1073 (5.44) | 1019 (5.16) |
| Health Care Utilization | ||||||||||||
| Any hospitalization | 808 (5.62) | 816 (5.67) | 1935 (6.14) | 1919 (6.09) | 475 (4.77) | 481 (4.83) | 1109 (4.76) | 1084 (4.65) | 486 (5.46) | 497 (5.59) | 1216 (6.16) | 1208 (6.12) |
| Any hospitalization within prior 30 days | 257 (1.79) | 266 (1.85) | 742 (2.36) | 750 (2.38) | 173 (1.74) | 174 (1.75) | 440 (1.89) | 416 (1.78) | 163 (1.83) | 176 (1.98) | 468 (2.37) | 466 (2.36) |
| N hospital days (mean, SD) | 0.29 (1.87) | 0.28 (1.90) | 0.32 (1.95) | 0.32 (2.15) | 0.22 (1.59) | 0.22 (1.45) | 0.23 (1.64) | 0.21 (1.44) | 0.28 (1.82) | 0.32 (2.61) | 0.33 (2.02) | 0.34 (2.44) |
| N physician visits (mean, SD) | 3.84 (2.91) | 3.78 (2.93) | 3.84 (3.00) | 3.80 (2.92) | 3.66 (2.83) | 3.56 (2.82) | 3.63 (2.85) | 3.53 (2.79) | 3.79 (2.89) | 3.68 (2.78) | 3.80 (3.00) | 3.72 (3.01) |
| N distinct non-insulin antidiabetic prescriptions (mean, SD) | 2.39 (0.88) | 2.40 (0.88) | 2.38 (0.88) | 2.37 (0.87) | 2.18 (0.77) | 2.19 (0.77) | 2.24 (0.80) | 2.24 (0.80) | 1.94 (0.65) | 1.95 (0.65) | 1.94 (0.66) | 1.94 (0.65) |
| N distinct prescriptions (mean, SD) | 8.46 (4.41) | 8.37 (4.32) | 8.58 (4.51) | 8.51 (4.44) | 8.02 (4.29) | 7.85 (4.10) | 8.17 (4.39) | 8.04 (4.18) | 7.88 (4.41) | 7.78 (4.19) | 8.01 (4.53) | 7.92 (4.30) |
| Number laboratory tests ordered (mean, SD) | 2.21 (2.07) | 2.16 (2.00) | 2.19 (2.05) | 2.17 (2.01) | 2.10 (1.93) | 2.06 (1.91) | 2.07 (1.90) | 2.04 (1.86) | 2.18 (2.10) | 2.14 (1.92) | 2.16 (2.04) | 2.16 (2.05) |
| Laboratory tests | ||||||||||||
| Patients with HbA1c available | 1359 (9.45) | 1420 (9.88) | 4239 (13.46) | 4497 (14.28) | 853 (8.56) | 879 (8.82) | 3021 (12.96) | 3064 (13.14) | 836 (9.40) | 972 (10.93) | 2683 (13.60) | 2755 (13.96) |
| HbA1c, % | 8.57 (2.06) | 8.62 (2.09) | 8.78 (7.39) | 8.85 (4.80) | 8.69 (2.02) | 8.78 (2.26) | 8.78 (6.49) | 8.88 (2.10) | 8.44 (2.17) | 8.88 (2.26) | 8.61 (6.44) | 9.07 (3.67) |
| Patients with creatinine available | 1464 (10.18) | 1468 (10.21) | 4581 (14.55) | 4718 (14.98) | 896 (8.99) | 874 (8.77) | 3236 (13.88) | 3168 (13.59) | 908 (10.21) | 953 (10.71) | 2903 (14.71) | 2855 (14.47) |
| Creatinine, mg/dL | 0.99 (0.32) | 0.96 (0.25) | 1.02 (0.34) | 0.98 (0.28) | 0.97 (0.30) | 0.96 (0.25) | 1.00 (0.31) | 0.98 (0.28) | 0.97 (0.31) | 0.94 (0.27) | 1.01 (0.33) | 0.96 (0.28) |
| eGFR, mL/min/1.73m2 | 100.93 (23.93) | 103.41 (20.84) | 96.29 (26.19) | 99.28 (23.24) | 102.62 (22.46) | 103.59 (21.67) | 97.36 (25.28) | 98.44 (23.62) | 102.59 (22.49) | 104.60 (21.48) | 97.98 (25.49) | 100.97 (22.86) |
| Patients with total cholesterol available | 1290 (8.97) | 1291 (8.98) | 4015 (12.75) | 4144 (13.16) | 791 (7.94) | 822 (8.25) | 2873 (12.32) | 2842 (12.19) | 806 (9.06) | 923 (10.38) | 2557 (12.96) | 2620 (13.28) |
| Total cholesterol, mg/dL | 181.44 (48.40) | 182.07 (51.12) | 182.20 (54.02) | 181.76 (50.41) | 184.39 (49.50) | 184.79 (54.86) | 183.26 (51.99) | 184.26 (50.50) | 184.16 (47.62) | 188.08 (54.59) | 184.60 (52.13) | 188.08 (56.11) |
| Patients with LDL available | 1183 (8.23) | 1193 (8.30) | 3711 (11.78) | 3824 (12.14) | 720 (7.22) | 766 (7.69) | 2636 (11.31) | 2656 (11.39) | 737 (8.29) | 855 (9.61) | 2350 (11.91) | 2419 (12.26) |
| LDL, mg/dL | 97.71 (37.84) | 98.87 (36.66) | 98.53 (38.67) | 98.40 (36.79) | 99.55 (38.17) | 99.27 (37.44) | 99.61 (38.74) | 99.34 (36.47) | 100.10 (37.86) | 102.88 (38.07) | 101.04 (38.46) | 102.02 (38.40) |
DPP-4i: dipeptidyl peptidase-4 inhibitors; SD: standard deviation; CABG: coronary artery bypass grafting; PTCA: percutaneous transluminal coronary angioplasty; ACE: Angiotensin-converting enzyme; ARBs: angiotensin II receptor blockers; HbA1c: hemoglobin A1c; eGFR: estimated glomerular filtration rate; LDL: low-density lipoprotein
Analysis
We cross-tabulated patient characteristics for each pair of linagliptin and the respective comparator. For each sequential cohort, we estimated an exposure PS using multivariable logistic regression predicting the initiation of linagliptin vs. each comparator, conditional upon over 100 pre-defined baseline characteristics (eTable-2).[28] We 1:1 PS-matched patients using the nearest neighbor methodology with a maximum caliper of 0.05 of the PS and additional matching within calendar quarter, such that patients initiating linagliptin were only eligible to be matched to patients initiating a comparator in the same calendar quarter.[29, 30] Post-matching covariate balance between treatments was assessed by the calculating absolute standardized differences for each covariate. Values greater than 0.1 were considered meaningful imbalances.[31] Post-matching PS model c-statistics provided a measure for balance across all covariates with a c close to 0.5 indicating excellent balance.
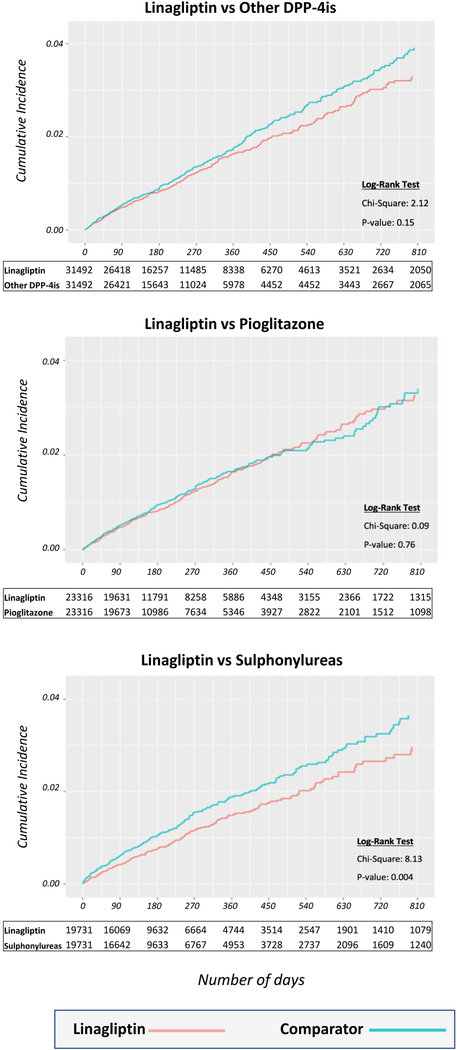
For each comparison and for all outcomes, hazard ratios (HR) with 95% confidence intervals (CI) were estimated in the PS-matched cohorts using unstratified Cox regression.[32] Analyses for both outcomes were conducted in each data source separately and then pooled across the data sources using a fixed-effects meta-analysis.[33] The cumulative incidence since drug initiation of the composite cardiovascular outcomes comparing PS-matched linagliptin and other agents initiators was plotted in Kaplan-Meier survival curves and the logrank test calculated.
Several sensitivity analyses were conducted to assess the robustness of our primary findings. First, to address potential exposure misclassification and potential informative censoring, we extended the exposure effect window until 30 days, instead of 90 days, after the end of the last prescription’s supply, and we applied an intention-to-treat type analysis, which carried forward the initial exposure until the occurrence of a study event, plan disenrollment, admission to a nursing home, or the end of the study period, disregarding treatment discontinuation or switching.[34] Second, to improve adjustment for potential unmeasured confounding, we implemented high-dimensional propensity score (hdPS) matching, which enriched the original PS with 500 additional empirically identified covariates.[35, 36] Third, to further align patients on a similar time scale for diabetes progression, and to reduce imbalances related to kidney disease for which metformin would be contraindicated, we restricted the analyses to patients with past or current use of metformin. Fourth, to account for potential differences in baseline glucose control between treatment groups, we re-estimated the original PS adding HbA1c level in addition to the other baseline covariates in the subgroup of patient with HbA1c values available, about 35% in Optum Clinformatics and 5% in IBM MarketScan). Lastly, we conducted subgroup analyses stratified by age (<65 and ≥65 years), gender, and presence of cardiovascular disease at baseline. All analyses were done using SAS 9.4 Statistical Software (SAS Institute Inc., Cary, NC).
Repeated analyses
As the objective of this study was to assess the incremental evidence as data accumulate, for the primary outcome we present the point estimates from each pre-specified analysis to show how these changed sequentially. We reported HRs and 95% CIs at 6 distinct time points over a 4.7-year period. No adjustments for multiple testing over time were made.[37]
Results
After applying inclusion and exclusion criteria, three pairwise 1:1 PS-matched cohorts of patients initiating linagliptin or other DPP-4i (N=31,492 pairs), linagliptin or pioglitazone (N=23,316 pairs), and linagliptin or sulfonylureas (N=19,731 pairs) were accumulated from May 2011 through December 2015 (eFigure 1). PS-matched linagliptin initiators approximately doubled from the first pre-specified analysis (May 2011 through June 2013), which identified a total of 14,379, 9,966, and 8,895 new linagliptin users, per comparator group respectively (Figure 1). With the exception of an increasing propensity of patients with concomitant kidney disease to be initiated on linagliptin and a decreasing likelihood for linagliptin to be initiated as first-line monotherapy, the patient characteristics after PS matching did not change meaningfully over the course of the monitoring program (Table 1). For each pre-specified analysis, patient characteristics showed absolute standardized differences smaller than 0.1 indicating good balance (eTables 2–4) with post-matching c-statistics smaller than 0.6 (eTable 5). All three comparisons groups had similar follow-up (mean follow-up from 0.73 to 0.79 years) with similar reasons for censoring (eTable 6 and eTable 7).
Figure 1. Sequentially updated propensity score matched cohorts of linagliptin initiators vs. three comparators, mean follow-up time, number of composite cardiovascular outcomes events, hazard ratios and 95% confidence intervals.
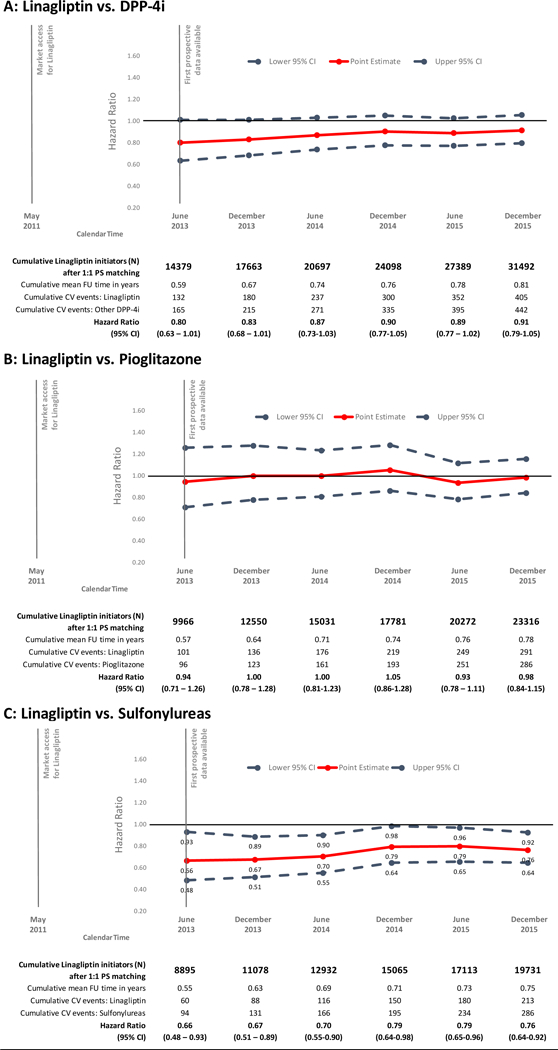
DPP-4is: dipeptidyl peptidase-4 inhibitors; PS: propensity score; FU: follow-up; CV: cardiovascular; CI: confidence intervals; DPP-4is: dipeptidyl peptidase-4 inhibitors
For the primary composite cardiovascular outcome, estimates remained overall stable over the course of the monitoring period for all three comparisons (Figure 1). In the first sequential analysis (data up to June 30, 2013) for the primary composite cardiovascular outcome, we identified 132 versus 165 events among initiators of linagliptin versus other DPP-4i (15.6 versus 19.6/1,000 person-years; HR= 0.80; 95% CI = 0.63 to 1.01); 101 versus 96 events among initiators of linagliptin versus pioglitazone (17.87 versus 19.5/1,000 person-years, HR= 0.94; 0.71 to 1.26); and 60 versus 94 events among initiators of linagliptin versus sulfonylureas (12.3 versus 19.1/1,000 person-years; HR= 0.66; 0.48 to 0.93).
By the sixth sequential analysis (data up to December 31, 2015) we had identified 405 versus 442 CV events among initiators of linagliptin versus other DPP-4i (16.2 versus 17.9/1,000 person-years; HR= 0.91; 0.79 to 1.05); 291 versus 286 events among initiators of linagliptin versus pioglitazone (16.3 versus 16.8/1,000 person-years; HR= 0.98; 0.84 to 1.15); and 213 versus 286 events among initiators of linagliptin versus sulfonylureas (14.6 versus 18.7/1,000 person-years; HR= 0.76; 0.64 to 0.92; see Table 2). The cumulative incidence since drug initiation of the composite cardiovascular outcomes comparing the PS-matched linagliptin and other comparator groups, are shown in Figure 2. The effects are similarly observed in both databases (eTable 8).
Table 2.
Mean follow-up time, number of events, incidence rates, hazard ratios and 95% confidence intervals for composite cardiovascular outcome and individual components of the composite outcome in propensity score matched analyses
| Linagliptin | Other DPP-4i | Linagliptin | Pioglitazone | Linagliptin | Sulfonylureas | |
|---|---|---|---|---|---|---|
| No. Patients | 31492 | 31492 | 23316 | 23316 | 19731 | 19731 |
| Mean follow-up in years (SD) | 0.79 (0.75) | 0.79 (0.76) | 0.77(0.71) | 0.73(0.68) | 0.74(0.71) | 0.77(0.75) |
| Composite CV outcome | ||||||
| Events | 405 | 442 | 291 | 286 | 213 | 286 |
| IR/1000 person-years (95% CI) | 16.19(14.69–17.85) | 17.86(16.27–19.61) | 16.28(14.51–18.26) | 16.81(14.97–18.88) | 14.58(12.75–16.68) | 18.74(16.69–21.04) |
| Hazard ratio (95% CI) | 0.91 (0.79–1.05) | Ref. | 0.98 (0.84–1.15) | Ref. | 0.76 (0.64–0.92) | Ref. |
| Myocardial infarction | ||||||
| Events | 161 | 165 | 113 | 121 | 87 | 115 |
| IR/1000 person-years (95% CI) | 6.40(5.48–7.47) | 6.62(5.68–7.71) | 6.29(5.23–7.56) | 7.07(5.92–8.45) | 5.93(4.81–7.32) | 7.48(6.23–8.98) |
| Hazard ratio (95% CI) | 0.97 (0.78–1.20) | Ref. | 0.89 (0.69–1.15) | Ref. | 0.79 (0.60–1.04) | Ref. |
| Stroke | ||||||
| Events | 122 | 123 | 86 | 77 | 59 | 87 |
| IR/1000 person-years (95% CI) | 4.84(4.05–5.78) | 4.93(4.13–5.88) | 4.78(3.87–5.90) | 4.50(3.60–5.63) | 4.01(3.11–5.18) | 5.65(4.58–6.97) |
| Hazard ratio (95% CI) | 0.98 (0.76–1.26) | Ref. | 1.07 (0.79–1.46) | Ref. | 0.71 (0.51–0.98) | Ref. |
| Unstable angina | ||||||
| Events | 93 | 124 | 74 | 84 | 47 | 85 |
| IR/1000 person-years (95% CI) | 3.69(3.01–4.52) | 4.97(4.17–5.93) | 4.11(3.27–5.16) | 4.91(3.96–6.08) | 3.20(2.40–4.26) | 5.53(4.47–6.84) |
| Hazard ratio (95% CI) | 0.74 (0.57–0.97) | Ref. | 0.84 (0.61–1.15) | Ref. | 0.57 (0.40–0.82) | Ref. |
| Coronary revascularization | ||||||
| Events | 196 | 209 | 142 | 138 | 105 | 136 |
| IR/1000 person-years (95% CI) | 7.80(6.78–8.97) | 8.40(7.33–9.62) | 7.91(6.71–9.32) | 8.08(6.84–9.55) | 7.16(5.91–8.67) | 8.86(7.49–10.48) |
| Hazard ratio (95% CI) | 0.93 (0.76–1.13) | Ref. | 0.98 (0.78–1.24) | Ref. | 0.81 (0.62–1.04) | Ref. |
DPP-4i: dipeptidyl peptidase-4 inhibitors; SD: standard deviation; IR: incidence rate; CI: confidence intervals
Figure 2. Propensity score matched Kaplan-Meier curves for cumulative incidence of composite cardiovascular outcome events since treatment initiation for the three comparisons.
DPP-4is: dipeptidyl peptidase-4 inhibitors
For the individual components of the composite cardiovascular outcome, results were largely consistent though the estimates were less precise due to the smaller number of patients. Compared with initiators of other DPP-4i or pioglitazone, linagliptin initiators had similar risk of myocardial infarction, stroke and coronary revascularization, and a trend to lower risk of unstable angina. For the comparison with sulfonylureas, all individual endpoint results generally pointed towards a numerical lower risk associated with the initiation of linagliptin. (Table 2).
Our findings remained consistent in sensitivity analyses that (1) defined the exposure effect window until 30 days after the end of the last prescription’s supply, (2) used an intention-to-treat type approach, (3) implemented hdPS-matching, (4) restricted to patients with past or current use of metformin, and (5) further adjusted for baseline HbA1c level, though with wider confidence intervals (eFigure 2). Results from subgroup analyses by age (<65 and ≥65 years), gender, and presence of cardiovascular disease at baseline, were mostly consistent with the findings from the total study population (eFigure 2).
Discussion
We report six interim analyses from a monitoring program for the cardiovascular safety of linagliptin in commercially-insured patients with T2D spanning the first 4.5 years after it first became available. Our results showed that linagliptin had similar safety on a cardiovascular composite outcome compared to other members of the DPP-4i class and compared to pioglitazone. Notably, we found that linagliptin was associated with a 24% decreased risk for the combined cardiovascular endpoint compared to sulfonylureas. These findings were largely consistent across sensitivity analyses and patient subgroups, though fewer events led to less precise estimates in many subgroups.
Two large cardiovascular outcome trials comparing linagliptin to either placebo (CARMELINA) or glimepiride, a sulfonylurea (CAROLINA), have just begun releasing large-scale information regarding the effects of linagliptin on cardiovascular endpoints. [12] Specifically, CARMELINA has shown that over a median follow-up period of 2.2 years, linagliptin was non-inferior to placebo with regard to the risk of a composite cardiovascular outcome (HR 1.02 95% CI 0.89, 1.17) in approximately 7,000 adults with high cardiovascular and renal risk. While randomized, controlled trials represent the gold standard for establishing the efficacy of medications, they generally exclude considerable portions of the potentially treatable population, thus limiting the generalizability of their study findings. A well-designed non-interventional study excels in the ability to provide real-world evidence on safety and effectiveness of treatments in routine clinical care settings, thus complementing results from RCTs of interest.
In CARMELINA and CAROLINA the average age was 66 and 64 years, respectively, and approximately 60% of patients were males. CARMELINA included patients with long-standing T2D and cardiovascular and/or kidney disease, whereas CAROLINA includes an earlier T2D population with cardiovascular risk factors or disease. However, both trials excluded patients with recent acute cardiovascular events (<3 month), BMI more than 45kg/mμ, and previous treatment with some other antidiabetic agents, e.g., GLP-1 receptor agonists and insulin in CAROLINA, GLP-1 receptor agonists, SGLT-2 inhibitors or glitazones in CARMELINA.[10, 11] Our study reflects routine care among commercially-insured US populations with T2D, and includes a broad spectrum of T2D patients. Therefore, we believe our study provides valuable and complementary real-world evidence on the effects of linagliptin in the setting of patterns of medication use and patient selection that reflect routine clinical care. In our study, the average age was 55 years, with approximately 20% of the population older than 65 years, and almost 60% of the participants were males. Individuals with history of cardiovascular disease, including recent acute cardiovascular events, represented about 10% of the sample and between 8% and 9% of patients used insulin at baseline.
Our results are consistent with a population-based study leveraging the Korean Health Insurance Review and Assessment Service database, which assessed cardiovascular outcomes for linagliptin as compared with sulfonylureas in over sixty thousand 1:1 PS matched pairs with T2D.[38] The study found reductions in the risk of stroke (HR=0.71; 0.62 to 0.82) and myocardial infarction (HR=0.77; 0.59 to 1.02) associated with linagliptin use. Another study based on the Taiwan National Health Insurance Research Database evaluated the association between linagliptin and major cardiovascular events in a population of 1,203 patients with T2D after an episode of acute coronary syndrome or ischemic stroke. Compared with patients who did not receive any incretin-based therapy, linagliptin users had a similar risk of major cardiovascular events (HR=1.06; 0.66 to 1.68).[39] These results complement our findings.
The observed cardiovascular risk reduction associated with linagliptin compared to sulfonylureas may be driven by a potential increased risk associated with sulfonylureas. Sulfonylureas may cause weight gain, fluid retention, and hypoglycemia, which are potential mediators of cardiovascular events.[40] Several non-randomized studies have found these agents to be associated with an increased risk of cardiovascular events[41]; this finding remained consistent after studies with major design-related biases were excluded.[42]
Nevertheless, residual confounding by some unmeasured characteristics is possible, although it is unlikely to be of a meaningful extent. To minimize this possibility, we used PS matching including over 100 baseline characteristics between the exposure groups, including proxies of diabetes progression and detailed history of diabetes medications, implemented hdPS matching, to further enrich the PS, and adjusted analyses for HbA1c among the subset of patients with HbA1c values. All analyses produced consistent results. In a validation study based on a subset of the population with linked laboratory test results and electronic health records, that quantified the residual confounding due to relevant clinical risk factors not observed in claims (e.g., BMI, duration of diabetes, HbA1c, estimated GFR), we showed convincing balance between the exposure groups for the clinical parameters.[23] The high rate of medication discontinuation for glucose-lowering medications in routine care limits the robust estimation of long-term effects beyond one year after initiation. However, depending on the specific comparison, between 5,000 and 9,000 PS matched patients were still on their initial treatment at 1.5 years of follow-up, providing reassurance regarding the assessment of long-term cardiovascular effects.
We purposefully did not adjust 95% confidence intervals for the repeated analyses at each pre-specified assessment. This monitoring system was set up to inform the medical community in a timely manner on the best available effect estimate as the data accumulated over time. Unlike a clinical trial, non-experimental studies based on large utilization databases cannot prevent people from receiving a substandard treatment once the treatment effects in routine care have been established. Thus, the periodic analyses in database studies are not performed to evaluate potential earlier study termination, nor any other time dependent actions such as regulatory decisions, but rather to provide the most timely and accurate information when treating individual patients.[18, 43, 44]
Conclusions
In a pre-specified analysis from a five-year monitoring program involving >100,000 commercially-insured patients with T2D, linagliptin had similar cardiovascular safety compared to other DPP-4i and pioglitazone, and a reduced cardiovascular risk compared to sulfonylureas. Together with other recent examples, [18, 43, 44] this monitoring program demonstrates the value of routinely collected health care data in a framework of principled epidemiological analyses to prospectively monitor the effectiveness and safety of approved medical products.
Supplementary Material
Acknowledgments:
We thank Dr. John Seeger for his contribution in an early phase of this study
Source of Support: This study was supported by a research grant to the Brigham and Women’s Hospital from Boehringer-Ingelheim. The study was conducted by the authors independent of the sponsor. The authors retained the right of publication and determined the final wording of the manuscript. EP was supported by a career development grant K08AG055670 from the National Institute on Aging. MK was supported by the National Institute of General Medical Sciences, grant RO1GM108999. EP is investigator of investigator-initiated grants to the Brigham and Women’s Hospital from GSK, not directly related to the topic of the submitted work. SS is the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Bayer and Vertex unrelated to the topic of this study. He is a consultant to WHISCON and to Aetion, a software manufacturer of which he owns equity. His interests were declared, reviewed, and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies.
EP was supported by a career development grant K08AG055670 from the National Institute on Aging. She is the investigator of investigator-initiated grants to the Brigham and Women’s Hospital from GSK, not directly related to the topic of the submitted work.
MK was supported by the National Institute of General Medical Sciences, grant RO1GM108999.
SS is the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Bayer and Vertex unrelated to the topic of this study. He is a consultant to WHISCON and to Aetion, a software manufacturer of which he owns equity. His interests were declared, reviewed, and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies.
This study was supported by a research grant to the Brigham and Women’s Hospital from Boehringer-Ingelheim. The study was conducted by the authors independent of the sponsor. The authors retained the right of publication and determined the final wording of the manuscript.
Footnotes
Conflicts of interest:
CG and JL have no conflicts of interest to disclose.
References
- 1.Ahren B, DPP-4 inhibitors. Best Pract Res Clin Endocrinol Metab, 2007. 21(4): p. 517–33. [DOI] [PubMed] [Google Scholar]
- 2.Schernthaner G, et al. , Safety and tolerability of linagliptin: a pooled analysis of data from randomized controlled trials in 3572 patients with type 2 diabetes mellitus. Diabetes Obes Metab, 2012. 14(5): p. 470–8. [DOI] [PubMed] [Google Scholar]
- 3.von Eynatten M, et al. , Efficacy and safety of linagliptin in type 2 diabetes subjects at high risk for renal and cardiovascular disease: a pooled analysis of six phase III clinical trials. Cardiovasc Diabetol, 2013. 12: p. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenstock J MN, Neubacher D, Seck T, Patel S, Woerle HJ, Johansen OE, Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol, 2015. 14(57). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S.F.D.A. TRADJENTA (linagliptin). Highlights of prescribing information. 2011; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201280lbl.pdf.
- 6.Go et al. , Heart disease and stroke statistics−−2014 update: a report from the American Heart Association. Circulation, 2014. 129(3): p. 28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scirica BM, et al. , Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med, 2013. 369(14): p. 1317–26. [DOI] [PubMed] [Google Scholar]
- 8.White WB, et al. , Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med, 2013. 369(14): p. 1327–35. [DOI] [PubMed] [Google Scholar]
- 9.Green JB, et al. , Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med, 2015. 373(3): p. 232–42. [DOI] [PubMed] [Google Scholar]
- 10.Rosenstock J, et al. , Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA((R))): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk. Cardiovasc Diabetol, 2018. 17(1): p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx N, et al. , Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)). Diab Vasc Dis Res, 2015. 12(3): p. 164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstock J, et al. , Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneeweiss S and Avorn J, A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol, 2005. 58(4): p. 323–37. [DOI] [PubMed] [Google Scholar]
- 14.Schneeweiss S, A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf, 2010. 19(8): p. 858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneeweiss S, et al. , Methods for comparative effectiveness research/patient-centered outcomes research: from efficacy to effectiveness. J Clin Epidemiol, 2013. 66(8 Suppl): p. S1–4. [DOI] [PubMed] [Google Scholar]
- 16.Schneeweiss S, Improving therapeutic effectiveness and safety through big healthcare data. Clin Pharmacol Ther, 2016. 99(3): p. 262–5. [DOI] [PubMed] [Google Scholar]
- 17.Gagne JJ, et al. , A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol, 2011. 64(7): p. 749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toh S, et al. , Prospective Postmarketing Surveillance of Acute Myocardial Infarction in New Users of Saxagliptin: A Population-Based Study. Diabetes Care, 2018. 41(1): p. 39–48. [DOI] [PubMed] [Google Scholar]
- 19.Yih WK, et al. , Prospective influenza vaccine safety surveillance using fresh data in the Sentinel System. Pharmacoepidemiol Drug Saf, 2016. 25(5): p. 481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JS, et al. , Early detection of adverse drug events within population-based health networks: application of sequential testing methods. Pharmacoepidemiol Drug Saf, 2007. 16(12): p. 1275–84. [DOI] [PubMed] [Google Scholar]
- 21.Seeger JD and Daniels G, Commercial insurance databases, in Pharmacoepidemiology, Strom BLK SE and Hennessy S, ed., Editors. 2012, Wiley&Sons: Philadelphia. [Google Scholar]
- 22.Ray WA, Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol, 2003. 158(9): p. 915–20. [DOI] [PubMed] [Google Scholar]
- 23.Patorno E, et al. , Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes Obes Metab, 2018. 20(4): p. 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyota Y, et al. , Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J, 2004. 148(1): p. 99–104. [DOI] [PubMed] [Google Scholar]
- 25.Wahl PM, et al. , Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf, 2010. 19(6): p. 596–603. [DOI] [PubMed] [Google Scholar]
- 26.Tirschwell DL and Longstreth WT Jr., Validating administrative data in stroke research. Stroke, 2002. 33(10): p. 2465–70. [DOI] [PubMed] [Google Scholar]
- 27.Saczynski JS, et al. , A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf, 2012. 21 Suppl 1: p. 129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin DB, Estimating causal effects from large data sets using propensity scores. Ann Intern Med, 1997. 127(8 Pt 2): p. 757–63. [DOI] [PubMed] [Google Scholar]
- 29.Rassen JA, et al. , One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf, 2012. 21 Suppl 2: p. 69–80. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat, 2011. 10(2): p. 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med, 2009. 28(25): p. 3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox DR, Regression models and life tables. Journal of the royal statistical society. Series B (Methodological), 1972. 34(2): p. 187–220. [Google Scholar]
- 33.Jones DR, Meta-analysis: weighing the evidence. Stat Med, 1995. 14(2): p. 137–49. [DOI] [PubMed] [Google Scholar]
- 34.Patorno E, et al. , Observational studies of the association between glucose-lowering medications and cardiovascular outcomes: addressing methodological limitations. Diabetologia, 2014. 57(11): p. 2237–50. [DOI] [PubMed] [Google Scholar]
- 35.Schneeweiss S, et al. , High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology, 2009. 20(4): p. 512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rassen JA, et al. , Covariate selection in high-dimensional propensity score analyses of treatment effects in small samples. Am J Epidemiol, 2011. 173(12): p. 1404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin D, et al. , Sequential surveillance for drug safety in a regulatory environment. Pharmacoepidemiol Drug Saf, 2018. [DOI] [PubMed] [Google Scholar]
- 38.Kim YG, et al. , Dipeptidyl Peptidase-4 Inhibitors and Risk of Heart Failure in Patients With Type 2 Diabetes Mellitus: A Population-Based Cohort Study. Circ Heart Fail, 2017. 10(9). [DOI] [PubMed] [Google Scholar]
- 39.Li YR, et al. , Linagliptin and cardiovascular outcomes in type 2 diabetes after acute coronary syndrome or acute ischemic stroke. Cardiovasc Diabetol, 2018. 17(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tahrani AA, Barnett AH, and Bailey CJ, Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol, 2016. 12(10): p. 566–92. [DOI] [PubMed] [Google Scholar]
- 41.Pladevall M, et al. , Cardiovascular risk associated with the use of glitazones, metformin and sufonylureas: meta-analysis of published observational studies. BMC Cardiovasc Disord, 2016. 16: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azoulay L and Suissa S, Sulfonylureas and the Risks of Cardiovascular Events and Death: A Methodological Meta-Regression Analysis of the Observational Studies. Diabetes Care, 2017. 40(5): p. 706–714. [DOI] [PubMed] [Google Scholar]
- 43.Gagne JJ, et al. , Active safety monitoring of newly marketed medications in a distributed data network: application of a semi-automated monitoring system. Clin Pharmacol Ther, 2012. 92(1): p. 80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer F, et al. , Safety and Effectiveness of Direct Oral Anticoagulants Versus Vitamin K Antagonists: Pilot Implementation of a Near-Real-Time Monitoring Program in Italy. J Am Heart Assoc, 2018. 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.