Abstract
Purpose:
To report the diurnal variation in choroidal vascularity index (CVI) in subfoveal (SF-CVI) and peripapillary area in healthy eyes.
Methods:
The study was a cross-sectional study including 12 healthy subjects. Swept-source optical coherence tomography scans were taken at 9 am, 11 am, 1 pm, 3 pm, and 5 pm. Subfoveal choroidal thickness (SFCT) and CVI were calculated using automated segmentation techniques and previously validated algorithms. Systemic parameters including systolic blood pressure (SBP), diastolic blood pressure, mean arterial pressure, and mean ocular perfusion pressure were calculated and correlated with SFCT and CVI.
Results:
A total of 12 eyes (right eye) of 12 patients (mean age: 26 ± 3.77 years) were analyzed. The mean (±standard deviation) amplitude of SFCT and SF-CVI variation was 35.91 ± 14.8 μm (range, 15–69 μm) and 0.05 ± 0.02 (range, 0.02–0.08). The mean CVI showed a significant diurnal variation in the temporal quadrant of the peripapillary region (P = 0.02).
Conclusion:
SFCT and SF-CVI showed a significant diurnal variation in amplitude (peak–trough analysis) and SF-CVI correlated well with SBP suggestive of a direct influence of blood pressure on choroidal vascularity. The mean peripapillary CVI in the temporal quadrant also showed a significant diurnal variation with no significant change in other quadrants.
Keywords: Choroidal thickness, choroidal vascularity index, diurnal variation, optical coherence tomograph
The choroid is a dense vascular layer of the eye located between the retina and sclera and is responsible for a majority of the ocular blood supply.[1] The choroidal imaging has received considerable attention in the past decade due to the advancements in imaging techniques, especially optical coherence tomography (OCT) including enhanced depth imaging and swept-source (SS)-OCT.[2,3] The choroidal blood flow is characterized by the absence of autoregulation and is prone to changes in systemic [systolic blood pressure (SBP) or diastolic blood pressure (DBP)] or ocular parameters [intraocular pressure (IOP)].[4,5] These manifest in the form of change in choroidal thickness (CT), volume, and probably choroidal vascularity index (CVI; described as the ratio of luminal and total choroidal area) as well.[6,7]
CT has been studied extensively in normal and diseased states and shows significant variation in various pathologies. Interestingly, CT has also been noted to show a diurnal variation with amplitude ranging from 19 to 33 μm in various studies.[6,7,8] These changes in CT due to diurnal variation need to be considered while studying other ocular and nonocular factors (age, axial length, refractionand blood pressure) affecting CT. Tan et al. have reported higher diurnal variation in CT in eyes with thicker choroid (>300 μm).[7]
CVI is a recently described choroidal parameter suggestive of the vascularity of choroid and represents the contribution of luminal area of choroidal blood vessels to the total CT.[9,10,11,12] Hypothetically, the quantum of change in CVI may be lesser compared with CT considering CVI as a ratio in which both the numerator and denominator are expected to change to a considerable extent.
A majority of the previous studies focused on the subfoveal CT (SFCT) and SF-CVI; however, SFCT and SF-CVI may not be representative of the entire choroid.[6,7,8,13,14,15] The thickness and vascularity of the choroid are highest subfoveally with a taper toward periphery. We have earlier reported the wide-field CVI in healthy eyes.[16] The macular choroid measured as twice the disc–fovea distance was found to have a significantly lower CVI compared with the other quadrants. The choroidal measurements were done at specific time frames between 9 am and 12 pm in a previous study.[16] Whether SF-CVI follows a diurnal variation similar to SFCT is not known. Moreover, subfoveal changes in CT and CVI may not be similar at peripapillary or peripheral choroid. In view of the above shortcomings in the literature, we studied the diurnal variation in SFCT and CVI in the subfoveal and peripapillary regions in normal eyes.
Methods
The study undertaken was a single-center, prospective, noninterventional study involving healthy eyes and was conducted at a tertiary center in Southern India. The study was approved by the institutional review board (LEC 01-18-006). All the methods adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to the procedures.
Twelve healthy adults with age more than 18 years and no known ocular or systemic comorbidities with a best-corrected visual acuity of 20/20 were included in the study. Exclusion criteria were as follows: (i) any history of prior ocular surgeries, (ii) OCT images in which choroidoscleral interface was not clearly identified, (iii) preexisting ocular structural’ abnormalities which affect the quality of OCT images, (iv) refractive error ≥±3 diopters (D), (v) caffeine intake prior to scan acquisition, (vi) history of smoking, (vii) axial length of less than 20 or more than 26 mm, and (viii) not willing to sign the informed consent.
All the subjects underwent comprehensive eye examination prior to the enrolment. Systemic examination included measurements of SBP, DBP, and pulse rate. IOP and axial length readings were recorded using Goldmann applanation tonometer and optical biometry (IOLMaster; Carl Zeiss Meditec, La Jolla, CA, USA), respectively. Blood pressure measurements were done using automated blood pressure monitor (Omron HEM 7120, Tokyo, Japan).
OCT measurements were done using an SSOCT device (Topcon Corporation, Tokyo, Japan) which uses a wavelength of 1050 nm and has a scan acquisition rate of 100,000 A scans per second. All scans were performed over 6 × 6 mm2 area consisting of 256 unaveraged horizontal cross-sectional scans per acquisition. Along with volume scans, 12 mm length horizontal and vertical raster scans were performed over the fovea and disc. The line scans passing through the disc were compared with the fundus photograph so that the scans passed through the midline of the optic disc thereby creating four equal segments of the optic disc. These voxel arrays were exported as 8-bit grayscale images.
SS-OCT scans were performed at 2-h intervals from 9 am to 5 pm in undilated state under mesopic light conditions. A total of five readings (9 am, 11 am, 1 pm, 3 pm, and 5 pm) were taken at the SF level and peripapillary region. Automated CT measurements were done from the outer border of retinal pigment epithelium to CSI. Shadow compensation and choroid binarization were done using Girard's and Vupparaboina's method, respectively.[17,18] Image binarization was done using adaptive histogram equalization, exponential enhancement, nonlinear enhancement, and thresholding (Otsu's method), the details of which have already been discussed in our previous work.[18,19,20] Automated detection of the choroidal inner and outer boundaries helped to identify and demarcate the region of interest. The total choroidal area and the area of the dark pixels (luminal area) were then calculated to finally derive CVI. CVI was measured as per our previous publications as a ratio of luminal area to total choroidal area [Figs. 1 and 2].[16,9,21] These scans were shadow compensated which provide more brightness with less masking due to the retinal blood vessels thereby providing a truer picture of the choroidal vascularity.[18] These procedures were performed as custom mode in MATLAB [version 8.5.0.197613 (R2015a); MathWorks, Natick, MA, USA]. We defined SF-CVI as the CVI in an area of length 1.5 mm on a line scan at the subfoveal level based on a previous description.[12] Similarly, peripapillary CVI was defined on a line scan from the outer border of the optic disc in all the four quadrants. Representative images showing automated segmentation, binarization, and CVI calculation involving both subfoveal and peripapillary areas are shown in Figs. 1 and 2.
Figure 1.
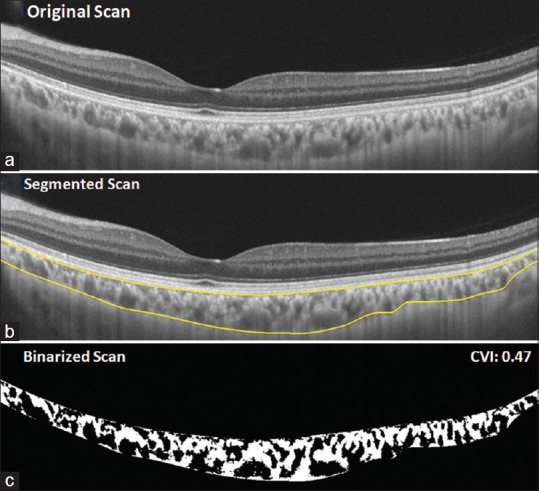
(a) The original swept-source optical coherence tomography (SS-OCT) image. (b) Automated segmentation (yellow lines) delineating the choroid defined from outer border of retinal pigment epithelium (RPE)–Bruch's complex to choroidoscleral interface. Image binarization was performed on the segmented area to determine choroidal vascularity index (CVI). (c) Dark and bright areas represent luminal and stromal areas, respectively
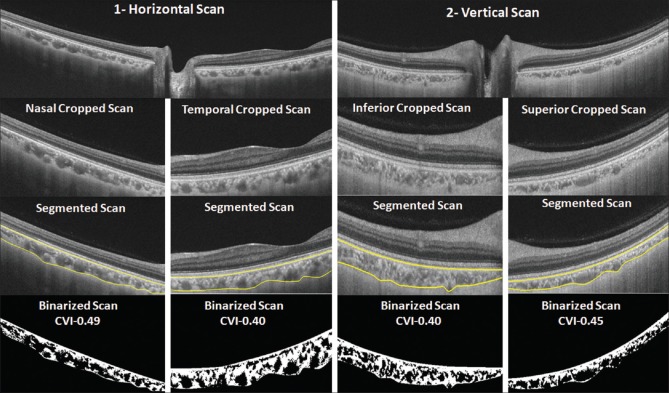
Figure 2.
Choroidal vascularity index (CVI) measurements in peripapillary area using swept-source optical coherence tomography (SS-OCT) line scans in both horizontal and vertical meridians (left, horizontal; right, vertical). Region of interest was cropped from the original OCT image. Automated segmentation was performed (yellow lines) followed by image binarization and CVI calculation similar to the description in Figure 1
Other measurements such as IOP, axial length measurements, and BP were done before the OCT measurements. The derived measurements including mean arterial pressure (MAP) and mean ocular perfusion pressure (MOPP) were calculated using the following formulae. MAP was the sum of DBP and product of one-third of the difference between SBP and DBP. MOPP was calculated as ⅔(MAP − IOP). The measurements were done for both eyes of all 12 patients. However, only the right eye readings were taken for statistical analysis considering the volume of data involved.
Statistical analysis
Statistical analysis was done using SPSS version (SPSS version 22.0; SPSS Inc, Chicago, IL, USA). The mean and standard deviation (SD) of baseline parameters were calculated. The comparison of CT and CVI at different time intervals was done using repeated measures analysis of variance (ANOVA). P value less than 0.05 with a 95% confidence interval (CI) was considered to be statistically significant.
Results
A total of 24 eyes of 12 subjects with a mean age of 26 ± 3.77 years were studied, and only the right eye of the study cohort was used for statistical analysis. The study cohort included four males and eight females. The mean (±SD) refraction was −0.25 ± 0.54D (range, −1.5D to + 0.75D). The axial length and IOP were measured at different time intervals as depicted in Fig. 3. The mean axial length measured at baseline was 23.42 ± 0.91 mm. Baseline recorded IOP at 9 am was 14 ± 2.82 mmHg.
Figure 3.
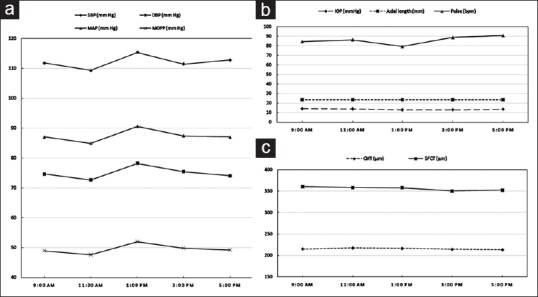
(a) The change in systemic parameters such as systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial (MAP), and mean ocular perfusion pressure (MOPP) at various time points from 9 am to 5 pm. (b and c) The graph depicting the diurnal variation in intraocular pressure (IOP), axial length, pulse rate, subfoveal choroidal thickness, and central macular thickness with time
Diurnal variation in SFCT and SF-CVI
There was a significant variation in SFCT (comparing the maximum and minimum SFCT in the right eye of each individual) across the time frames from 9 am to 5 pm (95% CI of the difference: 25.29–44.04; P = 0.0001). The mean SFCT was highest at baseline at 9 am, and the mean change in CT from 360.33 ± 83.05 μm at 9 am to 352.58 ± 88.72 μm at 5 pm using repeated measures ANOVA was not significant (P = 0.41) as shown in Table 1. In comparison, the diurnal variation in SF-CVI (maximum and minimum in each individual) was also significant (95% CI of the difference: 0.03–0.06; P = 0.0001). However, repeated measures ANOVA with Greenhouse–Geisser correction comparing mean SF-CVI at different time points was not significant (P = 0.43) as shown in Fig. 3.
Table 1.
Diurnal variation in various ocular and systemic parameters across various time frames from 9 am to 5 pm
| Variables | 9 am | 11 am | 1 pm | 3 pm | 5 pm | P |
|---|---|---|---|---|---|---|
| IOP (mmHg) | 14±2.83 | 13.83±2.89 | 13±3.13 | 13.08±3.39 | 13.5±3.08 | 0.38 |
| Axial length (mm) | 23.42±0.91 | 23.43±0.91 | 23.45±0.91 | 23.43±0.91 | 23.44±0.90 | 0.18 |
| SFCT (μm) | 360.33±83.05 | 358.25±91.90 | 357.91±89.37 | 350.25±91.51 | 352.58±88.72 | 0.41 |
| Macular CVI | 0.42±0.03 | 0.42±0.04 | 0.42±0.02 | 0.42±0.03 | 0.41±0.03 | 0.43 |
| Peripapillary CVI | ||||||
| Nasal | 0.49±0.04 | 0.48±0.03 | 0.49±0.02 | 0.49±0.02 | 0.49±0.02 | 0.83 |
| Temporal | 0.42±0.02 | 0.44±0.02 | 0.43±0.02 | 0.44±0.02 | 0.44±0.02 | 0.02* |
| Superior | 0.45±0.03 | 0.44±0.02 | 0.45±0.02 | 0.45±0.03 | 0.45±0.03 | 0.59 |
| Inferior | 0.45±0.03 | 0.45±0.04 | 0.45±0.04 | 0.45±0.03 | 0.45±0.02 | 0.99 |
| CMT (μm) | 214.83±12.44 | 217.25±10.39 | 216.33±11.44 | 214.33±12.16 | 213.5±11.72 | 0.21 |
| Pulse (per min) | 84.58±7.12 | 86.41±11.79 | 79.33±8.55 | 88.83±10.17 | 90.83±11.21 | 0.02 |
| SBP (mmHg) | 111.83±14.31 | 109.33±11.01 | 115.33±25.34 | 111.42±14.53 | 112.82±15.01 | 0.68 |
| DBP (mmHg) | 74.67±10.44 | 72.67±7.95 | 78.25±12.31 | 75.42±11.49 | 74.08±11.06 | 0.46 |
| MAP (mmHg) | 87.06±11.47 | 84.89±8.41 | 90.61±16.02 | 87.42±12.21 | 87.0±10.31 | 0.48 |
| MOPP (mmHg) | 48.95±6.69 | 47.61±4.72 | 51.99±9.98 | 49.8±7.63 | 49.25±6.46 | 0.36 |
IOP=Intraocular pressure, SFCT=Subfoveal choroidal thickness, CVI=Choroidal vascularity index, CMT=Central macular thickness, SBP=Systolic blood pressure, DBP=Diastolic blood pressure, MAP=Mean arterial pressure, MOPP=Mean ocular perfusion pressure. *Significant P values are highlighted
The mean (±SD) amplitude of SFCT and SF-CVI variation was 35.91 ± 14.8 μm (range, 15–69 μm) and 0.05 ± 0.02 (range, 0.02–0.08). Comparison of intraindividual variation in SFCT readings was done in view of high interindividual variation in SFCT. This revealed that 7 of 12 (58.3%) eyes had the highest SFCT at 9 am with a nadir at 3 pm in 6 of 12 (50%) of the eyes.
Diurnal variation in peripapillary CVI
The comparison of mean CVI in the temporal quadrant of peripapillary region showed a significant variation in CVI (P = 0.02), whereas nasal (P = 0.83), superior (P = 0.59), and inferior quadrants (P = 0.99) were not statistically significant [Fig. 4]. The pair-wise comparison revealed significant difference between 9 and 11 am readings only in the temporal quadrant (P = 0.046).
Figure 4.
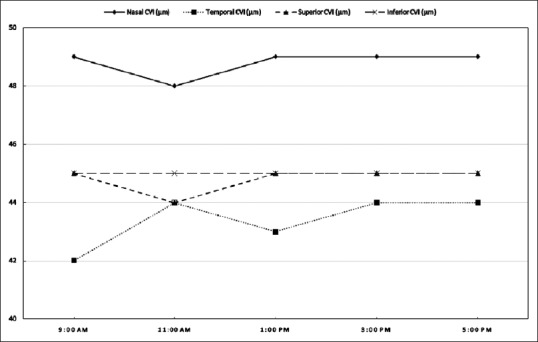
The graphical representation of the variation in peripapillary choroidal vascularity index (CVI) (shown as percentage) in all the four quadrants
Baseline SFCT and SF-CVI were not significant predictors of amplitude of SFCT and CVI variation (P = 0.45 and P = 0.70, respectively). CMT comparison using repeated measures ANOVA in the various time points was not significant (P = 0.21). The different systemic parameters such as SBP, DBP, MAP, and MOPP at various time points are represented in Fig. 3. Regression analysis comparing the amplitude of SFCT variation with age (P = 0.87), refraction (P = 0.87), axial length (P = 0.66), and other parameters including variation in SBP (P = 0.08), DBP (P = 0.51), MAP (P = 0.35), and MOPP (P = 0.49) were not significant. Similar comparison of the amplitude of CVI variation to variation in SBP revealed significant results (R = 0.61, P = 0.03). Other parameters including age (P = 0.46), refraction (P = 0.31), axial length (P = 0.52), DBP (P = 0.74), MAP (P = 0.45), and MOPP (P = 0.69) were not significantly correlated with amplitude of CVI variation.
Discussion
We studied the diurnal variation in SFCT and CVI in healthy eyes at the subfoveal level and each quadrant of the peripapillary area. Diurnal changes in subfoveal or peripapillary CVI have not been reported earlier. The comparison of the mean SFCT and SF-CVI for all the time frames was not significant. The mean SF-CVI remained consistent throughout the time intervals. However, the analysis of variation in only the maximum and minimum SFCT and SF-CVI in each individual showed a significant diurnal variation in SFCT and SF-CVI, respectively. This comparison of these two specific values was undertaken because of high interindividual differences with each individual having a different pattern of CT variation.
Previous authors have studied diurnal variation in SFCT in humans and other species such as chick and marmoset.[6,7,14,22,23,24] Among the studies involving the human choroid, various authors have shown that SFCT was maximum in the night or early morning which reached a nadir during the evening.[6,7] However, these observations have not been consistent with Chakraborty et al. and Toyokawa et al. showing an increase in SFCT as the day progressed.[14,25] Our results showed highest SFCT at 9 am with least SFCT at 3 pm. This variation in SFCT bears similarity to some of the previous studies.[6,7,8]
SF-CVI as described earlier was expected to carry a lesser diurnal variation when compared with SFCT. However, the results were contrary to our hypothesis. This can be explained by a proportionate increase in the stromal area compared with the luminal area leading to an overall increase in the total choroidal area.
There was no significant correlation between amplitude of SFCT and SF-CVI variation with baseline SFCT and SF-CVI, respectively. This result is contrary to that reported by Tan et al. The authors have shown a diurnal variation of 10.5 and 40 μm in eyes with SFCT of ≤300 and ≥400 μm, respectively, suggestive of a significant variation in CT in eyes with thicker choroid.[7] Studies have also shown a negative correlation between CT and axial length which was not evident in our study.[7,8]
Authors have reported conflicting results of variation in CT with SBP.[6,7,8] We found no significant correlation of diurnal variation in CT with SBP similar to the study by Lee et al.[8] However, Tan et al. and Usui et al. have reported a positive and negative correlation of SBP with CT, respectively.[6,7] On the other hand, CVI was found to have a significant correlation with variation in SBP (r = 0.61). This suggests that due to lack of autoregulation in the choroidal circulation, the change in systemic factors such as rise in SBP may lead to higher influx of blood due to compensatory dilatation and may directly lead to an increase in the area of the vascular lumen. However, the relationship between CVI or CT and arterial blood pressure (SBP, DBP, and MAP) is not linear due to a complex interplay between arterial blood pressure and IOP. An increase of up to 67% in MOPP has been shown to increase the choroidal vascular resistance translating to an increase of only 12% in the choroidal blood flow.[26] The timing of CT measurements adds another variable to a list of multiple factors influencing CT such as axial length, refraction, gender, age, and ethnicity. Researchers should therefore maintain uniformity in measuring SFCT or CVI at a specific point of time as the amplitude of intraindividual variation in CT and SF-CVI using OCT can be as high as 90 μm, and 0.08, respectively.[8] Brown et al. and Chakraborty et al. using optical biometry have shown diurnal variation in CT with amplitude ranging from 59.5 ± 24.2 and 29 ± 16 μm, respectively.[13,14] The mean amplitude of SFCT in our study cohort was 35.91 ± 14.8 μm (range, 15–69 μm), whereas the mean amplitude of SF-CVI variation was 0.05 ± 0.02 (range, 0.02–0.08).
We also compared the diurnal variation in peripapillary CVI in all quadrants. The variation in mean CVI in the temporal quadrant of the peripapillary region was significant with a statistical difference between two readings at 9 and 11 am in the pairwise comparisons. On the contrary, no significant differences of CVI were noted at different time points in the rest of the peripapillary area.
The strengths of this study are the analysis of variation in CVI in both subfoveal and peripapillary regions which has not been studied earlier. We used an automated, previously validated algorithms for CT and CVI calculation. We particularly used algorithm with shadow compensation which helps to obtain more accurate results on choroidal vascularity, which was never done in previous studies. The main drawback of this particular study is small sample size involving healthy individuals of younger age group. Therefore, variation in CT in elderly individuals or diseased eye was not available. The CT measurements were done during day time and variation in CT during the night was not studied, and therefore it is difficult to comment on the true peak or trough values of CT and CVI. The multitude of automated algorithms and the lack of studies comparing different algorithms pose difficulty in identifying the most accurate algorithm among them.[12,18,22,27,28] Moreover, choroidal vascularity obtained from these algorithms is still different from the true histological sections.[1,29]
Conclusion
In conclusion, we report a significant diurnal variability of SFCT and SF-CVI using peak–trough comparison in healthy eyes. However, the mean SFCT and SF-CVI did not show a significant difference. The changes in SF-CVI showed a significant positive correlation with SBP. The temporal quadrant in peripapillary area showed a significant variation in CVI. Prospective studies with large sample size may provide a better idea of the robustness of CVI as a choroidal parameter compared with CT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11:2183–9. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 3.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–5. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye (Lond) 1990;4:319–25. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- 5.Iwase T, Yamamoto K, Ra E, Murotani K, Matsui S, Terasaki H. Diurnal variations in blood flow at optic nerve head and choroid in healthy eyes: Diurnal variations in blood flow. Medicine (Baltimore) 2015;94:e519. doi: 10.1097/MD.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usui S, Ikuno Y, Akiba M, Maruko I, Sekiryu T, Nishida K, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012;53:2300–7. doi: 10.1167/iovs.11-8383. [DOI] [PubMed] [Google Scholar]
- 7.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–6. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 8.Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW. Diurnal variation in choroidal thickness in relation to sex, axial length, and baseline choroidal thickness in healthy Korean subjects. Retina. 2014;34:385–93. doi: 10.1097/IAE.0b013e3182993f29. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal R, Wei X, Goud A, Vupparaboina KK, Jana S, Chhablani J. Influence of scanning area on choroidal vascularity index measurement using optical coherence tomography. Acta Ophthalmol. 2017;95:e770–5. doi: 10.1111/aos.13442. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal R, Li LK, Nakhate V, Khandelwal N, Mahendradas P. Choroidal vascularity index in Vogt-Koyanagi-Harada disease: An EDI-OCT derived tool for monitoring disease progression. Trans. Vis SciTechnol. 2016;5:7. doi: 10.1167/tvst.5.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36:1646–1. doi: 10.1097/IAE.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090. doi: 10.1038/srep21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JS, Flitcroft DI, Ying GS, Francis EL, Schmid GF, Quinn GE, et al. In vivo human choroidal thickness measurements: Evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–9. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 15.Osmanbasoglu OA, Alkin Z, Ozkaya A, Ozpınar Y, Yazici AT, Demirok A. Diurnal choroidal thickness changes in normal eyes of Turkish people measured by spectral domain optical coherence tomography. J Ophthalmol. 2013;2013:687165. doi: 10.1155/2013/687165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SR, Invernizzi A, Rasheed MA, Cagini C, Goud A, Vupparaboina KK, et al. Wide-field choroidal vascularity in healthy eyes. Am J Ophthalmol. 2018;193:100–5. doi: 10.1016/j.ajo.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Girard MJ, Strouthidis NG, Ethier CR, Mari JM. Shadow removal and contrast enhancement in optical coherence tomography images of the human optic nerve head. Invest Ophthalmol Vis Sci. 2011;52:7738–48. doi: 10.1167/iovs.10-6925. [DOI] [PubMed] [Google Scholar]
- 18.Vupparaboina KK, Dansingani KK, Goud A, Rasheed MA, Jawed F, Jana S, et al. Quantitative shadow compensated optical coherence tomography of choroidal vasculature. Sci Rep. 2018;8:6461. doi: 10.1038/s41598-018-24577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Systems, Man, Cybernetics. 1979;9:62–6. [Google Scholar]
- 20.Vupparaboina K, Richhariya A, Chhablani J, Jana S. Optical coherence tomography imaging: Automated binarization of choroid for stromal-luminal analysis. IEEE Int Conference on Signal Inform Process (IConSIP), 2016:1–5. [Google Scholar]
- 21.Vupparaboina KK, Nizampatnam S, Chhablani J, Richhariya A, Jana S. Automated estimation of choroidal thickness distribution and volume based on OCT images of posterior visual section. Comput Med Imaging Graph. 2015;46:315–27. doi: 10.1016/j.compmedimag.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–81. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 23.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 24.Nickla DL, Wildsoet CF, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–28. [PubMed] [Google Scholar]
- 25.Toyokawa N, Kimura H, Fukomoto A, Kuroda S. Difference in morning and evening choroidal thickness in Japanese subjects with no chorioretinal disease. Ophthalmic Surg Lasers Imaging. 2012;43:109–14. doi: 10.3928/15428877-20120102-06. [DOI] [PubMed] [Google Scholar]
- 26.Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest Ophthalmol Vis Sci. 1997;38:2338–43. [PubMed] [Google Scholar]
- 27.Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014;55:3893–99. doi: 10.1167/iovs.14-14447. [DOI] [PubMed] [Google Scholar]
- 28.Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1901–8. doi: 10.1016/j.ophtha.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLeod DS, Lutty GA. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994;35:3799–811. [PubMed] [Google Scholar]