Abstract
Purpose:
To study the outcomes of therapeutic penetrating keratoplasty in fungal keratitis.
Methods:
This retrospective, observational, interventional case series involved an audit of 198 consecutive eyes that underwent therapeutic penetrating keratoplasty (ThPK) for fungal keratitis at L V Prasad Eye Institute between January 2008 and December 2010 was performed. The data on demographics, clinical characteristics, intraoperative, and late postoperative complications were noted. The primary outcome measure was eradication of infection and postoperative anatomical success. Secondary outcome measures were graft survival, risk factors, clinical features, and management of recurrent fungal keratitis post ThPK.
Results:
Mean follow-up after ThPK was 24 ± 17 months. A total of 178 (89.9%) eyes had complete eradication of fungal infection, whereas 20 (10.1%) eyes developed recurrence. Anatomical restoration was achieved in majority of cases (192 eyes; 97%). Larger infiltrate size was associated with a higher risk of recurrence of infection. The median graft survival rate was 5.9 months. The graft survival was better for grafts <8 mm versus those with >8 mm (P = 0.026) and not found significantly related to the species of fungus. Twenty-seven eyes underwent re-grafting: penetrating keratoplasty in 14 eyes, and Descemet's stripping endothelial keratoplasty in 13 eyes.
Conclusion:
As larger infiltrate prior to therapeutic keratoplasty had much higher risk of recurrences; timely surgical intervention should be considered in cases not responding to medical therapy. Alternative strategies of management of postoperative inflammation need to be considered to prevent graft failures.
Keywords: Fungal keratitis, keratoplasty, therapeutic penetrating keratoplasty
Fungal keratitis is an important cause of corneal blindness in India.[1,2] The infection usually occurs following ocular trauma and contamination of corneal lesion by soil particles and vegetative matter. It is also believed that the incidence of fungal keratitis is increased due to the widespread use of broad-spectrum antibiotics and steroids.[3] Fungal keratitis is often difficult to manage with medical therapy alone.[4,5] One of the important reasons for poor response to medical treatment is the delay in diagnosis and lapse in early institution of the therapy. A major limiting factor in the management of fungal keratitis is the absence of fungicidal medications. All antifungal medications for keratitis are fungistatic and so the treatment success with medications alone is limited.[6] Hence, therapeutic penetrating keratoplasty is required in cases where antifungals fail to eradicate the infection.
The success of keratoplasty in the active stage of infection has several limitations, especially in fungal keratitis. An important cause of failure of keratoplasty is the recurrence of fungal infection after surgery with reported rates ranging from 5% to 14%.[2,7] As corticosteroids favor the growth of fungus, the initiation of topical corticosteroids is delayed in the postoperative period. Consequently, the inflammation in the initial postoperative period is prolonged, thereby affecting the graft outcomes.
The purpose of the present study was to analyze the outcomes of therapeutic penetrating keratoplasty performed consecutively for nonresponding fungal keratitis.
Methods
The study was approved by the Institutional Ethics Committee and followed the tenets of the Declaration of Helsinki. Out of a total of 1661 cases of fungal keratitis treated between January 2008 and December 2010, 198 (11.9%) consecutive patients underwent therapeutic penetrating keratoplasty (ThPK) at tertiary eye care center and were included in the study. Data collected included demographics, microbiology of ulcers, surgical details, such as graft size, details of donor tissue, duration of follow-up, and information on recurrent keratitis, such as time of recurrence, clinical features, and management of recurrent keratitis.
The standard practice pattern at the institute for the management of keratitis is described here briefly. All eyes diagnosed to have infection on their first visit to the clinic, irrespective of their past diagnosis and treatment, underwent a routine microbiological evaluation. The diagnosis of fungal keratitis was made after a clinical slit lamp examination, potassium hydroxide (KOH) mount, and smear examination. Following a microbiological confirmation on smears and/or culture, antifungal treatment was instituted comprising of topical natamycin 5% suspension every hourly and oral ketoconazole twice a day. If the corneal ulcer deteriorated (defined as increase in size of the infiltrate and exudates in the anterior chamber) or did not show evidence of improvement on periodic follow-up on third or fourth visit on clinical examination, ThPK was considered. All patients underwent a B-scan ultrasonography at the time of initial visit and before corneal transplantation to exclude spread of infection into the posterior segment.
ThPK was performed under either a peribulbar block or general anesthesia. Intraoperatively, care was taken to size the graft 0.5–1 mm more than the area of the infiltrate in its widest dimensions. In a routine ThPK, after removal of the diseased cornea, anterior chamber angle and iris surface were irrigated carefully with saline to remove any exudates and hypopyon and careful inspection was done at the edges of the host to ensure that the area of infiltrate was completely excised. In eyes with total corneal infiltrate, peritomy was done to inspect the sclera of any contiguous spread and part of infected sclera (as much as feasible to preserve the angle anatomy) was included in the trephination. Corneal grafts were secured with 16–24 interrupted 10–0 nylon sutures.
At the time of ThPK, the infected corneal button was divided into two parts. One part of the button was sent in a dry bottle for microbiological evaluation. In the microbiology facility, the dry corneal button was split into four under laminar flow and inoculated in blood agar, Sabouraud's dextrose agar, brain heart infusion, and non-nutrient agar. The second half was sent in formalin 1% for histopathological analysis.
The postoperative care comprised of frequent topical instillation of antifungal agents and oral ketoconazole for 2 weeks. Assessment was made for any recurrence during the first two postoperative weeks and initiation of topical corticosteroids (prednisolone acetate 1%) 4 to 6 hourly was done only after a period of 10–14 days only when the eye was noted to have no evidence of residual or recurrent infection on clinical slit lamp examination. In cases when recurrence was suspected, corticosteroids were deferred; and topical and oral antifungals were continued. Topical and oral antiglaucoma medications were added in the postoperative period in those with a raised intraocular pressure.
In cases with suspected recurrence of infection on clinical examination, corneal scrapings were taken whenever possible, examined as wet mounts with KOH, and subjected to fungal culture and strain identification. The finding of fungal filaments from any of the above examinations served as confirmation of fungal recurrence. Recurrence of fungal infection was managed with medical treatment with antifungals and/or surgical intervention in the form of anterior chamber wash or repeat therapeutic keratoplasty in some cases.
The software Origin v 7.0 (OriginLab Corporation, Northampton, MA, USA) was used to perform the statistical analysis. Normality of the continuous data and homoscedasticity were evaluated using Shapiro–Wilk and Levene tests, respectively. Mean (± standard deviation) and median (along with interquartile range, IQR) were used to describe the parametric and nonparametric data, respectively. Categorical data were described in proportions. Kaplan–Meier survival analysis was performed to estimate the probability of graft survival following ThPK. Cox proportional hazards regression analysis was done to evaluate the risk factors of graft failure. The recurrence rate after ThPK and the presence of different risk factors were compared with Chi-square analysis. A P value of <0.05 was considered statistically significant.
Results
Demographics and preoperative characteristics
Table 1 summarizes the demographic details and preoperative characteristics of the study patients. There was a preponderance of males in the study population. All patients had infiltrate involving the full thickness of the cornea. The mean size of the infiltrate prior to therapeutic penetrating keratoplasty was 7.73 ± 2.56 mm. Majority (~80%) of the patients had infiltrate size over 6 mm. The median time interval between initial presentation to decision of surgery was 17 (9–29 days).
Table 1.
Demographics and preoperative characteristics of the patient population
| Demographics | |
| Patients (n) | 198 |
| Age (years), mean±standard deviation | 45±14.3 |
| Duration between symptoms and therapeutic penetrating keratoplasty (days), median (interquartile range) | 17 (9-29) |
| Infiltrate Characteristics | |
| <6 mm | 41 (20.7%) |
| 6-8 mm | 77 (38.9%) |
| >8 mm | 80 (40.4%) |
| Scleral involvement | 1 (0.5%) |
| Hypopyon | 54 (27.3%) |
| Perforation | 8 (4%) |
| Full thickness | 198 (100%) |
| Hypopyon and AC exudates | 11 (5.6%) |
| Graft size (mm), median (interquartile range) | 10.5 (9.5-11.5) |
| Lens status | |
| Clear | 63 (31.8%) |
| Cataract | 21 (10.6%) |
| Pseudophakia | 1 (0.5%) |
| Hazy view or no view | 113 (57.1%) |
| Posterior segment involvement (prior to surgery) | 0 (0%) |
Microbiological characteristics
In all 198 eyes, initial microbiological smear examination revealed fungal filaments. Microbiological cultures were obtained from either the initial corneal scrapings and/or half corneal button after keratoplasty.
Out of 198 eyes, corneal scrapings showed positive cultures 189 (95.5%) eyes. The cultures from half corneal button were positive for 140 eyes and concordant with the species of fungus isolated from corneal scrapings preoperatively in those where it was isolated.
The species identification obtained from either cultures of the corneal scrapings and/or growth from half corneal buttons revealed Aspergillus flavus in 64 eyes (32.3%), Acremonium spp in 35 eyes (17.7%), Fusarium spp in 31 eyes (15.7%), Dematiaceous fungi in 10 eyes (5.1%), Curvularia spp in 2 eyes (1%), Cladosporium spp in 1 eye (0.5%), Scedosporium spp in 1 eye (0.5%), and unidentified hyaline fungus in 54 eyes (27.2%). One eye had an unidentified hyaline fungus coinfection with Pseudomonas aeruginosa.
Surgery and donor details
The median donor graft size was 10.5 mm (IQR, 9.5–11.5 mm). The median age of the donor was 72 years (IQR, 62–80 years). The mean donor corneal endothelial cell density was 2232 ± 396.5 cells/mm2 (range, 1043–3436 cells/mm2). The donor corneas were stored in McCarey Kaufman medium and the mean donor death to preservation time was 3 days (range 1–4 days). Nineteen eyes (9.6%) had inadvertent additional intraoperative intervention along with keratoplasty; extracapsular cataract extraction (ECCE) in 14 eyes and ECCE along with anterior vitrectomy in 5 eyes. All eyes had 16 to 24 intact 10–0 nylon interrupted sutures.
Early postoperative outcome measures
Out of 198 eyes, 178 patients (89.9%) had complete eradication of fungal infection, whereas 20 eyes (10.1%) eyes developed recurrence. Anatomical restoration was achieved in majority of cases (192 eyes; 97%). Recurrence of fungal infection was noted at a mean duration of 15 ± 9.3 days (range, 1–28 days). The larger size of infiltrate and larger size of graft was a risk factor for recurrence (P = 0.007 and P = 0.02). There was no significant correlation of recurrence of infection with the species of fungus (P = 0.7).
Table 2 (and Supplemental data) describes the clinical features and eventual outcomes of those with recurrence of fungal keratitis after ThPK. Five eyes that had recurrence responded favorably to medical treatment and achieved clear grafts until 4.9 months. Eight eyes underwent anterior chamber wash along with medical treatment. Of these, seven responded favorably with anatomical restoration and one eye became phthisical. Four eyes underwent a repeat ThPK. Anatomical restoration was favorable with clear grafts in all four eyes after repeat therapeutic keratoplasty. Two had visual acuity better than 20/80 and the other two had visual acuity of 20/600. Two eyes underwent pars plan vitrectomy, three eyes had to be eviscerated due to extensive scleral and posterior segment spread of the infection. Anatomical restoration was successful in 13/20 (65%) eyes with recurrence, remaining 7/20 (35%) eyes ended with phthisis/anophthalmos.
Table 2.
Clinical characteristics, management and outcomes of recurrent fungal infection after therapeutic penetrating keratoplasty
| Characteristics | Results |
|---|---|
| Age (mean±standard deviation) | 42.9±11.8 years |
| Gender, male:female | 1.2:1 |
| Time interval of recurrence | |
| 1-7 days | 8 eyes (40%) |
| 8-20 days | 5 eyes (25%) |
| >20 days | 7 eyes (35%) |
| Size of donor graft | |
| 8-9 mm | 4 eyes (20%) |
| 9-11 mm | 10 eyes (50%) |
| >11 mm | 6 eyes (30%) |
| Site of initial recurrence | |
| Anterior chamber | 7 eyes (35%) |
| Graft-host junction | 5 eyes (25%) |
| Anterior chamber + graft-host junction | 5 eyes (25%) |
| Anterior chamber + posterior segment involvement | 4 eyes (20%) |
| Microbiology characteristics (Smear+/-Culture) | |
| Acremonium spp | 1 eye (5%) |
| Aspergillus flavus | 2 eyes (10%) |
| Dematiaceous fungus | 1 eye (5%) |
| Fusarium spp | 1 eye (5%) |
| Fungal filaments on smear (no growth) | 11 eyes (55%) |
| Management | |
| Medical therapy alone | 5 eyes (25%) |
| Medical therapy + anterior chamber wash | 8 eyes (40%) |
| Medical therapy + repeat ThPK | 4 eyes (20%) |
| PPV + intravitreal injection (Amphoptericin B) | 2 eyes (10%) |
| Evisceration | 3 eyes (15%) |
| Eventual outcomes | |
| Anatomical restoration | 13 eyes (65%) |
| Phthisis | 4 eyes (20%) |
| Anophthalmos | 3 eyes (15%) |
Median follow up of 178 eyes with successful eradication of infection after ThPK was 1.3 years (range, 1 month to 5.6 years). At 1–2 months, 130 eyes had clear grafts (73%), 31 had persistent graft edema (primary graft failures, 17.4%), 7 were diagnosed to have a rejection episode (3.9%), and 10 eyes were lost to follow-up (5.6%). At 3 months follow-up, out of the 130 clear grafts, 71 (54.6%) developed graft edema (secondary graft failures). At 1 year, 22 eyes maintained clear grafts (12.3%), 11 eyes (6.1%) at 4 years, and 7 eyes (3.9%) at 5 years. Of those with edematous grafts at 3 months (n = 71), 27 eyes underwent re-grafting: penetrating keratoplasty in 14 eyes and Descemet's stripping endothelial keratoplasty in 13 eyes.
A total of 10 eyes had raised intraocular pressure in the initial 3 months of postoperative period (2 weeks- 5 eyes, 1 month- 3 eyes, and 3 months- 2 eyes) and were managed with topical and oral antiglaucoma medications. All the eyes were continued on topical antiglaucoma medications till their last follow-up visits except for one eye where irido-zonulo-hyalindo vitrectomy was performed to control intraocular pressures. Seven eyes developed secondary bacterial infection after 1 month, which resolved with medical treatment in three eyes and necessitated repeat therapeutic PK in four.
Graft survival and associated risk factors
Fig. 1 shows the overall probability of graft survival after ThPK. The probabilities of survival were 49% ± 3.9%, 29.5% ± 3.7%, 20.5% ± 3.4%, 13.7% ± 3%, and 10.6% ± 2.8% at 6 months, 1, 2, 3, and 4 years, respectively. The median graft survival rate was 5.9 months. The presumed risk factors for failure were analyzed and the variables were categorized as follows: size of infiltrate (<6 mm, 6–8 mm and >8 mm), size of graft (8–9 mm, 9–11 mm, and >11 mm), species of fungus (Acremonium, Aspergillus, Fusarium, others, and unidentified hyaline fungus), additional cataract surgery (presence/absence), and socio-economic status (higher/lower).[8] Univariate cox proportional hazards regression analysis showed size of infiltrate (hazard ratio = 1.36; P = 0.01) and graft size (hazard ratio = 1.39; P < 0.0001) to be significant risk factors for failure. Multivariate cox proportional hazards regression analysis showed size of the infiltrate to be the significant risk factor for failure (hazard ratio = 1.37; P = 0.0003).
Figure 1.
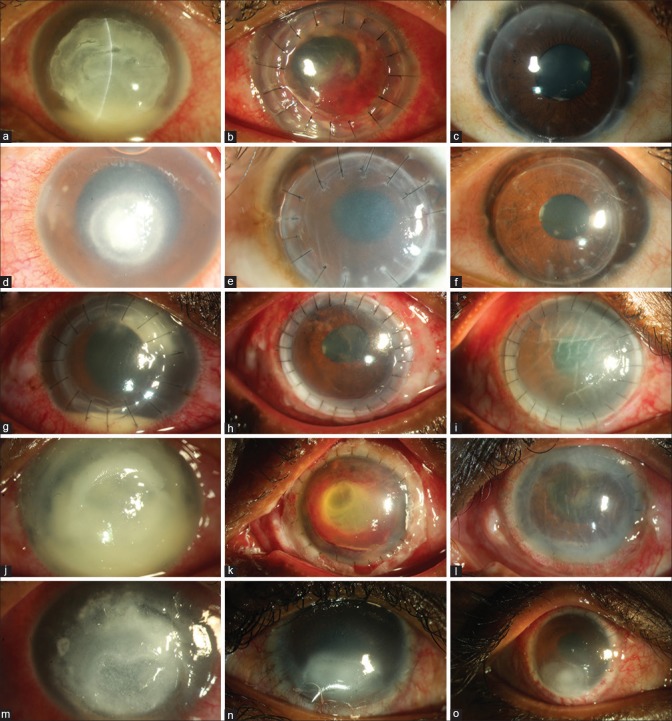
Representative images of five patients: Pt 1 (a-c) had 8.5 × 8.5 mm infiltrate, 5 years post ThPK, the graft was clear, BCVA 20/20; Pt 2 (d-f) had a nonhealing fungal infection, underwent ThPK which developed a rejection episode at 10 days. Endothelial keratoplasty restored BCVA of 20/25; Pt 3 (g-i) had a recurrence, managed by repeat ThPK. The graft failed due to acute rejection at 14th day; Pt 4 (j-l) had total corneal involvement. At 5 weeks, the graft is edematous with disorganized anterior segment; Pt 5 (m-o) had 9.5 × 10 mm infiltrate (a) and underwent large ThPK. Postoperatively the graft had persistent epithelial defect that healed after tarsorrhaphy
Visual outcomes
The preoperative visual acuity ranged from perception of hand movements to counting fingers at 3 m; as all patients had infiltrate involving visual axis. The median postoperative best-corrected visual acuity (BCVA) for all clear grafts was 20/100 at 3 months (n = 59), 20/60 at 6 months (n = 19), 20/40 at 1 year (n = 9), and 20/50 at 5 years (n = 7). The eyes with infiltrate size <9 mm (n = 8) had better postoperative BCVA (better than or equal to 20/50) in 4 years follow-up period.
Fig. 2 shows the representative images of five patients with varied outcomes after ThPK (details in the figure legends).
Figure 2.
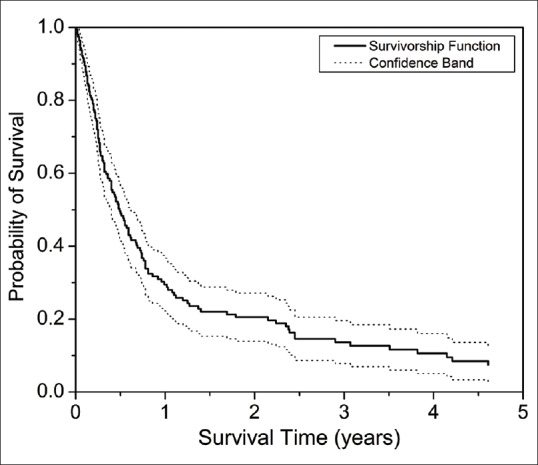
Kaplan Meir Survival analysis after ThPK
Discussion
The outcomes of therapeutic penetrating keratoplasty for fungal keratitis depend on multitude factors. The primary goal of the surgery is to eradicate the infection by taking care of macroscopically visible infected cornea, anterior chamber, and angle exudates. Visual rehabilitation in these eyes is a secondary goal as graft survival in the presence of active and severe infection is poor.[9] Also, due to a mismatch between the supply and demand of donor corneas with healthy endothelium, many of these eyes may receive donor corneas with relatively poorer endothelial cell counts than those eyes operated for noninfective causes. As steroids cannot be started immediately in the initial days postoperatively, many end up having a poor visual outcome due to eventual disorganization of the anterior segment and the angle structures, which limits future keratoplasty. Additionally, inability to start steroids in the early postoperative period puts these grafts at a high risk of rejection and vascularization, which further limits the subsequent interventions for visual restoration in these eyes. This study provides data on microbiological cure rates, graft survival, recurrence rate, and visual outcomes in a consecutive series of fungal keratitis cases referred to and treated at a single tertiary eye care center over a 3-year period (2008–2010).
Clinical characteristics, management and outcomes of patients with Recurrence after therapeutic PK (Refer to Table 2)
| Case no. | Age/gender | Site of initial recurrence | Time interval between Th PK and recurrence | Donor graft size (mm) | Clinical Course on medical therapy (natamycin 1 h and oral Azoles) | Surgical intervention for recurrence | Microbiology characteristics (smear+/- culture) | Final outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 46/m | GHJ | 27 days | 9.5 | Resolved on medical therapy | Nil | Fungus filaments | Anatomical restoration, BCVA -20/200 at 4 months |
| 2 | 36/m | GHJ | 7 days | 12 | Rapid spread to posterior segment (Endophthalmitis) | Evisceration | Fungus filaments | Anophthalmos |
| 3 | 40/f | AC | 13 days | 9 | Lost to follow up after recurrence noted, patient reviewed at 2 months later | Nil | Not available | Phthisis (noted at 2 months) |
| 4 | 35/m | GHJ + AC | 6 days | 11.5 | Resolved on medical therapy | AC wash + intracameral injection of amphotericin B | Fungus filaments | Phthisis |
| 5 | 30/f | GHJ + AC | 24 days | 11.5 | No response to medical therapy | Repeat ThPK | Acremonium species | Clear repeat graft, BCVA 20/50 at 2 months |
| 6 | 77/m | AC | 22 days | 11 | Resolved on medical therapy | AC wash | Dematiaceous fungus | Clear graft, with BCVA -20/800 at 2 months |
| 7 | 50/f | GHJ | 25 days | 9.5 | No response | Repeat ThPk | Fusarium species | BCVA 20/1200 at 1 month |
| 8 | 22/f | AC | 6 days | 11.5 | Slow response | AC wash + intracameral injection of amphotericin B | Fungal filaments | Edematous graft with BCVA HM at 5 months |
| 9 | 60/m | AC | 25 days | 9.5 | Slow response | AC wash + intracameral injection of amphotericin B | Fungal filaments | Clinical cure, BCVA HM at 1 month |
| 10 | 35/m | AC | 6 days | 9 | Slow response | ECCE + AV + AC reformation + intracameral amphoptericin-B | Fungal filaments | Failed graft |
| 11 | 48/m | GHJ + AC + PS | 28 days | 10 | Slow response | TA BCL + AC wash + intravitreal amphotericin B | Fungal filaments | Phthisis at 1 month |
| 12 | 35/f | GHJ + AC | 14 days | 8.5 | Resolved on medical therapy | Nil | Fungal filaments | Edematous graft, HM at 2 months |
| 13 | 45/f | AC + PS | 1 day | 8.5 | Posterior segment spread (Endophthalmitis) | PPV + IOAB + Intravitreal Ampho B | Not available | Phthisis |
| 14 | 45/f | AC + PS | 11 days | 11 | Posterior segment spread (Endophthalmitis) | Evisceration | Fungal filaments | Anophthalmos |
| 15 | 50/f | AC + PS | 11 days | 12 | Posterior segment spread (Endophthalmitis) | PPV + IOAB + Intravitreal Ampho B, Evisceration | Not available | Anophthalmos |
| 16 | 40/m | AC | 5 days | 9.5 | Partial response | AC wash + intracameral injection of amphotericin B | Not available | Failed graft at 3 months |
| 17 | 35/f | AC | 6 days | 12 | Partial response | AC wash + intracameral injection of amphotericin B | Fungus filaments | Failed graft at 2 months |
| 18 | 35/m | GHJ + AC | 14 days | 10.5 | Partial response | Repeat ThPK | Aspergillus flavus | Edematous graft, BCVA 20/600 at 6 months |
| 19 | 50/m | GHJ | 7 days | 8.75 | Resolved on medical treatment | Nil | Fungal filaments | BCVA 20/125 at 1 month |
| 20 | 43/m | GHJ | 25 days | 11.5 | Partial response | Repeat ThPK | Aspergillus flavus | Clear Repeat graft, BCVA 20/80 at 2 months |
We observed that therapeutic penetrating keratoplasty was required in 198 out of 1661 eyes with fungal keratitis seen during the study period, which were nonresponsive to medical therapy. This shows that although natamycin sulphate is effective in treating majority of fungal infections, a significant number of eyes that were initially treated with medical therapy needed therapeutic keratoplasty (11.9%).
In our study, anatomical restoration was achieved in majority of eyes (191/198 eyes; 96.4%) which is slightly better than that reported by Sharma et al.[2] and Chen et al.[10] (84.6%–88.5%). The recurrence rate was slightly higher compared to other published studies. The reasons for these could be related to the disease being managed surgically at an advanced stage of the infection. The recurrence risk was related to the size of the infiltrate, but there was no relationship between the species of fungus and the risk of recurrence.
The overall graft survival rates in our study are lower compared to other studies (Rogers, 2013; Xie, 2001; Sharma et al., 2013 [Table 3]).[11,12] Graft survival was 12.3% as compared to 79.3% in a study by Xie et al. We believe there were two reasons for a lower graft survival. First, all our patients had full thickness corneal infection and majority of the grafts in our study were large sized [Table 1]. Second, during the study period (2008–2010), many patients with fungal keratitis may have received donor corneas only fair to good endothelial grading (endothelial cell densities between 1500 and 2000 cells/mm2) to manage nonresolving fungal keratitis in view of relative scarcity of donor corneas in those years for optical keratoplasties.[13] In current times of last 4 years, due to an increase in the donor cornea collection at the local eye bank facility, the practice has changed to utilizing a donor cornea with better endothelial health (endothelial cell densities above 2000 cells/mm2). The outcomes of this study can be a used as a reference for comparison of outcomes of therapeutic penetrating keratoplasty after fungal keratitis at centers with similar practices (study ongoing).
Table 3.
Comparison of the present study with existing literature
| Characteristics | Xie et al., 2001 | Rogers et al., 2013* | Sharma et al., 2013 | Present study |
|---|---|---|---|---|
| Number of transplants | 108 | 32 | 506 (106/506 eyes had Fungal keratitis) | 198 |
| Study period | 4 years (1996-1999) | 10 years (2001-2011) | 10 years (1999-2009) | 3 years (2008-2010) |
| Country | China | USA | India | India |
| Age (years) | <20: 3.7% 21-40: 48.2% 41-60: 39.8% >60: 8.3% |
Median: 59 Range: 16-86 |
Mean: 45.6 Range: 14-72 |
Mean±SD: 45±14.3 |
| Male: Female | 1.8:1 | 0.9:1 | 0.7:1 | 1.9:1 |
| Infiltrate size (mm) | <6: 13.9% 6.1-8: 36.1% >8: 50% |
Minimum median diameter of infiltrate 1.50 mm | Mean 8.19±2.13 | <6: 20.7% 6-8: 38.9% >8: 40.4% |
| Donor tissue size (mm) | 7.25-8: 40% 8.5-9: 25.9% >9: 33.3% |
Not available | <9: 44% 9-11: 39% >11: 16.6% |
8-9 mm - 17.6% 9-11 mm - 54.5% >11 mm - 24.7% |
| Organism |
Aspergillus spp: 14.4% Candida spp: 9.3% Fusarium spp: 65% Penicillium spp: 4.1% Unidentified: 7.2% |
Alternaria spp: 9.4% Aspergillus spp: 12.5% Candida spp: 28.1% Fusarium spp: 34.4% Other filamentous fungi: 12.5% Other yeasts: 3.1% |
Fungal: 20.9% (Aspergillus spp. Most common) Bacterial: 31% Mixed Bacterial and Fungal: 6.9% Acanthamoeba: 1.6% |
Aspergillus flavus: 32.3% Acremonium spp: 17.7% Fusarium spp: 15.7% Cladosporium spp: 0.5% Unidentified hyaline: 27.2% Curvularia spp: 1% Dematiaceous fungi: 5.1% Scedosporium spp: 0.5% |
| Clear cornea | 79.6% at an average follow-up of 18.3 months | 53.1% at a median follow-up of 25 months | Not available | 12.3% at a follow up of 12 months, 10.6% at 24 months and 6.1% at 48 months |
| Recurrence rate | 7.4% | 9.4% | Reinfection noted in 12.6% | 10.1% |
| Graft failure | 13.9% at 24 months | 46.9% at 70 months | Not available | 71.7% at 60 months |
*Eyes that were treated with medical therapy and therapeutic keratoplasty were included in the comparison
The primary goal of therapeutic keratoplasty being eradication of infection, the practice pattern is to withhold steroids until 2 weeks of therapeutic penetrating keratoplasty. The disadvantage of withholding steroids is that many eyes develop a disorganized anterior segment, peripheral anterior synechiae, posterior synechiae, and graft edema during this time. Second intervention for visual rehabilitation is considered only in those patients where the anterior segment characteristics are favorable for a good visual outcome. Those with severely disorganized anterior segment have poor outcomes with a second keratoplasty and hence are usually best left alone. In this study, the second intervention for visual rehabilitation was performed in a small percentage of eyes. Twenty-seven (15%) eyes received a re-graft for visual restoration following therapeutic penetrating keratoplasty. One important reason for the small percentage of eyes undergoing a second intervention could be due to the advanced stage of infection in majority of our study patients.
Conclusion
In conclusion, our study reports the outcomes of ThPK specifically in fungal keratitis as the clinical course and management of these eyes differ from bacterial infections. Medical management with antifungal treatment is effective in majority of patients; however, therapeutic penetrating keratoplasty is needed to treat those with nonresponding fungal keratitis (~12%). Although anatomical restoration is good following therapeutic penetrating keratoplasty, visual restoration and secondary rehabilitation is limited in these eyes. Early surgical intervention in recalcitrant cases when smaller grafts can be planned can help in improving the graft survival. The management strategies after ThPK in fungal keratitis are consistently same over the past decades. Alternative medical therapies should be investigated to suppress the initial postoperative inflammation with efficacy like corticosteroids without the adverse effects, so that graft survival and subsequent visual rehabilitation of these eyes can be improved.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Khor WB, Prajna VN, Garg P, Mehta JS, Xie L, Liu Z, et al. The Asia cornea society infectious keratitis study: A prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol. 2018;195:161–70. doi: 10.1016/j.ajo.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Sharma N, Jain M, Sehra SV, Maharana P, Agarwal T, Satpathy G, et al. Outcomes of therapeutic penetrating keratoplasty from a tertiary eye care centre in northern India. Cornea. 2014;33:114–8. doi: 10.1097/ICO.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishnan T, Constantinou M, Jhanji V, Vajpayee RB. Factors affecting treatment outcomes with voriconazole in cases with fungal keratitis. Cornea. 2013;32:445–9. doi: 10.1097/ICO.0b013e318254a41b. [DOI] [PubMed] [Google Scholar]
- 4.Sharma N, Sachdev R, Jhanji V, Titiyal JS, Vajpayee RB. Therapeutic keratoplasty for microbial keratitis. Curr Opin Ophthalmol. 2010;21:293–300. doi: 10.1097/ICU.0b013e32833a8e23. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan M. Infective keratitis: A challenge to Indian ophthalmologists. Indian J Ophthalmol. 2007;55:5–6. doi: 10.4103/0301-4738.29487. [DOI] [PubMed] [Google Scholar]
- 6.FlorCruz NV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database Syst Rev. 2015:CD004241. doi: 10.1002/14651858.CD004241.pub4. doi: 10.1002/14651858. CD004241.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi W, Wang T, Xie L, Li S, Gao H, Liu J, et al. Risk factors, clinical features, and outcomes of recurrent fungal keratitis after corneal transplantation. Ophthalmology. 2010;117:890–6. doi: 10.1016/j.ophtha.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed A, Chaurasia S, Murthy SI, Ramappa M, Vaddavalli PK, Taneja M, et al. Endothelial keratoplasty: A review of indications at a tertiary eye care centre in South India. Asia Pac J Ophthalmol (Phila) 2014;3:207–10. doi: 10.1097/APO.0b013e3182a75304. [DOI] [PubMed] [Google Scholar]
- 9.Anshu A, Parthasarathy A, Mehta JS, Htoon HM, Tan DT. Outcomes of therapeutic deep lamellar keratoplasty and penetrating keratoplasty for advanced infectious keratitis: A comparative study. Ophthalmology. 2009;116:615–23. doi: 10.1016/j.ophtha.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Chen WL, Wu CY, Hu FR, Wang IJ. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am J Ophthalmol. 2004;137:736–43. doi: 10.1016/j.ajo.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Rogers GM, Goins KM, Sutphin JE, Kitzmann AS, Wagoner MD. Outcomes of treatment of fungal keratitis at the University of Iowa Hospitals and Clinics: A 10-year retrospective analysis. Cornea. 2013;32:1131–6. doi: 10.1097/ICO.0b013e3182883e9d. [DOI] [PubMed] [Google Scholar]
- 12.Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85:1070–4. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saini JS, Reddy MK, Jain AK, Ravindra MS, Jhaveria S, Raghuram L. Perspectives in eye banking. Indian J Ophthalmol. 1996;44:47–55. [PubMed] [Google Scholar]