Abstract
Purpose:
To measure the wall-to-lumen ratio (WLR) and the vascular wall cross-sectional area (WCSA) of retinal arterioles by an Adaptive Optics (AO) retinal camera using semi-automated software and comparing them between control and hypertensive population.
Methods:
This was a cross-sectional observational study including a hypertensive group and a control group. Subjects were examined and their medical history recorded. Retinal arteriolar morphometry was assessed by rtx1 AO retinal camera using AOdetect Artery semiautomated software. Main Outcome Measures: WLR and WCSA were measured on the basis of retinal arteriolar wall thickness (W1, W2), lumen diameter (LD) and vessel diameter (VD). Influence of age and arterial hypertension on the WLR and WCSA were examined.
Results:
A total of 150 human subjects were included out of which 110 were controls and 40 were hypertensives under treatment. There was statistically significant difference in the age, systolic and diastolic blood pressures between the control and hypertensive groups (P < 0.01). We found no significant correlation between age and WLR (R2 = 0.049, P > 0.05) or age and WCSA (R2 = 0.045, P > 0.05). We observed a significant difference in WLR and WCSA measurements between control and hypertensive groups (P < 0.01). On measuring intra-observer variability (IOV) we found excellent consistency.
Conclusion:
AO retinal imaging allows a direct measurement of the retinal vessel wall and LD with excellent IOV. WLR and WCSA reflect the remodelling process and can be used to further aid the early detection and monitoring of systemic hypertension.
Keywords: Adaptive optics imaging, cross-sectional area of the vascular wall, hypertension, wall-to-lumen ratio
Retinal imaging is limited by the size of the entrance and the same exit pupil.[1,2] The ability to retrieve the outgoing rays and form an image was possible with the development of the fundus camera.[3] Scanning laser ophthalmoscopy (SLO) utilizes the principle of illuminating only a small spot of the retina using a collimated laser light directed through the very centre of the pupil and capturing the reflections from its entire surrounds within the pupil confines.[4] This allows for usage of much lower levels of light energy with better image quality. Optical coherence tomography (OCT) gives cross-sectional images of retinal tissue to a submicron axial resolution using a low coherence interference technique combined with a broadband light source.[5] Despite these technological advancements, imaging of the retina was limited by the ‘optical aberrations’ of the eye, also expressed as ‘wave-front aberrations’. This limits the capacity of optical systems’ lateral resolution. Wave-front aberrations may be low order (LOA: defocus or astigmatism) or high order (HOA: coma, trefoil and spherical aberration).[6] HOAs still pose a limit to the lateral resolution capability, as they are non-stable, and hence recognition and correction have to be continuous to allow for excellent resolution.
Adaptive optics (AO) technology aims to correct these ocular aberrations and enhance performance of the optical systems. AO was first used in astronomical telescopes to allow for correction of atmospheric aberrations.[7] The three principal components of such an AO system include a wave-front sensor (typically Hartman-Shack), a deformable mirror and a customized software. The wave-front sensor measures the native aberrations of the eye in vivo. The deformable mirror uses a complex system of actuators to adjust several small mirrors to compensate for the aberrations measured. The interaction between the two is controlled by the specialized software.[6,8] AO in retinal imaging could be used with flood illumination or combined with both the SLO and OCT to produce high-quality images whose lateral resolution permits cone discrimination and also allows the study of blood vessels and cells.[9,10] By compensating for the aberrations caused by irregularities of the optics of the eye, lateral (transverse) resolutions to the order of 1-2 microns can be achieved, thereby allowing near histopathological resolution.[11]
Arterial hypertension is an important risk factor for vascular eye diseases, cardiovascular and cerebral events.[12] Chronically elevated blood pressure leads to chronic vasoconstriction of small arteries.[13] This leads to vascular remodelling,[14,15] that is, a decreased lumen diameter and a thickening of the arteriolar walls resulting in an increased wall-to-lumen ratio (WLR). The remodelling of arterioles is an early step in arterial hypertension-associated vessel alteration[16] and target organ disease, which was first demonstrated in an increased media-to-lumen ratio in biopsies of subcutaneous vessels.[17] Since retinal and cerebral vessels emerge from similar anatomic, physiologic and embryologic background,[18] retinal vascular abnormalities could be used as a predictor of stroke events.[19] An autopsy study in patients with strokes confirmed a close correlation between cerebral and retinal vascular changes.[20] To distinguish between two types of vessel wall alterations, eutrophic and hypertrophic remodelling,[14] the WLR and the cross-sectional area of the vascular wall (WCSA) should be considered. Eutrophic remodelling refers to decreased vessel lumen diameter with no changes in WCSA; this is usually seen in acute elevations of blood pressure. Hypertrophic vascular remodelling shows increased WLR and thickening of vessel wall thickness (increased WCSA); this is seen in chronically uncontrolled systemic blood pressure.[21]
Hence, we aim to measure the vascular parameters in normotensive and hypertensive individuals using AO imaging and study the difference in the vascular parameter measurements in these two groups.
Methods
The study was carried out according to the principles outlined in the Declaration of Helsinki of 1975, as revised in 2000. We obtained the approval of the institutional ethics committee and review board. Phakic patients older than 18 years with a clear ocular media (patients with early nuclear sclerosis, not compromising quality of images, were also included) and no ocular or systemic diseases other than arterial hypertension were included in the study. Patients’ past medical records and investigations were perused to determine the absence of systemic disorders other than systemic hypertension. Another group with no systemic or ocular conditions was recruited as controls. In all, 80% of our study population were south Indians, 14% were from northern and western parts of India and 6% from the eastern states. All patients were clearly explained the procedure, and a written consent prior to inclusion was obtained from all.
All patients underwent detailed ophthalmic examination including a dilated fundus evaluation. The dilatation was obtained with 1% topical tropicamide (Tropicamet, Sun Pharmaceutical Industries). All patients recruited for the study were rested for about 15 minutes prior to imaging, during which time they were explained the procedure. Prior to the AO image acquisition, the blood pressure (BP) of each patient was measured in the sitting position using an automated oscillometric device using an arm cuff (Omron HEM-7132 Blood Pressure Monitor with Fit Cuff). Enface AO fundus images were obtained using a commercially available flood-illumination AO retinal camera (rtx1; Imagine Eyes, Orsay, France). Briefly, the rtx1 camera measures and corrects wave-front aberrations with a 750 nm super luminescent diode source and an AO system operating in a closed loop. A 4 × 4 fundus area (i.e., approximately 1.2 × 1.2 mm in emmetropic eyes) is illuminated at 840 nm by a temporally low coherent light-emitting diode flashed flood source, and a stack of 40 fundus images is acquired in 4 seconds by a charge-coupled device camera. The measured refraction and axial length (GALILEI G6 Lens Professional, Zeimer Ophthalmic Systems, Switzerland) was integrated into the camera. Gaze was oriented by an external target in order to capture the region of interest. The region of interest was a segment of the supero-temporal artery of the right eye, at least 250 mm long with an inner diameter of at least 50 mm, devoid of bifurcations, within one disc diameter from the disc [Fig. 1]. A total of five such images were taken. BP was obtained again after image acquisition.
Figure 1.
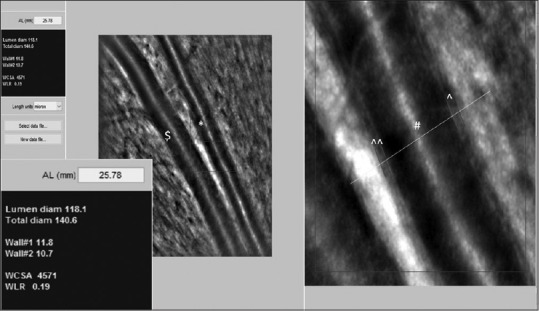
Retinal arteriole and venule taken by AO-backed retinal camera. Analysis of the superotemporal arteriole parameters of the 23-year-old healthy female patient: A point on the vessel is manually selected for analysis, the rest is performed automatically by a semi-automated software AOdetect Artery 2.0b13 (Imagine Eyes, Orsay, France). Automatic detection of the vessel walls is done by detection of a rapid contrast change in the intensity across the Vernier line. The software gives the results in a side window. Inset: Magnified view of the side window with the measured six parameters. Scale bar = 100 μm ($: retinal venule; *: retinal arteriole; ^: W1; ^^: W2; #: lumen of retinal arteriole)
Image acquisition and analysis
Brief description of the image processing method
Each series of 40 images acquired by the AO camera is processed using software programs provided by the system manufacturer (Imagine Eyes, Orsay, France). These images are registered and averaged to produce a final image with an improved signal-to-noise ratio. Image processing included image enhancement by applying a median filter followed by a nonlinear diffusion filter to allow smoothening of the blood vessels while preserving the contrast along their edges and image segmentation based on mathematical morphology, k-means clustering and active contours models relying on parallelism information, in order to extract the lumen (internal diameter) and outer diameters of the vessel. Finally, the wall thickness was defined as wall thickness = (outer diameter-internal diameter)/2, and the ratio of total parietal thickness over the LD defined the WLR.
All five AO images of each patient were analysed using a semi-automated software AOdetect Artery 2.0b13 (Imagine Eyes, Orsay, France). AOdetect artery can read any file format exported by the machine. Once a point on the vessel is manually selected for analysis, the rest is performed automatically by the software. Automatic detection of the internal and external limits of the walls is done by detection of a rapid contrast change in the intensity across the Vernier line. The analysis at a particular cross-section of the vessel is done corresponding to the manually selected point and is perpendicular to the vessel at that selected point. The software gives the results in a side window. Units can be in microns, pixels or arcmin, with the latter two not dependent on the eye's axial length. Lumen diam: Lumen diameter (LD), Total diam: External diameter (ED) = LD + Wall1 + Wall2, wall dimension (W1, W2), WCSA: Wall cross-sectional area: Area encompassed by vessel circumference-lumen area: ([ED/2]2-[LD/2]2) and WLR: Wall-to-lumen ratio: (W1 + W2)/LD [Fig 1].
We averaged the findings of each of the five images of a patient. We also calculated the intra-observer variability (IOV) by measuring the above parameters thrice in a single image. All the data was entered for analysis in the commercial software SPSS (version 22.0; SPSS Inc. Chicago, IL). Normal distribution of the data was tested using the Kolmogorov-Smirnov test. Results were expressed as the mean ± SD for normally distributed data, and as the median for not normally distributed data. Arterial hypertension was defined as a categorical variable. Age, retinal arteriolar wall thickness, vessel diameter, WLR and WCSA were evaluated as numeric variables and converted into categorial variables where it was needed. Cronbach's alpha was measured to study the IOV for WCSA and WLR parameters. For comparison between groups, the Mann-Whitney-U test was used. The level of significance was set at P < 0.05. Correlation analysis was performed using Pearson's correlation coefficient for normally distributed data and Spearman-Rho for not normally distributed data.
Results
We included a total of 150 patients. Of these, 40 patients had been diagnosed with systemic arterial hypertension and were on treatment for the same and the rest 110 patients had no known systemic ailments.
Demography
Age
The mean age of our group was 42 ± 14.03 years. There was a statistically significant difference in the age of control population and the group with systemic hypertension (mean age of controls: 37.5 ± 13 years; mean age of patients with systemic hypertension: 54.83 ± 8.03 years; P < 0.01) [Table 1].
Table 1.
Demographic parameters of study population and morphometric analysis of retinal arteriole in control and hypertensive population with AO imaging
| Control population | Hypertensive population | P | |
|---|---|---|---|
| Number | 110 | 40 | |
| Demographic parameters of study population | |||
| AGE (MEAN, SD) in years | 37.5, 13 | 54.83, 8.03 | <0.01 |
| GENDER (M: F) | 43:67 | 27:13 | |
| SYSTOLIC BP, mmHg (MEAN, SD) | 118.5, 8.1 | 134.1, 9.9 | <0.01 |
| DIASTOLIC BP, mmHg (MEAN, SD) | 76.9, 4.5 | 86.8, 7.8 | <0.01 |
| Morphometric analysis of retinal arterioles | |||
| WLR (MEAN, SD) | 0.23, 0.03 | 0.30, 0.07 | <0.01 |
| WCSA (MEAN, SD) | 4180.03, 1029.51 | 5087.98, 1402.24 | <0.01 |
Gender
We had 43 males and 67 females in the control group. The hypertensive group consisted of 27 male and 13 female patients [Table 1].
Axial length (AXL)
There was no statistically significant difference in AXLs of right eyes between the control and hypertensive population (controls: 23.39 ± 0.96 mm vs hypertensives: 23.28 ± 0.92 mm, P = 0.58).
Blood pressure
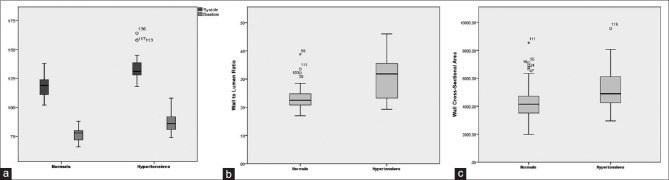
There was a statistically significant difference in both systolic and diastolic pressures between the controls and hypertensives (mean systolic BP: 118.5 mmHg ± 8.1 vs 134.1 mmHg ± 9.9, P < 0.01; mean diastolic BP: 76.9 mmHg ±4.5 vs 86.8 mmHg ± 7.8, P < 0.01) [Table 1 and Fig. 2a].
Figure 2.
Box-plot analysis: Differences between normal (control) [n = 110] and hypertensive [n = 40] population. The thick horizontal line within the box marks the median the lower and the upper boundaries of the box indicate the 25th and 75th percentiles. The whiskers below and above the box indicate the 2.5th and the 97.5 percentiles. The single dots indicate outliers, the asterisks extreme values. (a) Systolic and diastolic BP: normals - systolic/diastolic: 119/78 mmHg, hypertensives - systolic/diastolic: 131/86 mmHg (P < 0.01). (b) WLR: normals - 0.225, hypertensives - 0.3185 (P < 0.01). (c) WCSA: normals - 4144.85 mm2, hypertensives - 4895 mm2 (P < 0.01)
Retinal arteriolar morphology
Wall-to-lumen ratio (WLR)
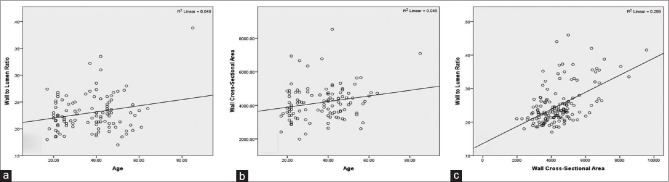
In the 110 controls, we found no significant correlation between age and WLR for the normal population (R2 = 0.049, P > 0.05) [Fig. 3a].
Figure 3.
(a and b) WLR and WCSA did not correlate significantly with age in normal population (R2 = 0.049; P > 0.05 and R2 = 0.045; P > 0.05, respectively). (c) WLR correlated significantly with the cross-sectional area of the vascular wall (R2 = 0.289; P < 0.01) (n = 150)
We found a statistically significant difference for WLR between the two groups with WLR significantly higher in the hypertensive group (controls: 0.23 ± 0.03 vs hypertensive group: 0.30 ± 0.07, P < 0.01) [Table 1 and Fig. 2b].
Wall cross-section area (WCSA)
In the 110 controls, we found no significant correlation between age and WCSA for the normal population (R2 = 0.045, P > 0.05) [Fig. 3b].
We found a statistically significant difference for WCSA between the two groups with WCSA being significantly higher in the hypertensive group (controls: 4180.03 mm2 ± 1029.51 vs hypertensive group: 5087.98 mm2 ± 1402.24, P < 0.01) [Table 1 and Fig. 2c].
We found a significant correlation between WLR and WCSA (Fig. 3c. R2 linear = 0.289; P < 0.01).
Intra-observer variability (IOV): IOV was calculated for vessel WCSA and WLR, and we found excellent consistency with Cronbach's alpha being 0.97 and 0.96, respectively.
Discussion
AO retinal imaging is a novel non-invasive way of evaluating qualitative and quantitative vascular morphology at a near histological level.[22] There was a statistically significant difference in systolic and diastolic pressures between the normal and hypertensive groups in our study. We found a high WLR and WCSA in our group of patients with hypertension compared to the normal population. This suggests that in patients with systemic hypertension there were retinal vascular remodelling processes which were attended by an arteriolar vessel wall thickening and a decreased lumen diameter, resulting in an increased WLR. An AO retinal camera allows a direct anatomical measurement of the vessel wall and lumen diameter. The AOdetect Artery software, a semi-automated method used to measure vessel parameters, offers a low IOV. Other methods to assess retinal vessel parameters include the functional method of Scanning Laser Doppler Flowmetry (SLDF), which measures the retinal vessel endothelium and the corpuscle free edge flow.[16,21,23,24] The WLR was higher in patients with a history of cerebrovascular event, compared to hypertensives on treatment and normotensives.[25] Some studies suggest that an assessment of retinal arteriolar structure by WLR may reflect vascular target organ damage.[21,23]
Two important vascular parameters which can help differentiate between eutrophic and hypertrophic vascular remodelling are WCSA and WLR.[14] Meixner et al.[26] reported that the median age-adjusted WLR was significantly higher in the hypertensive group with no significant difference in the age adjusted WCSA between the hypertensive and normotensive group. This finding suggests eutrophic rather than hypertrophic remodelling, which is supported by the fact that all hypertensive patients in their study suffered from mild hypertension without severe retinal angiopathy. Although data regarding the duration of hypertension in our patients was not reliable, a majority of them showed increased WLR and WCSA (hypertrophic remodelling) suggestive of disease chronicity or a persistently elevated blood pressure despite treatment. The data from the study conducted by Meixner et al. suggests that age has a dominant effect on the WLR, which is consistent with the results of Koch et al.,[27] who showed that age and mean arterial blood pressure accounted for 43% of the variability of WLR.
Gallo et al.,[28] in their study of 1500 patients, showed that age and BP independently increased the WLR by thickening the arteriolar wall. In our study, we found an increase in WLR and WCSA with age in our normal population but it was not statistically significant. The same group showed that a reversal of the vascular remodelling processes was possible in controlled hypertensives and it seemed to include short-term functional and long-term structural changes.[29,30]
To acquire high-quality images for analysis can be challenging as AO imaging requires good fixation. Low-quality images are usual in subjects with cataract or bad fixation. The AOdetect Artery software needs further validation and although we found an excellent consistency in intra-observer measurements, IOV of the same needs to be assessed. As only one retinal arteriole of one eye per patient was assessed, we cannot be certain that the changes in that arteriole are reflective of all other retinal vessels.
We included patients with systemic hypertension who already were on antihypertensive medical treatment. In future studies, it would be interesting to distinguish between treated and treatment naïve arterial hypertensive subjects, and to examine patients with severe arterial hypertension. All patients in this study were assumed to have essential hypertension based on the information declared by the subjects. One of the main limitations of the study is the small number. Larger studies with a well-matched comparison between normotensives and hypertensives will throw further light on the vascular remodelling process in health and disease.
Conclusion
AO retinal camera is a non-invasive tool to evaluate the retinal vessel WLR. WCSA and WLR reflect the remodelling process and can be used to further aid the early detection and monitoring of systemic hypertension. Changes in retinal vasculature can further help us understanding the health of the cerebral vasculature.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Colenbrander A. Principles of ophthalmoscopy. In: Tasman W, Jaeger EA, editors. Duane's Ophthalmology. Vol. 1. 2013 ed. chap 63. 2012. [Google Scholar]
- 2.Miller D TE, Atebara NH. Ophthalmic instrumentation. In: Yanoff M, Duker JS, editors. Ophthalmology, chap 2.10. 2008. [Google Scholar]
- 3.Meyer-Schwickerath GR. Ophthalmology and photography. Am J Ophthalmol. 1968;66:1011–9. doi: 10.1016/0002-9394(68)90809-x. [DOI] [PubMed] [Google Scholar]
- 4.Mainster MA, Timberlake GT, Webb RH, Hughes GW. Scanning laser ophthalmoscopy. Clinical applications. Ophthalmology. 1982;89:852–7. doi: 10.1016/s0161-6420(82)34714-4. [DOI] [PubMed] [Google Scholar]
- 5.Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–29. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 6.Lombardo M, Lombardo G. Wave aberration of human eyes and new descriptors of image optical quality and visual performance. J Cataract Refract Surg. 2010;36:313–31. doi: 10.1016/j.jcrs.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Babcock HW. Adaptive optics revisited. Science. 1990;249:253–7. doi: 10.1126/science.249.4966.253. [DOI] [PubMed] [Google Scholar]
- 8.Lombardo M, Serrao S, Ducoli P, Lombardo G. Eccentricity dependent changes of density, spacing and packing arrangement of parafoveal cones. Ophthalmic Physiol Opt. 2013;33:516–26. doi: 10.1111/opo.12053. [DOI] [PubMed] [Google Scholar]
- 9.Duncan JL, Zhang Y, Gandhi J, Nakanishi C, Othman M, Branham KE, et al. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3283–91. doi: 10.1167/iovs.06-1422. [DOI] [PubMed] [Google Scholar]
- 10.Torti C, Povazay B, Hofer B, Unterhuber A, Carroll J, Ahnelt PK, et al. Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina. Opt Express. 2009;17:19382–400. doi: 10.1364/OE.17.019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Roorda A. Evaluating the lateral resolution of the adaptive optics scanning laser ophthalmoscope. J Biomed Opt. 2006;11:014002. doi: 10.1117/1.2166434. [DOI] [PubMed] [Google Scholar]
- 12.Lavy S, Melamed E, Cahane E, Carmon A. Hypertension and diabetes as risk factors in stroke patients. Stroke. 1973;4:751–9. doi: 10.1161/01.str.4.5.751. [DOI] [PubMed] [Google Scholar]
- 13.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, et al. Vascular remodeling. Hypertension. 1966;28:505–6. [PubMed] [Google Scholar]
- 14.Korsgaard N, Aalkjaer C, Heagerty AM, Izzard AS, Mulvany MJ. Histology of subcutaneous small arteries from patients with essential hypertension. Hypertension 22. 1993:523–6. doi: 10.1161/01.hyp.22.4.523. [DOI] [PubMed] [Google Scholar]
- 15.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension: Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- 16.Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest.) form of target organ damage in mild essential hypertension? J Hypertens. 2001;19:921–30. doi: 10.1097/00004872-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 17.De Ciuceis C, Porteri E, Rizzoni D, Rizzardi N, Paiardi S, Boari GE, et al. Structural alterations of subcutaneous small-resistance arteries may predict major cardiovascular events in patients with hypertension. Am J Hypertens. 2007;20:846–52. doi: 10.1016/j.amjhyper.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004;351:2310–7. doi: 10.1056/NEJMra032865. [DOI] [PubMed] [Google Scholar]
- 19.Henderson AD, Bruce BB, Newman NJ, Biousse V. Hypertension-related eye abnormalities and the risk of stroke. Rev Neurol Dis. 2011;8:1–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Goto I, Katsuki S, Ikui H, Kimoto K, Mimatsu T. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke. 1975;6:263–9. doi: 10.1161/01.str.6.3.263. [DOI] [PubMed] [Google Scholar]
- 21.Baleanu D, Ritt M, Harazny J, Heckmann J, Schmieder RE, Michelson G. Wall-to-lumen ratio of retinal arterioles and arteriole-to-venule ratio of retinal vessels in patients with cerebrovascular damage. Invest Ophthalmol Vis Sci. 2009;50:4351–9. doi: 10.1167/iovs.08-3266. [DOI] [PubMed] [Google Scholar]
- 22.Roorda A, Romero-Borja F, Donnelly W III rd, Queener H, Hebert T, Campbel M. Adaptive optics scanning laser ophtalmoscopy. Opt Express. 2002;10:405–12. doi: 10.1364/oe.10.000405. [DOI] [PubMed] [Google Scholar]
- 23.Michelson G, Wärntges S, Baleanu D, Welzenbach J, Ohno–Jinno A, Pogorelov P, et al. Morphometric age-related evaluation of small retina vessels by scanning laser doppler flowmetry. Determination of a vessel wall index. Retina. 2007;27:490–8. doi: 10.1097/01.iae.0000243032.33738.f7. [DOI] [PubMed] [Google Scholar]
- 24.Ritt M, Harazny JM, Ott C, Schlaich MP, Schneider MP, Michelson G, et al. Analysis of retinal arteriolar strucuture in never-treated patients with essential hypertension. J Hypertens. 2008;26:1427–34. doi: 10.1097/HJH.0b013e3282ffdc66. [DOI] [PubMed] [Google Scholar]
- 25.Harazny JM, Ritt M, Baleanu D, Ott C, Heckmann J, Schlaich MP, et al. Increased wall: lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007;50:623–9. doi: 10.1161/HYPERTENSIONAHA.107.090779. [DOI] [PubMed] [Google Scholar]
- 26.Meixner E, Michelson G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research. Graefes Arch Clin Exp Ophthalmol. 2015;253:1985–95. doi: 10.1007/s00417-015-3115-y. [DOI] [PubMed] [Google Scholar]
- 27.Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet-Riffaud P, Girerd X, et al. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens. 2014;32:890–8. doi: 10.1097/HJH.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo A, Mattina A, Rosenbaum D, Koch E, Paques M, Girerd X. Retinal arteriolar remodeling evaluated with adaptive optics camera: Relationship with blood pressure levels. Ann Cardiol Angeiol (Paris) 2016;65:203–7. doi: 10.1016/j.ancard.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum D, Koch E, Girerd X, Rossant F, Pâques M. Imaging of retinal arteries with adaptative optics, feasibility and reproducibility. Ann Cardiol Angeiol (Paris) 2013;62:184–8. doi: 10.1016/j.ancard.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum D, Mattina A, Koch E, Rossant F, Gallo A, Kachenoura N, et al. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J Hypertension. 2016;34:1115–22. doi: 10.1097/HJH.0000000000000894. [DOI] [PubMed] [Google Scholar]