Abstract
Intraperitoneal administration of the cytokine interleukin-1β (IL-1β) induces brain-mediated sickness symptoms that can be blocked by subdiaphragmatic vagotomy. Intraperitoneal IL-1β also induces expression of the activation marker c-fos in vagal primary afferent neurons, suggesting that IL-1β is a key component of vagally mediated immune-to-brain communication. The cellular sources of IL-1β activating the vagus are unknown, but may reside in either blood or in the vagus nerve itself. We assayed IL-1β protein after intraperitoneal endotoxin [lipopolysaccharide (LPS)] injection in abdominal vagus nerve, using both an ELISA and immunohistochemistry, and in blood plasma using ELISA. IL-1β levels in abdominal vagus nerve increased by 45 min after LPS administration and were robust by 60 min. Plasma IL-1β levels increased by 60 min, whereas little IL-1β was detected in cervical vagus or sciatic nerve. IL-1β-immunoreactivity (IR) was expressed in dendritic cells and macrophages within connective tissues associated with the abdominal vagus by 45 min after intraperitoneal LPS injection. By 60 min, some immune cells located within the nerve and vagal paraganglia also expressed IL-1β-IR. Thus, intraperitoneal LPS induced IL-1β protein within the vagus in a time-frame consistent with signaling of immune activation. These results suggest a novel mechanism by which IL-1β may serve as a molecular link between the immune system and vagus nerve, and thus the CNS.
Keywords: dendritic cells, macrophages, rat, ELISA, immunohistochemistry, endotoxin, cytokines, vagus
Macrophages, dendritic cells, and other immune cells detect pathogens such as bacteria or viruses and respond by releasing proinflammatory mediators, such as the cytokine interleukin-1β (IL-1β). IL-1β acts both to coordinate the peripheral immune response and to signal the CNS (Dunn, 1993; Maier et al., 1993). Such pathogen-induced IL-1β release activates the CNS to orchestrate a cascade of endocrine, autonomic, and behavioral processes collectively termed the acute phase response. This response functions broadly to render physiological conditions of the host inhospitable to the pathogen. The precise mechanisms by which IL-1β signals the CNS are unknown, but possibilities include direct detection of immune mediators by barrier cells of the brain (e.g., circumventricular organs and endothelial cells) (Van Dam et al., 1992; Saper and Breder, 1994;Ericsson et al., 1997) or by activation of primary afferent neurons of the vagus nerve (Watkins et al., 1995b).
Subdiaphragmatic vagotomy inhibits a variety of acute phase responses resulting from intraperitoneal administration of either IL-1β or lipopolysaccharide (LPS; bacterial endotoxin, which induces the expression of IL-1β) (Watkins et al., 1994, 1995a; Bret-Dibat et al., 1995; Gaykema et al., 1995; Hansen and Krueger, 1997). In addition, the neuronal activation marker c-fos is expressed in primary afferent neurons of the vagus after intraperitoneal administration of either LPS (Gaykema et al., 1998) or IL-1β (Goehler et al., 1998). Furthermore, sensory structures associated with the abdominal vagus (vagal paraganglia) express binding sites for IL-1 ligands (Goehler et al., 1997). These lines of evidence support the idea that vagal afferents are in fact activated by immune-related stimuli, notably IL-1β, and relay this information to the brain.
Vagal signaling of immune activation must occur fairly rapidly, because the induction of centrally mediated acute phase responses such as fever and hyperalgesia occurs within 90 min (Watkins et al., 1994,1995a) and the vagal afferent neurotransmitter glutamate (Schaffar et al., 1997) is released in vagal afferent terminal fields within 60 min of intraperitoneal LPS administration (Mascarucci et al., 1998). If IL-1β is a constituent of a vagal signaling pathway, it must be induced within this time frame, in either blood or perivagal immune cells. Accordingly, we determined the time course of IL-1β protein expression in plasma and vagus nerve after intraperitoneal LPS administration. To determine whether LPS induction of IL-1β is specific to the abdominal vagus rather than a general feature of peripheral nerves, we compared IL-1β expression in the abdominal vagus with that in the cervical vagus and sciatic nerves. IL-1β protein levels were assessed across tissues and time points using an ELISA. The cell types (e.g., perivagal immune cells) expressing IL-1β were determined using immunohistochemistry.
MATERIALS AND METHODS
Quantitative assessment of IL-1β with ELISA
Subjects and tissue collection. Adult male Sprague Dawley viral-free rats (Harlan Sprague Dawley, Indianapolis, IN; 350–400 gm) were used. The subjects were maintained on a 12 hr light/dark cycle (lights on 7:00 A.M.-7:00 P.M.) with standard laboratory chow and water available ad libitum. Temperature in the colony room was maintained at 23°C. All procedures were in accordance with protocols approved by the University of Colorado Institutional Animal Care and Use Committee.
Rats were injected intraperitoneally with LPS (0111:B4; 100 μg/kg; Sigma, St. Louis, MO; lot L4391). At either 30, 45, or 60 min after injection, the animals (n = 8 per group) were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), the thoracic cavity was opened, and a sterile sample of cardiac blood was collected endotoxin-free in heparinized vials containing 17.55 mg EDTA (Becton Dickinson, Franklin Lakes, NJ) and immediately placed on ice until plasma could be collected. The animals were then quickly perfused transcardially with saline. The ventral abdominal vagus nerve including the hepatic branch, a 1–1.5 cm section of the cervical vagus just distal to the carotid bifurcation, and a 2 cm section of the proximal sciatic nerve distal to the pelvis and proximal to the knee, were dissected out and immediately frozen on dry ice. Nerves and plasma were collected from additional rats in an identical manner 30 (n = 4) or 60 min (n = 5) after equivolume vehicle (pyrogen-free physiological saline, 1 ml/kg). All nerve samples were stored at −70°C before sonication. Blood samples were kept on ice and centrifuged at the end of collection at 4°C. Plasma was stored at −20°C until ELISA.
Tissue processing. Each nerve sample was added to 0.25–0.5 ml of Iscove’s culture medium containing 5% fetal calf serum and a cocktail enzyme inhibitor (in mm: 100 amino-n-caproic acid, 10 EDTA, 5 benzamidine HCl, and 0.2 phenylmethyl sulfonyl fluoride). Total protein was mechanically dissociated from tissues using an ultrasonic cell disrupter (Heat Systems, Farmingdale, NY; model MS-50). Sonication consisted of 10 sec of cell disruption at the setting 10. Sonicated samples were centrifuged at 10,000 × g, at 4°C, for 10 min. Supernatants were removed and stored at 4°C until assayed.
Bradford protein assays. Bradford protein assays were performed to determine total protein concentrations in nerve sonication samples. Total protein levels were measured using a version of the Coomassie blue protein assay adapted from Bradford (1976), as previously described (Nguyen et al., 1998). All data were expressed in terms of micrograms of protein per 100 μl.
ELISA. The assays were performed using a commercially available rat IL-1β ELISA kit (R & D systems, Minneapolis MN), as previously described (Nguyen et al., 1998). This ELISA kit utilizes a rabbit anti-rat IL-1β polyclonal antibody (Ab) that can recognize both recombinant (r) as well as natural rat IL-1β. The manufacturer did not observe significant cross-reactivity of this Ab to rHuman IL-1 receptor type I, IL-1 receptor type II, IL-1ra, rRat IL-1α, IL-2, IL-4, interferon-γ, tumor necrosis factor-α, rMouse IL-1α, and IL-1ra. The recovery rate of IL-1β with this assay is between 50 and 70% (our unpublished observations). The results were expressed as picograms of IL-1β per 100 μg of total protein.
Data analysis. The data were analyzed using ANOVA, followed by post hoc t tests (Bonferroni-Dunn) for differences between the groups.
Nerve-associated IL-1β immunoreactivity
Subjects and tissue collection. Animals (as above), were injected with 100 μg/kg LPS. Either 30 (n = 8), 45 (n = 10), or 60 (n = 10) min after injection they were anesthetized and perfused transcardially with saline, as above. Additional groups of vehicle-treated animals were perfused either 30 or 60 min after injection. The nerve tissues were dissected in an identical manner as for the ELISA, as above, and immersion-fixed in 4% paraformaldehyde/0.1 m phosphate buffer (PB), pH 7.4, for 3–5 hr. They were cryoprotected overnight in 22% sucrose/PB, sectioned on a cryostat at 20 μm, and thaw-mounted on electrically charged glass slides (Fisher Scientific, Houston, TX).
Antisera. The thaw-mounted cryostat sections of nerves were processed for immunoreactivity (IR) using antisera directed against IL-1β [SILK6: mouse monoclonal IgG; the epitope is amino acid sequence 123–143 of the mature rat IL-1β (Schotanus et al., 1995) from Department of Pharmacology, Vrije Universiteit, Amsterdam, The Netherlands]. In addition, consecutive sections were processed for the following markers, to aid in identification of the cells expressing IL-β-IR: tyrosine hydroxylase (TH; Incstar, Stillwater, MN), MHC class II antigen (using OX-6, mouse monoclonal antibody; Biosource International, Camarillo, CA) and a macrophage marker (ED-1, mouse monoclonal IgG; Biosource International). TH is a marker for glomus cells in paraganglia (Berthoud et al., 1995) and was used solely to confirm the relationship of IL-1β-IR positive cells with the vagus nerve and paraganglia. TH staining is not shown. According to the manufacturer (Biosource International), OX-6 labels the class II monomorphic antigen rat/mouse RT1B in dendritic cells and some macrophages, whereas ED-1 recognizes a 90–100 kDa antigen present in lysosomal membranes of macrophages and some dendritic cells.
Single-label immunohistochemistry. Sections were washed in PBS and immersed in PBS containing 0.3% hydrogen peroxide and 0.1% sodium azide for 30 min to suppress endogenous peroxidase. Then the slides were rinsed and incubated in 10% normal goat serum (NGS) in PBS containing 0.5% Triton X-100 (PBS-T) for 1 hr. With intermittent washes in PBS, the sections were sequentially incubated in avidin and biotin (Vector AB blocking kit, both diluted 1:10 in PBS-T, each 1 hr; Vector Laboratories, Burlingame, CA) to prevent staining of endogenous biotin. Then the sections were incubated in primary mouse monoclonal anti-rat/human IL-1β (SILK6, 1:100), mouse monoclonal OX-6 (1:100), biotinylated mouse monoclonal ED-1 (1:300), or mouse monoclonal anti-TH (1:1000) overnight at room temperature (RT). The next day the slides were washed and incubated in secondary biotinylated goat anti-mouse IgG antibody (1:100; Jackson ImmunoResearch, West Grove, PA) overnight at RT, except those sections incubated in biotinylated ED-1. All antibodies were diluted in PBS-T containing 0.1% sodium azide and 10% NGS. Finally, sections were incubated in avidin–biotin complex (Vector Elite kit, 1:100 in PBS-T, 2 hr; Vector Laboratories) and rinsed in Tris HCl, pH 7.6. Peroxidase staining was performed using 3,3′-diaminobenzidine (Sigma) as a chromogen dissolved in Tris HCl (50 mg/100 ml) using glucose oxidase (Sigma; type V-S, 0.02%) and β-d-glucose (0.1%) to generate hydrogen peroxide (Shu et al., 1988). One series of sections was processed as above except that the primary antibody was omitted from the incubation buffer (omission controls). The slides were dried overnight, counterstained with cresyl violet, and coverslipped before viewing with an Olympus Vanox II bright-field microscope. Images were collected with a COHU CCD camera coupled to an Apple PowerMac 7200 equipped with NIH Image software (version 1.60) and stored on a Zip cartridge. The obtained micrographs were exported to and labeled using Adobe Photoshop. Except for small adjustments of brightness and contrast, the images were not altered.
Double-label immunofluorescence. Sections were washed in PBS and incubated in 10% NGS in PBS-T for 1 hr. The sections were then incubated in a mixture of rabbit polyclonal antiserum against IL-1β (1:100; Endogen, Woburn, MA) and either mouse monoclonal anti-mouse monoclonal OX-6 (1:100) or mouse monoclonal ED-1 (1:200) overnight at RT. The antisera were diluted in PBS-T containing 0.1% sodium azide and 10% NGS. The next day sections were rinsed and incubated in a mixture of FITC-conjugated goat anti-mouse IgG (1:100; Jackson ImmunoResearch) and Texas Red-conjugated goat anti-rabbit IgG (1:100; Jackson ImmunoResearch) overnight at RT. The slides were then rinsed in several changes of PBS, air-dried, and coverslipped in Vectashield mounting medium (Vector Laboratories). Adjacent series of sections were subjected to the same protocol with the omission of one of the two primary antisera to verify the absence of cross-reactivity. Another series of sections were incubated in the same mixture of primary antisera, diluted in PBS-T and 10% normal donkey serum, and then immersed in a different mixture of secondary antisera (purified for minimal cross-reactivity), and with the fluorescent tags reversed: Texas Red-conjugated donkey anti-mouse IgG, Fab2 fragment (1:100; Jackson ImmunoResearch), and FITC-conjugated donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch). The sections were examined in a Nikon epifluorescence microscope equipped with filters to excite either FITC or Texas Red, or both.
RESULTS
ELISA analysis
IL-1β protein content of the abdominal vagus nerve increased over time after intraperitoneal LPS (F(1,23) = 13.73; p < 0.001) (Fig.1A) IL-1β levels were very low (mean = 0.13 pg/100 μg protein) at 30 min, but were elevated by 45 min (mean = 3.03 pg/100 μg protein;p < 0.05). IL-1β levels were robust by 60 min (mean = 12.69 pg/100 μg protein; p < 0.0001). In the abdominal vagus nerves of saline-treated animals, IL-1β levels remained very low (mean = 0.08 pg/100 μg protein) at both times tested (Fig. 1A).
Fig. 1.
Interleukin-1β content as assessed using ELISA in abdominal vagus, cervical vagus, and proximal sciatic nerve tissues (A) and blood plasma (B) 30, 45, and 60 min after intraperitoneal injection of 100 μg/kg LPS or saline. Only the abdominal vagus shows the induction of IL-1β as early as 45 min. IL-1β content in plasma is significantly elevated by 60 min. *p < 0.05; **p < 0.0002.
IL-1β levels in plasma increased over time after intraperitoneal LPS (F(1,31) = 5.9; p < 0.02) as shown in Figure 1B. IL-1β levels in plasma (Fig.1B) remained low but detectable in saline- (mean = 3.7 pg/ml), and LPS-treated animals (means = 1.5–3.8 pg/ml) until 60 min after LPS injection, at which time plasma IL-1β content was significantly elevated (mean = 18.66 pg/ml) compared with saline controls (p < 0.0002).
In contrast, cervical vagus nerve and sciatic nerve IL-1β levels were low or undetectable in both saline- and LPS-treated animals (Fig.1A), and thus there were no significant effects of LPS treatment over time (cervical vagus: F(1,23)= 0.53, NS; sciatic nerve: F(1,23) = 1.3, NS).
Patterns of immune marker immunoreactivity in nerves
Histological differences between the tissues
The ventral abdominal vagus, and especially the hepatic branch, is characterized by abundant connective tissue, through which run several small blood vessels and lymph ducts (L. Goehler, unpublished observations) (Prechtl and Powley, 1987). This connective tissue also contains large aggregates of lymphoid/myeloid-like cells of varying morphology [nerve-associated lymphoid/myeloid cells (NALC)]. In contrast, connective tissue associated with the cervical vagus is somewhat more limited, and around the sciatic nerve it is restricted to a thin layer around the nerve sheath.
Expression of IL-1β immunoreactivity
The results from the immunohistochemical studies mirror those from the ELISA analysis. IL-1β-IR was not observed in saline-treated rats at any time point. Whereas IL-1β-IR was induced in a time-dependent manner in the abdominal vagus, very little IL-1β-IR was induced in cells associated with either the cervical region of the vagus or the sciatic nerve. A summary of immunohistochemical observations is shown in Table 1.
Table 1.
Summary of immunohistochemical observations of IL-1β, OX-6, and Ed-1 immunoreactivity in nerves after intraperitoneal LPS treatment
| Minutes | |||
|---|---|---|---|
| 30 | 45 | 60 | |
| Abdominal vagus | |||
| IL-1 | – | ++ | +++ |
| OX-6 | +++ | +++ | ++++ |
| ED-1 | + | ++ | +++ |
| Cervical vagus | |||
| IL-1 | – | – | – |
| OX-6 | + | + | + |
| ED-1 | + | + | ++ |
| Sciatic | |||
| IL-1 | – | – | – |
| OX-6 | + | + | + |
| ED-1 | + | + | ++ |
–, Not observed; +, present; ++, present in moderate amounts; +++, present in high amounts; ++++, present in very high amounts.
IL-1β-IR became evident in cells throughout the abdominal vagus, including the hepatic branch, by 45 min after LPS injection. Cells expressing IL-1β at this time point were scattered throughout the connective tissue, but appeared more numerous along the connective tissue edges (Fig. 2A) and within the NALC. The level of expression of IL-1β-IR was somewhat variable at this time point; some animals expressed limited IL-1β-IR, whereas others exhibited robust induction of IL-1β.
Fig. 2.
LPS-induced IL-1β-IR in tissue associated with the abdominal vagus nerve. A, Cells in perivagal NALC expressing IL-1β-IR (dark cytoplasmic stainingindicated with arrows) 45 min after intraperitoneal LPS administration. B–D, IL-1β-IR dendritiform cells in connective tissue adjacent to the abdominal vagus nerve 60 min after LPS. B shows a high magnification of an IL-1β-IR dendritiform cell. E, F, Darkly stained IL-1β-positive cells in perivagal NALC (E, F, arrow), most of which are round in shape. Some dendritiform IL-1β-IR cells are present between nerve fibers at edge of vagus nerve (F,arrowheads). Scale bars: A, 25 μm;B, 10 μm; C, 50 μm; D, 25 μm; E, 50 μm; F, 50 μm.NALC, Nerve-associated lymphoid/myeloid cells;VN, vagus nerve.
By 60 min after LPS injection, many more cells expressed IL-1β-IR, and the intensity of staining was much greater (Fig.2B–F). Cells expressing IL-1β-IR appeared to consist primarily of cells of the dendritic or macrophage phenotype. Cells with similar characteristics to those expressing MHC class II-IR (Fig. 2B–D, and see below) expressed IL-1β-IR within the connective tissue surrounding the nerve, as well as within the perineurium. Other cells expressing IL-1β-IR included small cells with a distribution, size, and morphology consistent with macrophages (Fig.2E,F, and see below, ED-1-IR). Many darkly stained cells could be seen among the NALC (Fig.2E,F). At this time point, a few cells expressing IL-1β-IR extended within the nerve fibers (Fig.2F), and some small cells with thin processes were seen within many paraganglia (Fig.3E). The dendritiform processes of these cells appeared similar to MHC-II-IR also seen in paraganglia (Fig. 3D, and see below). Other IL-1β-IR appeared to envelope the unlabeled type I (glomus cells), and thus may represent type 2 cells (described by Morgan et al., 1976). Variability was minimal, as all LPS-treated animals expressed robust IL-1β-IR.
Fig. 3.

A–C, MHC class II-IR dendritiform cells (dark reaction product) within the abdominal vagus nerve (A), cervical vagus nerve (B), and proximal sciatic nerve (C). Note the difference in density of immunostained cells, which is highest in the abdominal vagus (A) and lowest in the proximal sciatic nerve (C). MHC class II-IR is present in both saline- and LPS-treated rats. D, E, Immune cells in abdominal vagal paraganglia constitutively expressing MHC class II-IR (D) interspersed among vagus nerve fibers and glomus cells in a paraganglion, and those with similar morphology expressing LPS-induced IL-1β-IR (E) surrounding unlabeled glomus cells. F, ED-1-IR in cells of perivagal NALC 60 min after LPS treatment, showing macrophage-like (round or oval-shaped) morphology. Counterstaining with cresyl violet provides light background staining in A–D. Scale bars, 50 μm. NALC, Nerve-associated lymphoid/myeloid cells; PG, paraganglion; VN, vagus nerve.
Expression of MHC class II-IR (OX-6)
MHC class II-IR occurred in all three (abdominal vagus, cervical vagus, and sciatic) nerves in saline-treated animals, as well in those treated with LPS (Fig. 3A–C). MHC class II-IR was particularly abundant in the abdominal vagus, where cells formed a plexus extending along and between the vagus nerve fibers. They were dense within the connective tissue, as well as the nerve trunk, forming the appearance of a network. They occurred in the NALC as well, where they extended processes around and between the other cells. Many MHC class II-IR cells were found along and within the perineurium. Some MHC class II-IR cells extended across the surface of the vagal paraganglia, where their processes penetrated between the glomus cells (Fig. 3D). The cells expressing MHC class II-IR were comparatively large (10–20 μm) dendritiform, and possessed highly variable cell bodies with irregular shapes (Figs. 3,4A). Some of these cells were nearly round, with one or two prominent dendrites, whereas others were elongate or fusiform. All cells showed strong cytoplasmic expression of MHC class II-IR. These characteristics suggest that most of these cells were dendritic cells (Steinman, 1991; Banchereau and Steinman, 1998). MHC class II-IR cells displayed a similar pattern in the cervical vagus nerve and sciatic nerve, where their distributions were much more sparse (Fig. 3B,C), even in cervical vagus nerve samples that contained connective tissue.
Fig. 4.
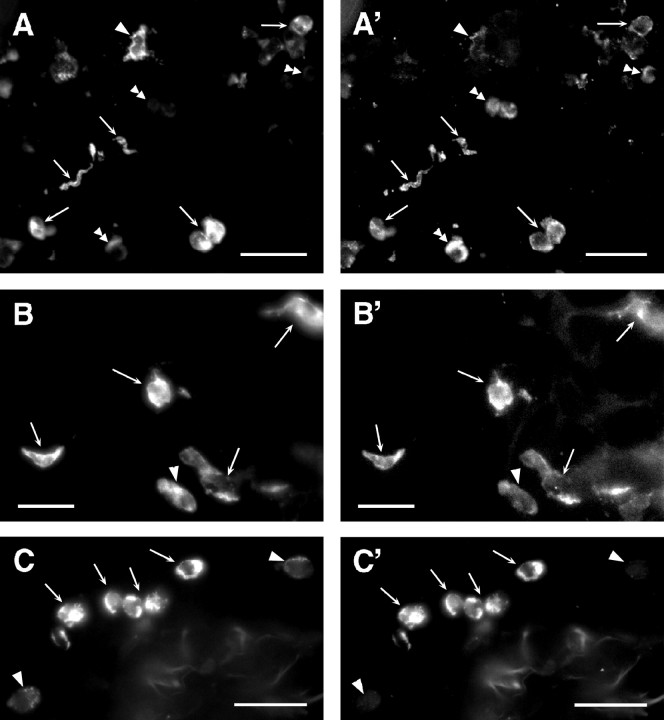
Colocalization of immunofluoresence for IL-1β (A′–C′), MHC-II (A, B), and ED-1 (C) in connective tissue surrounding the abdominal vagus. A, B′,Arrows indicate cells showing strong immunofluorescence for both MHC-II (A, B) and IL-1β (A′, B′). Subcellular localization of the two antigens is not always overlapping.Single arrowheads indicate strongly MHC class II-positive cells that lack prominent labeling for IL-1β.Double arrowheads (A, A′) point to cells that show clear IL-1β-IR but have weak or no MHC class II-IR. C, C′, Cells show strong dual labeling for ED-1 (C, arrows) and IL-1β (C′, arrows). Arrowheadspoint to weak ED-1-positive cells that lack IL-1β-IR. Scale bars, 25 mm.
Expression of ED-1-IR
ED-1-IR occurred in the sciatic, cervical vagus, and abdominal vagus nerves at low levels in saline-treated animals and increased expression was induced by 60 min after LPS injection. However, ED-1-positive cells were far more numerous in the abundant connective tissue of the abdominal vagus. These cells were small (5–10 μm), with a slightly irregular shape (Fig. 3F). They were located along the connective tissue edges, where they formed small and large aggregates. ED-1-positive cells were also distributed among the NALC (Fig. 3F) where they appeared to be major constituents. Some ED-1-IR occurred along the nerve perineurium, especially at 45 and 60 min after LPS injection.
Double-label immunofluorescence
In sections of vagus nerve incubated with antisera against IL-1β and either MHC class II or ED-1, double-labeled cells were observed (Fig. 4). Cells double positive for IL-1β and MHC class II were large (>10 μm) and varied morphologically. Some cells were nearly round, but with a distinctly irregularly shaped nucleus (Fig.4A,A′,B,B′), whereas others were spindle-shaped with a dendritic appearance (Fig.4B,B′). Cells double positive for IL-1β and ED-1 occurred in clumps of smaller cells (6 μm diameter) primarily in the connective tissue or NALC (Fig.4C,C′).
DISCUSSION
The results of this study demonstrate that IL-1β protein is induced in the abdominal vagus nerve within 1 hr after intraperitoneal LPS administration, as assessed by ELISA and immunohistochemistry. The cells expressing IL-1β immunoreactivity are primarily dendritic-type cells that are located along the nerve perineurium, connective tissue, and NALC, and in macrophage-like cells located in clusters also within the associated connective tissue and NALC. In addition, IL-1β immunoreactivity is also induced in cells located between vagal nerve fibers and in paraganglia. These observations are consistent with the idea that IL-1β is rapidly expressed by immune cells after detection of pathogens and that these cells then signal the abdominal vagus by releasing cytokines such as IL-1β.
Rapid induction of perivagal IL-1β is consonant with findings from other studies implicating IL-1β as a key mediator in immune signaling from the peritoneum to the brain (Dunn, 1993; Maier et al., 1993). Intraperitoneal injection of IL-1β activates vagal afferents (Goehler et al., 1998) and initiates brain-mediated acute phase responses that can be blocked by either pretreatment with systemic IL-1 receptor antagonist or subdiaphragmatic vagotomy (Maier et al., 1993, Schotanus et al., 1993; Watkins et al., 1994). Glutamate, a likely neurotransmitter for a majority of vagal afferents (Schaffar et al., 1997), is released in the nucleus of the solitary tract (the site of termination for vagal primary afferent nerve fibers) after intraperitoneal injection of either LPS or IL-1β (Mascarucci et al., 1998). Taken together with our observation of rapid induction of perivagal IL-1β after LPS treatment, these findings reinforce the idea that a major pathway for the actions of IL-1β on the brain is via the abdominal vagus nerve.
Site specificity of IL-1β expression in immune cells associated with nerves
The abdominal vagus nerve was the only nerve tested in which IL-1β was induced within 60 min after intraperitoneal LPS treatment. These observations indicate that rapid expression of intraperitoneal LPS-induced IL-1β is not a general feature of peripheral nerves.
Although IL-1β was not detected in either the cervical vagus or proximal sciatic nerve after intraperitoneal LPS treatment, these nerves do clearly contain small populations of resident immune cells, as evidenced by the presence of MHC class II-positive cells observed between the nerve fibers of both the cervical vagus and sciatic nerves. ED-1-positive macrophage-like cells were also observed in connective tissue associated with these nerves, the numbers of which appeared to increase moderately after LPS treatment. This increase in ED-1 expression may represent migration of activated macrophages to this tissue or may reflect the possibility that LPS in the systemic circulation by 60 min after the intraperitoneal injection (our unpublished observations) was sufficient to activate some of the resident cells. The functions of these nerve-associated immune cells are unclear, but some possibilities include initiation of immune responses to injury or infection (Clatworthy et al., 1995; Banchereau and Steinman, 1998) and/or they may serve trophic or regulatory functions, as has been suggested for dendritic-type cells in endocrine organs such as pituitary and gonads (Hoek et al., 1997).
The lack of IL-1β expression in the proximal sciatic and the cervical vagus after LPS may relate to the fact that both these nerves consist primarily of axons of nerve fibers that terminate a distance away and may not normally be exposed to immune stimuli. In contrast, the subdiaphragmatic vagus is located at the distal end of the nerve, closer to terminal fields of vagal afferents, and thus in closer proximity to likely sites of immune activation (e.g., by ingested or translocated pathogens, as well as intraperitoneal LPS), than are the other two nerve regions. It is also possible that immune cells in the cervical region of the vagus and/or the sciatic nerve failed to express IL-1β because they were not exposed to LPS. However, intraperitoneal LPS at this dose or similar doses (Lenczowski et al., 1998; our unpublished observations) can be measured in the general circulation. The time points investigated in this experiment were intentionally short, and it is possible that IL-1β may in fact be induced in other nerves, but later than in the abdominal region of the vagus nerve.
Source of IL-1β relevant to vagal afferents
In this study we show that intraperitoneal LPS induces significant IL-1β immunoreactivity in plasma by 60 min. IL-1β in plasma may therefore signal immune activation to vagal afferents. However, because IL-1β is induced earlier (by 45 min) in perivagal immune cells, it seems likely that additional sources of IL-1β relevant to the vagus are located within the nerve or its associated tissues and are probably dendritic cells and macrophages. These observations speak to a continuing controversy regarding the source of IL-1β and other cytokines that activate acute phase responses. In some studies, acute phase responses are evident in the absence of detectable levels of cytokines in the circulation (Kluger, 1991). Even when endotoxin or cytokines are detected in plasma, their levels or timing do not always correlate with acute phase activation after intraperitoneal administration of immune stimuli (Kluger, 1991). These observations have led to the suggestion that the relevant signals are not in the circulation, but rather arise in the peritoneum or other discrete tissue sites (Carlson, 1997). However, levels of IL-1β obtained from peritoneal lavage also do not correlate with brain-mediated illness responses (Lenczowski et al., 1998). The results from the present study offer a potential explanation for these seemingly confusing observations. Perivagal immune cells respond rapidly to intraperitoneal LPS, and they are located near enough to the vagal afferents and/or paraganglia that IL-1β may not need to enter either the general circulation or the peritoneal fluid to exert its effects.
Immune cells in paraganglia
The constitutive presence of MHC class II-IR in cells within the vagal paraganglia suggest that immune cells are normal residents of these structures. In addition, IL-1β is induced in immune-like, nonglomus cells by 60 min after intraperitoneal LPS administration. Glomus cells, many of which are innervated by vagal afferents (Berthoud et al., 1995), can express binding sites for IL-1 ligands (Goehler et al., 1997). The codistribution of glomus-type cells potentially expressing binding sites for IL-1 with immune-type cells capable of producing IL-1β provides a possible pathway by which immune-derived IL-1β, or other mediators, leads to vagal activation.
Dendritic cells and macrophages as sentinels for the nervous system
The presence of numerous dendritic cells in such close association with vagal nerve fibers and paraganglia reinforces the idea that one function of the vagus nerve is to signal immune activation in internal tissues to the CNS. Steinman (1991) has described dendritic cells as the “sentinels of the immune system”. Dendritic cells are typically distinguished by their highly irregular cell shape, numerous and often extensive processes (dendrites), and high constitutive and cytoplasmic expression of MHC class II molecules (Cella et al., 1997; Banchereau and Steinman, 1998). They are specialized to detect pathogens, thus they express pinocytic and phagocytic capability, as well as receptors for a variety of pathogens (Cella et al., 1997). They are well situated to serve as immune sentinels because they are distributed throughout the body (Banchereau and Steinman, 1998). When dendritic cells detect pathogens, they migrate in lymph to the draining lymph nodes, where they activate T-cells (Ni and O’Neill, 1997). In fact, dendritic cells are the only cells capable of activating naive T-cells (Banchereau and Steinman, 1998). Thus, dendritic cells are critical for the initiation of primary immune responses. Their prominent location within and around vagal nerve fibers and their expression of IL-1β after LPS administration suggests that they may serve as immune sentinels for the nervous system as well.
Macrophages, which also responded to intraperitoneal LPS by expressing IL-1β are also well suited to act as immune-to-brain sentinels. In addition to important clearance and primary defense functions (e.g., phagocytosis and release of peroxide and superoxide), macrophages help orchestrate primary immune responses (Goerdt et al., 1996). Macrophages express receptors for pathogen-associated molecules, such as LPS, and are active phagocytes, thus able to respond to a wide variety of pathogens (Goerdt et al., 1996). Their location within vagal nerve connective tissue and induction of IL-1β after LPS injection suggests that one function of IL-1β produced by these cells may be to signal the vagus nerve.
Conclusions
Intraperitoneal LPS induces perivagal immune cells to rapidly express IL-1β immunoreactivity. These results are consistent with a model of immune-to-brain communication such that immune activation within the peritoneal cavity stimulates dendritic cells and macrophages to synthesize and release cytokines such as IL-1β, which then activate vagal afferents. Vagal afferents then, via their terminations in the nucleus of the solitary tract, activate CNS-mediated illness consequences. In this way, immune cells may signal the brain using a paracrine action to stimulate neural, in addition to humoral, immune-to-brain communication pathways. It may be that this neural pathway is especially important as a rapid signaling pathway in the early phases of host defense.
Footnotes
This work was supported by National Institutes of Health Grants MH55283, MH01558, and MH00314 and internal funds of the Vrije Universiteit Amsterdam.
Correspondence should be addressed to Dr. Lisa E. Goehler, Department of Psychology, Muenzinger Hall, Box 345, University of Colorado, Boulder, CO 80309.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:425–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud H-R, Kressel M, Neuhuber WL. Vagal afferent innervation of rat abdominal paraganglia as revealed by anterograde DiI-tracing and confocal microscopy. Acta Anat. 1995;152:127–132. doi: 10.1159/000147691. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 4.Bret-Dibat JL, Bluthé R-M, Kent S, Kelley K, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9:242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- 5.Carlson DE. Adrenocorticotropin correlates strongly with endotoxemia after intravenous but not after intraperitoneal inoculations of E. coli. Shock. 1997;7:65–69. [PubMed] [Google Scholar]
- 6.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 7.Clatworthy AL, Illich PA, Castro GA, Walters ET. Role of peri-axonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci Lett. 1995;184:5–8. doi: 10.1016/0304-3940(94)11154-b. [DOI] [PubMed] [Google Scholar]
- 8.Dunn AJ. Role of cytokines in infection-induced stress. Ann NY Acad Sci. 1993;697:189–202. doi: 10.1111/j.1749-6632.1993.tb49932.x. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaykema RPA, Dijkstra I, Tilders FJH. Subdiaphragmatic vagotomy suppresses endotoxin-induced activation of the hypothalamic corticotrophin-releasing hormone neurons and ACTH secretion. Endocrinology. 1995;136:4717–4720. doi: 10.1210/endo.136.10.7664696. [DOI] [PubMed] [Google Scholar]
- 11.Gaykema RPA, Goehler LE, Tilders FJH, Bol JGJM, McGorry M, Fleshner M, Maier SF, Watkins LR. Bacterial endotoxin induces Fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation. 1998;5:234–240. doi: 10.1159/000026343. [DOI] [PubMed] [Google Scholar]
- 12.Goehler LE, Relton JK, Dripps D, Keichle R, Tartaglia N, Maier SF, Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 13.Goehler LE, Gaykema RPA, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804:306–310. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- 14.Goerdt S, Kodelja V, Schmuth M, Orfanos CE, Sorg C. The mononuclear phagocyte-dendritic cell dichotomy: myths, facts, and a revised concept. Clin Exp Immunol. 1996;105:1–9. doi: 10.1046/j.1365-2249.1996.d01-740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1β. Am J Physiol. 1997;273:R1246–R1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- 16.Hoek A, Allaerts W, Leenan PJN, Schoemaker J, Drexhage HA. Dendritic cells and macrophages in the pituitary and gonads. Evidence for their role in the fine regulation of the reproductive endocrine response. Eur J Endocrinol. 1997;136:8–24. doi: 10.1530/eje.0.1360008. [DOI] [PubMed] [Google Scholar]
- 17.Kluger MJ. Fever: the role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenczowski MJP, Schmidt ED, Van Dam A-M, Gaykema RPA, Tilders FJH. Individual variation in hypothalamus-pituitary-adrenal responsiveness of rats to endotoxin and IL-1β. Ann NY Acad Sci. 1998;856:139–147. doi: 10.1111/j.1749-6632.1998.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 19.Maier SF, Wiertelak EP, Martin D, Watkins LR. Interleukin-1 mediates the behavioral hyperalgesia produced by lithium chloride and endotoxin. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- 20.Mascarucci P, Perego C, Terrazino S, De Simoni MG. Glutamic acid release in the nucleus tractus solitarius induced by peripheral endotoxin and interleukin-1. Neuroscience. 1998;86:1285–1290. doi: 10.1016/s0306-4522(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 21.Morgan M, Pack RJ, Howe A. Structure of cells and nerve endings in abdominal vagal paraganglia of the rat. Cell Tissue Res. 1976;169:467–484. doi: 10.1007/BF00218147. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni K, O’Neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol. 1997;75:223–230. doi: 10.1038/icb.1997.35. [DOI] [PubMed] [Google Scholar]
- 24.Prechtl JC, Powley TL. A light and electron microscopic examination of the vagal hepatic branch of the rat. Anat Embryol. 1987;176:115–126. doi: 10.1007/BF00309759. [DOI] [PubMed] [Google Scholar]
- 25.Saper CB, Breder CD. The neurological basis of fever. N Engl J Med. 1994;330:1880–1886. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 26.Schaffar N, Rao H, Kessler JP, Jean A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res. 1997;778:302–308. doi: 10.1016/s0006-8993(97)01058-5. [DOI] [PubMed] [Google Scholar]
- 27.Schotanus K, Tilders FJH, Berkenbosch F. Human recombinant interleukin-1 receptor antagonist prevents ACTH, but not interleukin-6 responses to bacterial endotoxin in rats. Endocrinology. 1993;133:2461–2468. doi: 10.1210/endo.133.6.8243265. [DOI] [PubMed] [Google Scholar]
- 28.Schotanus K, Holtkamp GM, Meloen RH, Puijk WC, Berkenbosch F, Tilders FJH. Domains of rat interleukin-1b involved in type I receptor binding. Endocrinology. 1995;136:332–339. doi: 10.1210/endo.136.1.7530194. [DOI] [PubMed] [Google Scholar]
- 29.Shu S, Ju G, Fan L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 31.Van Dam A-M, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- 32.Watkins LR, Weirtelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 33.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995a;183:17–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review and analysis of alternative mechanisms. Life Sci. 1995b;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]