Abstract
Purpose:
To measure levels of collagen-derived antiangiogenic factors (arresten, canstatin, tumstatin, endostatin) and matrix metalloproteinases (MMP-2 and MMP-9) in anterior lens epithelial cells (LECs) and anterior capsules of children with cataract and persistent fetal vasculature (PFV) as cases and cataract without PFV as controls.
Methods:
Anterior capsules harboring LECs were collected from pediatric cataract patients with (n = 13) and without PFV (n = 13) during surgery. Samples were immediately subjected to RNA extraction and cDNA preparation. Quantitative real time PCR was performed to determine the mRNA levels of antiangiogenic factors and matrix metalloproteinases. GAPDH (Glyceraldehyde 3-Phosphate Dehydrogenase) and β Actin were used as the housekeeping control. The mRNA levels were expressed as a ratio, using the delta-delta method for comparing the relative expression results between controls and cases. The non-parametric Mann-Whitney U test was applied for statistical evaluation. P values < 0.05 were statistically significant.
Results:
The relative mRNA levels of arresten, canstatin, tumstatin, endostatin, MMP-2 and MMP-9 in cases were 6.20E-03 ± 0.003, 1.49E-01 ± 0.02, 1.70E-01 ± 0.007, 3.20E-03 ± 0.003, 1.11E-03 ± 0.0009 and 3.72E-04 ± 0.0001. The mRNA levels of arresten was 1.6 times lower (P = 0.01) while mRNA levels of MMP-2, tumstatin and canstatin were 4, 2.5, and 2.3 times higher in cases than in controls. No change was observed in mRNA levels of MMP-9 and endostatin (P = 0.82).
Conclusion:
A significant difference in the levels of arresten, canstatin, tumstatin, and MMP-2 was found in LECs with PFV.
Keywords: Antiangiogenic factors, lens epithelial cells, persistent fetal vasculature
At birth, regression of blood vessels is crucial for the maintenance of lens transparency. In mammalian eyes, the pupillary membrane and hyaloid vessels, including the hyaloid artery, tunica vasculosa lentis and vasa hyaloidea propria, nourish the immature lens, retina, and vitreous. These vessels are known to regress by the fifth month of gestation in humans.[1] Failure of the tunica vasculosa to regress leads to an ocular pathology called persistent fetal vasculature (PFV), which can lead to cataract formation and impaired visual function [Fig. 1].[2,3] In severe cases, the retro-lenticular fibrovascular tissue contracts and pulls on the ciliary processes. The ciliary processes are typically elongated and are easily seen with dilatation [Fig. 2]. The fibrovascular tissue adherent to the posterior lens capsule sometimes results in cataract formation and sometimes in intraocular hemorrhage. Typically, it is associated with microphthalmia and leukocoria. The broad clinical manifestations of PFV depend on the anatomic region involved, i.e., anterior PFV, posterior PFV, or both. In Anterior PFV vascular abnormality is located in the posterior lens capsule. In posterior PFV the remnant vascular stalk is seen arising from the optic disc and radiating out in all directions and it can occur in combination with anterior and posterior segment abnormalities, i.e., vitreous stalk, preretinal membranes, optic hypoplasia, retinal folds, and retinal detachment.[4] The present study uses the pathological condition of the lens, that is, persistent fetal vasculature (PFV), as a model to study the factors affecting the regression of fetal vasculature.
Figure 1.
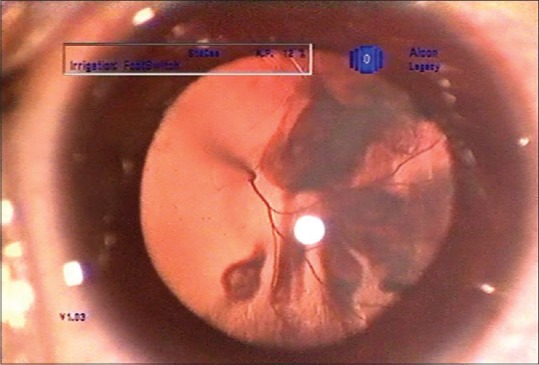
Clinical image of cataract with PFV
Figure 2.
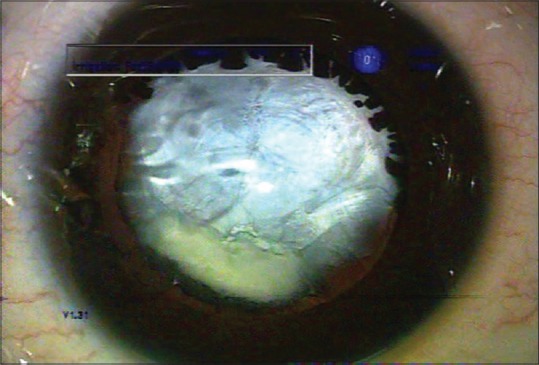
Elongated ciliary processes seen with dilatation
The basement membrane or the extracellular matrix (ECM) of the lens is known as the capsule. It is lined with epithelial cells in the anterior and equatorial capsules while the cells lining the posterior capsule disappear before birth. The lens capsule mainly consists of collagen IV with 6 genetically distinct α (IV) chains: α 1 (IV) to α 6 (IV). The non-collagenous (NC) domains of several different collagens have anti-angiogenic properties.[5,6] The NC-1 domains of the α 1, α 2, α 3, and α 6 chains of collagen IV inhibit angiogenesis and tumor growth in vivo.[7,8,9,10] These domains are called arresten, canstatin, tumstatin and endostatin, respectively. We hypothesized that these collagen-derived antiangiogenic factors present in the ECM (lens capsule) of lens epithelial cells (LECs) may have been altered in the condition of PFV cases. To our knowledge, there is no published study documenting the role of collagen derived anti-angiogenic factors in persistent fetal vasculature from lens epithelial cells in humans. The aim of the present study is to evaluate these antiangiogenic factors (arresten, canstatin, tumstatin, and endostatin) and regulatory molecules (MMP-2, MMP-9) in PFV cases.
Methods
Patient selection
Research procedures were conducted in accordance with Institutional Ethical Board guidelines and the Declaration of Helsinki. A total of 161 pediatric cataract surgeries were performed during the period March 2013 to March 2015. Of these, a total of 13 cases were diagnosed with PFV. All the cases had purely anterior PFV. All the cases had posterior capsule plaque and fibrovascular tissue attached to the posterior capsule. In 4 cases ghost vessels were seen emanating from posterior capsule into the vitreous. However, in these 4 eyes there was no vascular remnant arising from the optic disc. In no eye we could find anterior capsule changes in the form of fibrosis or vascularization. Cases with posterior PFV and mixed PFV were excluded from the study. The ideal controls would be transparent lenses in children. As it is ethically not possible to use such subjects as controls, we selected children who had cataract with absence of PFV. None of the control eyes were having anterior or posterior capsular changes in the form of fibrosis or vascularization. Cases and controls were both age and gender-matched.
Sample collection and processing
A continuous capsulorhexis was performed during cataract removal and the central portion of the lens capsule (approximately 5 mm in diameter) harboring LECs was collected in a balanced salt solution. Samples for quantitative Polymerase Chain Reaction (PCR) were collected in TRIZOL (Invitrogen) for RNA expression within 30 minutes of surgery and stored at - 26°C until processed further. Both RNA and protein were extracted from each sample according to the manufacturer's protocol. RNA was used for cDNA preparation.
Quantification of m-RNA by real-time PCR
Total RNA was extracted from samples using TRIZOL (Invitrogen) according to the manufacturer's protocol. 500 ng of total RNA was reverse transcribed at 25°C for 5 minutes, at 42°C for 60 minutes and then at 70°C for 5 minutes in 20 μl of 0.5 μg of oligo (dT), 20 pmol primer, 3 mM MgCl2, 0.05 mM dNTPs, 4 μl of Improm-II™ 5 × reaction buffer (Promega), 1 unit Recombinant RNasin® Ribonuclease Inhibitor and 1 μl Improm-II™ Reverse Transcriptase (Promega). For quantitative PCR, amplifications were performed on LightCycler®480 (Roche Applied Science) in 20 μl reaction using 2 μl cDNA, 10 μl SYBR Green I Master (Roche Molecular Biochemicals) and gene-specific primers at 2.5 μM concentration. The amplification was carried out at 95°C for 10 minutes before the first cycle, at 95°C for 15 seconds and at 60°C for 60 seconds repeated 40 times. Quantitative PCR for arresten, canstatin, tumstatin, endostatin, MMP-2 and MMP-9 was done and β-actin and GAPDH (Glyceraldehyde 3-Phosphate Dehydrogenase) were used as the housekeeping gene. cDNA was amplified using the primers from SA Biosciences. The relative expression normalized to the housekeeping gene was calculated using LightCycler®480 Software (Roche Molecular Biochemicals). For this analysis we have represented the ΔCt value of mRNA expression, which is inversely proportional to the amount of target nucleic acid in the sample that means lower value will indicate higher levels of the target molecule.
Statistical analysis
Statistical comparisons were performed using non-parametric tests such as the Kruskal-Wallis and Mann-Whitney tests.
Results
Table 1 shows the patient demographics of all patients with PFV. Mean age of all cases and controls was 41.15 months (3–132 months) and 26.64 months (1–108 months), respectively. Out of 13 cases, 4 (30.77%) were girls and 9 (69.23%) were boys, while out of 13 controls 8 (61.53%) were boys and 5 (38.46%) were girls.
Table 1.
Demographic data
| Controls | Cases | |
|---|---|---|
| Mean age (in months) | 26.64 (1 month-108 months) | 41.15 (3 months-132 months) |
| Gender | ||
| Female | 38.46% | 30.77% |
| Male | 61.53% | 69.23% |
The relative mRNA levels of arresten, canstatin, tumstatin, endostatin, MMP-2, and MMP-9 in cases were 6.20E-03 ± 0.003, 1.49E-01 ± 0.02, 1.70E-01 ± 0.007, 3.20E-03 ± 0.003, 1.11E-03 ± 0.0009 and 3.72E-04 ± 0.0001, respectively [Table 2 and Fig. 3]. The relative mRNA levels of arresten, canstatin, tumstatin, endostatin, MMP-2, and MMP-9 in controls were 1.48E-02 ± 0.006, 3.25E-01 ± 0.01, 5.51E-02 ± 0.024, 3.00E-04 ± 0.0002, 4.76E-03 ± 0.002and 3.72E-04 ± 0.0001, respectively [Table 2]. The mRNA levels of arresten was 1.6 times lower (P = 0.01) while mRNA levels of MMP-2, tumstatin and canstatin were 4, 2.5 and 2.3 times higher in cases than in controls. No change was observed in mRNA levels of MMP-9 and endostatin (P = 0.82).
Table 2.
∆Ct value of relative m-RNA levels in controls and cases
| Controls | Cases | P | |
|---|---|---|---|
| Arresten | 1.48E-02±0.006 | 6.20E-03±0.003 | 0.01 |
| Canstatin | 3.25E-01±0.01 | 1.49E-01±0.02 | 0.02 |
| Tumstatin | 5.51E-02±0.024 | 1.70E-01±0.007 | 0.01 |
| Endostatin | 3.00E-04±0.0002 | 3.20E-03±0.003 | 0.29 |
| MMP-2 | 4.76E-03±0.002 | 1.11E-03±0.0009 | 0.02 |
| MMP-9 | 3.72E-04±0.0001 | 3.72E-04±0.0001 | 0.82 |
Figure 3.
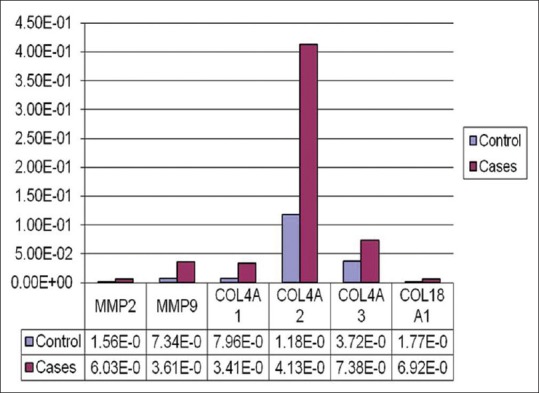
Graphical presentation of the relative levels of MMP2,MMP 9,COL4A1(Arrestin),COL4A2 (Canstatin), COL4A3 (Tumstatin) and COL18A1 (Endostatin)
Discussion
The aim of the present study is to identify the role of antiangiogenic factors (arresten, canstatin, tumstatin, and endostatin) and regulatory molecules (MMP-2 and MMP-9) in controlling regression of fetal vasculature. For this, the study uses a model of persistent fetal vasculature (PFV). In children, PFV typically manifests as unilateral cataract. It is rarely bilateral and is usually associated with microphthalmia, cataract, retinal traction or dysplasia, and elongation of the ciliary processes.[2,11] In a study reported by Solebo et al., they have observed the incidence of PFV to be 24% in children with congenital cataract, out of which 46% had unilateral and 8% had bilateral cataract.[12] Age of presentation in our study was ranging from 3 months to 132 months (mean: 41.15 months), which is corresponding to the study done by Mullner-Eidenbock A et al. who reported age of presentation ranging from 2 weeks to 15 years in 31 cases of unilateral cataract with PFV.[13] This wide range of age at presentation can be attributed to the fact that all the cases in our study had unilateral cataract with PFV which is likely to go unnoticed because of the normal functioning other eye. The lens capsule is mainly composed of type 4 collagen. Therefore, we decided to study type 4 collagen derived antiangiogenic factors from lens epithelial cells. To study the impact of these factors we decided to have single subset of PFV; therefore, we included cases with only anterior PFV. Furthermore, cases with anterior PFV will usually present with cataract, so it is easier for us to take sample of anterior capsule during cataract surgery. As the amount of tissue available for analysis was quite little, detection of antiangiogenic factors using western blotting was not possible. Recently, a technology using antibody coated bead-based fluorescent multiplex assay is used to quantify multiple proteins from a single sample. However, we did not have access to this technology. Hence, we decided to study m-RNA levels of these factors.
The regression of tunica vasculosa lentis has been extensively studied using animal models. They have provided some insight into the molecular and cellular mechanisms driving the Hyaloid Vascular System (HVS) regression including tunica vasculosa lentis. Several factors including angiopoietin-2, p53 tumor suppressor gene, arf tumor suppressor gene, γ and β crystallin and vascular endothelium derived growth factors have been studied in animal models.[14,15,16,17,18,19] Mice lacking plasma levels of endostatin caused delay in normal regression of hyaloid vessels.[20] However, the potential discrepancies between all the mice models reported and the human disease are that most cases of persistent fetal vasculature are not bilateral nor are they uniformly severe. To the best of our knowledge, no study has been published in literature evaluating the role of anti-angiogenic factors in PFV in humans. Hence, it would be valuable if these factors were analyzed in clinical cases of PFV.
It has been established that angiogenesis, the formation of new capillaries from a pre-existing capillary network, is highly regulated by the action of pro- and anti-angiogenic factors.[21] It is known that angiogenesis is influenced by several factors, including type IV collagen derived angiogenesis inhibitors like arresten, canstatin, tumstatin, and endostatin. Arresten efficiently inhibits the proliferation, migration, and tube formation of different types of endothelial cells.[8,9,22] Boosani et al. suggest that arresten may play a potential role in arresting the progression of retinal neovascularization in various pathological conditions of retina in adult eyes.[23]
Canstatin is a potent inhibitor of angiogenesis with a distinct anti-tumor activity.[2] Tumstatin was identified as the 28 kDa NC1 domain of the α3 chain of type IV collagen (α3[IV] NC1). Tumstatin was most potent in inhibiting the proliferation of endothelial cells and causing apoptosis when compared with the other α (IV) chain NC1 domains.[24,25] In type IV collagen α3 chain knockout (COL4A3−/−) mice, which are also deficient in Tumstatin, an increased pathological angiogenesis and accelerated tumor growth is observed.[26] Esipov et al. provided evidence of anti-vascular effects of T8 (Tumstatin peptide) on a newly formed vessel network in a corneal neovascularization model.[27]
Endostatin is a protein with a relative molecular weight of 20 kDa.[28] It may directly affect cells undergoing angiogenesis or regulate the secretion of various growth factors and it has a stronger effect on undifferentiated blood vessels as compared to differentiated blood vessels.[29,30,31]
MMP-2 and MMP-9 play a crucial role in the initiation and progression of vessel formation.[32] It has been suggested that these molecules display both pro and anti-angiogenic activity.[33,34] By degrading ECM, these molecules control angiogenesis during the early and in later stages.[35,36,37] They are capable of degrading ECM proteins and play a significant role in a number of ocular pathologies including retinopathy of prematurity, diabetic retinopathy, and choroidal neovascularization of age-related macular degeneration.[38] Boosani et al. suggested that arresten has inhibitory effects on MMP 2 activation during angiogenesis.[23] In the present study, our results of high m-RNA levels of MMP-2 and low m-RNA levels of arresten in PFV cases conquered with this study.
In our study, m-RNA levels of arresten were low but m-RNA levels of tumstatin and canstatin were high. We speculate that low levels of arresten resulted in the compensatory higher levels of tumstatin and canstatin. It is possible that arresten plays a decisive role in regression of fetal vasculature. There was no change in the m-RNA levels of endostatin. It is possible that endostatin plays a role in the formation of vessels but no decisive role in regression of fetal vasculature. We found no significant difference in mRNA levels of MMP-9 between cases and controls. Alterations of levels of various angiogenic and anti-angiogenic factors lead to failure of regression of fetal vasculature resulting in persistent fetal vasculature.
Implications
Regression of fetal vasculature is an important phenomenon involved in maintaining the clarity of crystalline lens. At present, we are dependent on animal models to understand the detailed process of regression. Studying clinical cases of PFV in children could be used as a model system to research it further. Such a clinical model of PFV could also be used to understand interplay of various molecules and proteins in angiogenesis as well as regression. Its implication in tumor formation and other eye pathologies resulting new vessel formation is obvious. Moreover, genes responsible for such changes in antiangiogenic factors in PFV cases should be identified and studied further.
Limitations
Studying these factors in the PFV fibrovascular tissue in addition to LECs could have been more informative. We have taken cases with only anterior PFV, so this is applicable to only anterior PFV cases. Role of same factors needs to be established in cases of posterior or mixed PFV. Since low levels of arresten in PFV cases has been noted for the first time it needs to be validated by further studies in future.
Conclusion
We found m-RNA levels of arresten were significantly low. An imbalance in the levels of anti-angiogenic factors arresten, canstatin, tumstatin, and MMP-2 may be responsible for the clinical condition of PFV with cataract.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–87. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg MF. Persistent fetal vasculature (PFV): An integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV). LIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1997;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- 3.Silbert M, Gurwood AS. Persistent hyperplastic primary vitreous. Clin Eye Vis Care. 2000;12:131–7. doi: 10.1016/s0953-4431(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Fan DB, Zhao YT, Li Y, Cai FF, Zheng GY. Surgical treatment and visual outcomes of cataract with persistent hyperplastic primary vitreous. Int J Ophthalmol. 2017;10:391–9. doi: 10.18240/ijo.2017.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85–9. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Sudhakar A, Boosani CS. Signaling mechanisms of endogenous angiogenesis inhibitors derived from type IV collagen. Gene Regul Syst Bio. 2007;1:217–26. doi: 10.4137/grsb.s345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyberg P, Xie L, Sugimoto H, Colorado P, Sund M, Holthaus K, et al. Characterization of the anti-angiogenic properties of arresten, an alpha1beta1 integrin-dependent collagen-derived tumor suppressor. Exp Cell Res. 2008;314:3292–305. doi: 10.1016/j.yexcr.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, et al. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J Clin Invest. 2005;115:2801–10. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5, beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 11.Reese AB. Persistent hyperplastic primary vitreous. Am J Ophthalmol. 1955;40:317–31. doi: 10.1016/0002-9394(55)91866-3. [DOI] [PubMed] [Google Scholar]
- 12.Solebo AL, Russell-Eggitt I, Cumberland P, Rahi JS. Congenital cataract associated with persistent fetal vasculature: Fndings from IoL under 2. Eye. 2016;30:1204–9. doi: 10.1038/eye.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullner-Eidenbock A, Amon M, Moser E, Klebermass N. Persistent fetal vasculature and minimal fetal vascular remnants 1: A frequent cause of unilateral congenital cataracts. Ophthalmology. 2004;111:906–13. doi: 10.1016/j.ophtha.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–23. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 15.Reichel MB, Ali RR, D’Esposito F, Clarke AR, Luthert PJ, Bhattacharya SS, et al. High frequency of persistent hyperplastic primary vitreous and cataracts in p53-deficient mice. Cell Death Differ. 1998;5:156–62. doi: 10.1038/sj.cdd.4400326. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda S, Hawes NL, Chang B, Avery CS, Smith RS, Nishina PM. Severe ocular abnormalities in C57BL/6 but not in 129/Sv p53-deficient mice. Invest Ophthalmol VisSci. 1999;40:1874–8. [PubMed] [Google Scholar]
- 17.Thornton JD, Swanson DJ, Mary MN, Pei D, Martin AC, Pounds S, et al. Persistent hyperplastic primary vitreous due to somatic mosaic deletion of the arf tumor suppressor. Invest Ophthalmol Vis Sci. 2007;48:491–9. doi: 10.1167/iovs.06-0765. [DOI] [PubMed] [Google Scholar]
- 18.Rutland CS, Mitchell CA, Nasir M, Konerding MA, Drexler HC. Microphthalmia, persistent hyperplastic hyaloid vasculature and lens anomalies following overexpression of VEGF-A188 from the alphaA-crystallin promoter. Mol Vis. 2007;13:47–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Gehlbach P, Hose S, Lei B, Zhang C, Cano M, Arora M, et al. Developmental abnormalities in the Nuc1 rat retina: A spontaneous mutation that affects neuronal and vascular remodeling and retinal function. Neuroscience. 2006;137:447–61. doi: 10.1016/j.neuroscience.2005.08.084. [DOI] [PubMed] [Google Scholar]
- 20.Duh EJ, Yao YG, Dagli M, Goldberg MF. Persistence of fetal vasculature in a patient with Knobloch syndrome: Potential role for endostatin in fetal vascular remodeling of the eye. Ophthalmology. 2004;111:1885–8. doi: 10.1016/j.ophtha.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Hamano Y, Kalluri R. Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun. 2005;333:292–8. doi: 10.1016/j.bbrc.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 22.Boosani CS, Sudhakar A. Cloning, purification, and characterization of a non-collagenous anti-angiogenic protein domain from human alpha1 type IV collagen expressed in Sf9 cells. Protein Expr Purif. 2006;49:211–8. doi: 10.1016/j.pep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Boosani CS, Nalabothula N, Sheibani N, Sudhakar A. Inhibitory effects of arresten on bFGF induced proliferation, migration and matrix metalloproteinase-2 activation in mouse retinal endothelial cells. Curr Eye Res. 2010;35:45–55. doi: 10.3109/02713680903374208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745–50. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- 25.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, et al. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275:21340–8. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 26.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esipov R, Beyrakhova K, Likhvantseva V, Stepanova E, Stepanenko V, Kostromina M, et al. Antiangiogenic and antivascular effects of a recombinant tumstatin-derived peptide in a corneal neovascularization model. Biochimie. 2012;94:1368–75. doi: 10.1016/j.biochi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 28.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: An endogenous inhibitor of angiogenesis and tumour growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 29.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong XP, Xiao TH, Dong H, Jiang N, Zhao XG. Endostar combined with cisplatin inhibits tumour growth and lymphatic metastasis of lewis lung carcinoma xenografts in mice. Asian Pac J Cancer Prev. 2013;14:3079–83. doi: 10.7314/apjcp.2013.14.5.3079. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Yang S, Hao J, Yuan X, Luo W, Jiang L, et al. Endostar attenuates melanoma tumour growth via its interruption of b-FGF mediated angiogenesis. Cancer Lett. 2015;359:148–54. doi: 10.1016/j.canlet.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–28. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 34.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 35.Kalluri R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 36.Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–14. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 38.Garner A. Ocular angiogenesis. Int Rev Exp Pathol. 1986;28:249–306. [PubMed] [Google Scholar]


