Abstract
Hippocampal cells often fire prolonged bursts of action potentials, resulting in dynamic modulation of postsynaptic responses; yet long-term potentiation (LTP) has routinely been studied using only single presynaptic stimuli given at low frequency. Recent work on neocortical synapses has suggested that LTP may cause a “redistribution of synaptic strength” in which synaptic responses to the first stimulus of a presynaptic burst of action potentials are potentiated with later responses depressed. We have examined whether this redistribution occurs at hippocampal synapses during LTP. Using prolonged bursts that result in maximal short-term depression of later responses within the burst, we found that LTP resulted in a uniform potentiation of individual responses throughout the burst rather than a redistribution of synaptic strength. This occurred both at Schaffer collateral–CA1 synapses and at CA3–CA3 synapses, the latter being activated and monitored using paired recordings. Thus in the hippocampus, LTP preserves the fidelity of postsynaptic responses to presynaptic bursts by a uniform increase rather than a redistribution of synaptic strength, a finding that suggests there are important differences between neocortex and hippocampus in how long-term changes in synaptic strength are used to encode new information.
Keywords: long-term potentiation, redistribution of synaptic strength, short-term synaptic depression, short-term synaptic facilitation, presynaptic bursts, electrophysiology, hippocampus, rat
The normal firing pattern of many neurons in the CNS consists of bursts of action potentials (Ranck, 1973; O’Keefe, 1976; Wilson and McNaughton, 1993), leading to synaptic responses that may undergo both facilitation and depression, often in sequence (Dobrunz and Stevens, 1997; Brenowitz et al., 1998). Facilitation may be essential for the conversion of a temporal into a spatial code (Buonomano and Merzenich, 1995) and may also play a role in increasing the reliability of synaptic transmission (Dobrunz and Stevens, 1997), enabling bursts to function as the most basic element of the neural code (Lisman, 1997). Depression may be caused by depletion of synaptic vesicles, activation of inhibitory presynaptic autoreceptors, failure of action potential propagation, desensitization of postsynaptic receptors, or a combination of these (Luscher et al., 1994; Dobrunz and Stevens, 1997; Brenowitz et al., 1998) and may serve to control synaptic gain by attenuating responses to afferents with statically elevated firing rates (Abbott et al., 1997; Tsodyks and Markram, 1997)
In the hippocampus, bursts of action potentials are commonly observed during in vivo recordings (Ranck, 1973; O’Keefe, 1976;Wilson and McNaughton, 1993). In fact, hippocampal CA3 pyramidal cells have an intrinsic bursting capability (Kandel and Spencer, 1961; Wong and Prince, 1981). Excitatory synapses made by CA3 cells onto other CA3 pyramidal cells and onto CA1 pyramidal cells are notable for exhibiting long-term potentiation (LTP), paired-pulse facilitation, and, with longer stimulus trains, short-term depression (Dobrunz and Stevens, 1997).
Although most studies of LTP have monitored synaptic strength using single presynaptic stimuli, a recent study has demonstrated the importance of examining the effects of LTP with more prolonged, complex stimuli (Markram and Tsodyks, 1996). Temporally pairing action potentials in synaptically connected pairs of layer 5 pyramidal cells in the somatosensory cortex resulted in LTP, as measured with the response to the first action potential in presynaptic bursts. However, there was no net change in synaptic strength when all of the responses to the presynaptic bursts were considered; the responses to the early stimuli in the bursts were potentiated, whereas the responses to the later stimuli were depressed. This shift in the synaptic response pattern was termed a “redistribution of synaptic strength” (Markram and Tsodyks, 1996).
An important question is whether this redistribution of synaptic strength during LTP is a general feature of all excitatory synapses in the mammalian brain (Zador and Dobrunz, 1997). Because the excitatory synapses onto CA1 pyramidal cells in the hippocampus have provided the most detailed information currently available on the mechanisms of LTP (Bliss and Collingridge, 1993; Nicoll and Malenka, 1995), we decided to address this question at these synapses. We used patterns of presynaptic bursts causing both facilitation and depression of the synaptic responses but found no evidence of a redistribution of synaptic strength during LTP. Instead, we found that the fidelity of the synaptic responses was preserved by a uniform potentiation to all stimuli in the bursts. We therefore conclude that LTP at hippocampal synapses and LTP at neocortical synapses involve distinct mechanisms with distinct functional implications for the ways in which these two structures encode new information.
MATERIALS AND METHODS
We prepared and recorded from transverse hippocampal slices using standard procedures (Selig et al., 1995). Slices (500–600 μm) from 13- to 18-d-old Sprague Dawley rats were allowed to recover for a minimum of 1.5 hr before being transferred to a submerged recording chamber where they were superfused with artificial CSF (ACSF) maintained at 24–26°C and saturated with 95% O2/5% CO2. Our standard ACSF for these experiments contained 119 mm NaCl, 2.5 mm KCl, 4.0 mm CaCl2, 1.0 mmMgSO4, 1.0 mmNaH2PO4, 26.2 mmNaHCO3, 11 mm glucose, and 0.1 mm picrotoxin, pH 7.4. Some of the experiments (see Figs.1, 2) were conducted in 4.0 mm MgSO4. When recording from CA3, we found it necessary to double the concentration of divalents in our standard ACSF (to 8.0 mmCaCl2 and 2.0 mm MgSO4) to prevent spontaneous bursting (Frankenhaeuser and Hodgkin, 1957; Miles and Wong, 1983, 1987). When recording from the CA1 region, we made two cuts perpendicular to the pyramidal cell layer to prevent propagation of epileptiform activity from the CA3 and subicular regions. Perforated-patch recordings (Rae et al., 1991) were made using pipettes (1–2 MΩ) filled with a solution containing 130 mmCsMeSO3, 8.0 mm NaCl, 10 mmHEPES, and 0.2 mm EGTA, pH 7.2 with CsOH (290–300 mOsm). Amphotericin B (1.2 mg/ml; Sigma, St. Louis, MO) dissolved in DMSO (0.6% final concentration) was added to this solution, triturated, and used to backfill pipettes. Experiments were begun only after the access resistance had stabilized (typically 12–20 MΩ). Cells were voltage clamped at −60 mV without correction for the liquid junction potential. For some experiments (see Fig. 8), whole-cell recordings from the presynaptic cell were obtained in current clamp (−60 mV) using pipettes (2–4 MΩ) filled with a solution containing 130 mm Kgluconate, 8.0 mm NaCl, 10 mmHEPES, 0.2 mm EGTA, 4 mm Mg ATP, and 1 mm NaGTP, pH 7.2 with KOH (290–300 mOsm).d-AP-5 (50 μm) was obtained from Tocris. Adenosine (1–2 μm) and kynurenic acid (250 μm) were obtained from Sigma. 6-Nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX; 5 μm) was obtained from Precision Biochemicals (Colton, CA) and dissolved in DMSO (0.05% final concentration).
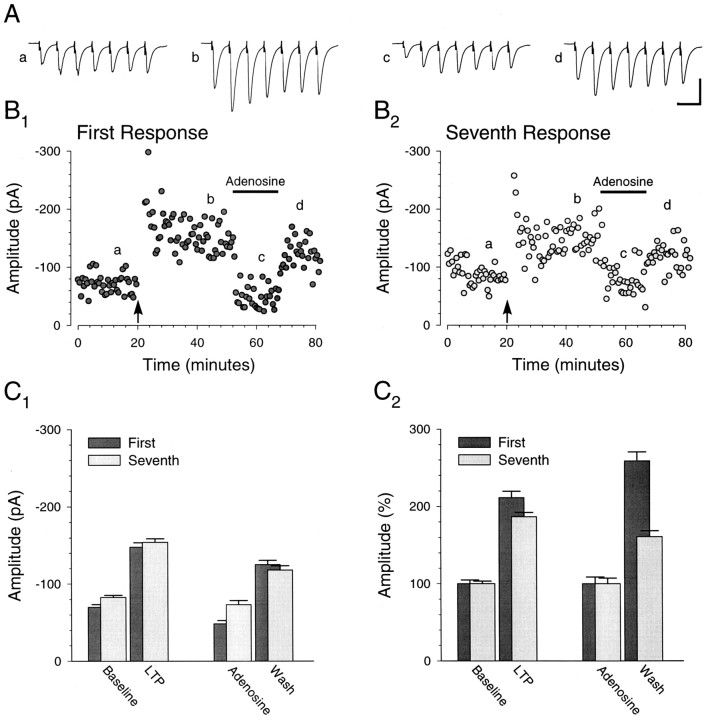
Fig. 1.
Example of synaptic response to short presynaptic bursts after LTP. A, Averages of 20 consecutive traces in response to short-burst stimulation (25 Hz; 7 stimuli) at the times indicted by theletters a–d in B. Calibration: 100 pA, 50 msec. B, Typical experiment in which pairing presynaptic stimulation with postsynaptic depolarization (filled arrow) results in a stable increase in the amplitude of the response to the first (B1) as well as the seventh (B2) stimulus in the burst. Adenosine (1 μm) was perfused during the time indicated by thehorizontal bar. After wash, the amplitude of both the first and seventh response was augmented. C, Average amplitudes of the first and seventh responses immediately before pairing (Baseline), 20 min after pairing (LTP), during adenosine (Adenosine), and immediately after wash of adenosine (Wash). Absolute response amplitudes (C1) and the first and seventh response amplitudes normalized to their respective baseline amplitudes (C2) are shown. All values are represented as the mean ± SEM. Allpanels illustrate data from the same cell.
Fig. 2.
Fidelity of the synaptic response to short presynaptic bursts is preserved after LTP.A, Summary graph (n = 5) showing that the amplitude of the response to the first (A1) and seventh (A2) stimulus in the short presynaptic bursts (25 Hz; 7 stimuli) undergoes a similar, stable potentiation with pairing (filled arrow).B, Average response amplitude to each stimulus before (○) and after (•) pairing normalized to the amplitude of the first response before pairing. Inset, The same data but with the amplitudes of all responses after pairing normalized to the average amplitude of the first response after pairing. C, Average amplitude of first and seventh response immediately before pairing (Baseline) and 20 min after pairing (LTP). Amplitudes are normalized to the amplitude of the first response during the baseline (C1). The first and seventh response amplitudes are normalized separately to their respective baseline amplitudes (C2). Potentiation of the first and seventh response amplitudes was not significantly different (286 ± 42 and 256 ± 38%, respectively;n = 5; p > 0.05, pairedt test). D, The same experiments with adenosine (1–2 μm) applied after pairing. After wash, there was an increase in the amplitude of both the first and seventh responses (n = 4). All panelsillustrate data from the same set of cells (n = 5).
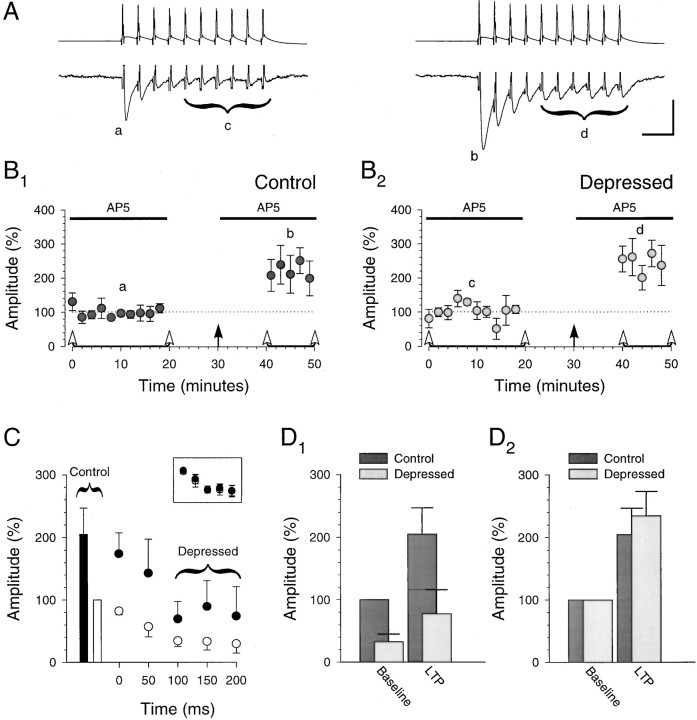
Fig. 8.
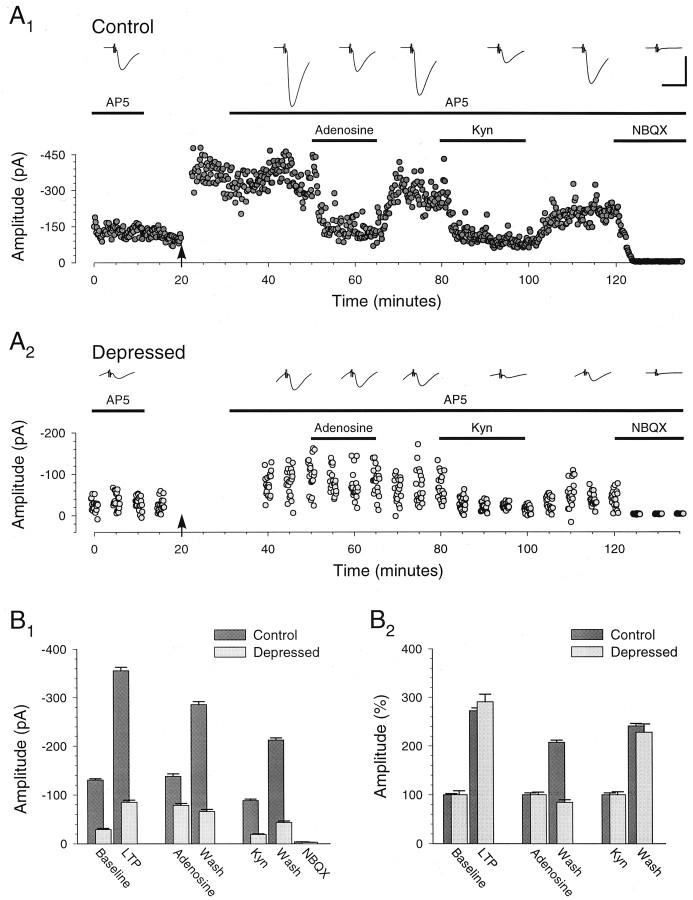
Fidelity of the synaptic response to presynaptic bursts is preserved after LTP when recording from pairs of CA3 pyramidal cells. A, Individual example showing averages of 40 consecutive unitary responses (lower traces) to presynaptic bursts (upper traces; 40 Hz; 10 stimuli) given at the times indicted by the letters a–d in B. Calibration: 100 mV, 10 pA, 50 msec. B, Summary graph (n = 4) showing that the unitary control responses (B1) and the unitary depressed responses (B2; last six responses in burst) undergo a similar, stable potentiation after induction ofLTP with pairing (filled arrow). Bursts were induced in the presynaptic cell every 30 sec at the times indicated by the open arrowheads. C, Unitary control responses (bars, ■ and ▪) and unitary responses to burst (circles, ○ and •) during the baseline (open symbols, ■ and ○) and after pairing (closed symbols, ▪ and •).Inset, The same data but with the amplitudes of all responses after pairing normalized to the average amplitude of the control response after pairing (each point represents the responses to two stimuli in the burst). It is evident that the unitary responses to bursts underwent a uniform potentiation, thus maintaining fidelity to the original synaptic response. D, Averages of unitary control and unitary depressed response amplitudes during the baseline and after LTP. Amplitudes are normalized to the control response amplitude during the baseline (D1). Control and depressed response amplitudes are normalized to their respective amplitudes during the baseline (D2). They underwent a similar degree of potentiation (205 ± 42 and 234 ± 39%, respectively; n = 4). B–D, Data from the same set of connected CA3 cell pairs (n = 4), including the pair whose responses are illustrated in A.
Slices were stimulated with 100 μsec monophasic current pulses using monopolar, stainless steel stimulating electrodes placed in the stratum radiatum immediately adjacent to or within the presumed dendritic arbor (Ishizuka et al., 1995) of the cell from which the recording was being made. Slices were stimulated every 30 sec in some of the experiments (see Figs. 1, 2) and every 10 sec in the remaining experiments. Bursts of stimuli were given every 30 sec in some of the experiments (see Figs. 1, 2, 8) and every 5 min in the remaining experiments. The rest of the stimuli were single pulses. LTP was induced by pairing 120 stimuli at 1 Hz while voltage clamping the postsynaptic cell at +10 mV. Only cells exhibiting potentiation (>40% measured 30 min after the induction protocol) were considered for further analysis. Responses were amplified, low-pass filtered at 1 kHz, sampled at 2 kHz, and analyzed on-line. Response amplitude was taken as the difference between a 5 msec baseline and a 10 msec period surrounding the peak of the response.
Control responses in the graphs include both the responses to single stimuli and the responses to the first stimuli of the bursts. Depressed responses in the graphs span the last several responses in the bursts (see and compare Figs. 3A, 8A). In several experiments (see Figs. 3-7), we have expanded the time course of the depressed responses of individual bursts for illustrative purposes (see Fig. 6A2 for example). Unless otherwise noted, bar graphs represent the average response over a period of 10 min. In some figures (see Figs.4A2, 5A2), we have time aligned and averaged the last 20 responses of successive bursts to arrive at the single traces shown. For all traces, the stimulus artifact is omitted for clarity. Summary graphs were prepared by subtracting the isolated stimulus artifact (NBQX, d-AP-5, or picrotoxin) when available from all other responses, setting the baseline period to 100%, normalizing all responses to this baseline, averaging adjacent responses in a given experiment, and then averaging across experiments. Data in the graphs and text are presented as the mean ± SEM.
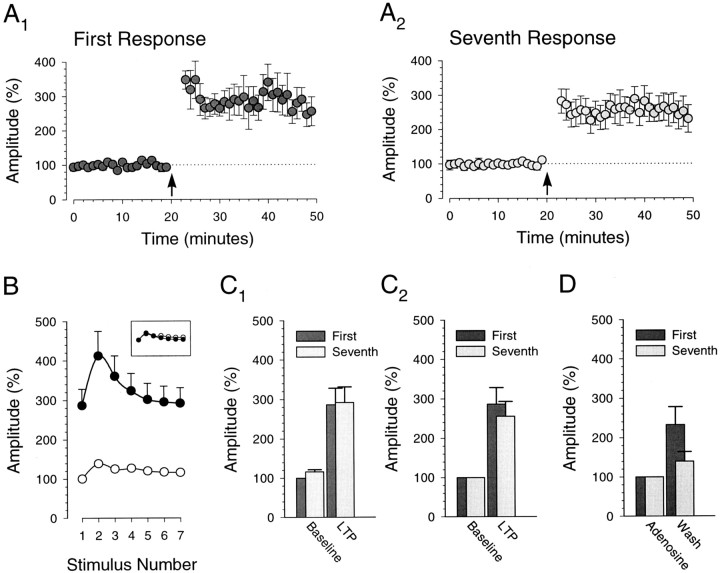
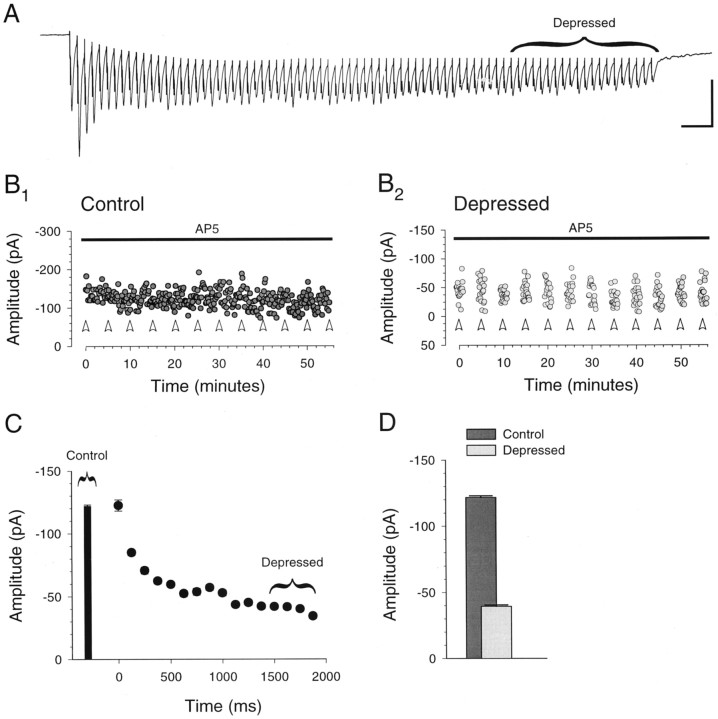
Fig. 3.
Prolonged presynaptic bursts of stimuli are necessary to depress the synaptic response. A, Example of the response to a prolonged presynaptic burst (40 Hz; 80 stimuli). The trace shows the average of 12 consecutive burst responses given every 5 min in the presence of 50 μmd-AP-5. Calibration: 100 pA, 100 msec. B, The amplitudes of the control (B1) and depressed (B2) responses, showing that these amplitudes were stable over time. Control responses (B1) consist of responses to single stimuli (every 10 sec) as well as the first response in each burst. Depressed responses (B2) are the last 20 responses in each burst, as shown in A. Bursts were given at the times indicated by the open arrowheads. Note the different amplitude scales in B1and B2. C, Average amplitude of the control response as well as the average amplitude of each group of five successive responses (125 msec) in the 12 bursts.D, Average control and depressed response amplitudes. All panels illustrate data from the same cell.
Fig. 4.
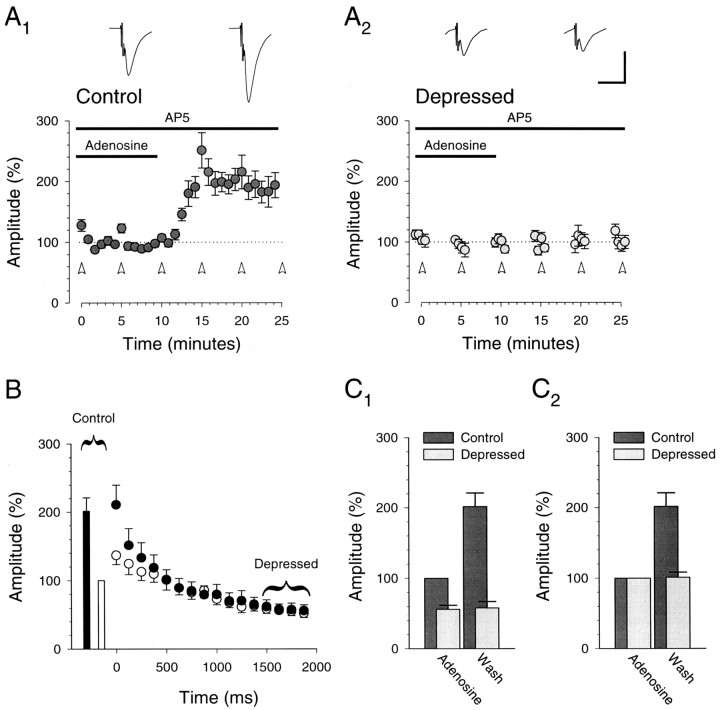
Depressed synaptic responses are unaffected by adenosine. A, Depressed synaptic responses are unaffected by adenosine, a presynaptic neuromodulator, suggesting that under these conditions the burst is maximally depressing (n = 10). Control responses (A1) increase after washing adenosine (1–2 μm), but depressed responses (A2; last 20 responses in burst) remain unchanged. Traces are averages of 60 responses (10 min) and are centered over the time when the averages were taken. Open arrowheads designate bursts (40 Hz; 80 stimuli). B, Control responses (bars, ■ and ▪) and responses to the burst (circles, ○ and •) during adenosine (open symbols, ■ and ○) and after washing (closed symbols, ▪ and •) are shown. The effect of adenosine was evident only in the responses to the first 10–20 stimuli in the burst (each point represents the responses to five stimuli in the burst).C, Averages (10 min) of control and depressed responses in adenosine and after wash are shown. Amplitudes are normalized to the control response amplitude in adenosine (C1) and to the average amplitudes in adenosine (C2). Allpanels illustrate data from the same set of cells (n = 10).
Fig. 5.
Example of the synaptic response to prolonged bursts after LTP, adenosine, and kynurenic acid.A, Typical experiment in which the induction ofLTP with pairing (filled arrow) results in a stable potentiation of the control responses (A1) as well as the depressed responses elicited by the prolonged presynaptic bursts (A2; 40 Hz; 80 stimuli). Adenosine (2 μm), kynurenic acid (Kyn; 250 μm), and NBQX (5 μm) were perfused during the times indicated by the horizontal bars. Adenosine depressed the control responses without affecting the depressed responses. Kynurenic acid depressed both responses. Traces are averages of 60 responses and arecentered over the time when the averages were taken. Calibration: A1,A2, 200 pA, 20 msec. Note, however, the different amplitude scales in A1 andA2. B, Averages of control and depressed response amplitudes with LTP, adenosine, kynurenic acid, and NBQX. Absolute amplitudes are summarized (B1). Control and depressed response amplitudes are normalized to their respective amplitudes during the baseline, in adenosine, and in kynurenic acid (B2). All panelsillustrate data from the same cell.
Fig. 6.
Fidelity of the synaptic response to prolonged bursts is preserved after LTP.A, Summary graph (n = 8) showing that the control responses (A1) and the depressed responses (A2) undergo a similar, stable potentiation after the induction ofLTP with pairing (filled arrow). Bursts were given at the times indicated by the open arrowheads (40 Hz; 80 stimuli). B, Control responses (bars, ■ and ▪) and responses to burst (circles, ○ and •]) during the baseline (open symbols, ■ and ○) and after pairing (closed symbols, ▪ and •). Inset, The same data but with the amplitudes of all responses after pairing normalized to the average amplitude of the control response after pairing (each point represents the responses to five stimuli in the burst). It is evident that the potentiation during LTPwas uniform for responses to all stimuli of the burst, thus maintaining fidelity of the synaptic burst response. C, Averages of control and depressed response amplitudes during the baseline and afterLTP. Amplitudes are normalized to the control response amplitude during the baseline (C1). Control and depressed response amplitudes (C2) are normalized to their respective ampli-tudes during the baseline and are not significantly different after LTP (275 ± 30 and 215 ± 32%, respectively; n = 8;p > 0.05, paired t test). Allpanels illustrate data from the same set of cells (n = 8).
Fig. 7.
Synaptic responses to prolonged bursts are altered by adenosine but not by kynurenic acid. A, After LTP, control responses (A1) increase after washing adenosine (1–2 μm), but depressed responses (A2; last 20 responses in burst) are unaffected (n = 6). Open arrowheadsdesignate bursts (40 Hz; 80 stimuli). B, Control responses (bars, ■ and ▪) and responses to burst (circles, ○ and •) during adenosine (open symbols, ■ and ○) and after washing (closed symbols, ▪ and •) are shown. Each point represents the responses to five stimuli. C, Averages of control and depressed response amplitudes in adenosine and after wash are shown. Amplitudes are normalized to the control response amplitude in adenosine (C1) and to the average amplitudes in adenosine (C2).D, After LTP, both control (D1) and depressed responses (D2; last 20 responses in burst) increase after washing kynurenic acid (Kyn; 250 μm; n = 6). Open arrowheads designate bursts (40 Hz; 80 stimuli). Thebreak in the graphs represents 5–10 min.E, Control responses (bars, ■ and ▪) and responses to burst (circles, ○ and •) during kynurenic acid (open symbols, ■ and ○) and after washing (closed symbols, ▪ and •) are shown.Inset, The same data are shown but with the amplitudes of all responses after washing normalized to the average amplitude of the control response after washing (each point represents the responses to five stimuli in the burst). It is evident that the responses to bursts underwent a uniform increase in gain. F, Averages of control and depressed response amplitudes in kynurenic acid and after wash. Amplitudes are normalized to the control response amplitude in kynurenic acid (F1) and to the average amplitudes in kynurenic acid (F2). All panelsillustrate data after LTP from the same cells (n = 6), a subset of those shown in Figure 6.
RESULTS
LTP and burst-induced facilitation
In an initial set of experiments, we induced LTP in CA1 pyramidal cells while monitoring synaptic transmission with brief bursts of stimulation (25 Hz; seven stimuli) to the Schaffer collateral/commissural afferents in the stratum radiatum. Using the perforated-patch recording technique (Rae et al., 1991) allowed us both to achieve relatively low access resistances (12–20 MΩ) and to avoid washout of LTP, which commonly occurs when whole-cell recordings with extended baselines are made (Malinow and Tsien, 1990).
Figure 1 shows a typical example and Figure 2 shows a summary (n = 5) of results from this experiment. After obtaining a 20 min baseline, we induced LTP with 120 single stimuli given at 1 Hz while holding the cells at +10 mV. The response to each stimulus in the burst potentiated (Figs. 1A,2B) for the duration of the recordings (Figs.1B, 2A). Moreover, although a modest difference in the level of potentiation for the first and seventh responses was seen in the individual example, the level of potentiation for the first and seventh responses was not significantly different when all cells were considered (286 ± 42 and 256 ± 38%, respectively; n = 5; p> 0.05, paired t test; Fig.2A,C2). In fact, all responses during the burst underwent a similar potentiation (Fig.2B, inset). Thus, the fidelity of the synaptic response to brief, facilitatory bursts of presynaptic stimuli is preserved by a uniform potentiation of responses within the burst after LTP.
This pattern of change in synaptic strength is very different from that seen with a redistribution of synaptic strength (Markram and Tsodyks, 1996), in which the synaptic response to the first stimulus in the burst is potentiated but the responses to stimuli near the end of the burst are relatively unaffected. Because redistribution of synaptic strength was seen in the neocortex by the use of current-clamp recordings, we considered the possibility that the responses near the end of the burst failed to show an increase with LTP because the current was shunted through voltage-gated channels that were more strongly activated after potentiation. However, when we repeated these experiments in current clamp, a similar increase in the response to the first and seventh stimuli of the burst was still evident (n = 3; data not shown), suggesting that shunting does not explain the difference between these results and those of Markram and Tsodyks (1996).
An important difference between the synaptic responses illustrated in Figures 1 and 2 and those observed between pairs of neocortical cells (Markram and Tsodyks, 1996) is that the presynaptic burst in CA1 was facilitatory rather than depressing. This is an important distinction because only with higher frequency stimuli that elicited short-term depression did neocortical LTP manifest itself as a redistribution rather than a uniform increase in synaptic strength (Tsodyks and Markram, 1997). We therefore asked what constitutes an adequate stimulus for deciding whether LTP is accompanied by a redistribution of synaptic strength.
Two models of the synaptic responses to presynaptic bursts of stimuli are helpful in understanding the answer to this question (Abbott et al., 1997; Tsodyks and Markram, 1997). These models predict that with a sufficient number of presynaptic stimuli delivered at a rate beyond a certain frequency of stimulation, termed the “limiting frequency,” the amplitude of the synaptic response will be inversely proportional to the frequency of stimulation, and thus the average postsynaptic response will be constant. Such presynaptic bursts therefore result in the maximal short-term depression of the postsynaptic response. Functionally, the simplest way of conceptualizing the limiting frequency is that frequency of stimulation at which the rate of synaptic vesicle recycling eventually determines the synaptic response size. This will occur when the presynaptic burst is sufficiently long and of a sufficiently high frequency such that the releasable pool of vesicles enters a depleted state (Dobrunz and Stevens, 1997). Such a burst was required to observe a pure redistribution of synaptic strength (Markram and Tsodyks, 1996) and can be identified by modulating release probability and by verifying that the amplitudes of the responses near the end of the burst are unaffected.
To determine whether the brief presynaptic bursts we used in Figure 1were maximally depressing, because anything less could explain our failure to observe a redistribution of synaptic strength, we applied and washed out adenosine to modulate release probability (Prince and Stevens, 1992). To facilitate the comparison of the effects of this pharmacological manipulation with those of LTP, we examined what happened after the washout of adenosine. Washout had the expected effect on the first response, causing an increase in amplitude (Fig.1B1). However, the seventh response also increased (Fig.1B2). Adenosine was applied in three of the other four cells in which brief presynaptic bursts were used to monitor LTP. On average, the seventh response again increased (Fig. 2D), suggesting that under these experimental conditions brief bursts of presynaptic stimuli are not maximally depressing bursts. Therefore these experiments, as well as the results of a previous study of LTP using brief facilitatory bursts in the hippocampus (Pananceau et al., 1998), do not eliminate the possibility that a redistribution of synaptic strength accompanies hippocampal LTP; the presynaptic bursts may have been insufficient to reveal any sort of redistribution. It was therefore important to arrange the experimental conditions so that the presynaptic bursts we gave elicited maximally depressed synaptic responses.
Burst-induced depression
To enhance our ability to elicit maximally depressed responses, we lowered [Mg2+] from 4.0 to 1.0 mm, increased the frequency of the presynaptic bursts from 25 to 40 Hz, and increased the number of stimuli from 7 to 80. Figure3 shows that these prolonged bursts depressed the response amplitude by a factor of three to four, which is similar to the magnitude of depression observed with much shorter presynaptic bursts in neocortical cell pairs (Markram and Tsodyks, 1996). Bursts were given once every 5 min, the shortest interval at which the burst responses remained stable, and were elicited in the presence of d-AP-5 (50 μm), an NMDA receptor antagonist, to prevent induction of LTP (Fig.3B2). The last 20 responses in each prolonged burst were grouped for analysis because their amplitude was fairly constant (Fig. 3A,C). Interleaved with the bursts were single stimuli that allowed us to monitor the nondepressed, or control, responses (Fig.3B1).
To determine whether these prolonged bursts were in fact maximally depressing, we compared the burst responses in the presence and absence of adenosine. Because one of the goals of these experiments was to compare what happens during LTP with the effects of increasing the probability of transmitter release, we again examined the recovery from the depressing action of adenosine (1–2 μm; Fig.4A,C2). Washout resulted in an increase of 202 ± 20% (n= 10) in the amplitude of the control responses to single stimuli but had no effect on the amplitude of the depressed responses at the end of the burst (101 ± 7%; n = 10). Therefore, these prolonged bursts satisfy the requirements for presynaptic stimulation that elicits maximally depressed synaptic responses.
LTP and burst-induced depression
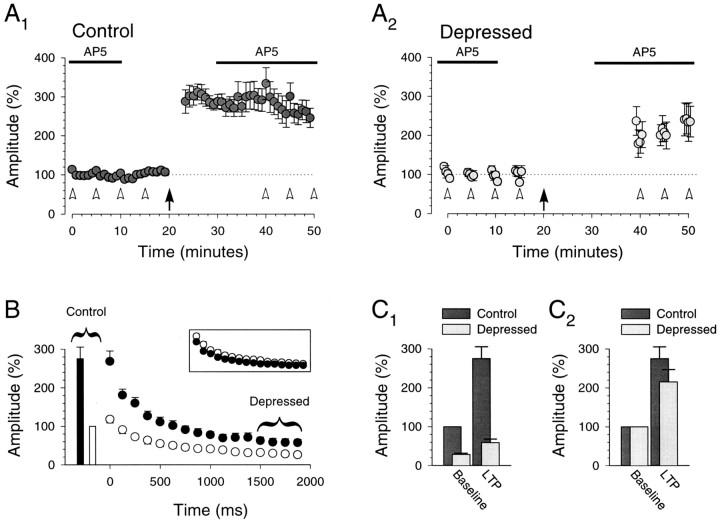
Having verified that this prolonged stimulation is maximally depressing, we returned to the question of whether hippocampal LTP results in a redistribution of synaptic strength. A typical example of this experiment is shown in Figure 5. After obtaining a 20 min baseline during which prolonged presynaptic bursts were given every 5 min and at the end of whichd-AP-5 was washed out (Fig.5A1,A2), we induced LTP. d-AP-5 was then reapplied, and bursts were restarted. Figure 5, B1 andB2, illustrates that the depressed responses at the end of the burst exhibited an increase in amplitude that was equivalent to that of the control responses (control responses, 272 ± 6%; depressed responses, 290 ± 16%). To ensure that in this cell these prolonged bursts were indeed maximally depressing, we applied adenosine (2 μm) that, consistent with the experiment illustrated in Figure 4, dramatically decreased the control responses but had no effect on the depressed responses. These two manipulations (LTP and adenosine) performed on the same set of synapses strongly suggest that a redistribution of synaptic strength does not occur during hippocampal LTP. As an additional control, we also applied kynurenic acid (250 μm), a low-affinity glutamate receptor antagonist. This had the expected effect in that, like LTP, both the control and depressed response amplitudes changed in parallel after washout of the drug (control responses, 241 ± 5%; depressed responses, 228 ± 17%; Fig.5A,B2). At the end of the experiment we applied NBQX, a specific AMPA receptor antagonist, which abolished both the control and the depressed responses (Fig.5A,B1).
LTP was obtained in seven additional cells in which prolonged bursts were given (Fig. 6). Although there was a tendency for the control responses to undergo more potentiation than the depressed responses, on average the increase in the two was not significantly different (control responses, 275 ± 30%; depressed responses, 215 ± 32%; n = 8;p > 0.05, paired t test; Fig.6A,C2). In fact, the increases in response amplitudes throughout the burst were similar (Fig. 6B, inset), indicating that the fidelity of the synaptic response, even with these prolonged bursts, is preserved during LTP.
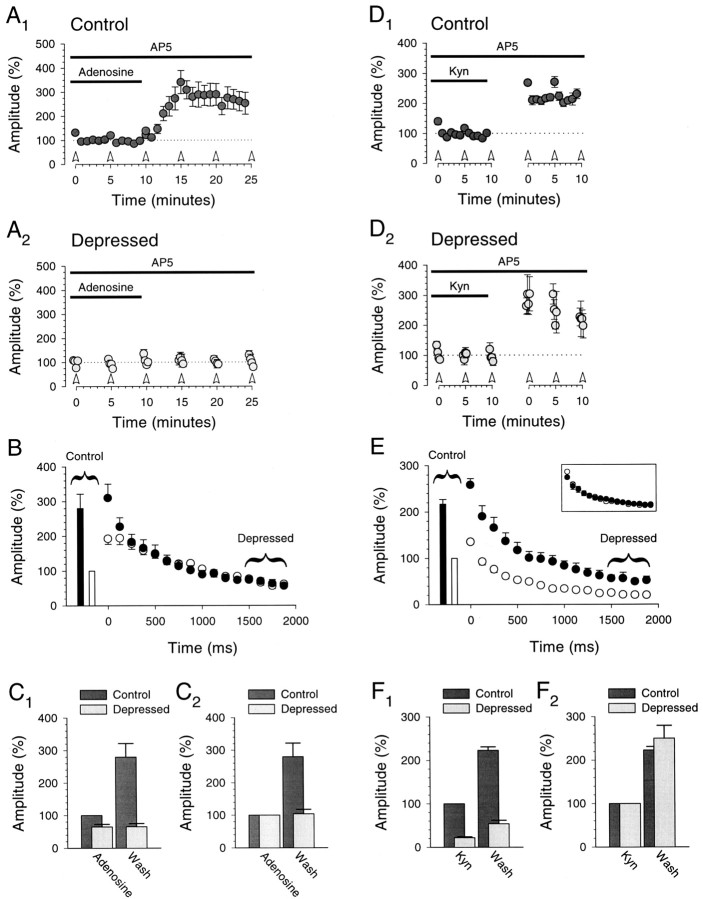
In six of the eight cells we also applied adenosine (Fig.7A–C) at a concentration (1–2 μm) that, after washout, gave us an increase in the control response amplitude similar to that obtained during LTP. Again, consistent with the example shown in Figure 4, when we washed out adenosine, the control response amplitude increased dramatically, whereas the depressed response amplitude was unaffected (control responses, 280 ± 42%; depressed responses, 104 ± 14%;n = 6; Fig.7A,C2). Thus in the same cells in which LTP caused an equivalent increase in responses throughout the burst (i.e., failed to result in a redistribution of synaptic strength), we were able to demonstrate that depressed responses were unaffected by a presynaptic manipulation of transmitter release, thus proving that the bursts were maximally depressing. In these same cells we also applied and then washed out kynurenic acid to assess how a pure postsynaptic manipulation would affect the responses to prolonged bursts (Fig. 7D–F). Again, we chose a concentration of kynurenic acid (250 μm) that, after washout, gave us an increase in the control response similar to that obtained during LTP. As would be expected, these results closely paralleled the LTP results obtained earlier in the same cells (Fig. 6). The increase in the amplitude of the control and depressed responses was similar (control responses, 223 ± 8%; depressed responses, 250 ± 30%; n = 6; Fig.7D,F2) as was the increase for all responses in the bursts (Fig. 7E,inset).
These experiments strongly suggest that LTP at excitatory synapses on CA1 pyramidal cells is fundamentally different from the LTP observed in the neocortex (Markram and Tsodyks, 1996). However, it is conceivable that because we stimulated extracellularly and recorded population synaptic responses, rather than stimulating the presynaptic cell directly as is done in paired recordings, a redistribution of synaptic strength may have occurred in our experiments without our detecting it because of inconsistent extracellular stimulation of the afferent fibers during the bursts. To address this issue, we attempted to record from connected pairs of hippocampal pyramidal cells.
LTP and burst-induced depression at synapses between pairs of hippocampal pyramidal cells
Recording from connected pairs of hippocampal pyramidal cells in slices is a difficult undertaking for many reasons, most notable of which is the low level of synaptic connectivity (MacVicar and Dudek, 1979; Miles and Wong, 1986; Sayer et al., 1990; Foster and McNaughton, 1991; Malinow, 1991; Smith et al., 1995). We were further handicapped by the need to record from the postsynaptic cell using the perforated-patch technique to obtain an adequate baseline of burst responses for analysis. We first attempted to record from connected CA3–CA1 pyramidal cell pairs. We examined 29 cell pairs but did not observe a single connection. We therefore turned our attention to CA3 pyramidal cell pairs in which the basic properties of LTP are indistinguishable from those of LTP between CA3 and CA1 pyramidal cells (Zalutsky and Nicoll, 1990; Pavlidis and Madison, 1997; Debanne et al., 1998).
We recorded from a total of 209 pairs of CA3 pyramidal cells, finding 26 pairs that were synaptically connected. Initially we used the same experimental conditions that were used for the CA1 experiments. However, the CA3 region exhibited spontaneous bursting. This was prevented by doubling the concentrations of the divalent cations to reduce overall excitability (Frankenhaeuser and Hodgkin, 1957). It was also necessary to reduce the number of stimuli in each burst, because we found the unitary responses were generally depressed to a stationary level after three to four stimuli. We therefore shortened the bursts to 10 stimuli (at 40 Hz) and gave them every 30 sec. The last six unitary responses in the burst were designated as the depressed responses. The control responses consisted of the unitary responses to the first stimulus in each burst combined with unitary responses to interleaved single stimuli.
Figure 8 shows the results from the four pairs of CA3 pyramidal cells in which it was possible to complete the experiment. As was the case for the population synaptic responses recorded in CA1 pyramidal cells, the unitary control response amplitude and the unitary depressed response amplitudes exhibited a similar potentiation after the induction of LTP (205 ± 42 and 234 ± 39%, respectively; n = 4; Fig.8B,D2). Indeed the amplitude of all unitary responses in the burst underwent a similar potentiation (Fig. 8C, inset). Thus, even though the presynaptic burst stimulation necessary to elicit depressed responses in these CA3 cell pairs was similar to that used in the neocortical cell pairs (Markram and Tsodyks, 1996), these CA3–CA3 synapses exhibited a form of LTP distinct from the redistribution of synaptic strength seen with neocortical LTP.
DISCUSSION
It is well established that the firing pattern of neurons in the CNS varies dramatically over time, ranging from single isolated spikes to prolonged high-frequency bursts of action potentials (Ranck, 1973;Connors and Gutnick, 1990; Lisman, 1997). It is also well documented that the probability of release and thus the strength of synaptic transmission are markedly influenced by the pattern of presynaptic firing (Magleby, 1987; Zucker, 1989). Therefore a fundamental issue in understanding the neural encoding of information is to determine the consequences, if any, that long-lasting changes in synaptic strength might have on short-term synaptic dynamics. We have examined this issue by asking whether NMDA receptor-dependent LTP in the hippocampus involves a redistribution of synaptic strength with a presynaptic burst, as has been observed at neocortical synapses (Markram and Tsodyks, 1996), or instead involves a uniform potentiation of responses to the stimuli of the burst.
To address this issue we first recorded synaptic responses in CA1 pyramidal cells in response to brief presynaptic bursts that elicited facilitated responses. After the induction of LTP, the responses exhibited a uniform potentiation rather than a redistribution of synaptic strength, a finding that differs from that obtained at neocortical synapses (Markram and Tsodyks, 1996). These results are in complete agreement with a recent study that examined the effects of LTP on the synaptic responses to brief (80–200 msec), facilitatory bursts (Pananceau et al., 1998). There, it was also found that all of the responses to the stimuli of the train were potentiated equally during LTP (i.e., no redistribution took place). Both sets of results using brief presynaptic bursts are consistent with and follow from previous studies, which have found a lack of interaction between LTP at CA1 synapses and paired-pulse facilitation (McNaughton, 1982; Manabe et al., 1993; Asztely et al., 1996; but see Schulz, 1997; Sokolov et al., 1998). However, prolonged bursts of presynaptic stimuli result in short-term synaptic depression, which clearly uses processes not evoked by either paired pulses or brief, facilitatory bursts (Dobrunz and Stevens, 1997). That the processes resulting in synaptic depression may be affected by LTP is supported by the observation that at neocortical synapses, the extent of redistribution of synaptic strength is positively correlated with the extent of synaptic depression (Markram and Tsodyks, 1996). Thus a conclusive test of whether LTP at hippocampal synapses causes redistribution of synaptic efficacy requires that the presynaptic stimuli elicit synaptic responses that, like those in the neocortical experiments, are maximally depressed by the end of the train.
We therefore extended the length of the burst and modified the recording conditions to elicit maximal depression of the responses. After confirmation that the responses were indeed maximally depressed, we again found that LTP was accompanied by a uniform potentiation of successive responses to the stimuli of the burst. Finally, because of the possibility that a redistribution of synaptic strength was overlooked because of inconsistent extracellular stimulation of afferents, we examined LTP between pairs of hippocampal pyramidal neurons. Yet again, LTP was associated with a uniform potentiation of successive responses to the stimuli of the burst. Thus, in contrast to LTP in the neocortex (Markram and Tsodyks, 1996), NMDA receptor-dependent LTP in the hippocampus involves a uniform potentiation of responses to all patterns of stimulation, including both brief, facilitatory bursts and prolonged depressing bursts.
It should be noted that the burst response patterns between CA1 pyramidal cells (Figs. 1-7) and CA3 pyramidal cells (Fig. 8) differed. Although intrinsic differences in the properties of the presynaptic boutons contacting CA1 versus CA3 pyramidal cells or inconsistent stimulation of afferent fibers when extracellular stimulation was used (but see Allen and Stevens, 1994) may have contributed to this difference, we think that the different extracellular [Ca2+]/[Mg2+] ratio in the two experiments is an important factor. Regardless of the differences in the burst responses from the two sets of synapses, however, the important finding was that both synapses underwent LTP in which the individual synaptic responses were uniformly increased rather than redistributed. The similarity of these results may not be surprising because CA3–CA3 and CA3–CA1 synapses exhibit similar forms of NMDA receptor-dependent synaptic plasticity (Zalutsky and Nicoll, 1990;Pavlidis and Madison, 1997; Debanne et al., 1998).
Although the differences found between hippocampal and neocortical LTP are most likely indicative of a fundamental difference in the mechanisms responsible for LTP in these two brain regions, several differences in the experimental conditions between the two studies should be noted. These include the recording technique (perforated patch vs whole cell), the temperature at which experiments were conducted, the presence or absence of GABAAreceptor-mediated inhibition, and the extracellular divalent cation concentration. However, it seems unlikely that differences in these experimental parameters could account for the presence or absence of a redistribution of synaptic strength during LTP.
The simplest explanation for our results is that hippocampal synapses express a form of LTP that is primarily caused by some postsynaptic modification of glutamate receptor function and/or number. This would cause the observed uniform increase in the synaptic responses throughout the burst. In contrast, the most straightforward explanation for the redistribution of synaptic strength observed at neocortical synapses is that they express a form of LTP that involves significant alterations in presynaptic mechanisms controlling transmitter release (Markram and Tsodyks, 1996). For instance, an increase in the probability of transmitter release would enhance the responses to the early stimuli of the burst but would have minimal effects on depressed responses at the end of the burst.
What are some of the functional implications of the uniform potentiation of responses to bursts shown here? Abbott et al. (1997)hypothesize that depression serves to control synaptic gain by attenuating responses to afferents with statically elevated firing rates. If afferents exhibit a broad range of firing rates, some level of attenuation is obviously important. However, a redistribution of synaptic strength has no effect on the average postsynaptic contribution of an afferent input (Tsodyks and Markram, 1997). Thus at afferent rates of firing in which temporal summation and synaptic attenuation are significant factors, LTP in the form of redistribution will have little effect on either the action potential timing or firing rate of the postsynaptic cell. On the basis of our results, hippocampal LTP may serve to override synaptic attenuation under such circumstances so that the important inputs (those that are potentiated) can drive the postsynaptic cell more effectively. Separately, Lisman (1997)hypothesizes that at a given synapse only one of the stimuli in a burst result in a quantal response and that therefore bursts are the fundamental unit of the neural code. Under such circumstances, the response at a single synapse can be characterized by the conditional probability that a given stimulus in the burst will result in quantal release when all the previous stimuli fail to cause quantal release. LTP in the form of redistribution will serve to increase the probability that the single release event occurs early in the burst, thereby decreasing the probability that it will occur later. Thus the timing of the postsynaptic response to a burst (i.e., the timing of the single quantal release) will be advanced after the redistribution associated with neocortical LTP. The quantal size will be unaffected. As mentioned above, our results with hippocampal LTP are most easily explained by an increase in the postsynaptic response. Therefore, in Lisman’s model the single quantum will be released with the same timing (defined by the series of conditional probabilities) but will be of a greater magnitude. In this context, therefore, the effects of a redistribution of synaptic strength will be significant where the precise timing of the inputs affects the firing of the postsynaptic cell, whereas the effects of hippocampal LTP will be significant where the synaptic weights most affect the firing of the postsynaptic cell.
We have considered the interaction between LTP and short-term plasticity manifested in responses to bursts of stimuli. We have found that NMDA receptor-dependent LTP in the hippocampus involves an unconditional potentiation entirely independent of the temporal pattern of the input, thus preserving the fidelity of responses to dynamically modulated stimuli. Current evidence supports the hypothesis that LTP may be one of the biological substrates of learning and long-term memory (Stevens, 1998). It has recently become apparent that both burst-induced depression and burst-induced facilitation may also serve functional roles in the neural coding of information (Buonomano and Merzenich, 1995; Abbott et al., 1997; Lisman, 1997; Tsodyks and Markram, 1997). This study of the interaction between LTP and short-term plasticity should therefore help in understanding how synapses in the hippocampus undergo meaningful modifications that can be used for the encoding of new information.
Footnotes
D.K.S. is supported by a National Institutes of Health National Research Service Award Postdoctoral Training Fellowship. R.A.N. is a member of the Keck Center for Integrative Neuroscience and the Silvio Conte Center for Neuroscience Research and is supported by grants from the National Institutes of Health. R.C.M. is a member of the Center for the Neurobiology of Addiction and the Center for Neurobiology and Psychiatry and is supported by grants from the National Institutes of Health, by an Investigator Award from the McKnight Endowment Fund for Neuroscience, and by a grant from the Human Frontier Science Program. We thank Antonello Bonci and other members of the Malenka and Nicoll laboratories for many useful comments and discussions during the course of these experiments.
Correspondence should be addressed to Dr. R. Malenka, Department of Psychiatry, Langley Porter Psychiatric Institute, Box 0984, University of California, San Francisco, CA 94143.
REFERENCES
- 1.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 2.Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asztely F, Xiao MY, Gustafsson B. Long-term potentiation and paired-pulse facilitation in the hippocampal CA1 region. NeuroReport. 1996;7:1609–1612. doi: 10.1097/00001756-199607080-00016. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 5.Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABA(B) receptors. Neuron. 1998;20:135–141. doi: 10.1016/s0896-6273(00)80441-9. [DOI] [PubMed] [Google Scholar]
- 6.Buonomano DV, Merzenich MM. Temporal information transformed into a spatial code by a neural network with realistic properties. Science. 1995;267:1028–1030. doi: 10.1126/science.7863330. [DOI] [PubMed] [Google Scholar]
- 7.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 8.Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol (Lond) 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 10.Foster TC, McNaughton BL. Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus. 1991;1:79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- 11.Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. J Physiol (Lond) 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol. 1995;362:17–45. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- 13.Kandel ER, Spencer WA. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- 14.Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 15.Luscher C, Streit J, Lipp P, Luscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. II. Decrease of conduction reliability during repetitive stimulation. J Neurophysiol. 1994;72:634–643. doi: 10.1152/jn.1994.72.2.634. [DOI] [PubMed] [Google Scholar]
- 16.MacVicar BA, Dudek FE. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1979;184:220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- 17.Magleby KL. Short-term changes in synaptic efficacy. In: Edelman GM, Einar GW, Cowan WM, editors. Synaptic function. Wiley; New York: 1987. pp. 21–56. [Google Scholar]
- 18.Malinow R. Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science. 1991;252:722–724. doi: 10.1126/science.1850871. [DOI] [PubMed] [Google Scholar]
- 19.Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- 20.Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- 21.Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature. 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 22.McNaughton BL. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. J Physiol (Lond) 1982;324:249–262. doi: 10.1113/jphysiol.1982.sp014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles R, Wong RK. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- 24.Miles R, Wong RK. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol (Lond) 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miles R, Wong RK. Latent synaptic pathways revealed after tetanic stimulation in the hippocampus. Nature. 1987;329:724–726. doi: 10.1038/329724a0. [DOI] [PubMed] [Google Scholar]
- 26.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 27.O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 28.Pananceau M, Chen H, Gustafsson B. Short-term facilitation evoked during brief afferent tetani is not altered by long-term potentiation in the guinea-pig hippocampal CA1 region. J Physiol (Lond) 1998;508:503–514. doi: 10.1111/j.1469-7793.1998.503bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlidis P, Madison DV. Synaptic transmission and plasticity in pair recordings from hippocampal neurons. Soc Neurosci Abstr. 1997;23:365. [Google Scholar]
- 30.Prince DA, Stevens CF. Adenosine decreases neurotransmitter release at central synapses. Proc Natl Acad Sci USA. 1992;89:8586–8590. doi: 10.1073/pnas.89.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- 32.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 33.Sayer RJ, Friedlander MJ, Redman SJ. The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CA1 neurons in the hippocampal slice. J Neurosci. 1990;10:826–836. doi: 10.1523/JNEUROSCI.10-03-00826.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz PE. Long-term potentiation involves increases in the probability of neurotransmitter release. Proc Natl Acad Sci USA. 1997;94:5888–5893. doi: 10.1073/pnas.94.11.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selig DK, Hjelmstad GO, Herron C, Nicoll RA, Malenka RC. Independent mechanisms for long-term depression of AMPA and NMDA responses. Neuron. 1995;15:417–426. doi: 10.1016/0896-6273(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 36.Smith KL, Szarowski DH, Turner JN, Swann JW. Diverse neuronal populations mediate local circuit excitation in area CA3 of developing hippocampus. J Neurophysiol. 1995;74:650–672. doi: 10.1152/jn.1995.74.2.650. [DOI] [PubMed] [Google Scholar]
- 37.Sokolov MV, Rossokhin AV, Behnisch T, Reymann KG, Voronin LL. Interaction between paired-pulse facilitation and long-term potentiation of minimal excitatory postsynaptic potentials in rat hippocampal slices: a patch-clamp study. Neuroscience. 1998;85:1–13. doi: 10.1016/s0306-4522(97)00592-7. [DOI] [PubMed] [Google Scholar]
- 38.Stevens CF. A million dollar question: does LTP = memory? Neuron. 1998;20:1–2. doi: 10.1016/s0896-6273(00)80426-2. [DOI] [PubMed] [Google Scholar]
- 39.Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 41.Wong RK, Prince DA. Afterpotential generation in hippocampal pyramidal cells. J Neurophysiol. 1981;45:86–97. doi: 10.1152/jn.1981.45.1.86. [DOI] [PubMed] [Google Scholar]
- 42.Zador AM, Dobrunz LE. Dynamic synapses in the cortex. Neuron. 1997;19:1–4. doi: 10.1016/s0896-6273(00)80341-4. [DOI] [PubMed] [Google Scholar]
- 43.Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 44.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]