Abstract
We investigated how the brain switches between the preparation of a movement where a stimulus is the target of the movement, and a movement where a stimulus serves as a landmark for an instructed movement elsewhere. Monkeys were trained on a pro-/anti-saccade paradigm in which they either had to generate a pro-saccade toward a visual stimulus or an anti-saccade away from the stimulus to its mirror position, depending on the color of an initial fixation point. Neural activity was recorded in the superior colliculus (SC), a structure that is known to be involved in the generation of fast saccades, to determine whether it was also involved in the generation of anti-saccades. On anti-saccade trials, fixation during the instruction period was associated with an increased activity of collicular fixation-related neurons and a decreased activity of saccade-related neurons. Stimulus-related and saccade-related activity was reduced on anti-saccade trials. Our results demonstrate that the anti-saccade task involves (and may require) the attenuation of preparatory and stimulus-related activity in the SC to avoid unwanted pro-saccades. Because the attenuated pre-saccade activity that we found in the SC may be insufficient by itself to elicit correct anti-saccades, additional movement signals from other brain areas are presumably required.
Keywords: superior colliculus, eye movement, anti-saccade, stimulus–response mapping, sensorimotor transformation, oculomotor, motor preparation, saccade, visual fixation
Rapid saccadic eye movements are used to move the eyes to objects of interest. It is well known that the superior colliculus (SC) in the midbrain is an important structure for the generation of these fast saccades in which the stimulus is also the target of the movement (Wurtz and Goldberg, 1972; Schiller et al., 1987; Edelman and Keller, 1996; Dorris et al., 1997). The movement repertoire of primates, however, is not constrained to spatially congruent movements. Primates can acquire arbitrary stimulus–response mappings, where a stimulus can serve as a landmark for an instructed incongruent movement (for review, see Wise et al., 1996).
The frontal eye field (FEF), supplementary eye field (SEF), and dorsolateral prefrontal cortex (DPC) are considered to be the predominate brain regions involved in the generation of incongruent saccades. Patients with frontal cortex lesions (Guitton et al., 1985;Pierrot-Deseilligny et al., 1991) have difficulty correctly performing the anti-saccade task (Hallett 1978; Hallett and Adams, 1980; for review, see Everling and Fischer, 1998), in which they are instructed to suppress a saccade toward a suddenly appearing peripheral visual stimulus (pro-saccade) and to generate a saccade to its mirror position (anti-saccade). Recently, neurophysiological studies have also shown that SEF (Schlag-Rey et al., 1997) and DPC (Funahashi et al., 1993) neurons are active for anti-saccades. To date, the contribution of the SC to the generation of anti-saccades is unknown.
The primate SC receives direct projections from many cortical areas, including FEF, SEF, and DPC (Leichnetz et al., 1981; Fries, 1984; Stanton et al., 1988; Shook et al., 1990), and projects directly to preoculomotor neurons in the brain stem (for review, see Moschovakis et al., 1996). However, FEF and SEF also have direct projections to the preoculomotor region in the brain stem that bypass the SC (for review, see Schall, 1997). The combination of serial and parallel pathways in the saccadic system has led to two extreme hypotheses for the generation of anti-saccades. Guitton (1991) hypothesized that the anti-saccade movement signal generated in the FEF or SEF is relayed to the SC, which in turn sends the motor command to the brain stem saccade generator (“serial hypothesis”). In contrast, Forbes and Klein (1996) hypothesized that the cortical motor command for the anti-saccade is sent directly by the cortex to the brain stem (“bypass hypothesis”).
These two hypotheses predict different neural activity in the SC during the anti-saccade task: The serial hypothesis predicts similar activity of saccade-related neurons in the SC before pro-saccades and anti-saccades, whereas the bypass hypothesis predicts that saccade-related neurons in the SC are inactive for anti-saccades. To distinguish between these two hypotheses, we recorded single neuron activity in the SC of awake behaving rhesus monkeys trained to perform saccades in a task with randomly interleaved pro-saccade and anti-saccade trials.
Preliminary reports of some of these data have been published previously (Everling et al., 1998a,b)
MATERIALS AND METHODS
Preparation of experimental animals. Two adult male rhesus monkeys (Macaca mulatta), weighing 6 and 10 kg, were used in this study. All procedures were approved by the Queen’s University Animal Care Committee and were in compliance with the Canadian Council on Animal Care policy for the care and use of laboratory animals. Animals were prepared for chronic experiments in a single surgery. Eye movements were monitored by the magnetic search coil technique (Fuchs and Robinson, 1966; Judge et al., 1980). Scleral search coils, a head restraint, and a stainless steel recording chamber were implanted under ketamine/isoflurane anesthesia and aseptic conditions (for details, see Munoz and Istvan, 1998). The recording chamber was centered on the midline and tilted 38° posterior of vertical to allow recordings of both SCs.
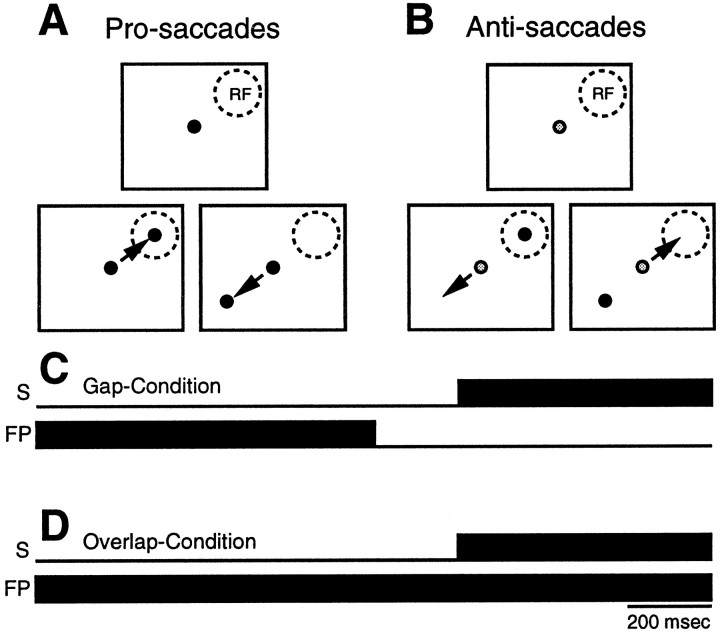
Behavioral paradigms. The monkeys were trained to perform a pro-/anti-saccade paradigm (Fig. 1). The experiments were conducted in a dark, sound-attenuated room. The monkeys were seated comfortably in a primate chair (Crist Instruments) with their head restrained. They faced a screen 86 cm in front of them that spanned 70° × 70° of the visual field. Isoluminant light-emitting diodes (green and red, 0.3 cd/m2) were back-projected onto the screen to generate visual stimuli. During the intertrial intervals, the screen was illuminated diffusely (1.0 cd/m2) to prevent the animals from becoming dark-adapted. Each trial started with the removal of the background light, and after a period of 250 msec, a fixation point (FP) appeared at the center of the screen. The monkeys were required to look at the FP and maintain fixation for 700 to 900 msec. A red FP was used to signal a pro-saccade trial (Fig. 1A) and a green FP was used to signal an anti-saccade trial (Fig. 1B). For half of the trials, the FP was extinguished 200 msec (gap period) before stimulus presentation (Fig. 1C,Gap-Condition). For the other half of the trials, the FP remained illuminated throughout the whole trial (Fig.1D, Overlap-Condition). Then, within a block of trials, a red visual stimulus was projected pseudorandomly with equal probability at one of two possible positions on opposite sides of the horizontal and vertical meridians. For saccade-related and visual-related neurons, one position was set to the location that yielded the optimal saccade-related or visual-related activity, respectively (contralateral to the side of recording). The other position was set to the mirror location on the opposite side of the horizontal and vertical meridians (ipsilateral side). The optimal response location of a saccade-related neuron and a visual-related neuron was identified in a block of gap pro-saccade trials in which the location of stimulus presentation was changed systematically. Saccade-related neurons in the SC can exhibit preparatory activity and visual activity in addition to their motor-related activity (Munoz and Wurtz, 1995a). Therefore, we refer in the following text to the optimal response location of a neuron as the neuron’s “response field,” instead of using the terms “receptive field” or “motor field.” For neurons with fixation-related activity, the stimulus was presented pseudorandomly 10° to the left and 10° to the right of the FP. Monkeys received a liquid reward if they maintained central fixation throughout the visual fixation and gap periods and then generated a saccade within 500 msec after stimulus presentation to the stimulus location on pro-saccade trials (as indicated by red FP) or to its mirror location on anti-saccade trials (as indicated by green FP), and then maintained fixation there for at least 200 msec.
Fig. 1.
Schematic representation of the pro-/anti-saccade paradigm. FP, Fixation point; RF, response field; S, stimulus. See Materials and Methods for details.
The on-line accuracy criterion for the saccades consisted of a window of ±60% of the length of the vector of the stimulus. This large window was necessary, because the endpoints of anti-saccades display great variability (Hallett, 1978; Smit et al., 1987). The accuracy criterion was more stringent off-line for the quantification of saccade-related activity (see below). On anti-saccade trials, a green stimulus was presented 600 msec after and at the mirror location of the red stimulus to provide the monkey with post-trial information about the correct location for the anti-saccade. During the recording of one neuron, between 12 and 20 trials of each of the eight conditions were presented in a pseudorandom order by combining the two types of mapping conditions (pro-saccade and anti-saccade) with the two fixation conditions (gap and overlap) and the two stimulus locations. The monkeys received water until satiation, after which they were returned to their home cages. Daily records were kept of the weight and health status of the monkeys, and additional water and fruit was provided as needed.
Recording techniques. Single neuron activity was recorded extracellularly in both SCs in the two monkeys with commercially available tungsten microelectrodes (Frederick Haer) with impedances of 0.5–5 MΩ at 1 kHz. Electrodes were driven by a hydraulic microdrive (Narishige, Tokyo, Japan) through stainless steel guide tubes that were held in position firmly by a Delrin grid inside the recording chamber (Crist et al., 1988). Single-neuron activity was sampled at 1 kHz after passing through a window discriminator (Bak Electronics), which excluded action potentials that did not meet both amplitude and time constraints.
A 486 personal computer running a real-time data acquisition system (REX) (Hays et al., 1982) was used to control the behavioral paradigms and visual displays and to store data. Horizontal and vertical eye positions were sampled at 500 Hz from one eye.
Data analysis. The off-line data analysis was performed on a Sun Sparc2 workstation with the use of a computer program that marked the beginning and end of a saccade based on velocity and acceleration threshold criteria (Waitzman et al., 1991). The saccades marked on each trial were verified by an experimenter and corrected if necessary. Because saccades with saccadic reaction times (SRTs) below 80 msec had a 50% chance of being in the correct direction on pro-saccade trials, all such saccades were excluded as anticipation. Trials with SRTs above 500 msec were excluded as no response trials.
To evaluate the relationship between neuronal discharge and specific events (stimulus appearance, saccade initiation), rasters of neuronal discharge and continuous activation waveforms were constructed. The activation waveform was obtained by convolving each spike with an asymmetric function that resembled a postsynaptic potential (Hanes and Schall, 1996; Thompson et al., 1996; Dorris and Munoz, 1998;Everling et al., 1998a):
| Equation 1 |
where the activation level A as a function oft varies according to τg, the time constant for the growth time that was set to 1 msec, and τd, the time constant for the decay time that was set to 20 msec. This asymmetric activation waveform, which is designed to mimic an excitatory postsynaptic potential, is physiologically more plausible than the plain Gaussian activation function (Richmond and Optican, 1987), because a spike exerts an influence only forward and not backward in time.
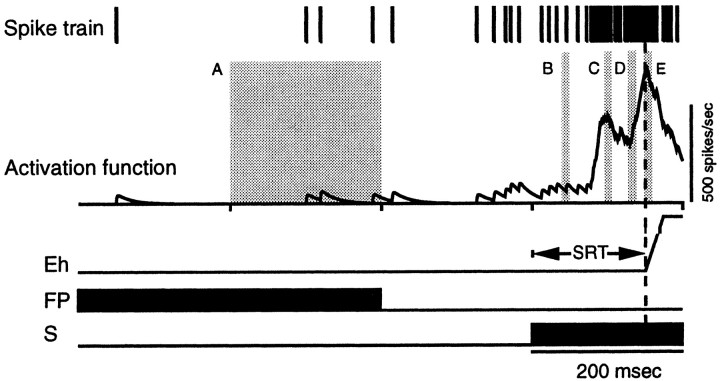
For the quantification and comparison of neuronal activity between pro-saccade and anti-saccade trials on a single trial basis, each spike train was replaced with the postsynaptic activation function (Eq. 1) with a binwidth of 1 msec (Fig. 2). The mean activity of a single neuron was calculated by averaging the single trial activities.
Fig. 2.
Quantification of neuronal activity on a single trial. A–E, Analysis epochs (see Materials and Methods for details). Eh, Horizontal eye position;FP, fixation point; S, stimulus;SRT, saccadic reaction time.
Neuronal activity was quantified in several epochs (Fig. 2). To quantify the neuronal activity during the instruction period(fixation of the red or green FP) on pro-saccade and anti-saccade trials, the activity during the 400–200 msec interval before presentation of the stimulus was calculated for correct pro-saccade and anti-saccade trials (Fig. 2, A). Overlap trials and gap trials were combined, because during this epoch the stimulus conditions and the instructions were identical for overlap and gap trials.
To quantify the gap-related changes in neuronal activity on pro-saccade and anti-saccade trials, the mean activity level in the interval 40–50 msec after the appearance of the stimulus was measured on gap trials (Fig. 2, B). All SC neurons with stimulus-related responses had latencies >50 msec, as determined by the Poisson spike train analysis developed by Thompson et al. (1996). This technique identified on individual trials the time at which the neural activity deviated significantly from that expected from a random Poisson distribution. We never found stimulus-related responses <50 msec on any individual trials. Therefore, the activity during the interval 40–50 msec after stimulus presentation reflected the activation level before stimulus presentation and was not influenced by any changes related to the appearance of the stimulus. To determine the change in neural activity from the activity during the instruction period, the average activity in the interval 400–200 msec before stimulus presentation was subtracted from the average activity in the interval 40–50 msec after stimulus presentation.
The activity of SC neurons during the gap period is correlated with SRT (Dorris and Munoz, 1995, 1998; Dorris et al., 1997). To examine further the relationship between SRT and prestimulus activity, trial-by-trial correlations (Pearson product–moment correlation coefficientr) between SRT and prestimulus activity level in the interval 40–50 msec after stimulus presentation were calculated for saccade-related neurons with a low-frequency prestimulus activity (see “Neuron classification” below) on pro-saccade gap trials and anti-saccade gap trials.
To quantify the magnitude of the stimulus-related activityof neurons with stimulus-related responses on pro-saccade trials and anti-saccade trials, the largest peak of activity in the interval between 60 and 120 msec after stimulus presentation was determined (Fig. 2, C). The average activation was measured in a 10 msec interval extending from 5 msec before to 5 msec after the peak, and the prestimulus activation in the interval 40–50 msec after stimulus presentation was subtracted as the baseline activity from this value. These analyses were performed on overlap trials with the exclusion of all saccades with SRTs below 125 msec (express saccades) (Fischer and Boch, 1983; Edelman and Keller, 1996; Paré and Munoz, 1996) to avoid a contamination of the stimulus-related activity with the saccade-related activity. Neurons that increased their activity >50 spikes/sec above the prestimulus level in the interval 60–120 msec after stimulus presentation were considered to have a visual response.
To quantify the pre-saccade activity, the average level of activity was measured in the interval 10–20 msec before saccade initiation (Fig. 2, D). We consider this interval to encompass the activity that triggers saccade initiation, because microstimulation experiments have shown that stimulation of the SC evokes saccades with latencies of ∼20 msec during visual fixation (Robinson, 1972). The latencies are reduced to ∼8 msec when the stimulation occurs during a saccade in midflight (Miyashita and Hikosaka, 1996). We also tested the intervals 20–30 and 0–10 msec before saccade initiation and obtained similar results.
The endpoints of anti-saccades display a much greater variability than the endpoints of visually guided pro-saccades in humans (Hallett, 1978;Smit et al., 1987). This imposed a potential confound for our analysis, because the magnitude of the saccade-related activity of SC neurons depends greatly on the saccadic vector. Saccade-related neurons display a maximum discharge for saccades into the center of their response field, and the magnitude of the saccade-related response decreases when the vector of the saccade deviates from this location (Sparks and Mays, 1980; Munoz and Wurtz, 1995a). To minimize this confound, we considered for this analysis only pro-saccades and anti-saccades that landed within a radius that deviated only ±20% of the optimal vector of the saccade.
To quantify the magnitude of the saccade-related activity of saccade-related neurons on pro-saccade trials and anti-saccade trials, the largest peak of activity in the interval ±20 msec around saccade initiation was determined for each neuron in the overlap condition (Fig. 2, E). Then, the average activity was measured in a 10 msec interval extending from 5 msec before to 5 msec after the peak. Also for this analysis, we used only saccades that landed within a radius that deviated less than ±20% of the optimal vector of the saccade (see above).
Only neurons with at least five correct anti-saccade trials and five correct pro-saccade trials were considered for these analyses. All of the data are expressed as mean ± SEM. For the sample of neurons, the neural activity on pro-saccade trials was compared with the neural activity on anti-saccade trials with a paired Student’s ttest or, if a test of normal distribution failed (Kolmogorov–Smirnov test), with the nonparametric Wilcoxon signed-rank test. For individual neurons, the neural activity on pro-saccade trials was compared with the neural activity on anti-saccade trials with an unpaired Student’st test. Significance was accepted at the p< 0.05 level.
Neuron classification. Neurons in the SC were separated into four different classes (visual, fixation, buildup, and burst) on the basis of criteria described previously (Goldberg and Wurtz, 1972; Munoz and Wurtz, 1993a, 1995a; Dorris et al., 1997). This classification was based on the data obtained in the pro-saccade gap condition. The dorsal surface of the SC was determined as the electrode depth where visual background activity was first noticed. To be classified as a visual neuron in the superficial layers of the SC, neurons had to be located 0–1.5 mm below the dorsal surface of the SC and had to possess the following discharge characteristics: (1) an increase in stimulus-related activity 50 spikes/sec above baseline for stimulus presentations into the neuron’s response field and (2) no saccade-related activity. To be classified as a fixation neuron, neurons had to be located from 1 to 3 mm below the dorsal surface of the rostrolateral pole of the SC and had to possess the following discharge characteristics: (1) tonic activity >10 spikes/sec during both the visual fixation and the end of gap periods, i.e., while the monkey fixated the FP even when it was removed momentarily and the monkey was required to maintain the same eye position (this test excluded visual neurons with a foveal receptive field); and (2) a pause in activity during all ipsiversive and most contraversive saccades (Munoz and Wurtz, 1993a). To be classified as a buildup neuron, a neuron had to be located 1–3 mm below the dorsal surface of the SC and possess the following discharge characteristics: (1) low-frequency prestimulus activity during the end of the gap epoch (Munoz and Wurtz, 1995a; Dorris et al., 1997) that was significantly greater than during the visual fixation epoch (paired t test, p< 0.05); and (2) saccade-related activity above 100 spikes/sec for pro-saccades into the neuron’s response field. To be classified as a burst neuron, a neuron had to located 1–3 mm below the dorsal surface of the SC and to possess the following discharge characteristics: (1) no significant increase in discharge during the gap period and (2) saccade-related activity above 100 spikes/sec for pro-saccades into the neuron’s response field. Both buildup and burst neurons may or may not exhibit visual responses.
RESULTS
Behavior
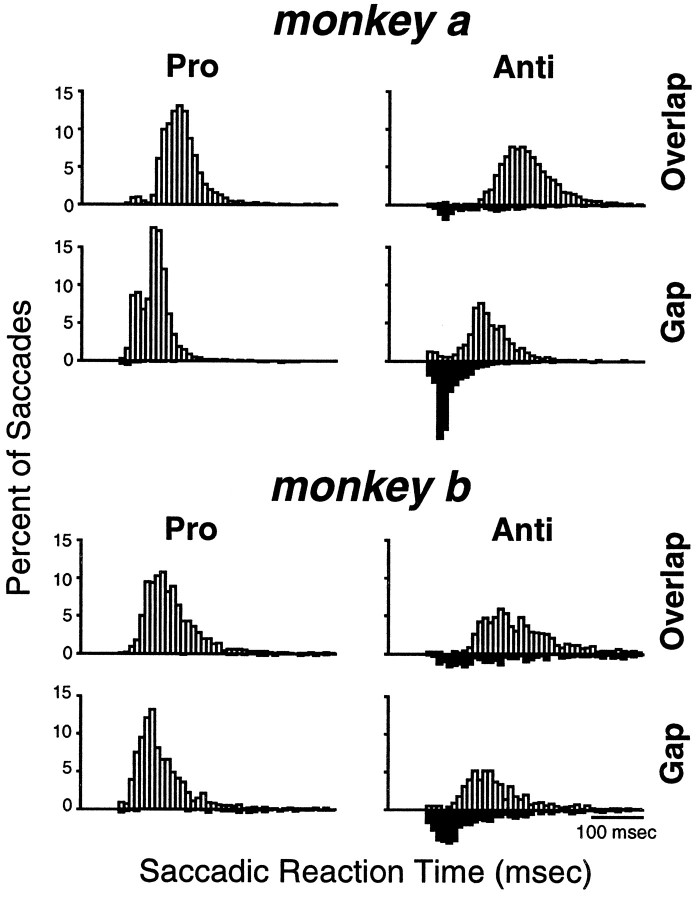
After completion of training in the pro-/anti-saccade paradigm, both monkeys had achieved a stable performance that was significantly above chance. Figure 3 shows the distribution of SRTs of both monkeys in the four task conditions obtained by pooling the saccades generated during the recording of the neurons in this study. Table 1 summarizes the mean SRTs and the percentage of errors in the different task conditions for the two monkeys. As in human subjects (Fischer and Weber, 1992), the SRTs for monkeys were longer for anti-saccades than for pro-saccades. The monkeys generated pro-saccades and anti-saccades with shorter SRTs in the gap condition than in the overlap condition. Moreover, the monkeys made more errors in the anti-saccade task in the gap condition compared with the overlap condition. A large proportion of these errors occurred at very short SRTs, in the range of express saccades. These errors were corrected on most of the trials. Incorrect responses in the pro-saccade task, i.e., saccades away from the stimulus, were very rare.
Fig. 3.
Distribution of saccadic reaction times for the two monkeys in the different conditions of the pro-/anti-saccade paradigm. The bin width is 10 msec. The white bars show correct responses, and the black bars show direction errors.
Table 1.
Mean saccadic reaction times and percentage of errors in the pro-/anti-saccade paradigm
| Pro-saccades | Anti-saccades | |||
|---|---|---|---|---|
| Monkey A | Monkey B | Monkey A | Monkey B | |
| Overlap | ||||
| Correct (msec) | 198 | 184 | 275 | 263 |
| Error (msec) | 244 | 385 | 181 | 231 |
| % error | 0.3 | 1.3 | 13.8 | 26.4 |
| Gap | ||||
| Correct (msec) | 147 | 164 | 199 | 218 |
| Error (msec) | 209 | 291 | 129 | 172 |
| % error | 2.7 | 4.9 | 41.6 | 43.8 |
Neuronal activity
Figure 4 shows the discharge of typical individual neurons. The activity of a single visual neuron recorded in the left SC in the pro-/anti-saccade paradigm is shown in Figure 4A–D. On trials in which the stimulus was presented in the neuron’s response field, the neuron displayed several phasic stimulus-related bursts in discharge on pro-saccade trials (Fig.4A,C, left panels) and anti-saccade trials (Fig. 4A,C, right panels). The magnitude of the stimulus-related response was reduced on anti-saccade trials. The neuron did not display any increase in discharge when the stimulus was presented ipsilateral to the neuron’s response field, regardless of whether the saccade was generated ipsilateral to the response field on pro-saccade trials (Fig. 4B,D, left panels) or in the response field on anti-saccade trials. (Fig.4B,D, right panels).
Fig. 4.
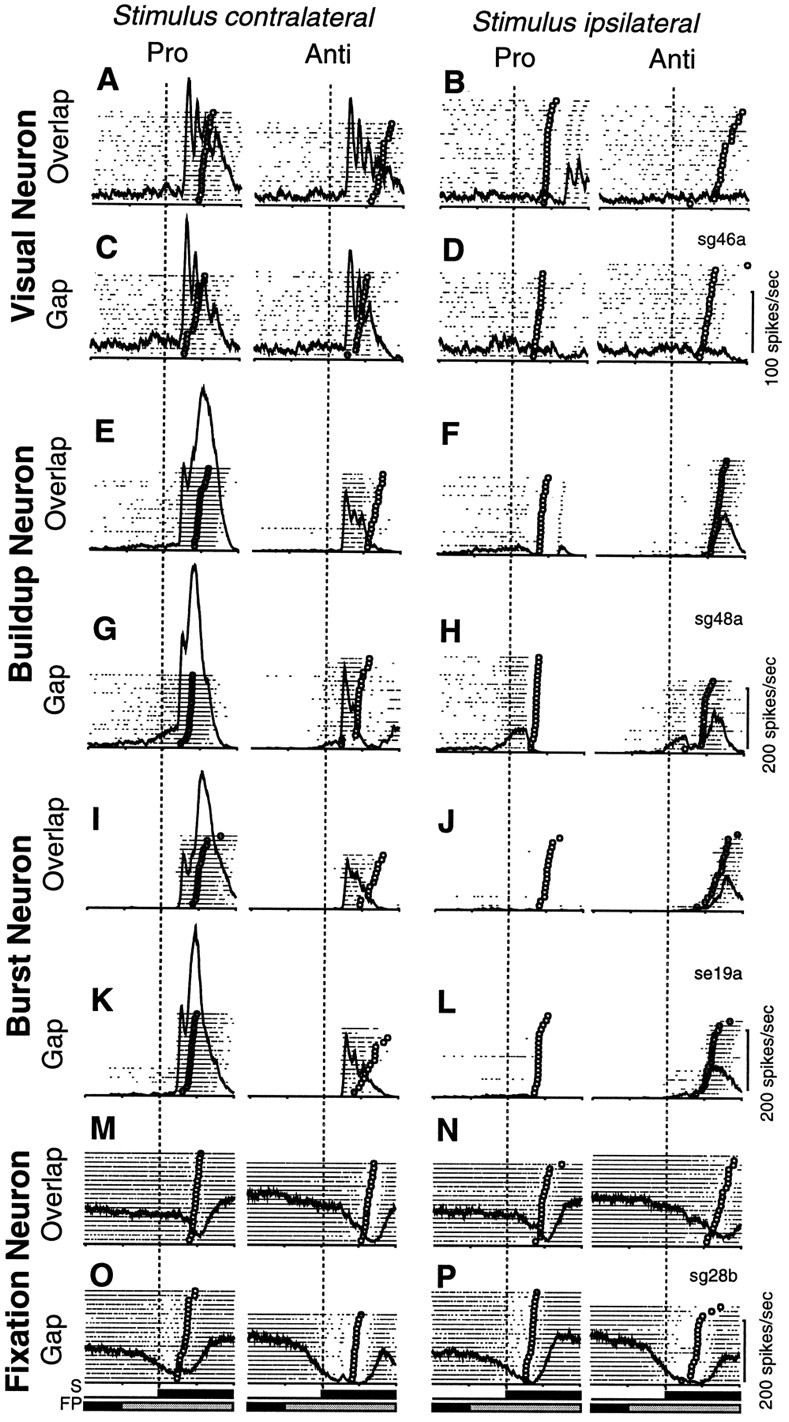
Activity of superior colliculus neurons in the pro-/anti-saccade paradigm. A–D, Activity of a visual neuron that was located in the left SC, 0.4 mm below the dorsal surface, and the stimulus was presented 7° right and 7° up (A, C, stimulus contralateral) or 7° left and 7° down (B, D, stimulus ipsilateral). Eachdot indicates the time of an action potential relative to stimulus presentation, and each row represents one trial. The trials are sorted according to saccadic reaction times (indicated by ○). Superimposed on the rasters is the average activation waveform. The presentation of visual stimuli (FP, fixation point;S peripheral stimulus) is represented byblack (gap and overlap condition) andgray (overlap condition) horizontal barsbelow the rasters. The time of stimulus presentation is indicated bydashed vertical lines. The activity is shown in overlap trials (A, B) and gap trials (C, D) for pro-saccades and anti-saccades. E–H, Activity of a buildup neuron that was located in the left SC, 1 mm below the dorsal surface, and the stimulus was presented 5° right (E, G, stimulus contralateral) or 5° left (F, H, stimulus ipsilateral). I–L, Activity of a burst neuron that was located in the left SC, 1.7 mm below the dorsal surface, and the stimulus was presented 8° right (I, K, stimulus contralateral) or 8° left (J, L, stimulus ipsilateral). M–P, Activity of a fixation neuron that was located in the right SC, 2.6 mm below the dorsal surface, and the stimulus was presented 10° left (M, O, stimulus contralateral) or 10° right (N, P, stimulus ipsilateral). Each time mark indicates 200 msec.
The activity of a single buildup neuron is shown in Figure4E–H. The neuron had low-frequency activity during the instruction period on pro-saccade trials (Fig.4E–H, left panels). This low-frequency activity was almost absent during the instruction period on anti-saccade trials (Fig. 4E–H, right panels). During the gap period, the neuron increased its discharge rate on pro-saccade trials (Fig. 4G,H, left panels) and on anti-saccade trials (Fig. 4G,H,right panels). The increase in low-frequency prestimulus activity, however, was higher on pro-saccade trials compared with anti-saccade trials. On trials when the stimulus was presented in the neuron’s response field (Fig. 4E,G), the neuron displayed a stimulus-related burst in discharge (first peak) on pro-saccade trials (Fig. 4E,G, left panels) and anti-saccade trials (Fig. 4E,G,right panels); however, the magnitude of the stimulus-related response was reduced, and the response decayed on anti-saccade trials. On pro-saccade trials (Fig.4E,G, left panels), the neuron then discharged a high-frequency burst of action potentials for saccades into its response field (second peak). On trials in which the stimulus was presented on the ipsilateral side (Fig.4F,H), the low-frequency prestimulus activity was truncated at the time when the neuron showed the stimulus-related burst in discharge after presentation of the stimulus in the neuron’s response field. The neuron then remained silent on pro-saccade trials (Fig. 4F,H, left panels), whereas the activity increased on anti-saccade trials (Fig. 4F,H,right panels), and the neuron displayed a saccade-related motor burst for anti-saccades into the response field. The magnitude of the saccade-related activity, however, was weaker for anti-saccades compared with pro-saccades (Fig. 4F,H, right panels vs Fig. 4E,G, left panels).
The activity of a single burst neuron is shown in Figure4I–L. The activity of the burst neuron was very similar to the activity of the buildup neuron, except that the burst neuron by definition did not display a significant increase in low-frequency prestimulus activity during the gap period (Fig.4K,L). It had a stimulus-related burst in discharge on trials in which the stimulus was presented into the neuron’s response field (Fig. 4I,K; first peak). The magnitude of this response was significantly greater on pro-saccade trials (Fig.4I,K, left panels) than on anti-saccade trials (Fig. 4I,K, right panels). The neuron then displayed a high-frequency burst in discharge associated with pro-saccades into its response field (Fig. 4I,K,left panels; second peak), whereas the activity decayed before saccade onset on anti-saccade trials (Fig.4I,K, right panels). On trials in which the stimulus was presented ipsilateral to the neuron’s response field, the neuron was silent on pro-saccade trials (Fig.4J,L, left panels), but it discharged a saccade-related burst of action potentials for anti-saccades into the response field (Fig. 4J,L, right panels). The saccade-related activity was weaker for anti-saccades than for pro-saccades.
The activity of a single fixation neuron in the pro-/anti-saccade paradigm is shown in Figure 4M–P. The neuron was tonically active during visual fixation of the FP in the instruction period on pro-saccade trials (Fig. 4M–P, left panels) and on anti-saccade trials (Fig. 4M–P,right panels), but this activity was higher on anti-saccade trials compared with pro-saccade trials. During the gap period, the neuron decreased its activity on pro-saccade trials (Fig.4O,P, left panels) and on anti-saccade trials (Fig. 4O,P, right panels). The neuron then displayed a discrete pause in discharge associated with pro-saccades (Fig. 4M–P, left panels) and anti-saccades (Fig. 4M–P, right panels).
We recorded sufficient data from 114 SC neurons (19 visual neurons, 22 fixation neurons, 40 buildup neurons, and 33 burst neurons) in two monkeys performing the pro-/anti-saccade paradigm for quantitative analysis. In the subsequent sections, we contrast the activity of neurons in the pro-/anti-saccade paradigm (1) during the instruction period (for visual, fixation, buildup, and burst neurons), (2) during the gap period (for fixation and buildup neurons), (3) between prestimulus neural activity and SRTs (for buildup neurons), (4) in magnitude of stimulus-related activity (for visual, burst, and buildup neurons), (5) in level of pre-saccade activity (for burst and buildup neurons), and (6) in magnitude of saccade-related activity (for burst and buildup neurons). For each stage of analysis, at least five responses were required in each category. Therefore the number of neurons tested in each analysis varied.
Instruction period-related neuronal activity
Each trial started with the presentation of a central FP, whose color was the instruction to generate either a pro-saccade or an anti-saccade on stimulus presentation. Therefore, the monkeys knew before stimulus presentation which type of motor response (pro-saccade or anti-saccade) was required on a given trial and could potentially use this information to prepare their response, although the direction of the response was not specified at that time.
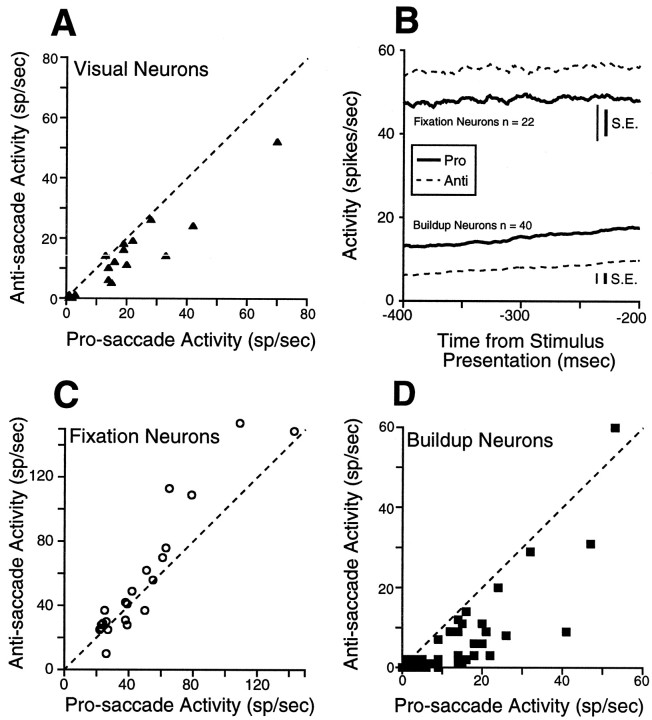
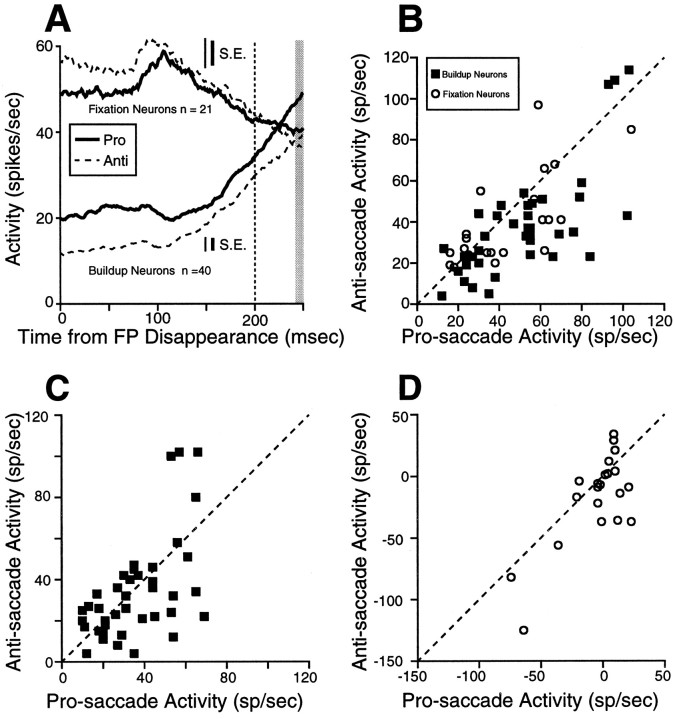
We found that fixation during the instruction period on anti-saccade trials was associated with a significant decreased activity of visual neurons in the superficial SC compared with fixation during the instruction period on pro-saccade trials (Fig.5A; Table2). Fixation neurons in the intermediate SC had a significant increase in activity on anti-saccade trials compared with pro-saccade trials (Fig. 5B,C; Table 2), whereas buildup neurons showed the opposite discharge behavior (Fig.5B,D; Table 2). No differences in instruction period-related discharge were observed between pro-saccade and anti-saccade trials in burst neurons (Table 2).
Fig. 5.
Activity during the instruction period for the sample of fixation neurons, buildup, and visual neurons.A, The mean discharge rate of individual visual neurons during the instruction period on pro-saccade trials is plotted against the mean activity during anti-saccade trials. Gap and overlap trials are combined. B, The thick solid linesrepresent the mean spike density on pro-saccade trials, and thedashed lines represent the mean spike density on anti-saccade trials; same as A for fixation (C) and buildup neurons (D). Dashed line, unity line (slope = 1). SE is the SEM discharge rate, averaged from the SEM computed in each bin (1 msec).
Table 2.
Comparison of discharge in pro-saccades versus anti-saccades
| Cell type | n | Discharge [mean ± SE (range) in spikes/sec] | Group statistics | Test | Individual statistics: cells with significant differences (%) | |||
|---|---|---|---|---|---|---|---|---|
| Pro-saccades | Anti-saccades | df | t | p | ||||
| Instruction period | ||||||||
| Visual | 19 | 18.7 ± 3.8 (1–70) | 13.1 ± 2.9 (0–52) | <0.0001 | W | 53 | ||
| Fixation | 22 | 48.5 ± 6.5 (22–143) | 55.8 ± 8.7 (10–154) | <0.05 | W | 45 | ||
| Burst | 33 | 10.2 ± 3.0 (0–71) | 9.4 ± 3.4 (0–88) | 0.49 | W | |||
| Buildup | 40 | 13.9 ± 2.0 (0–53) | 6.9 ± 1.8 (0–60) | <0.0001 | W | 73 | ||
| End of the gap period | ||||||||
| Fixation | 21 | 44.4 ± 5.1 (16–104) | 40.2 ± 4.9 (18–97) | 20 | 1.09 | 0.29 | T | |
| Buildup | 40 | 52.6 ± 4.2 (12–115) | 42.2 ± 5.2 (5–175) | 39 | 2.98 | 0.005 | T | 40 |
| Change in activity during the gap period | ||||||||
| Fixation | 21 | −5.2 ± 5.5 (−74–23) | −17.1 ± 8.1 (−125–34) | 20 | 2.21 | <0.05 | T | 33 |
| Buildup | 40 | 35.9 ± .28 (10–69) | 34.3 ± 3.9 (4–102) | 39 | 0.51 | 0.61 | T | |
| Magnitude of stimulus-related activity | ||||||||
| Visual | 18 | 217.1 ± 25.1 (97–499) | 185.1 ± 24.7 (66–505) | 17 | 2.99 | <0.01 | T | 61 |
| Burst | 16 | 110.1 ± 84.7 (51–249) | 84.7 ± 12.0 (30–179) | 15 | 3.40 | <0.005 | T | 25 |
| Buildup | 23 | 158.9 ± 17.2 (50–332) | 134.9 ± 15.9 (44–300) | 22 | 3.51 | <0.005 | T | 35 |
| Level of pre-saccade activity | ||||||||
| Burst | 11 | 148.4 ± 19.2 (43–288) | 38.5 ± 3.0 (3–102) | 10 | 5.86 | <0.0005 | T | 100 |
| Buildup | 20 | 244.4 ± 19.8 (102–410) | 85.3 ± 7.7 (27–151) | 19 | 8.46 | <0.0001 | T | 100 |
| Magnitude of saccade-related activity | ||||||||
| Burst | 11 | 273.1 ± 41.7 (98–574) | 98.0 ± 18.7 (15–192) | 10 | 5.82 | <0.001 | T | 100 |
| Buildup | 20 | 410.5 ± 32.7 (196–625) | 154.5 ± 15.2 (37–285) | 19 | 9.82 | <0.0001 | T | 100 |
W, Wilcoxon signed-rank test; T, Student’s ttest.
Gap period-related neuronal activity
Previous studies (Dorris and Munoz, 1995; Munoz and Wurtz, 1995a;Dorris et al., 1997; Everling et al., 1998c) have shown that neurons in the SC change their activity during the gap period on pro-saccade trials: fixation neurons decrease their activity and buildup neurons increase their activity. Half of the trials in the pro-/anti-saccade paradigm contained a gap period in which the FP disappeared 200 msec before the stimulus was presented.
Fixation neurons reduced their discharge rate during the gap period on pro-saccade trials and anti-saccade trials (Fig.6A) after a transient increase in discharge after FP disappearance in some fixation neurons (Everling et al., 1998c). The activity of fixation neurons did not differ significantly in this interval between pro-saccades and anti-saccades (Fig. 6B, ○; Table 2).
Fig. 6.
Activity during the gap period for fixation neurons and buildup neurons. A, The thick solid lines represent the mean spike density on pro-saccade trials, and the dashed lines represent the mean spike density on anti-saccade trials. The vertical dashed line indicates the time of stimulus presentation. B, The mean discharge rate of individual fixation and buildup neurons at the end of the gap period on pro-saccade trials is plotted against the mean activity at the end of the gap period on anti-saccade trials (shaded area in A). One additional buildup neuron (115 spikes/sec pro-saccade activity and 175 spikes/sec anti-saccade activity) was not plotted in B. C, Change in neuronal activity during the gap period for individual buildup neurons and (D) fixation neurons. Dashed line, unity line (slope = 1). SE is the SEM discharge rate, averaged from the SEM computed in each bin (1 msec).
Buildup neurons increased their activity during the gap period on pro-saccade trials and anti-saccade trials (Fig. 6A). The activity at the end of the gap period, however, was significantly different between pro-saccade trials and anti-saccade trials (Fig.6B, ▪; Table 2).
No differences in the change in neuronal activity were found from the instruction period to the end of the gap period between pro-saccade and anti-saccade trials in buildup neurons (Fig.6C; Table 2). Fixation neurons, however, had a significantly stronger decrease in discharge during the gap period on anti-saccade trials compared with pro-saccade trials (Fig. 6D; Table 2).
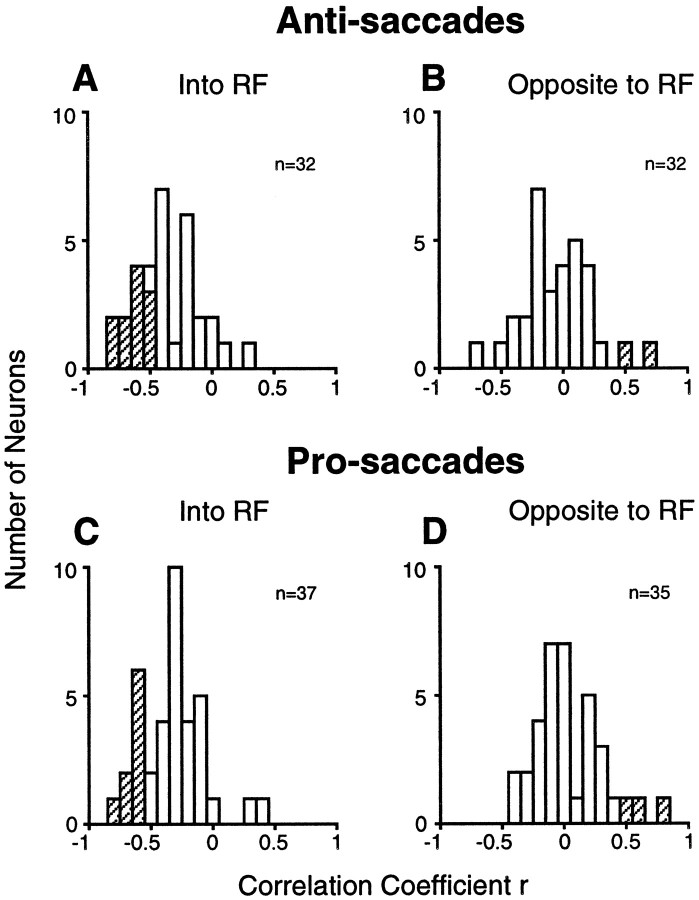
Relationship between SRT and neuronal activity
It was demonstrated previously that the prestimulus activity of buildup neurons predicts SRTs in pro-saccade gap tasks on a trial-by-trial basis (Dorris et al., 1997; Dorris and Munoz, 1998). The correlation of prestimulus activity with SRT was significantly greater for the condition when the stimulus was presented in the neuron’s response field as compared with when it was presented on the opposite side at the mirror position. This finding has been taken as evidence that the prestimulus activity of buildup neurons reflects motor preparation (Dorris and Munoz, 1998). However, because stimulus location and response location are identical in the pro-saccade task, it remained possible that the decrease in SRTs with an increase in prestimulus activity is the result of faster stimulus processing and not increased motor preparation. The anti-saccade task allows the dissociation between those two processes, because stimulus and response locations are different in this task. A negative correlation of the prestimulus activity of neurons contralateral to the saccade with SRTs would support the motor preparation hypothesis, whereas a negative correlation between the prestimulus activity of neurons contralateral to the stimulus would support the stimulus-processing hypothesis. Our results support the motor preparation hypothesis. For anti-saccades, the mean correlation coefficient between SRT and prestimulus activity of buildup neurons in the SC contralateral to the saccade (Fig.7A) was −0.31 ± 0.05 (range −0.77 to +0.31), whereas the mean correlation coefficient between SRT and prestimulus activity in the SC contralateral to the stimulus (Fig. 7B) was 0.00 ± 0.05 (range −0.63 to +0.76) (t test; t = 4.59, df = 62, p < 0.0001). We also calculated the mean correlation coefficients between prestimulus activity of buildup neurons and SRT for pro-saccades. For pro-saccades, the mean correlation coefficient between SRT and prestimulus activity of buildup neurons contralateral to the stimulus and saccade (Fig.7C) was −0.28 ± 0.04 (range −0.77 to +0.48), and the mean correlation coefficient between SRT and prestimulus activity in the SC ipsilateral to the stimulus and saccade (Fig. 7D) was 0.09 ± 0.05 (range −0.38 to +0.82) (t test;t = 5.86, df = 70, p < 0.0001). These values are similar to those reported previously (Table3). The correlation coefficients for pro-saccades and anti-saccades into the neuron’s response field (t test; t = 0.47, df = 67,p = 0.64) and the correlation coefficients for pro-saccades and anti-saccades to the mirror position of the neuron’s response field did not differ (t test; t = 1.33, df = 65, p = 0.19). Thus, these findings support the hypothesis that an increased prestimulus activity of buildup neurons in the SC shortens SRTs because of an increased motor preparation and not faster stimulus processing.
Fig. 7.
Distribution of correlation coefficients for the relationship between prestimulus activity and SRT in the gap condition for anti-saccades into the neuron’s response field (A), anti-saccades to the mirror position (B), pro-saccades into the response field (C), and pro-saccades to the mirror position (D). RF, Response field. Thehatched bars represent statistically significant correlations (p < 0.05).
Table 3.
Means of correlation coefficients between SRT and prestimulus neuronal activity
| Pro-saccades | Anti-saccades | |||
|---|---|---|---|---|
| Contralateral | Ipsilateral | Contralateral | Ipsilateral | |
| Dorris et al. (1997) | −0.23 | 0.01 | ||
| Dorris and Munoz (1998) | −0.43 | 0.18 | ||
| Present study | −0.28 | 0.09 | −0.31 | 0.00 |
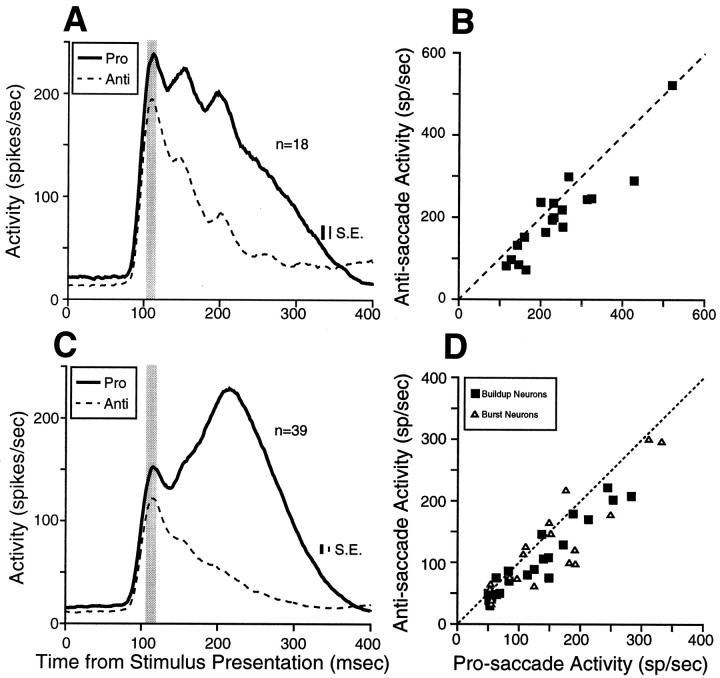
Magnitude of stimulus-related responses
The stimulus-related response of many SC neurons in the superficial layers is enhanced in a pro-saccade task when the stimulus is the target for a saccade compared with a fixation task when the maintenance of fixation on the FP is required (Goldberg and Wurtz, 1972; Mohler and Wurtz, 1976). In the anti-saccade task, the stimulus acts both as a landmark for the anti-saccade and as a distractor that can elicit an incorrect pro-saccade. To assess the influence of these task demands on the stimulus-related activity of SC neurons, we compared the peak of the stimulus-related response on pro-saccade trials and anti-saccade trials for visual neurons (Fig.8A,B) and saccade-related neurons (buildup and burst neurons) (Fig.8C,D). All visual neurons in the superficial layers of the SC increased discharge only when the stimulus was presented into their response field, regardless of the direction of the subsequent saccade (for an example, see Fig. 4). The stimulus-related response, however, was higher on pro-saccade trials compared with anti-saccade trials for the majority of the visual neurons (Fig. 8B; Table2).
Fig. 8.
Stimulus-related activity for the sample of visual neurons (A, B) and saccade-related neurons with stimulus-related responses (C, D) in the overlap condition. A, C, The thick solid linerepresents the mean spike density on pro-saccade trials, and the dashed line represents the mean spike density onanti-saccade trials. B, D, The stimulus-related activity of individual neurons on pro-saccade trials is plotted against the stimulus-related activity on anti-saccade trials (shaded area in A, C). Dashed line, unity line (slope = 1). SE is the SEM discharge rate, averaged from the SEM computed in each bin (1 msec).
Significant stimulus-related activity was found in 56% (23/41) of the buildup neurons and in 48% (16/33) of the burst neurons. All saccade-related neurons with stimulus-related responses had only a transient increase in discharge when the stimulus was presented into their response field, regardless of whether the monkeys generated pro-saccades to the stimulus or anti-saccades to the opposite side (for examples, see Fig. 4). However, of those saccade-related neurons with visual responses, the majority had a stronger stimulus-related response on pro-saccade trials compared with anti-saccade trials (Fig.8D; Table 2).
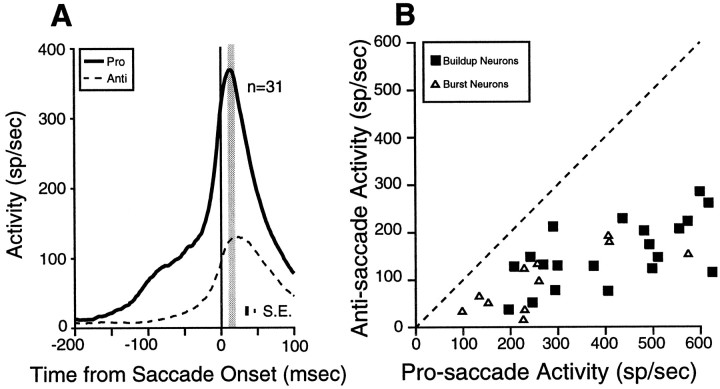
Pre-saccade activity
Two extreme hypotheses have been proposed for the contribution of the SC to the generation of anti-saccades: the serial hypothesis (Guitton, 1991) and the bypass hypothesis (Forbes and Klein, 1996). The bypass hypothesis predicts that saccade-related neurons in the SC ipsilateral to the stimulus are inactive for anti-saccades. To test this prediction, we compared the level of pre-saccade activity in burst and buildup neurons in the SC ipsilateral to the stimulus for pro-saccades and anti-saccades (Fig.9A). The majority of burst neurons (Fig. 9B, ▵; Table 2) and all buildup neurons (Fig. 9B, ▪; Table 2) had a higher discharge for anti-saccades than for pro-saccades in the SC ipsilateral to the stimulus. Thus, saccade-related neurons in the SC ipsilateral to the stimulus were active for anti-saccades. This finding does not support the bypass hypothesis.
Fig. 9.
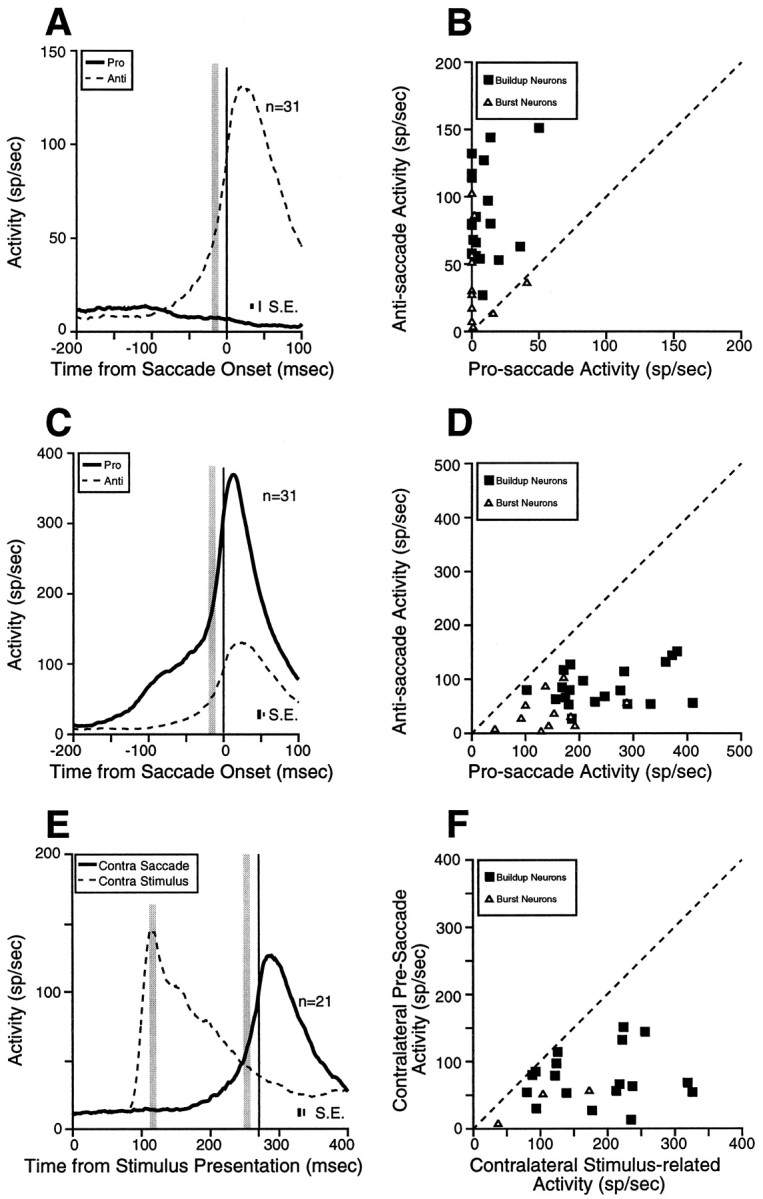
Comparisons of pre-saccade activity of saccade-related neurons in the overlap condition. A, Mean spike density in the SC ipsilateral to the stimulus on pro-saccade trials (thick solid line) and on anti-saccade trials (dashed line). B, The pre-saccade activity of individual buildup and burst neurons in the SC ipsilateral to the stimulus on pro-saccade trials is plotted against the pre-saccade activity on anti-saccade trials (shaded areain A). C, Mean spike density in the SC contralateral to the movement direction, on pro-saccade trials (thick solid line) and anti-saccade trials (dashed line). D, The pre-saccade activity of individual buildup and burst neurons in the SC contralateral to the movement on pro-saccade trials is plotted against the pre-saccade activity on anti-saccade trials (shaded area in C). E, Mean spike density in the SC contralateral to the movement on anti-saccade trials (thick solid line) and mean spike density in the SC contralateral to the stimulus on anti-saccade trials (dashed line). F, The stimulus-related activity of individual buildup and burst neurons in the SC contralateral to the stimulus on anti-saccade trials is plotted against the pre-saccade activity contralateral to the saccade on anti-saccade trials (shaded area in E). Dashed line in B, D, andF, unity line (slope = 1). SE is the SEM discharge rate, averaged from the SEM computed in each bin (1 msec).
The serial hypothesis predicts similar pre-activity of saccade-related neurons in the SC before pro-saccades and anti-saccades. To test this prediction, we compared the level of pre-saccade activity before saccade initiation in burst and buildup neurons in the SC contralateral to the saccade for pro-saccades and anti-saccades (Fig. 9C). Burst neurons and buildup neurons had a significantly higher level of pre-saccade activity on pro-saccade trials compared with anti-saccade trials (Fig. 9D; Table 2). This finding does not support the serial hypothesis.
Schall and Hanes (1996) demonstrated a fixed level of pre-saccade activity in individual FEF neurons for pro-saccades in a countermanding task. On the basis of this observation, they hypothesized that FEF neurons have fixed saccade thresholds and that saccades are elicited only when this threshold activity level is surpassed. Our findings show that the threshold activity level is different for pro-saccades and anti-saccades in the SC. If a fixed saccade threshold exists in individual SC saccade-related neurons in the anti-saccade task, then the level of pre-saccade activity before anti-saccades should never be reached by the stimulus-related activity on correct anti-saccade trials. To test this hypothesis, we compared the stimulus-related activity (without subtracting the baseline activity) in the SC contralateral to the stimulus with the level of pre-saccade activity in the SC contralateral to the saccade on anti-saccade trials in burst and buildup neurons with stimulus-related responses (Fig.9E,F). All burst neurons (n = 3) and all buildup neurons (n = 18) in the sample had higher stimulus-related activities than pre-saccade activities on anti-saccade trials (Fig. 9F). Significant differences were found in all three burst neurons, and in 72% (13/18) of the buildup neurons (t test; p < 0.05). For burst neurons, the mean stimulus-related activity was 104.7 ± 39.3 spikes/sec (range 37–173 spikes/sec), and the pre-saccade activity was 30.0 ± 15.6 spikes/sec (range 7–51 spikes/sec). For buildup neurons, the mean stimulus-related activity was 182.9 ± 18.4 spikes/sec (range 80–326 spikes/sec) and the pre-saccade activity was 75.9 ± 9.2 spikes/sec (range 13–151 spikes/sec) on anti-saccade trials (pairedt test; t = 5.37, df = 17,p < 0.0001). Thus, the stimulus-related activity of SC neurons was higher than the pre-saccade activity on anti-saccade trials, although the stimulus-related activity did not elicit a saccade.
Taken together, these results show that neurons in the SC are active during the generation of anti-saccades. However, the markedly weaker pre-saccade activity before anti-saccades and the finding that the stimulus-related activity was higher than the saccade-related activity on anti-saccade trials suggest that additional signals from other brain areas bypass the SC.
Magnitude of saccade-related responses
The anti-saccade task requires the generation of a saccade away from the visual stimulus, to an unmarked spatial location, whereas a pro-saccade is directed toward a visual stimulus. A common finding in all saccade-related neurons was that they discharged for saccades into their response field, whether the stimulus was presented in the response field (pro-saccades) or on the opposite side (anti-saccade).
Figure 10 shows the discharge of a typical burst neuron (A) and a typical buildup neuron (B) aligned on the onset of pro-saccades (left panel) and anti-saccades (right panel). Both neurons displayed stronger saccade-related responses for pro-saccades than for anti-saccades. It should be noted that although the metrics of pro-saccades and anti-saccades were matched for this comparison, the peak velocities were lower for anti-saccades than for pro-saccades (Fig. 10) [see also Smit et al. (1987); Van Gelder et al. (1997); Amador et al. (1998)].
Fig. 10.
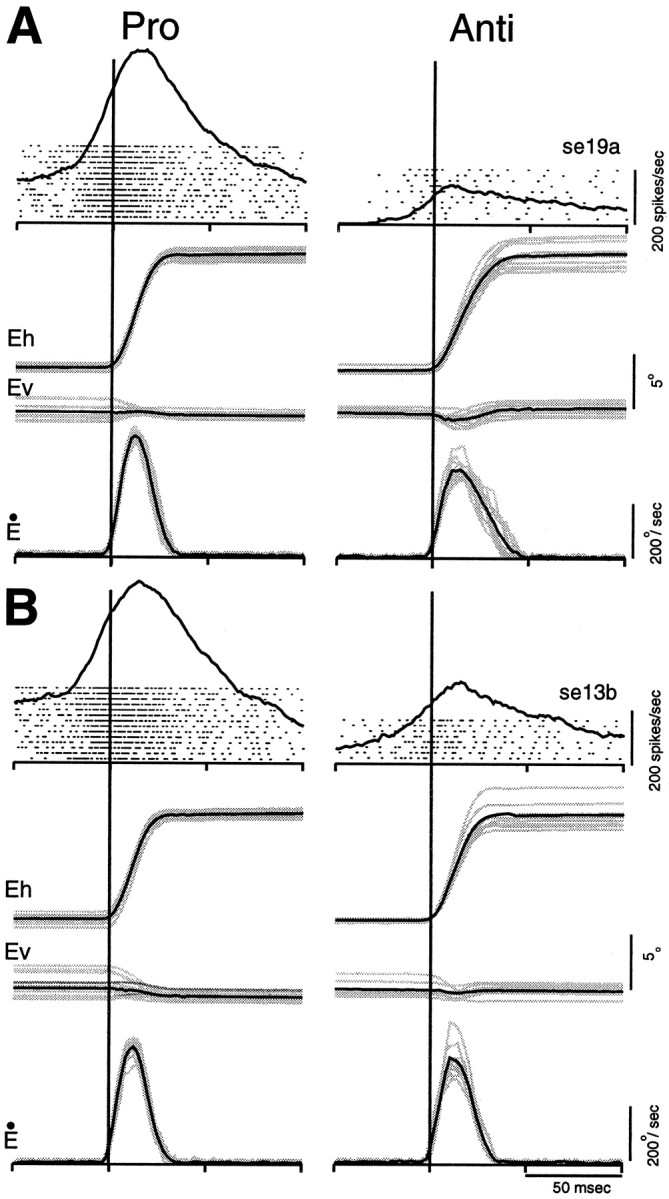
Comparison of saccade-related activity in two individual SC neurons for pro-saccades and anti-saccades.A, Neural activity of a burst neuron aligned on the onset (vertical lines) of pro-saccades (left panel) and anti-saccades (right panel). Each dot indicates the time of an action potential relative to saccade onset, and each row represents one trial. Superimposed on the rasters is the average activation waveform. The horizontal (Eh) and vertical (Ev) eye positions and the radial velocities (Ė) are shown below.Gray lines indicate individual trials and black lines indicate averages. B same asA, but for a buildup neuron.
To investigate the contribution of saccade-related neurons in the generation of anti-saccades, we compared the magnitude of the saccade-related responses of burst neurons and buildup neurons on pro-saccade trials with the magnitude of the saccade-related response on anti-saccade trials (Fig.11A). All burst neurons and all buildup neurons in our sample had significantly stronger saccade-related responses for pro-saccades compared with anti-saccades (Fig. 11B; Table 2). Thus, saccade-related neurons in the SC displayed a motor burst for anti-saccades into their response field, but the magnitude of this motor burst was significantly lower than for pro-saccades.
Fig. 11.
Saccade-related activity for the sample of saccade-related neurons in the overlap condition. A, Thethick solid line represents the mean spike density on pro-saccade trials, and the dashed line represents the mean spike density on anti-saccade trials. B, The saccade-related activity of individual buildup and burst neurons on pro-saccade trials is plotted against the saccade-related activity on anti-saccade trials (shaded area in A).Dashed line, unity line (slope = 1). SE is the SEM discharge rate, averaged from the SEM computed in each bin (1 msec).
DISCUSSION
In this study, we have provided important new insights into how anti-saccades are prepared. By examining the activity of neurons in the SC of monkeys performing a pro-/anti-saccade paradigm, we were able to show that anti-saccades were associated with decreased preparatory activity (Figs. 4, 5B,D) and increased fixation activity (Figs. 4, 5B,C) during the instruction period before stimulus presentation. Furthermore, neurons with stimulus-related activity responded to stimulus presentations into their response field (Figs. 4, 8), regardless of the direction of the impending movement. The magnitude of the stimulus-related response, however, was weaker before anti-saccades (Figs. 4, 8). The movement-related activity of SC neurons represented the motor signal for the eye movement (Fig. 4), regardless of the location of the visual stimulus. The magnitude of the movement-related activity, however, was markedly weaker for anti-saccades (Figs. 4, 9-11). Taken together, these results demonstrate that the instructional set to generate an anti-saccade modulates the activity of SC neurons. The low pre-saccade activity (Fig. 9) and the weak motor burst for anti-saccades (Figs. 10,11) suggest that although the SC does participate in anti-saccade generation, additional movement signals are necessary that presumably bypass the SC.
Prestimulus activity in the SC is modulated by behavioral context
The importance of SC fixation neurons in the maintenance of fixation has been shown by pharmacological (Munoz and Wurtz, 1992,1993b) and microstimulation studies (Munoz and Wurtz, 1993b). An increased post-saccadic enhancement in neuronal activity has also been found in some fixation neurons, which has been interpreted as a correlate for the refractory state of the saccadic system after visually guided saccades (Munoz and Wurtz, 1993a; Everling et al., 1998c). In the present study, we have demonstrated that many fixation neurons also modulate their activity depending on the instruction to generate either a pro-saccade or an anti-saccade. The anti-saccade instruction was associated with an increased activity of many fixation neurons and with reduced low-frequency activity of virtually all buildup neurons. Enhanced fixation activity during anti-saccade trials could lead to the reduced buildup activity via intracollicular inhibition (Munoz and Istvan, 1998).
The disappearance of the FP in gap pro-saccade tasks is associated with a decrease in the activity of SC fixation neurons (Dorris and Munoz, 1995; Dorris et al., 1997; Everling et al., 1998c) and an increase in low-frequency activity in SC buildup neurons (Munoz and Wurtz, 1995a;Dorris et al., 1997; Dorris and Munoz, 1998), which may be partly mediated by intracollicular inhibitory connections (Munoz and Istvan, 1998). Our results show that fixation neurons also decreased their activity on anti-saccade trials during the gap period to an activity level that did not differ from the activity level on pro-saccade trials. Therefore, the activity of fixation neurons alone cannot account for the attenuated activity in buildup neurons at the end of the gap period on anti-saccade trials compared with pro-saccade trials.
The intermediate layers of the SC receive direct afferents from many cortical areas (for review, see Sparks and Hartwich-Young, 1989). Moreover, cortical signals can reach the SC indirectly by a pathway that includes the caudate nucleus and the substantia nigra pars reticulata (SNpr) (for review, see Hikosaka and Wurtz, 1989). It is likely that the modulation of prestimulus activity during the instruction period and the gap period between pro-saccade trials and anti-saccade trials are shaped by these projections. Brain imaging studies in humans (Paus et al., 1993; O’Driscoll et al., 1995; Sweeney et al., 1996; Doricchi et al., 1997) have shown that the performance of an anti-saccade compared with a pro-saccade task is associated with an increased blood flow in many cortical and subcortical areas, e.g., DPC, FEF, SEF, cingulate, posterior parietal cortex, thalamus, and basal ganglia. An important tonic inhibitory input on SC neurons arises from the SNPr (Hikosaka and Wurtz, 1983,1985), which in turn is phasically inhibited by the caudate nucleus. An increased inhibition from the SNpr could account for the reduced activity of buildup neurons during the instruction and gap period. Interestingly, it has been shown that Huntington’s disease, which results from the loss of cholinergic neurons in the striatum, is associated with high rates of reflexive pro-saccades in the anti-saccade task (Lasker et al., 1987).
Recently, it has been demonstrated that a high prestimulus activity of buildup neurons at the site in the SC where the stimulus is represented is associated with reflexive saccades toward the stimulus in an anti-saccade task (Everling et al., 1998a). It was hypothesized that when a visual stimulus is presented in the response field of buildup neurons that already have a high level of prestimulus activity, the visual burst may trigger a saccade directly (see below). Taken together, the increased prestimulus activity of fixation neurons during the instruction period and the decreased prestimulus activity of buildup neurons on anti-saccade trials may reflect different preparatory sets (Evarts et al., 1984) that are necessary to preset the oculomotor system to allow for the preparation of an anti-saccade saccade by reducing the probability of an automatic pro-saccade.
Stimulus-related activity in the SC is modulated by behavioral context
Recently, it was demonstrated that stimulus-related activity was independent of movement direction in the primary motor cortex (Requin and Riehle, 1995; Riehle et al., 1997). In these studies, monkeys had to move a pointer toward a peripheral visual stimulus (congruent movement) or in the opposite direction (incongruent movement), depending on the color of the stimulus. These results were interpreted as evidence for an automatic activation of the congruent response (Riehle et al., 1997), and as evidence for a partial transmission from visual to motor cortical areas before the incongruent movement was programmed (Requin and Riehle, 1995).
We suggest that the stimulus-related activity of burst and buildup neurons in the SC on anti-saccade trials also represents an automatically activated congruent response. This assumption follows the recently proposed hypothesis that the visual response of SC neurons in the intermediate layers can be viewed as a motor burst that fails to elicit a saccade (Sommer, 1994; Edelman and Keller, 1996; Dorris et al., 1997). Although sensory in its origin, the visual burst and the motor burst of saccade-related neurons are simply action potentials from the same neuron, which makes it impossible for the recipient neurons to differentiate between a visual and a motor signal. The hypothesis is supported by two findings: (1) the stimulus-related response is lower than the saccade-related response in these neurons, and (2) express saccades, which have reaction times <125 msec, are preceded by only one burst, which occurs at the same time as the visual burst, but with a greater magnitude (Edelman and Keller, 1996; Dorris et al., 1997).
The weaker stimulus-related responses on anti-saccade trials than on pro-saccade trials could represent a mechanism that reduces the risk of generating a pro-saccade with express-saccade latency before the anti-saccade is programmed. Further work is needed to understand the sources of the modulation of stimulus-related responses in SC neurons. One possibility is that the responses are weaker on anti-saccade trials because of an increased inhibition of saccade-related neurons before stimulus presentation. Our findings of differences in the prestimulus activity between pro-saccade and anti-saccade trials support this possibility. Another possibility is a reduced visual input on anti-saccade trials. Our finding of reduced stimulus-related responses in superficial SC neurons may indicate a reduced activity in the visual cortex on anti-saccade trials, because the superficial SC receives afferent projections from the striate (Weyand et al., 1986) and extrastriate visual cortex (Huerta and Harting, 1984). Alternatively, the reduced stimulus-related response of SC neurons may be the result of increased inhibition signals at the time of stimulus presentation on anti-saccade trials. Schlag-Rey et al. (1997) reported that SEF neurons have stronger visual responses on anti-saccade trials compared with pro-saccade trials. They proposed that this could reflect a role of the SEF in the inhibition of quick reflexive saccades.
Role of the SC in the generation of anti-saccades
The serial hypothesis for anti-saccades (Guitton, 1991) proposed that SC neurons receive direct and indirect cortical inputs via the basal ganglia and in turn send the motor command for the anti-saccade to the preoculomotor neurons in the brain stem. In contrast, the bypass hypothesis (Forbes and Klein, 1996) proposed that the SC is inhibited during the preparation and generation of anti-saccades and that the motor command for the anti-saccade bypasses the SC by direct projections from frontal cortex to the brain stem. On the basis of our results, we can reject the bypass hypothesis. SC neurons provide a movement signal for anti-saccades. However, although SC saccade-related neurons are active for anti-saccades, their low level of pre-saccade activity before an anti-saccade is presumed to be insufficient to trigger the saccade.
The generation of a saccade requires the suppression of omnipause neurons (OPNs), which act as a tonic inhibitory gate for the brain stem saccade generator (for review, see Moschovakis et al., 1996). OPNs are inhibited polysynaptically by SC saccade-related neurons (Raybourn and Keller, 1997). The low pre-saccade discharge before an anti-saccade may be insufficient by itself to suppress OPNs, and additional suppression signals are likely needed. These additional signals may arise from FEF or SEF neurons that also project to the brain stem saccade burst generator (for review, see Schall, 1997). A compensatory trigger input from SEF neurons is supported by the higher level of pre-saccade activity in SEF neurons for anti-saccades compared with pro-saccades (Schlag-Rey et al., 1997).
If additional signals not only bypass the SC to trigger an anti-saccade but also during an anti-saccade, then these findings are likely to have important implications for current models of saccade generation and control. Several recent models have placed the SC within a feedback loop controlling saccade accuracy (Waitzman et al., 1988, 1991; Droulez and Berthoz, 1991; Munoz et al., 1991, 1996; Lefèvre and Galiana, 1992; Optican, 1994; Munoz and Wurtz, 1995b). In these models, the SC sends a motor error signal to the saccade generator in the pons, and a signal is fed back to the SC to allow a comparison between current and desired eye position to control the amplitude of the saccade. If movement signals bypass the SC during saccade generation, it would suggest that either the SC is located upstream of the feedback mechanism, or more likely, the feedback control mechanisms may be distributed and involve multiple structures and parallel systems (Quaia et al., 1998). Further experimentation is required to test these hypotheses explicitly.
In summary, we have shown that the instructional set to generate either a pro-saccade or an anti-saccade modulates the activity of SC neurons. The results further demonstrate that the SC generates a motor signal for the anti-saccade as predicted by the serial hypothesis, but additional trigger signals, which bypass the SC, are also likely needed for the generation of correct anti-saccades.
Footnotes
This work was supported by a Collaborative Research Grant from the National Sciences and Engineering Research Council of Canada to R.M.K. and D.P.M. S.E. was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft. M.C.D. was supported by a Queen’s Graduate Fellowship. D.P.M. is a research scholar of the EJLB foundation and a Medical Research Council of Canada Scientist. We thank A. Lablans for her outstanding assistance in monkey training, and K. Moore and D. Hamburger for computer assistance. I. T. Armstrong, B. D. Corneil, E. Olivier, and A. Spantekow commented on an earlier version of this manuscript. We gratefully acknowledge the helpful comments of the anonymous referee.
Correspondence should be addressed to Dr. Douglas Munoz, Department of Physiology, Queen’s University, Kingston, Ontario, Canada, K7L 3N6.
Dr. Everling’s present address: Department of Experimental Psychology, Oxford University, South Parks Road, Oxford OX1 3UD, UK.
REFERENCES
- 1.Amador N, Schlag-Rey M, Schlag J. Primate antisaccades. I. Behavioral characteristics. J Neurophysiol. 1998;80:1775–1786. doi: 10.1152/jn.1998.80.4.1775. [DOI] [PubMed] [Google Scholar]
- 2.Crist CF, Yamasaki DSG, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods. 1988;26:117–122. doi: 10.1016/0165-0270(88)90160-4. [DOI] [PubMed] [Google Scholar]
- 3.Doricchi F, Perani D, Inoccia C, Grassi F, Cappa SF, Bettinari V, Galati G, Pizzamigilio L, Fazio F. Neural control of fast-regular saccades and antisaccades, an investigation using positron emission tomography. Exp Brain Res. 1997;116:50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- 4.Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol. 1995;73:2558–2562. doi: 10.1152/jn.1995.73.6.2558. [DOI] [PubMed] [Google Scholar]
- 5.Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci. 1998;18:7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci. 1997;17:8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Droulez J, Berthoz A. A neural network model of sensoritopic maps with predictive short-term memory properties. Proc Natl Acad Sci USA. 1991;88:9653–9657. doi: 10.1073/pnas.88.21.9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelman JA, Keller E. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol. 1996;76:908–926. doi: 10.1152/jn.1996.76.2.908. [DOI] [PubMed] [Google Scholar]
- 9.Evarts EV, Shinoda Y, Wise SP. Neurophysiological approaches to higher brain functions. Wiley; New York: 1984. [Google Scholar]
- 10.Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- 11.Everling S, Dorris MC, Munoz DP. Reflex suppression in the anti-saccade task is dependent upon prestimulus neural processes. J Neurophysiol. 1998a;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- 12.Everling S, Dorris MC, Klein RM, Munoz DP. Superior colliculus neuronal activity in monkeys during pro- and anti-saccade tasks. Soc Neurosci Abstr. 1998b;24:163.1. [Google Scholar]
- 13.Everling S, Paré M, Dorris MC, Munoz DP. Comparison of the discharge characteristics of brain stem omnipause neurons and superior colliculus fixation neurons in monkey: implications for control of fixation and saccade behavior. J Neurophysiol. 1998c;79:511–528. doi: 10.1152/jn.1998.79.2.511. [DOI] [PubMed] [Google Scholar]
- 14.Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 1983;260:21–26. doi: 10.1016/0006-8993(83)90760-6. [DOI] [PubMed] [Google Scholar]
- 15.Fischer B, Weber H. Characteristics of “anti” saccades in man. Exp Brain Res. 1992;89:415–424. doi: 10.1007/BF00228257. [DOI] [PubMed] [Google Scholar]
- 16.Forbes K, Klein RM. The magnitude of the fixation offset effect with endogenously and exogenously controlled saccades. J Cognit Neurosci. 1996;8:344–352. doi: 10.1162/jocn.1996.8.4.344. [DOI] [PubMed] [Google Scholar]
- 17.Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 19.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- 21.Guitton D. Control of saccadic eye movements by the superior colliculus and basal ganglia. In: Carpenter RHS, editor. Eye movements. Macmillan; London: 1991. pp. 244–276. [Google Scholar]
- 22.Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- 23.Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 24.Hallett PE, Adams BD. The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Res. 1980;20:329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- 25.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 26. Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. 1982. W ESCON Conf Proc 2:1–10.
- 27.Hikosaka O, Wurtz RH. Visual and oculomotor functions of the monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- 28.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- 29.Hikosaka O, Wurtz RH. The basal ganglia. In: Wurtz RH, Goldberg ME, editors. The neurobiology of saccadic eye movements. Elsevier; Amsterdam: 1989. pp. 257–281. [Google Scholar]
- 30.Huerta MF, Harting JK. Connectional organization of the superior colliculus. Trends Neurosci. 1984;7:286–289. [Google Scholar]
- 31.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:553–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 32.Lasker AG, Zee DS, Hain TC, Folstein SE. Saccades in Huntington’s disease: initiation defects and distractibility. Neurology. 1987;44:2285–2289. doi: 10.1212/wnl.37.3.364. [DOI] [PubMed] [Google Scholar]
- 33.Lefèvre P, Galiana HL. Dynamic feedback to the superior colliculus in a neural network model of the gaze control system. Neural Networks. 1992;5:871–890. [Google Scholar]
- 34.Leichnetz GR, Spencer RF, Hardy SGP, Astruc K. The prefrontal corticotectal projection in the monkey: an anterograde and retrograde horseradish peroxidase study. Neuroscience. 1981;6:1023–1041. doi: 10.1016/0306-4522(81)90068-3. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita N, Hikosaka O. Minimal synaptic delay in the saccadic output pathway of the superior colliculus studied in awake monkey. Exp Brain Res. 1996;112:187–196. doi: 10.1007/BF00227637. [DOI] [PubMed] [Google Scholar]
- 36.Mohler CW, Wurtz RH. Organization of monkey superior colliculus: intermediate layer cells discharging before eye movements. J Neurophysiol. 1976;39:722–744. doi: 10.1152/jn.1976.39.4.722. [DOI] [PubMed] [Google Scholar]
- 37.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Proc Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 38.Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- 39.Munoz DP, Wurtz RH. Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J Neurophysiol. 1992;67:1000–1002. doi: 10.1152/jn.1992.67.4.1000. [DOI] [PubMed] [Google Scholar]
- 40.Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol. 1993a;70:559–575. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- 41.Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol. 1993b;70:576–589. doi: 10.1152/jn.1993.70.2.576. [DOI] [PubMed] [Google Scholar]
- 42.Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol. 1995a;73:2313–2333. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- 43.Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J Neurophysiol. 1995b;73:2334–2348. doi: 10.1152/jn.1995.73.6.2334. [DOI] [PubMed] [Google Scholar]
- 44.Munoz DP, Pêalisson D, Guitton D. Movement of neural activity on the superior colliculus motor map during gaze shifts. Science. 1991;251:1358–1360. doi: 10.1126/science.2003221. [DOI] [PubMed] [Google Scholar]
- 45.Munoz DP, Waitzman DM, Wurtz RH. Activity of neurons in monkey superior colliculus during interrupted saccades. J Neurophysiol. 1996;75:2562–2580. doi: 10.1152/jn.1996.75.6.2562. [DOI] [PubMed] [Google Scholar]
- 46.O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS. Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci USA. 1995;92:925–929. doi: 10.1073/pnas.92.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Optican LM. Control of saccade trajectory by the superior colliculus. In: Fuchs AF, Brandt T, Büttner U, Zee DS, editors. Contemporary ocular motor and vestibular research: a tribute to David A. Robinson. Thieme; Stuttgart, Germany: 1994. pp. 98–104. [Google Scholar]
- 48.Paré M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76:3666–3681. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]
- 49.Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses, a positron emission tomography study. J Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- 50.Pierrot-Deseilligny CP, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114:1472–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- 51.Quaia C, Aizawa H, Optican LM, Wurtz RH. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J Neurophysiol. 1998;79:2097–2110. doi: 10.1152/jn.1998.79.4.2097. [DOI] [PubMed] [Google Scholar]
- 52.Raybourn MS, Keller E. Colliculoreticular organization in primate oculomotor system. J Neurophysiol. 1977;40:861–878. doi: 10.1152/jn.1977.40.4.861. [DOI] [PubMed] [Google Scholar]
- 53.Requin J, Riehle A. Neural correlates of partial transmission of sensorimotor information in the cerebral cortex. Acta Psychologica. 1995;90:81–95. doi: 10.1016/0001-6918(95)00039-w. [DOI] [PubMed] [Google Scholar]
- 54.Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol. 1987;57:147–161. doi: 10.1152/jn.1987.57.1.147. [DOI] [PubMed] [Google Scholar]
- 55.Riehle A, Kornblum S, Requin J. Neural correlates of sensorimotor association in stimulus-response compatibility. J Exp Psychol Hum Percept Perform. 1997;23:1708–1726. doi: 10.1037//0096-1523.23.6.1708. [DOI] [PubMed] [Google Scholar]
- 56.Robinson DS. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- 57.Schall JD. Visuomotor areas of the frontal lobe. Cereb Cortex. 1997;12:527–638. [Google Scholar]
- 58.Schiller PH, Sandel JH, Maunsell JHR. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- 59.Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- 60.Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field. I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol. 1990;301:618–642. doi: 10.1002/cne.903010410. [DOI] [PubMed] [Google Scholar]
- 61.Smit AC, van Gisbergen JA, Cools ARA. A parametric analysis of human saccades in different experimental paradigms. Vision Res. 1987;27:1745–1762. doi: 10.1016/0042-6989(87)90104-0. [DOI] [PubMed] [Google Scholar]
- 62.Sommer MA. Saccades elicited during visual scan in the monkey. Vision Res. 1994;34:2023–2038. doi: 10.1016/0042-6989(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 63.Sparks DL, Mays LE. Movement fields of saccade-related burst neurons in the monkey superior colliculus. Brain Res. 1980;190:39–50. doi: 10.1016/0006-8993(80)91158-0. [DOI] [PubMed] [Google Scholar]
- 64.Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. In: Wurtz RH, Goldberg ME, editors. The neurobiology of saccadic eye movements. Elsevier; Amsterdam: 1989. pp. 213–255. [PubMed] [Google Scholar]
- 65.Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988;271:493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- 66.Sweeney JA, Mintun MA, Kwee S. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- 67.Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- 68.Van Gelder P, Lebedev S, Tsui WH. Peak velocities of visually and nonvisually guided saccades in smooth pursuit and saccadic tasks. Exp Brain Res. 1997;116:201–215. doi: 10.1007/pl00005750. [DOI] [PubMed] [Google Scholar]
- 69.Waitzman DM, Ma TP, Optican LM, Wurtz RH. Superior colliculus neurons provide the saccadic motor error signal. Exp Brain Res. 1988;72:649–652. doi: 10.1007/BF00250610. [DOI] [PubMed] [Google Scholar]
- 70.Waitzman DM, Ma TP, Optican LM, Wurtz RH. Superior colliculus neurons mediate the dynamic characteristics of saccades. J Neurophysiol. 1991;66:1716–1737. doi: 10.1152/jn.1991.66.5.1716. [DOI] [PubMed] [Google Scholar]
- 71.Weyand TG, Malpeli JG, Lee C, Schwark HD. Cat area 17. IV. Two types of corticotectal cells defines by controlling geniculate inputs. J Neurophysiol. 1986;56:1102–1108. doi: 10.1152/jn.1986.56.4.1102. [DOI] [PubMed] [Google Scholar]
- 72.Wise SP, di Pellegrino G, Boussaoud D. The premotor cortex and nonstandard sensorimotor mapping. Can J Physiol Pharmacol. 1996;74:469–482. doi: 10.1139/cjpp-74-4-469. [DOI] [PubMed] [Google Scholar]
- 73.Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. J Neurophysiol. 1972;35:587–596. doi: 10.1152/jn.1972.35.4.587. [DOI] [PubMed] [Google Scholar]