Abstract
Context
Anti–pituitary-specific transcriptional factor-1 (anti–PIT-1) antibody syndrome is characterized by acquired and specific deficiencies in growth hormone, prolactin, and thyroid-stimulating hormone. Although PIT-1–reactive cytotoxic T lymphocytes (CTLs) have been speculated to recognize anterior pituitary cells and to cause the injury in the pathogenesis of the syndrome, it remains unclear whether endogenous PIT-1 protein is processed through the proteolytic pathway and presented as an antigen on anterior pituitary cells.
Objective
To examine how PIT-1 protein is processed and whether its epitope is presented by major histocompatibility complex (MHC)/HLA class I on anterior pituitary cells.
Materials and Methods
Immunofluorescence staining and proximity ligation assay (PLA) were performed using anti–PIT-1 antibody and patients’ sera on PIT-1–expressing cell line GH3 cells and human induced pluripotent stem cell (iPSC)-derived pituitary tissues.
Results
PIT-1 was colocalized with MHC class I molecules, calnexin, and GM130 in the cytosol. PLA results showed that PIT-1 epitope was presented by MHC/HLA class I molecules on the cell surface of GH3 cells and iPSC-derived pituitary cells. The number of PIT-1/HLA complexes on the cell surface of pituitary cells in the patient was comparable with that in the control subject.
Conclusions
Our data indicate that PIT-1 protein is processed in the antigen presentation pathway and that its epitopes are presented by in MHC/HLA class I on anterior pituitary cells, supporting the hypothesis that PIT-1–reactive CTLs caused the cell-specific damage. It is also suggested that number of epitope presentation was not associated with the pathogenesis of anti–PIT-1 antibody syndrome.
Keywords: anti–PIT-1 antibody syndrome, hypopituitarism, hypophysitis, HLA class I, cytotoxic T cells
Anti–pituitary-specific transcriptional factor-1 (anti–PIT-1, also known as POU1F1) antibody syndrome is a recently established clinical entity that leads to hypopituitarism caused by autoimmunity to PIT-1 [1, 2]. This syndrome is characterized by acquired and specific defect in growth hormone (GH), prolactin (PRL), and TSH. PIT-1 plays an essential role in the differentiation of somatotrophs, lactotrophs, and thyrotrophs in the anterior pituitary and regulates the expression of GH, PRL, and TSH. Recently, it has been reported that breakdown of immune tolerance toward PIT-1 is caused by aberrant expression of PIT-1 protein in the thymoma [3]. In addition, circulating PIT-1–reactive cytotoxic T lymphocytes (CTLs) were detected in patients with anti–PIT-1 antibody syndrome, and the infiltration of these cells was observed in the pituitary, indicating that CTLs play a pivotal role in the development of the syndrome [4]. However, it remains unclear whether PIT-1 antigen is presented on anterior pituitary cells and how CTLs recognize these antigens.
CTLs exert their cytotoxicity by recognizing the peptides presented by major histocompatibility complex (MHC)/HLA class I molecules. MHC/HLA class I molecules are expressed on most tissues and in the pituitary [5]. Various autoimmune diseases, such as type 1 diabetes [6] and alopecia areata, an autoimmune hair loss disease [7], are considered to be caused by CTLs. CTLs express CD8, which is crucial for recognizing peptide–MHC/HLA class I complexes [8]. In the target cells, proteins are processed, and antigenic peptides are produced through the proteasome pathway [9]. Next, the generated peptides translocate into the lumen of the endoplasmic reticulum (ER), where MHC class I molecules are bound with peptide at their peptide-binding groove. These peptide–MHC class I complexes are recruited by cargo vesicles for transport to the plasma membrane thorough the Golgi apparatus [8, 9].
In this study, we investigated the antigen processing of PIT-1 and the presence of PIT-1 epitopes with MHC/HLA class I in anterior pituitary cells, which are important for elucidating the pathogenesis of anti–PIT-1 antibody syndrome. We also examined whether PIT-1 presentation was patient specific or not.
1. Materials and Methods
A. Patient
This study was approved by the ethics committee of Kobe University Graduate School of Medicine. All methods were performed in accordance with the guidelines. We recruited a patient with anti–PIT-1 antibody syndrome (case 1) [2]. The patient provided written informed consent for the experiment.
B. Culture of Cell Line and Induced Pluripotent Stem Cell–Derived Pituitary Cells
GH3 cells, a PIT-1–expressing rat pituitary tumor cell line, were obtained from the American Type Culture Collection (Manassas, VA). The cells were maintained in DMEM (Sigma, St. Louis, MO) containing 10% fetal calf serum. Patient-derived induced pluripotent stem cells (iPSCs) (AP01) were obtained from RIKEN Center for Biosystems Dynamics Research (Kobe, Japan), and control-derived iPSCs (201B7; cell ID HPS0063 [10] and 409B2; cell ID HPS0076 [11]) were purchased from RIKEN BioResource Research Center by Department of iPS cell Applications, Graduate School of Medicine, Kobe University, and were transferred. To generate patient-derived iPSCs, OCT3/4, SOX2, KLF4, L-MYC, LIN28, EBNA1, and mp53DD were transduced in peripheral blood leukocytes using episomal vectors as previously described [12]. The iPSCs were maintained on mitomycin C–treated SNL feeder cells [13] in DMEM/F12 (D6421; Sigma) supplemented with 20% (v/v) knockout serum replacement (KSR) (lot no. 1848845; Invitrogen, Lafayette, CO); 0.1 mM nonessential amino acids (M7154; Sigma); 2 mM l-glutamine, 5 ng/mL recombinant human basic FGF (064-04543; Wako, Tokyo, Japan); and 0.1 mM 2-mercaptoethanol under an atmosphere of 2% CO2. Induction of pituitary tissue was performed as previously described with some modifications [14]. In brief, iPSCs were dissociated into single cells with Accutase (Nacalai Tesque, Kyoto, Japan). Next, 10,000 cells were plated on each well of a 96-well, V-bottom, low-attachment plate (Sumitomo Bakelite, Tokyo, Japan) in growth factor–free, chemically defined medium [11] supplemented with 10% (v/v) KSR and 10 mM of the Rho-associated kinase inhibitor Y-27632 (034-24024; Wako). At day 6, 5 nM of BMP-4 (R&D Systems, Minneapolis, MN) and 2 µM of SAG (Cayman Chemical, Ann Arbor, MI) were added to the medium. At day 18, BMP-4 was withdrawn from the medium, and the aggregates were maintained under high O2 condition (40%). At day 30, aggregates were cultured in growth factor–free, chemically defined medium supplemented with 20% (v/v) KSR. These aggregates became LHX3-positive pituitary progenitor cells and differentiated into pituitary hormone–producing cells after 100 days of culture.
C. Immunofluorescence Staining
Immunofluorescence staining was performed on a 24-well dish. Fixation, permeabilization, and blocking were performed using an Image-iT™ Fixation/Permeabilization kit (Cat. # R37602; Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The following primary antibodies used were: rabbit polyclonal anti–PIT-1 antibody (X-7; Santa Cruz Biotechnology, Santa Cruz, CA) [15], in which full-length human PIT-1 protein was used as an antigen; rabbit anti-Calnexin antibody (C4731; Sigma) [16]; rabbit anti-GM130 (C-terminal) antibody (G7295; Sigma) [17]; mouse anti-MHC class I antibody (MCA51R; AbD Serotec, Kidlington, United Kingdom) [18]; mouse anti-HLA class I antibody (EMR8-5; Abcam, Cambridge, United Kingdom) [19]; and serum sample from a patient with anti–PIT-1 antibody syndrome (case 1 [2]) and healthy control subjects. The secondary antibodies goat anti-human–IgG–Alexa Flour 488 antibody [20], goat anti-mouse Alexa Flour 488 [21], donkey anti-mouse Alexa Flour 546 [22], donkey anti-mouse Alexa Flour 488 [23], and donkey anti-rabbit Alexa Flour 546 [24] were used for immunofluorescent signal detection. For the PIT-1 protein absorption test, each serum sample was incubated for 24 hours at 4°C with or without treatment with 100 μg/mL of recombinant human PIT-1 (rhPIT-1) protein (sc-4014; Santa Cruz Biotechnology) prior to use.
D. Proximity Ligation Assay
Proximity ligation assay (PLA) was performed using a Duolink in situ PLA kit (Cat. # DUO92014; Sigma Aldrich, St. Louis, MO). Briefly, after permeabilization and blocking, tissues were incubated with primary antibodies. After washing, PLA probe solution (anti-rabbit plus [25], anti-rabbit minus [26], anti-mouse plus [27], and anti-mouse minus [28]) was added to the tissues, which were then incubated for 60 minutes at 37°C. After the PLA probe solution was removed, ligation and amplification were performed per the manufacturer’s instructions.
E. Microscopy
All images were obtained using a fluorescence microscope (BZ-X710; Keyence, Osaka, Japan) [29] or a confocal microscope system (LSM 700; Carl Zeiss, Jena, Germany) [30]. The images were then reconstructed using advanced analysis software (BZ-H3A, Keyence [31]; Zen Lite, Carl Zeiss [32]).
F. Statistical Analysis
All analyses were performed using the JMP Statistical Database Software version 14.0.0 (SAS Institute, Cary, NC). Analyses were performed by three independent experiments. Comparison of PLA results between the patient and control groups was performed using the nonparametric Kruskal-Wallis test. Significance level was set at P < 0.05.
2. Results
A. PIT-1 Immunoreactivity Was Detected in the Nucleus and Cytosol of GH3 Cells
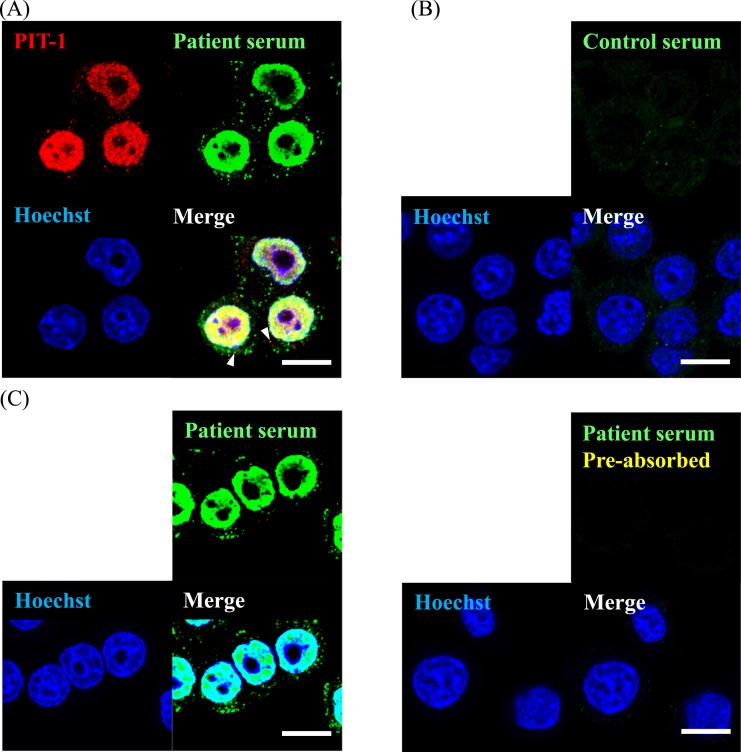
Detailed intracellular localization of PIT-1 protein was examined by immunofluorescence staining using anti–PIT-1 antibody [15], which recognizes whole protein of anti–PIT-1 and serum of patients with anti–PIT-1 antibody syndrome [2]. It has been shown that anti–PIT-1 antibody in serum exclusively recognizes PIT-1 protein, as analyzed by immunoblotting analysis using preabsorption test [2]. GH3 is a cell line derived from rat pituitary adenoma, and it expresses GH, PRL, and PIT-1 [33]. Anti–PIT-1 antibody was mainly localized in the nucleus (Fig. 1A), which is consistent with the fact that PIT-1 is a transcription factor. It was also detected in the cytosol and/or membrane, showing spotted signals. Immunofluorescence staining on a patient’s serum [2] showed similar results, with a part of the signals merged with the spot detected as anti–PIT-1 antibody [15]. In contrast, control serum did not show any signals (Fig. 1B).
Figure 1.
PIT-1 protein localized not only in nucleus but in cytosol and/or membrane in GH3 cells. (A) Immunofluorescence using the patient serum and polyclonal anti–PIT-1 antibody [15]. Arrowheads show merged signals detected by the patient serum [2] and polyclonal anti–PIT-1 antibody [15]. (B) Immunostaining of GH3 cells using control serum. (C) Absorption test using recombinant PIT-1 protein. Left panel shows the immunostaining without preabsorption and right panel shows that with preabsorption. Scale bar, 10 µm.
To examine the specificity of the serum samples, we performed an antigen absorption test using rhPIT-1 protein. After preincubation of serum with rhPIT-1, both the nuclear signal and the cytosolic/membrane signal disappeared (Fig. 1C). These data indicated that patients’ sera [2] specifically recognized PIT-1 protein, which was localized in the cytosol/membrane and nucleus of GH3 cells.
B. PIT-1 Protein Was Processed Through the MHC Class I Antigen Presentation Pathway in GH3 Cells
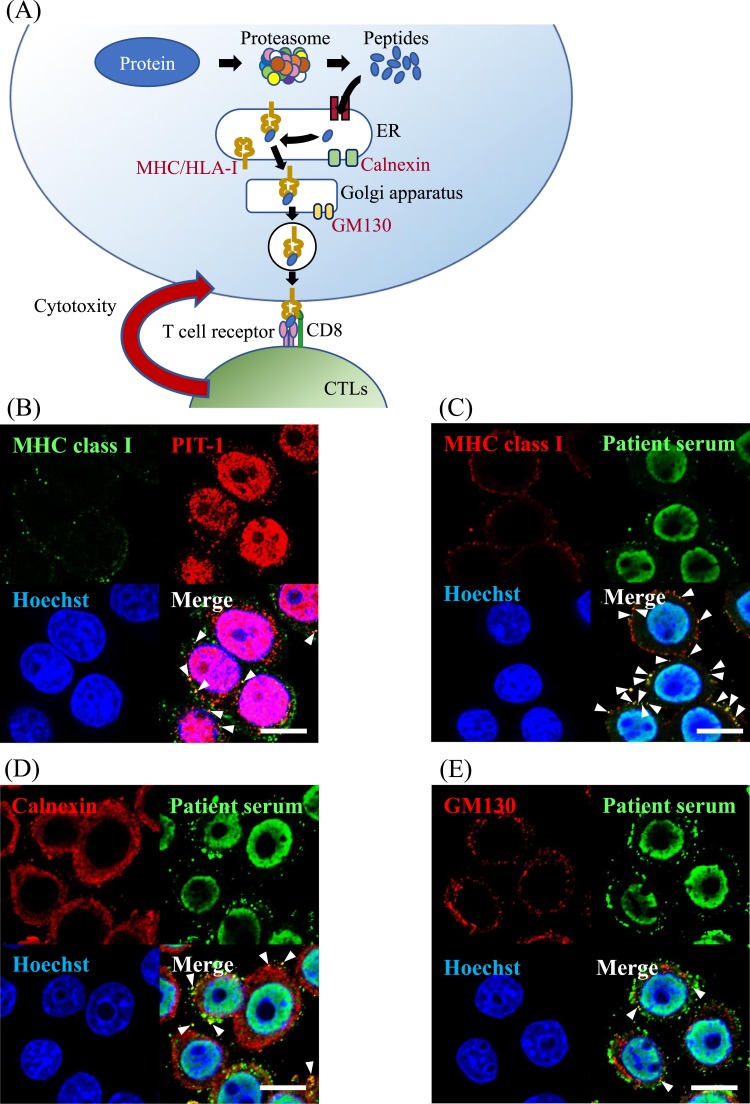
It has been shown that the antigenic peptides of peptide-MHC/HLA class I complexes are processed through the MHC class I antigen presentation pathway [8]. In this pathway, proteins are processed in the proteasome, and the generated peptides translocate into the ER (Fig. 2A). In the lumen of ER, the antigen peptides bind to the peptide-binding groove of MHC/HLA class I molecules. The resulting peptide-MHC/HLA class I complexes are then recruited by cargo vesicles for transport to the plasma membrane thorough the Golgi apparatus (Fig. 2A) [8]. The T cell receptor on CTLs recognizes peptide-MHC/HLA class I complexes in the plasma membrane and causes specific injury of the target cells.
Figure 2.
PIT-1 protein was processed in the antigen presentation pathway in GH3 cells. (A) Schematic diagram of MHC/HLA class I antigen presentation pathway. (B) PIT-1 detected by the anti–PIT-1 antibody [15] demonstrated a colocalization with MHC class I molecules. (C) PIT-1 detected by the patient serum [2] demonstrated a colocalization with MHC class I molecules. (D) PIT-1 colocalized with calnexin, which is an ER marker. (E) PIT-1 colocalized with GM130, which is a Golgi apparatus marker. Arrowheads indicate merged signals with each protein. Scale bar, 10 µm.
To investigate whether PIT-1 protein is processed through the MHC class I antigen presentation pathway, we performed immunofluorescence double staining using antibodies against calnexin (ER marker) [16], GM130 (Golgi apparatus marker) [17], and MHC class I molecules [18]. PIT-1 was colocalized with MHC class I molecules (Fig. 2B and 2C), calnexin (Fig. 2D), and GM130 (Fig. 2E), indicating that PIT-1 protein was presented in both the ER and Golgi apparatus and that PIT-1 protein was processed through the MHC class I antigen presentation pathway.
C. PIT-1 Epitope Was Colocalized With MHC/HLA Class I on the Cell Surface of GH3 Cells and Human Anterior Pituitary Cells Derived From iPSCs
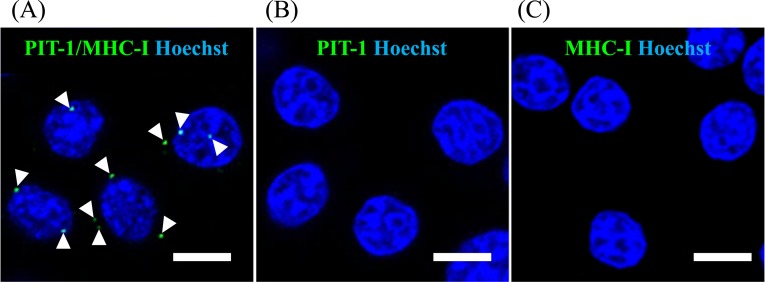
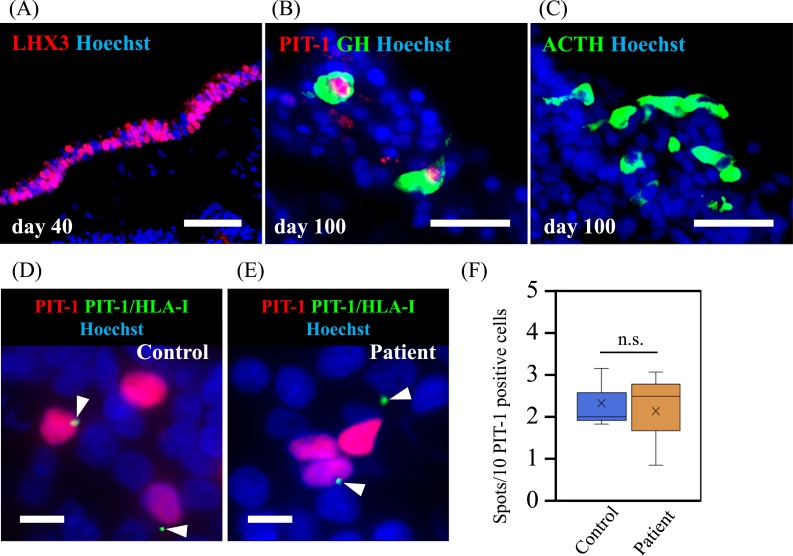
We analyzed whether PIT-1 epitope is presented by MHC/HLA class I molecules on the cell surface by PLA using GH3 cells and pituitary tissue derived from human iPSCs. PLA is a standard method used to detect the binding of HLA and peptide epitopes [34]. When the substrates linked to both antibodies come to close the distance for binding, bright spots are detected. PLA using anti–PIT-1 [15] and anti-MHC class I antibody [18] showed positive spots, indicating the presence of PIT-1 epitope on MHC class I molecules (Fig. 3A). There was no signal detected when PLA was conducted using only either anti–PIT-1 [15] or anti-MHC class I antibody [18] (Fig. 3B and 3C) as negative controls, indicating the specificity of the signals. We next analyzed the human pituitary tissue differentiated from human iPSCs as previously described [14]. After 40 days of induction of pituitary differentiation, a key transcription factor of anterior pituitary cells, LHX3, was expressed on the outer layer of the cells, indicating the differentiation into oral ectoderm that is a precursor of anterior pituitary cells (Fig. 4A) as previously described [14]. At day 100 of induction, PIT-1, GH (Fig. 4B), and ACTH (Fig. 4C) were expressed on the same layer, indicating that these cells were differentiated into anterior pituitary cells. PLA results showed colocalized signals on the surface of PIT-1–positive cells (Fig. 4D and 4E). The quantification analysis showed that the number of PIT-1/HLA class I complexes presented per PIT-1–expressing cells was not different between the control and patient iPSC-derived pituitary cells (Fig. 4F). These data indicated that at least some PIT-1 epitopes were presented by MHC/HLA class I in pituitary cells and that quantitative abnormalities were not associated with the development of the syndrome.
Figure 3.
PLA in GH3 cells showed the presentation of PIT-1 epitope by MHC class I. (A) PLA was performed using both the polyclonal anti–PIT-1 antibody [15] and anti-MHC class I antibody [18], demonstrating the presentation of PIT-1 epitope by MHC class I molecules. PLA was performed only using only anti–PIT-1 antibody [15] (B) or only anti-MHC class I antibody [18] (C). Scale bar, 10 µm.
Figure 4.
PLA in human pituitary cells differentiated from iPSCs showed the presentation of PIT-1 epitope by MHC class I. iPSCs derived from the patient with anti–PIT-1 antibody syndrome and from a control subject were induced for the differentiation into anterior pituitary cells. (A) LHX3 expression on day 40, indicating the differentiation into oral ectoderm, which is a precursor of anterior pituitary cells. (B) PIT-1 and GH expression and (C) ACTH expression on day 100. Scale bar, 50 µm. PLA showed the presentation of PIT-1 epitope by MHC class I molecules in the anterior pituitary cells derived from the patient (D) and the control subject (E). Scale bar, 20 µm. (F) Quantitative analysis showed a comparable number in the presentation between the patient and the control subject. Three technical replicates were performed. Kruskal-Wallis test was used.
3. Discussion
In anti–PIT-1 antibody syndrome, ectopic expression of PIT-1 in the thymoma causes the breakdown of immune tolerance to PIT-1, resulting in the production of PIT-1–reactive CTLs, which specifically injure anterior pituitary cells [2–4]. However, it remains unclear how CTLs recognize PIT-1 protein, considering that PIT-1 is a transcription factor and is supposed to be localized in the nucleus. It has been shown that all nucleated cells in the body display MHC class I molecules [5]. In general, self or nonself antigens derived from cytoplasmic or intranuclear sources are degraded by the proteasome into peptides and then presented by MHC class I molecules [35]. Considering the pathophysiology of anti–PIT-1 antibody syndrome, it is important to elucidate whether PIT-1 epitope is presented by MHC class I molecules on the cell surface and, if so, if this occurrence is patient specific.
In this study, we showed that PIT-1 protein was processed through the MHC class I presentation pathway and that PIT-1 epitope was presented by MHC class I molecules on the cell surface of rat GH3 cells and human anterior pituitary cells derived from iPSCs. These data clearly indicate that PIT-1 is presented by MHC class I molecules in the anterior pituitary cells, which can be specifically recognized by CTLs.
Our present data indicated that endogenous PIT-1 was spontaneously processed through the MHC class I presentation pathway. The number of PIT-1/MHC class I complexes was comparable between patient and control iPSC-derived pituitary cells, indicating that their presentation was not patient specific, although quantitative abnormalities cannot be ruled out. These data also suggest that the PIT-1 presentation to specific CTLs is essentially involved in the development of the syndrome. The results of PLA showed a relatively low number of PIT-1/MHC class I complexes both in GH3 cells and iPSC-derived pituitary cells, which may not be enough for the activation of CTL. We speculate that this presentation was at a basal level and that the presentation may be enhanced if stimuli such as nonspecific inflammation occurred. Nonetheless, these data clearly indicate that PIT-1/MHC class I complexes are present at the surface of pituitary cells.
In general, the antigenicity of a peptide is restricted by a certain HLA type. For example, HLA-DQ2 and HLA-DR3 are commonly observed in Addison disease, myasthenia graves, and celiac disease [36]. Although there was no common HLA typing found among patients with anti–PIT-1 antibody syndrome [2], a more detailed analysis may reveal a common HLA typing. It is also important to identify the precise epitopes in the PIT-1 protein, especially for CTLs, to clarify the detailed mechanisms.
It is unknown how CTLs activated by thymoma tissue migrate to pituitary tissue and attack these cells. The effector T cells (activated CTLs) generally circulate in the blood and recognize the site of inflammation that changes the condition of adhesion molecules [37]. Given that inflammation often takes place in the pituitary as hypophysitis, it is speculated that there may be an affinity for lymphocytes in the pituitary. It is also speculated that nonspecific inflammatory reaction in the pituitary may trigger the migration of CTLs.
The epitope of endogenous proteins, including transcription factors, is presented by MHC class I molecules in the anterior pituitary. These observations may explain the pathogenesis of other autoimmune pituitary diseases, such as isolated ACTH deficiency and autoimmune hypophysitis. It was reported that, in patients with isolated ACTH deficiency, infiltration of lymphocytes occurred in the anterior pituitary [38, 39] and anticorticotroph antibody was detected in the serum of the patients [40, 41]. A case of nivolumab-induced hypophysitis exhibiting isolated ACTH deficiency showed the DRB1*04:05-DQA1*03:03-DQB1*04:01 HLA haplotype, which was associated with susceptibility to autoimmune polyglandular syndrome with pituitary disorder [38]. It is speculated that if a specific epitope is presented in corticotroph, a portion of the pathogenesis of isolated ACTH deficiency may be explained by similar mechanisms as those of anti–PIT-1 antibody syndrome.
In summary, we showed that PIT-1 epitope is presented by HLA class I molecules on PIT-1–positive cells, supporting the hypothesis that anti–PIT-1 antibody is caused by PIT-1–reactive CTLs through injury to specific cells. Although further investigation is necessary, these findings unveiled a mechanistic insight to autoimmune pituitary diseases.
Acknowledgments
We thank C. Ogata and K. Imura for their excellent technical assistance and T. Aoi (Department of iPS cell Applications, Graduate School of Medicine, Kobe University, Kobe, Japan) for iPSC.
Financial Support: This work was supported by Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture Grants 17K16165 (to H.B.); Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture Grants 23591354, 15K094315, and 18K08514 (to G.I.); Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture Grants 23659477 and 26670459 (to Y.T.); Japan Agency for Medical Research and Development Grant 17bm0804012h0001 (to Y.T.); and funds from the Uehara Memorial Foundation and the Naito Foundation (to Y.T.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- CTL
cytotoxic T lymphocyte
- ER
endoplasmic reticulum
- GH
growth hormone
- iPSC
induced pluripotent stem cell
- KSR
knockout serum replacement
- MHC
major histocompatibility complex
- PIT-1
pituitary-specific transcriptional factor-1
- PLA
proximity ligation assay
- PRL
prolactin
- rhPIT-1
recombinant human pituitary-specific transcriptional factor-1
References and Notes
- 1. Iguchi G, Bando H, Takahashi Y. A novel clinical entity of autoimmune endocrinopathy: anti-PIT-1 antibody syndrome. Front Horm Res. 2017;48:76–83. [DOI] [PubMed] [Google Scholar]
- 2. Yamamoto M, Iguchi G, Takeno R, Okimura Y, Sano T, Takahashi M, Nishizawa H, Handayaningshi AE, Fukuoka H, Tobita M, Saitoh T, Tojo K, Mokubo A, Morinobu A, Iida K, Kaji H, Seino S, Chihara K, Takahashi Y. Adult combined GH, prolactin, and TSH deficiency associated with circulating PIT-1 antibody in humans. J Clin Invest. 2011;121(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bando H, Iguchi G, Okimura Y, Odake Y, Yoshida K, Matsumoto R, Suda K, Nishizawa H, Fukuoka H, Mokubo A, Tojo K, Maniwa Y, Ogawa W, Takahashi Y. A novel thymoma-associated autoimmune disease: anti-PIT-1 antibody syndrome. Sci Rep. 2017;7(1):43060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bando H, Iguchi G, Fukuoka H, Yamamoto M, Hidaka-Takeno R, Okimura Y, Matsumoto R, Suda K, Nishizawa H, Takahashi M, Tojo K, Takahashi Y. Involvement of PIT-1-reactive cytotoxic T lymphocytes in anti-PIT-1 antibody syndrome. J Clin Endocrinol Metab. 2014;99(9):E1744–E1749. [DOI] [PubMed] [Google Scholar]
- 5. Daar AS, Fuggle SV, Fabre JW, Ting A, Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38(3):287–292. [DOI] [PubMed] [Google Scholar]
- 6. Culina S, Lalanne AI, Afonso G, Cerosaletti K, Pinto S, Sebastiani G, Kuranda K, Nigi L, Eugster A, Østerbye T, Maugein A, McLaren JE, Ladell K, Larger E, Beressi JP, Lissina A, Appay V, Davidson HW, Buus S, Price DA, Kuhn M, Bonifacio E, Battaglia M, Caillat-Zucman S, Dotta F, Scharfmann R, Kyewski B, Mallone R; ImMaDiab Study Group. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol. 2018;3(20):eaao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lankat-Buttgereit B, Tampé R. The transporter associated with antigen processing: function and implications in human diseases. Physiol Rev. 2002;82(1):187–204. [DOI] [PubMed] [Google Scholar]
- 9. Schubert U, Antón LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404(6779):770–774. [DOI] [PubMed] [Google Scholar]
- 10. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 11. Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci USA. 2008;105(33):11796–11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31(3):458–466. [DOI] [PubMed] [Google Scholar]
- 13. McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62(6):1073–1085. [DOI] [PubMed] [Google Scholar]
- 14. Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, Oiso Y, Tsuji T, Sasai Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7(1):10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RRID: AB_2166884, https://scicrunch.org/resolver/AB_2166884.
- 16. RRID: AB_476845, https://scicrunch.org/resolver/AB_476845.
- 17. RRID: AB_532244, https://scicrunch.org/resolver/AB_532244.
- 18. RRID: AB_322394, https://scicrunch.org/resolver/AB_322394.
- 19. RRID: AB_1269092, https://scicrunch.org/resolver/AB_1269092.
- 20. RRID: AB_10894731, https://scicrunch.org/resolver/AB_10894731.
- 21. RRID: AB_10893722, https://scicrunch.org/resolver/AB_10893722.
- 22. RRID: AB_2534012, https://scicrunch.org/resolver/AB_2534012.
- 23. RRID: AB_2732856, https://scicrunch.org/resolver/AB_2732856.
- 24. RRID: AB_2534016, https://scicrunch.org/resolver/AB_2534016.
- 25. RRID: AB_2810940, https://scicrunch.org/resolver/AB_2810940.
- 26. RRID: AB_2810942, https://scicrunch.org/resolver/AB_2810942.
- 27. RRID: AB_2810939, https://scicrunch.org/resolver/AB_2810939.
- 28. RRID: AB_2713942, https://scicrunch.org/resolver/AB_2713942.
- 29. RRID:SCR_017202, https://scicrunch.org/resolver/SCR_017202.
- 30. RRID:SCR_017377, https://scicrunch.org/resolver/SCR_017377.
- 31. RRID:SCR_017375, https://scicrunch.org/resolver/SCR_17375.
- 32. RRID:SCR_013672, https://scicrunch.org/resolver/SCR_013672.
- 33. Bancroft FC, Levine L, Tashjian AH, Jr. Control of growth hormone production by a clonal strain of rat pituitary cells: stimulation by hydrocortisone. J Cell Biol. 1969;43(3):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin H, Arase N, Hirayasu K, Kohyama M, Suenaga T, Saito F, Tanimura K, Matsuoka S, Ebina K, Shi K, Toyama-Sorimachi N, Yasuda S, Horita T, Hiwa R, Takasugi K, Ohmura K, Yoshikawa H, Saito T, Atsumi T, Sasazuki T, Katayama I, Lanier LL, Arase H. Autoantibodies to IgG/HLA class II complexes are associated with rheumatoid arthritis susceptibility. Proc Natl Acad Sci USA. 2014;111(10):3787–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annu Rev Immunol. 1998;16(1):323–358. [DOI] [PubMed] [Google Scholar]
- 36. Thorsby E, Lie BA. HLA associated genetic predisposition to autoimmune diseases: Genes involved and possible mechanisms. Transpl Immunol. 2005;14(3-4):175–182. [DOI] [PubMed] [Google Scholar]
- 37. Khan U, Ghazanfar H. T lymphocytes and autoimmunity. Int Rev Cell Mol Biol. 2018;341:125–168. [DOI] [PubMed] [Google Scholar]
- 38. Richtsmeier AJ, Henry RA, Bloodworth JM, Jr, Ehrlich EN. Lymphoid hypophysitis with selective adrenocorticotropic hormone deficiency. Arch Intern Med. 1980;140(9):1243–1245. [PubMed] [Google Scholar]
- 39. Kubo S, Kitamura O, Orihara Y, Tsuda R, Hirose W, Nakasono I. Isolated adrenocorticotropic hormone deficiency: an autopsy case of adrenal crisis. A case report. Am J Forensic Med Pathol. 1997;18(2):202–205. [DOI] [PubMed] [Google Scholar]
- 40. De Bellis A, Pane E, Bellastella G, Sinisi AA, Colella C, Giordano R, Giavoli C, Lania A, Ambrosio MR, Di Somma C, Zatelli MC, Arvat E, Colao A, Bizzarro A, Bellastella A; Italian Autoimmune Hypophysitis Network Study. Detection of antipituitary and antihypothalamus antibodies to investigate the role of pituitary or hypothalamic autoimmunity in patients with selective idiopathic hypopituitarism. Clin Endocrinol (Oxf). 2011;75(3):361–366. [DOI] [PubMed] [Google Scholar]
- 41. Hannon MJ, O’Halloran DJ. Isolated acquired ACTH deficiency and primary hypothyroidism: a short series and review. Pituitary. 2011;14(4):358–361. [DOI] [PubMed] [Google Scholar]
- 42. Ohara N, Ohashi K, Fujisaki T, Oda C, Ikeda Y, Yoneoka Y, Hashimoto T, Hasegawa G, Suzuki K, Takada T. Isolated adrenocorticotropin deficiency due to nivolumab-induced hypophysitis in a patient with advanced lung adenocarcinoma: a case report and literature review. Intern Med. 2018;57(4):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]