Introduction
Lung isolation is a technique commonly used in thoracic surgery to improve surgical access, especially in patients undergoing minimally invasive procedures. Selective lobar blockade has been described for patients who cannot tolerate complete lung isolation and, less commonly, for patients who have undergone previous pneumonectomy.1, 2 Selective lobar blockade may also benefit patients with compromised oxygenation by reducing pulmonary shunting, compared with complete lung collapse.3 To our knowledge, there are no reports of selective segmental isolation in the literature. We present the case of a post pneumonectomy patient with a new primary malignancy scheduled for lung resection.
Case Report
A 65-year-old man presented with a history of locally advanced lung cancer of the left lung that had been treated three years earlier with induction chemoradiotherapy followed by left thoracotomy with left pneumonectomy. He had no evidence of disease on follow-up, until routine surveillance CT identified an enlarging 1-cm right lower lobe (RLL) nodule that was concerning for new primary lung cancer. He underwent a transbronchial biopsy, which revealed adenocarcinoma of lung origin. His medical history included obstructive lung disease and a smoking history of 40 pack-years. His weight was 89 kg, and the results of physical exam were unremarkable except for absent left-sided breath sounds. His exercise tolerance was >4 metabolic equivalents. Results of laboratory assessment were also unremarkable. EKG showed normal sinus rhythm, with a rate of 67 beats per minute. His pre-procedure (post pneumonectomy) forced expiratory volume in 1 minute was 2.18 L (58% predicted), and his diffusion capacity of the lung for carbon monoxide was 16.4 mL/mmHg/min (68% predicted). In addition to the CT, the patient underwent a PET/CT scan, which revealed a solitary hypermetabolic RLL nodule with no extrathoracic disease. The patient was offered local therapy with either surgery or stereotactic radiotherapy; he chose surgical resection. The operative plan was to attempt a minimally invasive, video-assisted thoracic surgery (VATS) robotic approach with intrathoracic CO2 insufflation, including low tidal volume ventilation, with the option to convert to thoracotomy if exposure was inadequate.
The patient was premedicated with midazolam, and an epidural catheter was inserted at T8/9. Standard monitors were applied, and anesthesia was induced using fentanyl, propofol, and vecuronium. The trachea was intubated with an 8.0-mm internal diameter endotracheal tube. A 9-French Uniblocker (Fuji Systems Corporation, Tokyo, Japan) was advanced into the RLL superior segment bronchus, under bronchoscopic guidance. To achieve the required angle to enter the superior segment bronchus, the tip of the bronchial blocker was prebent before insertion. The patient was positioned in the left lateral decubitus position. The position of the bronchial blocker was confirmed, and its balloon was inflated with 1 mL of air under direct vision. The patient received pressure-control ventilation with an inspiratory pressure of 15 cm H2O, tidal volume of 5 mL/kg predicted body weight, and positive end–expiratory pressure of 5 cm H2O. Anesthesia was maintained with sevoflurane in oxygen (FiO2=1.0), vecuronium, and a continuous epidural infusion of bupivacaine 0.05% and hydromorphone at 8 µg/mL.
With the patient in the left lateral decubitus position, the chest was explored by robotic VATS with concomitant CO2 insufflation of the chest (up to 8 mmHg). There was no hemodynamic instability or desaturation noted during the procedure. Exploration of the chest revealed ideal segmental atelectasis, which allowed mobilization of the lesion and successful limited resection via wedge resection (Figure). The duration of segmental blockade required for the wedge resection was 62 minutes. The patient was extubated without event at the conclusion of the procedure. He was discharged home on postoperative day 1.
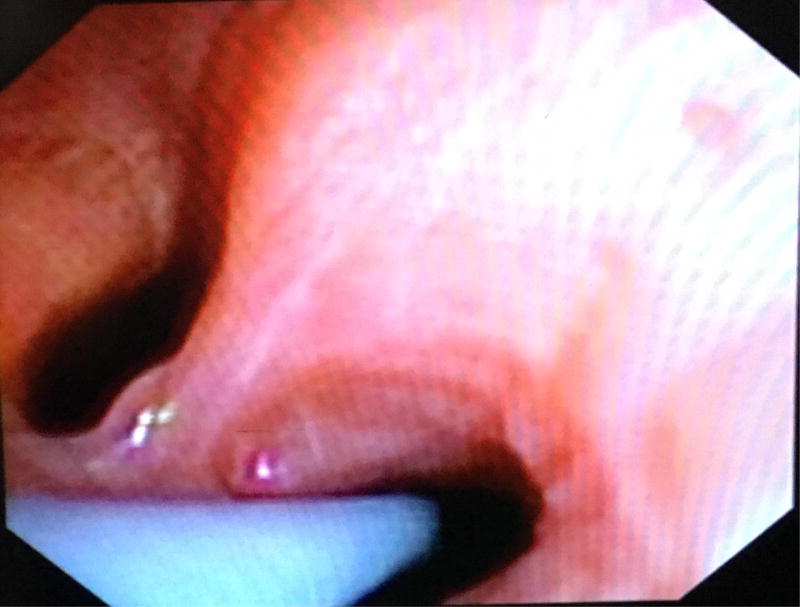
Figure 1.
Endobronchial blocker positioned in the right lower lobe superior segmental bronchus
Discussion
To our knowledge, this is the first reported case of segmental blockade in a post pneumonectomy patient. Segmental blockade may be useful in patients who have previously undergone pneumonectomy who are scheduled for further resection of a lesion in their remaining lung, as it can be challenging to maintain surgical exposure and oxygenation in these patients. Unlike segmental blockade, selective lobar bronchial blockade following contralateral pneumonectomy has been reported.1 The use of lobar blockade for pediatric patients undergoing VATS has also been described.4 Lobar blockade may be useful for providing improved surgical exposure, compared with segmental blockade; however, segmental blockade preserves more ventilation. To minimize the risk of bronchial injury from overinflation of the bronchial blocker balloon during bronchial blockade, appropriate balloon sizing and the use of minimal inflation volumes are recommended.
In a prospective, randomized study of 30 patients without previous thoracic surgery who underwent pulmonary lobectomy or wedge resection, Campos found that selective lobar blockade improved arterial oxygenation, compared with complete lung collapse.3 The effect of segmental blockade on arterial oxygenation has not been examined, compared with lobar blockade or complete lung collapse. We speculate that segmental blockade likely improves oxygenation when compared with lobar blockade or complete lung collapse, but do not have evidence to support this notion. This patient may have tolerated lobar blockade and we cannot be certain that segmental blockade was superior to lobar blockade in this case.
Blood gas analysis performed before and during segmental blockade would have been useful to confirm oxygenation and ventilation during segmental collapse. Due to this patient’s lack of other major medical comorbidities, short duration of the procedure and minimal lung collapse anticipated with segmental blockade, we chose to avoid arterial cannulation. Arterial blood gas analysis was not performed before or during the segmental blockade. The oxygen saturation monitored with pulse oxymetry and was 97–100% during segmental collapse. End tidal carbon dioxide during segmental collapse was 40–49 mmHg.
Low tidal volume ventilation and jet ventilation have been suggested as alternatives for patients who cannot tolerate selective lobar blockade.5 Jet ventilation maintains oxygenation during thoracoscopic surgery while facilitating surgical exposure and limiting lung motion. Low-frequency jet ventilation has also been described for a patient with a previous left pneumonectomy who was undergoing right upper lobectomy.5 Although this technique can maintain oxygenation, it may result in hypercapnia, which may contribute to intraoperative hypertension, tachycardia, and ventricular arrhythmias.
Segmental blockade combined with chest insufflation provided adequate exposure to facilitate robotic resection of an RLL nodule in a post pneumonectomy patient. We were able to achieve excellent selective lung isolation with segmental blockade, which allowed for minimally invasive RLL wedge resection and avoided the need for open thoracotomy.
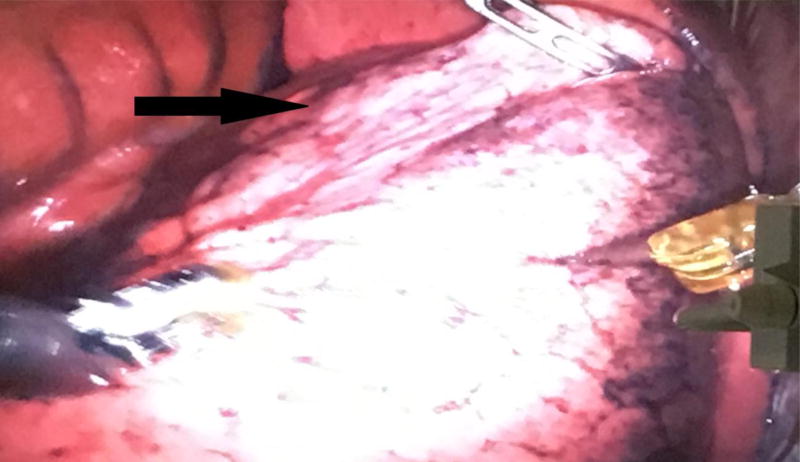
Figure 2.
Right lower lobe with superior segmental blockade. The arrow indicates the non-ventilated portion of right lower lobe
Acknowledgments
Financial support: This work was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Ng JM, Hartigan PM. Selective lobar bronchial blockade following contralateral pneumonectomy. Anesthesiology. 2003;98:268–270. doi: 10.1097/00000542-200301000-00041. [DOI] [PubMed] [Google Scholar]
- 2.Campos JH. Update on selective lobar blockade during pulmonary resections. Curr Opin Anaesthesiol. 2009;22:18–22. doi: 10.1097/ACO.0b013e32831a437a. [DOI] [PubMed] [Google Scholar]
- 3.Campos JH. Effects of oxygenation during selective lobar versus total lung collapse with or without continuous positive airway pressure. Anesth Analg. 1997;85:583–586. doi: 10.1097/00000539-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Yamada M, Honda I, et al. Selective lobar--bronchial blocking for pediatric video-assisted thoracic surgery. Anesthesiology. 2001;94:170–172. doi: 10.1097/00000542-200101000-00033. [DOI] [PubMed] [Google Scholar]
- 5.King BW, Gross KP. Right upper lobe resection after left pneumonectomy. Anesthesiology. 1994;81:771–773. doi: 10.1097/00000542-199409000-00031. [DOI] [PubMed] [Google Scholar]