Abstract
A recent systematic review (SR) and meta-analysis of human studies found an association between prenatal serum polybrominated diphenyl ethers (PBDE) concentrations and a decrease in the IQ of children. A SR of experimental developmental animal PBDE-mediated neurotoxicity studies was performed in the present study. Outcomes assessed included measures related to learning, memory, and attention, which parallel the intelli9ence-related outcomes evaluated in the human studies SR. PubMed, Embase, and Toxline were searched for relevant experimental nonhuman mammalian studies. Evaluation of risk of bias (RoB) and overall body of evidence followed guidance developed by the National Toxicology Program. Animal studies using varying designs and outcomes were available for BDEs 47, 99, 153, 203, 206, and 209 and the technical mixture DE-71. Study reporting of methods and results was often incomplete leading to concerns regarding RoB. A meta-analysis of 6 Morris water maze studies showed evidence of a significant increase in last trial latency (effect size of 25.8 [CI, 20.3 to 31.2]) in PBDE-exposed animals with low heterogeneity. For most endpoints, there were unexplained inconsistencies across studies and no consistent evidence of a dose-response relationship. There is a “moderate” level of evidence that exposure to BDEs 47, 99, and 209 affects learning. For other PBDEs and other endpoints, the level of evidence was “low” or “very low”. The meta-analysis led to stronger conclusions than that based upon a qualitative review of the evidence. The SR also identified RoB concerns that might be remedied by better study reporting.
Keywords: Developmental neurotoxicity, systematic review, learning and memory
Introduction
Polybrominated diphenyl ethers (PBDE) are a class of brominated hydrocarbons that have been used as flame retardants in a variety of products, such as textiles, plastics, electronic materials, and polyurethane foams for furniture (EPA 2010). The class is comprised of 209 congeners that share a brominated diphenyl ether molecule with up to 10 bromine atoms attached. Three classes of commercial formulations of PBDE mixtures were produced at one time, the pentaPBDEs, octaPBDEs, and decaPBDEs, but these chemicals are no longer produced or used in newly manufactured products in the United States. However, due to their persistence (Bramwell et al. 2016), these compounds remain ubiquitous in the environment with several PBDE present in air, soil, indoor building dust, and various foods on every continent (EPA (U.S. Environmental Protection Agency) 2010; Fayiga and Ipinmoroti 2017). The predominant congeners detected in environmental samples are BDE-47, BDE-99, and BDE-153.
PBDEs can undergo a variety of metabolic changes depending upon their structure and degree of bromine substitution (Stapleton et al. 2009). For example, rodents given 2,2′,4,4′,5-penta-bromodiphenyl ether (BDE-99) produce hydroxylated congeners (OH-BDE) and other metabolites (Chen et al. 2006; Qiu et al. 2007). BDE-99 undergoes more extensive metabolism when compared with either BDE-47, 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153), or 2,2′,3,3′,4,4′,5,5′,6,6′-deca-bromodiphenyl ether (BDE-209) (Chen et al. 2006; Staskal et al. 2006). Glucuronide and sulfate conjugates of dibromophenols and tribromophenols are also formed and excreted in urine and feces (Ho et al. 2015).
Human exposure to PBDE is well-documented (Bramwell et al. 2016). One approach to monitoring human exposure involves measurement of BDE, OH-BDEs, and methoxylated (MeO-BDEs) congeners in blood, milk, and other biological samples (EPA (U.S. Environmental Protection Agency) 2010; Wiseman et al. 2011; Butryn et al. 2015). The apparent half-lives of octaBDEs and decaBDEs in human serum are less than 3 months (Thuresson et al. 2006), whereas the half-lives for pentaBDEs may be 2 years or more (Geyer et al. 2004).
In experimental studies, PBDEs exert effects on the liver, the nervous system, and other organs; PBDEs and OH-BDEs have been associated with possible endocrine-disrupting effects (Cowens et al. 2015; EFSA 2011; Gill et al. 2016; Linares, Bellés, and Domingo 2015). The OH-BDE exhibit greater endocrine activity than their parent PBDE (Ptak et al. 2006, 2005). Certain PBDE and OH-BDE alter estrogen receptor alpha and estrogen receptor beta activity in rats (Ceccatelli et al. 2006), mice (Mercado-Feliciano and Bigsby 2008), and cultured cells exposed in vitro (Meerts et al. 2001). In vitro exposure to OH-BDEs also affects aromatase (CYP19) and steroid 17a- monooxygenase (CYP17) activities, enzymes involved in estrogen and androgen synthesis (Canton et al. 2005; Cantón et al. 2008; He et al. 2008; Karpeta et al. 2013). Changes in female sexual behavior and estrous cyclicity and alterations in the expression patterns of estrogen-regulated genes in sexually dimorphic brain regions occur in rats following in utero exposure to BDE-99 (Lilienthal et al. 2006; Faass et al. 2013). In addition, PBDEs affect thyroid hormone homeostasis. In animal models, investigators reported that OH-BDEs bind thyroid hormone receptors and serum thyroid hormone binding proteins, leading to lower free and total thyroxine (T4) concentrations (Darnerud and Sinjari 1996; Fowles et al. 1994; Linares, Belles, and Domingo 2015; Meerts et al. 2000; Siddiqi, Laessig, and Reed 2003).
The mechanisms of action through which developmental exposure to PBDEs alters neurobehavioral outcomes, such as IQ or attention in children or learning and memory in rodents, are incompletely understood. Thyroid hormone is critical for normal brain development (Horn and Heuer 2010) and thyroid hormone insufficiency during early development have been associated with reduced IQ (Ghassabian et al. 2014) and increased risk of ADHD behaviors (Modesto et al. 2015). There are other mechanisms through which developmental PBDE exposure might affect later cognitive or behavioral function including alterations in alter intracellular calcium signaling (Gassmann et al. 2014; Kim et al. 2011), increased oxidative stress (Costa et al. 2015), or altered gene or protein expression in key cellular targets (Dingemans, van Den Berg, and Westerink 2011; Kodavanti et al. 2015).
The potential effects of PBDEs on developing brain are receiving considerable attention and have been the focus of recent research (Chen et al., 2014a, 2014b; Gill et al. 2016) and reviews by the public-health community (Digemans et al., 2011). Lam et al. (2017) conducted a systematic review of epidemiological studies that evaluated prenatal exposure to PBDEs in relation to neurological effects in children. Data demonstrated that there was “sufficient evidence” of an association between serum PBDE concentrations and measures of intelligence and limited evidence for attention deficit/hyperactivity disorder (ADHD) and attention-related behavioral conditions in children (Lam et al. 2017). The purpose of the present study was to perform a systematic review (SR) of the evidence from animal studies to ascertain whether there is an association between PBDE exposure and neurodevelopmental impacts. This effort was intended to complement the recent SR of the human data, thus the present study focused on animal studies that measured the effects of exposure to PBDEs on learning, memory, attention, and response inhibition. These endpoints have comparability to the human intelligence and attention-related behavioral conditions reviewed by Lam et al. (2017). This SR was designed to answer the following question: is developmental exposure to PBDEs in nonhuman mammals associated with alterations in learning, memory, attention, or response inhibition? Therefore, animal studies that solely evaluated motor function, fear conditioning, and other behavioral tests that were not directly linked to learning, memory, attention, or response inhibition were considered outside the scope of the systematic review.
This review was initiated as part of a study by a committee of the National Academies of Science, Engineering, and Medicine (NASEM) that was formed to evaluate evidence of endocrine-related low dose toxicity. Here, evidence is highlighted from animal studies to demonstrate the application of SR methods to animal neurotoxicology data.
Methods
Problem formulation and protocol development
The specific review question for nonhuman animals was developed as part of problem formulation activities that would parallel the Lam et al. (2017) review. Learning and memory were selected for evaluation because these endpoints in nonhuman mammals were considered to have the closest parallels to intelligence measured in human studies; tests of attention and response inhibition were considered to parallel attention-related behaviors in humans. The protocol for the conduct of the SR was developed and peer reviewed in accordance with standard report review practices of the NASEM. The specific review question and PECO (Population, Exposure, Comparator, and Outcome) statement for the SR are as follows:
Review question: Is developmental exposure to PBDEs in nonhuman mammals associated with alterations in learning, memory, attention, or response inhibition?
Population: Nonhuman mammals
Exposure: PBDE refers to any single PBDE congener or combination of grouped congeners. Any developmental exposure to PBDEs, with no restrictions based on route of exposure or administered dose or concentration. To be considered “developmental,” the exposure occurred during any of the following periods: prior to conception in one or both parents, prenatal in the pregnant female (exposure to offspring in utero), or postnatal until sexual maturation.
Comparator: Nonhuman mammals exposed during development to different doses of PBDEs or vehicle-only treatment.
The protocol was based upon the method developed by the National Toxicology Program’s Office of Health Assessment and Translation (OHAT) for conducting SRs (hereto referred to as the OHAT method) (NTP 2015).
Literature searches and study screening
Searches for relevant existing SRs were performed first to avoid duplicating any recent work or work in progress. PubMed was searched for SRs published in 2013 or later, and the systematic-review protocol registries PROSPERO and CAMARADES were searched for relevant protocols on August 3, 2016.
PubMed, Embase, and Toxline databases were searched for relevant studies on the effects of developmental exposure to PBDEs on measures of learning, memory, attention, or response inhibition in nonhuman mammals on August 15, 2016. The search strategies for animal data have been published previously (NASEM, 2017). Briefly, search terms included chemical names (including CAS numbers), relevant mammalian species, and terms related to the endpoints of interest. All terms were searched using both controlled vocabulary [Medical Subject Headings (MeSH) in PubMed] and free text words in titles and abstracts. To be eligible for inclusion, studies had to comply with the criteria specified by the PECO statement and had to be peer reviewed, include original data, and published in the English language.
References were independently screened at the title and abstract level and at the full-text level for adherence to the PECO statement by two people (SM and EM) using DistillerSR (Evidence Partners Inc., Ottawa, Canada). At the title and abstract screening level, if there was disagreement between the reviewers or an abstract was not available, the reference was passed on to the full-text screening level for further review. At the full-text level, dis-agreements about whether to include a reference were discussed by the two reviewers to reach agreement (SM and EM); if consensus could not be reached, a third team member resolved the differences (DCD).
Data extraction
Data from the included studies were entered into the Health Assessment Workspace Collaborative (HAWC; https://hawcproject.org). Data extraction elements included funding, animal model, treatment, experimental methods, results, and other relevant information (see NASEM 2017; Appendix E, Section E-1d). Data regarding funding was drawn from text provided in the original study (e.g., acknowledgements). Data from figures were extracted using a commercially available digitizer software program (DigitizeIt Version 2.2.2, I. Bormann, Braunschweig, Germany). One individual entered data and a second person verified the entries (DCD, SM). All data entered into HAWC are available at the following link: https://hawcproject.org/assessment/352/.
Risk of bias (rob) of individual studies
RoB was assessed using the Office of Health Assessment and Translation (OHAT) RoB tool (NTP (National Toxicology Program) 2015) customized to address the research question for this review (Supplemental Table 1). Three RoB elements were considered more important than the others for assessing potential bias; these “key” elements included whether or not animals were randomly assigned to treatment groups, reliability of the outcome measures (including blinding of outcome assessors to treatment groups), and investigator control for litter effects in the experimental design or statistical approaches. The RoB assessment also considered blinding of researchers to study group during the study, characterization of the test chemical, concealment of allocation to study groups, completeness of reporting, and information regarding attrition and data exclusion. Two individuals independently assessed each study and answered all applicable RoB questions. Individual questions were rated as definitely low RoB, probably low RoB, probably high RoB, definitely high RoB, or not reported (NR). A rating of NR was considered to be equivalent to probably high risk RoB.
Meta-analysis
A meta-analysis of studies that evaluated the effects of exposure to PBDEs using the Morris Water Maze was performed in order to examine whether studies were inconsistent in their results. The Morris Water Maze was the test used the most frequently to test the neurobehavioral effects of PBDEs. The Morris water maze is a test of spatial learning for rodents that relies on distal cues to navigate from start locations around the perimeter of an open swimming arena to locate a submerged escape platform (Vorhees and Williams 2006). Spatial learning trials are usually conducted over several days, with one or more trials per day. Supplemental Table 2 provides additional details regarding the methods used in the studies selected for meta-analysis. The latency to find the platform in the last acquisition trial was the measure of learning used in the meta-analysis since it was routinely reported in the reviewed studies. Reference memory was also assessed in some studies at the end of learning (often one day after the last acquisition day), by giving a probe (transfer) trial, however, these data were not included in the meta-analysis.
There are no a priori data that suggest species differences in the sensitivity to PBDE neurotoxicity, so results for rats and mice were analyzed together. Given the sparse data on individual PBDEs, all BDEs were initially analyzed together. Thus, a meta-analysis using all the data from BDE-47, −99, −153, and −209 and the technical mixture, DE-71, was performed. For some studies, the standard deviations (SDs) were unreported or could not be digitized. Additional analyses of individual BDEs were conducted in cases where there were more than two data points. All studies were considered for inclusion in the meta analysis except Reverte et al. (2013), which used transgenic mice with a variant of the human APOE gene.
Effect sizes were calculated as the log10 ratio of the mean difference between the treatment group and the concurrent control multiplied by 100:
For small changes, this is approximately equal to the percent change (e.g., a 5% change corresponds to y = 5.1), but the resulting confidence interval is more symmetric and closer to normal (Hedges, Gurevitch, and Curtis 1999; Lajeunesse 2011). This normalization allows for treatment groups to be compared across studies and experiments. A standard random effects model was applied in the metaanalysis, using the Restricted Maximum Likelihood Estimate as implemented in the R package metafor (Viechtbauer 2005; 2010; Raudenbush 2009).
Here, yi was the observed effect size for group i; μ was the average true effect size; μ + ui was the true effect size for group i, which was normally distributed μi ~ N(0,tau2); and εi ~ N(0,vi) was the sampling error, where vi was calculated based on the reported sample sizes and standard deviations of the treatment and control groups. The average true effect μ was estimated along with its 95% confidence interval (CI) and z-score. For heterogeneity, tau2 was estimated, as well as the Q statistic and its p-value (whether there was statistically significant heterogeneity) and the I2 index (I2 = tau2/overall variance).
When normalized in this way treatment groups within a study are correlated because they are compared to a common control. Therefore, in one of the sensitivity analyses, effects were estimated using only the highest treatment group from each study. Additional sensitivity analyses were performed by sequentially excluding each study (all treatment groups for that study).
Meta-regression involved adding n, predictors xj,i to the random effects model in an attempt to explain the residual heterogeneity.
The meta-regression analyses focused on the dose-response relationship. Three models were used: linear: x1,I = dosei, log-linear: x1,I = log10 (dosei), and linear-quadratic: x1,I = dosei; x2,I = dose2i. For the linear and linear-quadratic models, the “intercept” term μ was omitted to ensure that there was no effect at dose = 0. Further for these models, the coefficients were rescaled in terms of the change per 10 mg/kg-day (e.g., y = b*[dose/10] + c*[dose/10]2) for ease of interpretation. Benchmark dose (BMD) estimates were calculated for an effect size of 5% (BMD5). The BMD5 was calculated using the linear or the linear-quadratic model, with the model selection based on the lowest Akaike information criterion (AICc) corrected for small sample size. The BMD5 was calculated only for the “fixed effect”—that is, the estimated mean response across studies.
Confidence rating and level of evidence conclusions
The OHAT method (NTP (National Toxicology Program) 2015) and expert judgment were used to rate confidence in the body of evidence on each congener and outcome and to draw conclusions about the level of evidence of a health effect (see Figure 1). The OHAT method is based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group guidelines (Guyatt et al. 2011; Rooney et al. 2014). The bodies of evidence on each congener started with an initial rating of “high” confidence based on design factors characteristic of experimental studies (e.g., controlled exposure) (NTP (National Toxicology Program) 2015). Several factors were then considered to determine whether the initial rating need to be downgraded or upgraded. Factors that decreased confidence in results and led to downgrading were RoB, unexplained inconsistency in results, indirectness or reduced applicability for the outcome of interest, imprecision (indication of a large standard deviation [SD]. E.g., SD > mean), and publication bias. Sources of funding were used to evaluate publication bias in terms of whether a particular sector funded more studies than another. Factors that increased confidence in results and may have upgraded a rating were a large magnitude of effect, evidence of a dose-response relationship, consistency across animal models (or species), consideration of residual confounding, and other factors that may have increased confidence in the association or effect (e.g., rare outcomes). Confidence ratings were independently assessed by two individuals; discrepancies were resolved by consensus and final consultation with all study authors. The 4 possible ratings were: high, moderate, low, or very low confidence in the body of evidence. The final confidence rating was subsequently translated into a “level of evidence” for each congener and outcome by considering whether there was evidence of a health effect. Using the OHAT scheme, 4 conclusions can be drawn if there is evidence of an effect (high, moderate, low, or inadequate) and two can be drawn if there is evidence of no effect (inadequate or evidence of no health effect).
Figure 1.
Overview of the process used to assess the overall confidence in the body of evidence.
Results
Search results
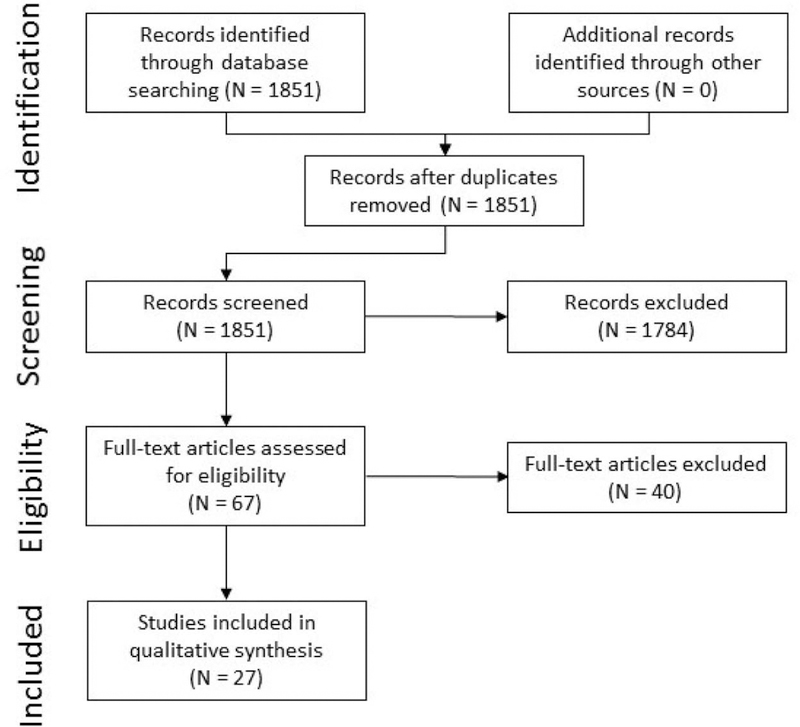
A search for recently published or ongoing SRs on developmental exposure to PBDEs and alterations in learning, memory, attention, or response inhibition in nonhuman mammals found no relevant reviews. A search of PubMed, Embase, and Toxline for relevant publications to address the PECO statement found 1,851 unique citations. A total of 67 publications met the criteria for full-text review, and 27 of those met the inclusion criteria for data extraction (see Figure 2) and were included in the review. No additional references were located by searching reference lists of included studies.
Figure 2.
Summary of the search and screening of the literature on the effects of developmental exposure to PBDEs on learning, memory, attention, or response inhibition in animals. Full text articles were excluded for the following reasons: no original data (n = 2); study did not report experimental PBDE exposure (n = 1); study did not include developmental exposure (n = 1); study did not assess or report quantitative measures of learning, memory, attention, or response inhibition (n = 9); not in English (n = 1); or other reason (n = 38). NOTE: the number of studies does not equal the total in the figure because the screeners sometimes excluded a study for different reasons.
The search identified studies on 6 BDE congeners (BDE-47, −99, −153, −203, −206, and −209) and one technical grade flame retardant mixture (DE-71). Studies on learning were available for all of these BDEs; studies of memory were available on 5 of the congeners (BDE-47, −99, −153, −203, and −209) and DE-71; a study of attention was available on DE-71. No studies on response inhibition were found for any of the BDEs. A wide range of behavioral tests was used to evaluate learning, memory, and attention in rodents exposed to PBDEs during development (see Table 1).
Table 1.
Studies Included in the PBDE systematic review. Reprinted with permission (NASEM, 2017).
| Study | Chemical | Species (strain) | Life Stage Exposed | Life Stage Assessed |
Test(s) | Doses (mg/kg-day) |
|---|---|---|---|---|---|---|
| Eriksson, Jakobsson, and Fredriksson 2001 | BDE-47 | Mouse (NMRI) | PND 10 | 5 months | Morris water maze | 0, 10.5 |
| He et al. 2009 | BDE-47 | Rat (Sprague-Dawley) | PND 10 | 2 months | Morris water maze | 0 5, 0, |
| He et al. 2011 | BDE-47 | Rat (Sprague-Dawley) | PND 10 | 2 months | Morris water maze | 0, 1, 5, 10 |
| Koenig et al. 2012 | BDE-47 | Mouse (C57Bl/6J) | 4 weeks before breeding to PND 21 | 8 weeks | Barnes spatial maze | 0, 0.03, 0.1, 1 |
| Ta et al. 2011 | BDE-47 | Mouse (C57BL/6J) | GD 0 - PND 21 | 8 weeks | Morris water maze | 0, 0.03, 0.1, 1 |
| Woods et al. 2012 | BDE-47 | Mouse (Mecp2 308+/−) Mouse (wild type) | GD 0 - PND 21 | PND 50–54 | Morris water maze | 0, 0.03 |
| Blanco et al. 2013 | BDE-99 | Rat (Sprague-Dawley) | GD 6 - PND 21 | PND 26–35 | Morris water maze | 0, 1, 2 |
| Cheng et al. 2009 | BDE-99 | Rat (Sprague-Dawley) | GD 6 - PND 21 | PND 34–36 | Morris water maze | 0, 2 |
| Eriksson, Jakobsson, and Fredriksson 2001 | BDE-99 | Mouse (NMRI) | PND 10 | 5 months | Morris water maze | 0, 12 |
| Fischer, Fredriksson, and Eriksson 2008 | BDE-99 | Mouse (NMRI) | PND 10 | 4 and 6 months | Morris water maze; radial arm maze | 0, 0.8 |
| Llansola et al. 2009 | BDE-99 | Rat (Wistar) | GD 2–9 or GD 11–19 | PND 68–70 | Y maze | 0, 30 (IP) |
| Zhao et al. 2014 | BDE-99 | Rat (Sprague-Dawley) | GD 1 - PND 21 | PND 34–36 | Morris water maze | 0, 0.2 |
| Viberg, Fredriksson, and Eriksson 2003 | BDE-153 | Mouse (NMRI) | PND 10 | PND 180 | Morris water maze | 0, 0.45, 0.9, 9 |
| Zhang et al. 2013 | BDE-153 | Rat (Sprague-Dawley) | PND 10 | PND 40 and 70 | Morris water maze; passive avoidance | 0, 1, 5, 10 (IP) |
| Viberg et al. 2006 | BDE-203 | Mouse (NMRI) | PND 3 or 10 | PND 90 | Morris water maze | 0, 16.8 |
| Viberg et al. 2006 | BDE-206 | Mouse (NMRI) | PND 10 | PND 90 | Morris water maze | 0, 18.5 |
| Biesemeier et al. 2011 | BDE-209 | Rat (Sprague-Dawley) | GD 6 - PND 21 | PND 22, PND 62 | Water T maze | 0, 1, 10, 100, 1000 |
| Buratovic et al. 2014 | BDE-209 | Mouse (NMRI) | PND 3 | 5 and 7 months | Morris water maze | 0, 3.4, 7.9 |
| Chen et al. 2014b | BDE-209 | Rat (Sprague-Dawley) | GD 1–14 | PND 25 | Morris water maze | 0, 10.0, 30, 50 0, 10, 30 |
| Reverte et al. 2013 | BDE-209 | Mouse (apoE2) Mouse (apoE3) Mouse (apoE4) | PND 10 | PND 120 and 360 | Morris water maze | |
| Reverte et al. 2014 | BDE-209 | Mouse (apoE2) Mouse (apoE3) Mouse (apoE4) | PND 10 | PND 150–180 |
Fear conditioning | 0, 10, 30 |
| Rice et al. 2009 | BDE-209 | Mouse (C57BL6/J) | PND 2–15 | PND 87 or PND 497 | Operant (fixed ratio; fixed interval; visual discrimination) | 0, 6, 20 |
| Verma, Singh, and Gandhi 2013 | BDE-209 | Mouse (Swiss albino) | PND 3–10 | PND 60–66 | Morris water maze; radial arm maze | 0, 20 |
| Verma, Singh, and Gandhi 2014 | BDE-209 | Mouse (Swiss albino) | PND 3–10 | NR | Morris water maze | 0, 20 |
| Bowers et al. 2015 | DE-71 | Rat (SpragueDawley) | GD 1 - PND 21 | PND 235 | Morris water maze | 0, 0.3, 3, 30 |
| de-Miranda et al. 2016 | DE-71 | Rat (Wistar) | PND 5–22 | PND 100 | Radial maze learning | 0, 30 |
| Driscoll, Gibson, and Hieb 2009 | DE-71 | Rat (Long-Evans) | GD 0 - PND 21 | PND 40–95 | Visual discrimination; attention task | 0, 3 or 0, 4.5 |
| Driscoll et al. 2012 | DE-71 | Rat (Long-Evans) | PND 6–12 | PND 40–95 | Visual task; attention task | 0, 5, 15 |
| Dufault, Poles, and Driscoll 2005 | DE-71 | Rat (Long-Evans) | PND 6–12 | PND 30 | Visual discrimination; attention task | 0, 30 |
Notes. Unless otherwise noted the studies involved oral exposure. GD, gestation day; IP, intraperitoneal; PND, postnatal day; NR, not reported.
Rob evaluation
Most studies had ratings of probably high or definitely high RoB for at least one of the key issues considered (e.g., lack of randomization of treatment), and most of the studies had multiple RoB issues, including not controlling for litter effects in the study design or analysis (Figures 3 and 4). Numerous studies received a “not reported” rating for one or more of the questions because either reporting of methods and results was incomplete or the appropriate method was not performed. For each body of evidence comprised of studies grouped by congener and outcome (e.g., separately for learning and memory), there were sufficient RoB concerns to support a downgrade by one (e.g., concern regarding a single key RoB element question) or two levels (concern regarding multiple key RoB questions and several other RoB issues) in overall confidence.
Figure 3.
Risk of bias heatmap of BDE-47, BDE-99, BDE-203, and BDE 206 studies of memory, learning, and attention in rodents. Modified from NASEM (2017).
Figure 4.
Risk of bias heatmap of BDE-153, BDE-209, and DE-71 studies of memory, learning, and attention in rodents. Modified from NASEM (2017).
Meta-analysis of morris water maze latency
The meta-analysis of rodent studies on the relationship between exposure to several BDEs and latency in the last learning trial of the Morris water maze showed a robust, consistent, and significant association between developmental exposure to PBDEs and decrements in this measure (Figures 5 and 6). Specifically, a significant overall effect of PBDE treatment was observed (effect size of 25.8 [CI, 20.3 to 31.2], corresponding to about a 30% change in latency). This effect was robust to leaving out individual studies or using only the highest dose group in each study (Supplemental Table 2). There was also low or no heterogeneity (25%, not significant in primary analysis; and < 35%, not significant in all sensitivity analyses) indicating that effects reported across studies were consistent. However, interpretation of this effect size is unclear because it is a summary across different BDE congeners and dose levels. With respect to dose, there was a positive, but not significant, trend from the meta-regression in logio(dose). Meta-regression using a linear model or a linear-quadratic model resulted in a significant linear term. The estimated BMD5 for change in latency was 5.1 mg/kg-day (95% confidence interval [CI]: 3.2, 13) for the linear model and 1.8 mg/kg-day (95% CI: 1.1, 4.5) for the linear-quadratic model. In both cases, however, heterogeneity was significant with I2 increased to > 70%, suggesting that the model did not explain differences across studies and dose. Overall, the meta-analysis results indicate that PBDE treatment consistently impairs learning in the Morris water maze, but did not provide evidence of the presence of a dose-response relationship.
Figure 5.
Forest plot of all studies of BDEs and latency in the last trial of the Morris water maze in rats and mice. Studies shown in black reported standard deviations while the studies in blue did not or the standard deviation could not be confidently digitized from the study figures. Dashed lines separate different congeners. Reprinted with permission (NASEM, 2017).
Figure 6.
Results of the meta-analysis of PBDEs and latency in the last trial of the Morris water maze in rats and mice sorted by congener and then by dose. Analysis was restricted to studies for which sample sizes were reported and standard deviations were either reported or could be digitized from figures presented in the publications. Dashed lines separate different congeners. The overall effect of treatment is shown at the bottom of the figure. Reprinted with permission (NASEM, 2017).
Confidence ratings
Upgrades were not used for any congener or outcome. There was no apparent evidence of a large magnitude of effect, or a dose-response relationship for any congener or outcome. The body of evidence was deemed inadequate for making judgments about cross-species consistency so no upgrade was applied for consistency across species or animal models in any case. Residual confounding primarily relates to observational studies (NTP, 2015) and not applied as a factor to upgrade the confidence rating for a data stream.
With few exceptions (Biesemeier et al. 2011; Driscoll et al. 2012), the reviewed studies were funded by governmental agencies so downgrades for publication bias were not applied for any evidence stream. Downgrades for indirectness were not applied for any congener or outcome because the behavioral tests used were considered direct measures of learning, memory, or attention. Downgrades for imprecision were also not used since there was little to no indications of excessively large standard deviations. Other factors (e.g., RoB concerns) that resulted in a downgrade in the confidence ratings for an individual congener and outcome are discussed below.
BDE-47 and learning and memory
There is moderate confidence in the body of evidence (Table 2) on developmental exposure to BDE-47 and effects on learning in rodents. As presented in Table 2, the initial high confidence was downgraded once for RoB concerns.
Table 2.
Profile of the body of evidence on pbdes and learning, memory, and attention, key:“—“ no concern or not present; “⊠” serious concern to downgrade confidence; “↑”sufficient evidence to upgrade confidence, modified from NASEM (2017).
| Initial confidence for each body of evidence (# of studies) |
Factors Decreasing Confidence |
Factors Increasing Confidence |
Final confidence rating |
Level of evidence for health effect |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RoB | Unexplained Inconsistency |
Indirectness | Imprecision | Publication Bias |
Large Magnitude |
Dose Response |
Residual Confounding |
Consistency Species/Model |
|||
| BDE-47 Learning High (2 rat,a 4 mouseb) | ↓ | — | — | — | — | — | — | — | — | Moderate | Moderate |
| BDE-47 Memory High (1 rat,c 4 mouseb) | ↓ | ↓ | — | — | — | — | — | — | — | Low | Low |
| BDE-99 Learning High (4 rat,d 1 mousee) | ↓ | — | — | — | — | — | — | — | — | Moderate | Moderate |
| BDE-99 Memory High (2 rat,f 1 mousee) | ↓ | — | — | — | — | — | — | — | — | Moderate | Inadequate |
| BDE-153 Learning High (1 rat,g 1 mouseh) | ↓ ↓ | — | — | — | — | — | — | — | — | Low | Low |
| BDE-153 Memory High (1 rat,g 1 mouseh) | ↓ ↓ | — | — | — | — | — | — | — | — | Low | Low |
| BDE-203 Learning High (1 mousei) | ↓ ↓ | ↓ | — | — | — | — | — | — | — | Very Low | Inadequate |
| BDE-203 Memory High (1 mousei) | ↓ ↓ | ↓ | — | — | — | — | — | — | — | Very Low | Inadequate |
| BDE-206 Learning High (1 mousei) | ↓ ↓ | ↓ | — | — | — | — | — | — | — | Very Low | Inadequate |
| BDE-209 Learning High (2 rat,j 6 mousek) | ↓ | — | — | — | — | — | — | — | — | Moderate | Moderate |
| BDE-209 Memory High (1 rat,l 5 mousem) | ↓ | ↓ | — | — | — | — | — | — | — | Low | Low |
| DE-71 Learning High (3 ratn) | ↓ ↓ | ↓ | — | — | — | — | — | — | — | Very Low | Inadequate |
| DE-71 Memory High (2 rato) | ↓ ↓ | ↓ | — | — | — | — | — | — | — | Very Low | Inadequate |
| DE-71 Attention High (3 ratp) | ↓ ↓ | ↓ | — | — | — | — | — | — | — | Very Low | Inadequate |
Eriksson, Jakobsson, and Fredriksson (2001); Ta et al. (2011); Koenig et al. (2012); Woods et al. (2012).
Eriksson, Jakobsson, and Fredriksson (2001); Rice et al. (2009); Reverte et al. (2013); Verma, Singh, and Gandhi (2013, 2014)); Buratovic et al. (2014).
Six studies of learning were available. Two studies in rats found several indications of decreased learning in the Morris water maze (e.g., prolonged latency to find the platform) after developmental exposure to BDE-47. Both studies were from the same lab (He et al. 2011, 2009). Three of the 4 mouse studies reported decreased learning in at least one test, strain, or gender and conducted by different research groups (Eriksson, Jakobsson, and Fredriksson 2001; Koenig et al. 2012; Ta et al. 2011; Woods et al. 2012). The mouse results were variable depending upon the tests administered, and range of test conditions contributed to difficulty in evaluating results across studies. For example, no clear pattern was identified to explain differences in response relative to a susceptible strain, gender, or dose. The overall body of evidence provides consistent evidence of diminished learning given the reduction observed in two rat studies and in at least one outcome in 3 of 4 mouse studies, although the heterogeneity was a potential concern. Qualitatively, the studies of learning in rodents appeared to have inconsistent results that might warrant a downgrade in confidence because of unexplained inconsistency. Nevertheless, meta-analysis on the latency in the last trial of the Morris water maze from several PBDE congeners provided additional evidence of the consistency of effect on learning and further supported the decision not to downgrade for unexplained inconsistency. Results of the meta-analysis suggest that the apparent qualitative inconsistency can be explained by differences in precision across studies.
There is low confidence in the body of evidence on developmental exposure to BDE-47 and effects on memory in rodents (the initial high confidence was downgraded for RoB concerns and unexplained inconsistency) (Table 2). There were 5 studies that tested memory in rodents after exposure to BDE-47. One study in rats (He et al. 2011) found decreased memory in the Morris water maze. Three of the mouse studies (Eriksson, Jakobsson, and Fredriksson 2001; Koenig et al. 2012; Ta et al. 2011) noted no adverse effects on memory, and a fourth study by Woods et al. (2012) reported decrements in memory in female Mecp2 308+/− mice, with no adverse effects on males or in C57BL6 mice of either gender.
BDE-99 and learning and memory
There is moderate confidence in the body of evidence on developmental exposure to BDE-99 and effects on learning and memory in rodents (initial high confidence was downgraded for RoB concerns) (Table 2). Five studies of the effects of exposure to BDE-99 on learning were available. Two of the three studies in rats demonstrated slower learning in the Morris water maze at a dose of 2 mg/kg-day (Blanco et al. 2013; Cheng et al. 2009). Zhao et al. (2014) reported no adverse effects at a lower dose (0.2 mg/kg-day) under similar exposure and testing conditions. In contrast, developmental exposure of Wistar rats at doses up to 30 mg/kg-day exerted no adverse effect on learning tested with a Y maze (Llansola et al. 2009). A single study (Fischer, Fredriksson, and Eriksson 2008) in NMRI mice also reported decrements in learning during the acquisition period in tests using either a radial maze or a Morris water maze at a dose of 0.8 mg/kg-day. Similar to the body of evidence for the effects of BDE-47 on learning, the meta-analysis on the latency in the last trial of the Morris water maze provided support for the decision not to downgrade for inconsistency. Three studies of BDE-99 and memory detected no adverse effects in several memory tests at doses of 0.2–2 mg/kg-day.
BDE-153 and learning and memory
There is low confidence in the body of evidence on developmental exposure to BDE-153 and effects on learning and memory (initial high confidence downgraded twice for serious RoB concerns) (Table 2). Two studies assessing learning were available, one in mice and one in rats. Viberg, Fredriksson, and Eriksson (2003) found longer latencies in the acquisition period in the Morris water maze when mice were exposed to BDE-153 at 0.9 or 9 mg/kg-day on PND 10. The rat study (Zhang et al. 2013) reported no adverse effect of BDE-153 at 10 mg/kg-day on performance in either the Morris water maze or the passive avoidance test when evaluated at PND 40 or PND 70. Similar to the bodies of evidence for BDE-47, BDE-99 and learning, the meta-analysis provided support for the decision not to downgrade for inconsistency.
The studies of learning also evaluated memory. The mouse study (Viberg, Fredriksson, and Eriksson 2003) observed longer latencies in the relearning period of mice tested at 6 months of age in the Morris water maze after exposure to BDE-153 at 0.9 or 9 mg/kg-day. The rat study (Zhang et al. 2013) reported increased swimming time in the Morris water maze 1 month after treatment with BDE-153 at 5 and 10 mg/kg-day when evaluated at PND 40 or PND 70 and memory impairment in the passive avoidance test at 10 mg/kg-day on PND 70.
BDE-203 and learning and memory
There is very low confidence in the body of evidence on developmental exposure to BDE-203 and effects on learning and memory in mice (initial high confidence downgraded twice for serious RoB concerns and once for unexplained inconsistency) (Table 2). One study was available on both end points (Viberg et al. 2006), and only a single dose (16.8 mg/kg) was tested in a single species (mouse). The results suggested that timing of exposure might exert an influence on effects because exposure to BDE-203 at 16.8 mg/kg-day on PND 10 affected learning during the acquisition period, whereas exposure on PND 3 had no marked effects on learning (Viberg et al. 2006). It was decided to downgrade confidence given that there was only a single study; it was not possible to establish or evaluate consistency because elements that would have strengthened the conclusions from a single study, such as multiple species, strains, or particularly large sample sizes (e.g., n = 50–100) were not available. This study found no adverse effect on memory following BDE-203 administration.
BDE-206 and learning
There is very low confidence in the body of evidence on developmental exposure to BDE-206 and learning in mice (initial high confidence downgraded twice for serious RoB concerns and once for unexplained inconsistency) (Table 2). Only a single study (Viberg et al. 2006) in NMRI mice was identified and no adverse effect on learning was seen at a single BDE-206 exposure level (16.8 mg/kg-day). Similar to BDE-203, confidence was downgraded given there was only the single study and therefore it was not possible to establish or evaluate consistency.
BDE-209 and learning and memory
There is moderate confidence in the body of evidence on developmental exposure to BDE-209 and effects on learning (initial high confidence downgraded for RoB concerns) (Table 2). Eight studies on learning were available. No marked effect on learning was seen in one study that evaluated Sprague-Dawley rat performance on a water T maze following exposure to BDE-209 at 1000 mg/kg-day (Biesemeier et al. 2011). Several studies (Buratovic et al. 2014; Eriksson, Jakobsson, and Fredriksson 2001; Reverte et al. 2013; Verma, Singh, and Gandhi 2014) failed to show significant effects on learning when it was assessed using the Morris water maze. However, other studies (Reverte et al. 2013; Verma, Singh, and Gandhi 2013) that also used the Morris water maze observed effects on learning when BDE-209 was tested at similar doses (10–30 mg/kg-day). In addition, studies using other test methods, including cued fear conditioning (Reverte et al. 2013) or performance on operant or visual discrimination tasks (Rice et al. 2009), also noted effects on learning.
There is low confidence in the body of evidence of BDE-209 on memory (initial high confidence downgraded for RoB concerns and unexplained inconsistency) (Table 2). Six studies on memory were available for BDE-209. Several mouse studies demonstrated effects on memory when assessed with the Morris water maze (Buratovic et al. 2014; Eriksson, Jakobsson, and Fredriksson 2001; Reverte et al. 2013) or radial arm maze (Verma, Singh, and Gandhi 2013). These studies used BDE-209 exposures that ranged from 3.4 to 20 mg/kg-day. However, other studies found no marked effects on memory using Morris water (Reverte et al. 2013) or radial arm mazes (Verma, Singh, and Gandhi 2014) and similar dose ranges. One study also failed to observe effects on memory in Sprague-Dawley rats given 1000 mg/kg-day and assessed using a water T maze (Biesemeier et al. 2011).
DE-71 and learning, memory, and attention
There is very low confidence in the body of evidence to evaluate whether developmental exposure to DE-71 affects learning, memory, or attention in rats (downgraded twice because of serious RoB concerns and once for unexplained inconsistency) (Table 2). There were three studies on learning, two on memory, and three on attention. The results of the three studies on learning were inconsistent and used different tests (Morris water maze, radial maze, and visual discrimination) and animals of different ages. One study (Dufault, Poles, and Driscoll 2005) reported increased errors in the visual discrimination task at the single dose tested (30 mg/kg-day on postnatal days 6–12). The other two studies found no marked effects of DE-71 on learning at the same dose using longer exposure windows (Bowers et al. 2015; de-Miranda et al. 2016); however, the animals in these studies were evaluated at older ages than were the rats tested by Dufault, Poles, and Driscoll (2005). The two studies of the effects of DE-71 on memory displayed inconsistent results and were evaluated in rats of different ages and with different tests (Morris water maze and radial maze). One study (de-Miranda et al. 2016) reported memory deficits in female Wistar rats (but not in males) in the radial maze at the single dose tested (30 mg/kg-day). Studies of DE-71 and attention were from a single lab (Driscoll, Gibson, and Hieb 2009; Driscoll et al. 2012; Dufault, Poles, and Driscoll 2005), and the majority of the tests reported no marked effects at doses up to 30 mg/kg-day across multiple tests (various attention tasks and a visual task).
Level of evidence conclusions
There is moderate confidence in the body of evidence on PBDEs and effects on learning in rodents based on studies on BDE-47, −99, and −209 and low confidence in the body of evidence on BDE-153. The moderate confidence rating coupled with evidence of an effect translates to a moderate level of evidence that developmental exposure to these congeners is associated with decrements in learning in rodents. Very low confidence ratings for the bodies of evidence on BDE-203 and −206 and DE-71 translate into a conclusion that there is inadequate evidence to assess whether exposure to these congeners or to the technical mixture is associated with decrements in learning in rodents. There is low confidence in the body of evidence on developmental exposure to PBDEs and effects on memory in rodents based on evidence for BDE-47, −153, and −209. Confidence in the body of evidence on BDE-99 was rated as being moderate, but the findings in those studies suggested a lack of association. A more robust database is needed to support a finding of a lack of significant effect (NTP (National Toxicology Program) 2015), such that the evidence was judged to be inadequate to reach a conclusion that developmental exposure to BDE-99 exerted no marked effect on memory. The only available data on attention and PBDEs was on DE-71. Confidence in the body of evidence was rated as being very low, thus there is inadequate evidence to assess whether exposure to this technical mixture is associated with effects on attention in rats.
Discussion
Our analyses illustrate the application of SR methods to a chemical hazard assessment. Systematic reviews use explicit, pre-specified methods to identify, select, assess, and summarize findings of similar but separate studies to answer a focused research question (IOM, 2011). The SR process is undertaken to (1) identify relevant studies on the agent of interest, (2) evaluate the studies identified, and (3) provide a qualitative and, where possible, a quantitative synthesis of the identified studies. Methods for conducting SRs are well established (Higgins and Green 2011; IOM 2011) and the methods have been adapted for use in toxicology (NTP (National Toxicology Program) 2015; Rooney et al. 2014; Woodruff and Sutton 2014). Detailed guidance for the conduct of toxicological SRs, including evaluation of the confidence in the evidence and data integration, have emerged in the past few years (NTP (National Toxicology Program) 2015; Rooney et al. 2014).
Challenges in the conduct of SRs of animal neurotoxicology studies exist. For example, information important to the evaluation of the quality of individual animal studies (e.g., randomly assignment, blinding of outcome assessors, control for litter effects) was often not reported in the publications evaluated in this review. This led to high RoB ratings for multiple questions for the individual studies and subsequent downgrading of the overall level of confidence in the bodies of evidence. For each PBDE congener and outcome considered, there were sufficient RoB concerns to support a downgrade by at least one level for all of the bodies of evidence. It is unclear if the NR rating represents a failure to consider elements evaluated in our assessment of study RoB, or a lack of reporting of otherwise acceptable study design and study conduct practices. Increased use of reporting standards developed for animal studies (Kilkenny et al. 2010) might likely reduce this concern, both in reporting and in making researchers aware of low RoB practices before conducting future studies. In addition, animal models, and exposure regimens varied among studies, which further complicated synthesis of the results.
Standardized neurobehavioral test batteries were not used to evaluate PBDE exposed rodents. Behavioral tests of learning and memory included the Barnes spatial maze (Koenig et al. 2012); passive avoidance (Zhang et al. 2013); Y maze (Llansola et al. 2009); water T maze (Biesemeier et al. 2011); radial arm maze (de-Miranda et al. 2016; Fischer, Fredriksson, and Eriksson 2008; Verma, Singh, and Gandhi 2013); visual discrimination (Dufault, Poles, and Driscoll 2005; Rice et al. 2009); operant conditioning test paradigms (Rice et al. 2009), and the Morris water maze. Even within individual tests, different methods of testing the animals and categorizing their responses were used, making it difficult to determine whether different studies shared similar or conflicting results and whether they assessed similar or different aspects of cognitive function.
The most commonly used test of learning and memory in association with exposure to PBDEs was the Morris water maze. Several behaviors were assessed using the Morris water maze, including spatial memory or reversal learning. Outcome measures included escape latency across trials, path length, swim speed, and animal orientation in relation to the platform location. For the purposes of this review, acquisition and reversal learning with the Morris water maze were considered tests of learning whereas performance in probe trials was considered an assessment of memory. Because of its widespread use in the reviewed studies, a series of meta-analyses of latency data was performed from the Morris water maze.
Our meta-analysis of latency data from the Morris water maze for several BDEs showed a significant overall effect of PBDE treatment that was robust to multiple sensitivity analyses. Moreover, this meta-analysis provided evidence of a possible relationship between PBDEs and decrements in learning that was not evident when datasets on individual BDEs were evaluated qualitatively. Nevertheless, the evidence did not exhibit a consistent dose-response gradient across PBDE congeners, as there was no significant trend in log10(dose) and the observed heterogeneity increased under linear and linear-quadratic meta-regression. When accounting for dose, this heterogeneity might be because different PBDEs have differing potencies for this effect, varying durations of dosing in the studies produces different cumulative doses, and test methods (e.g., number of daily water maze trials) varied among experiments. It is possible that using a different dose metric, such as cumulative dose or cumulative dose during a particular developmental window, might produce a dose-response gradient. The meta-analysis results also helped inform the confidence ratings of the body of evidence (in this case, by supporting a decision not to downgrade the evidence for unexplained inconsistency for some of the learning datasets) and supported the calculation of BMDs based on data from multiple studies.
The current analyses support the finding that there is a moderate level of evidence that developmental exposure to certain PBDE congeners is associated with decrements in learning and memory in rodents. Although not evaluated here, the NASEM report (2017) also assessed the human evidence and concluded that the epidemiological literature provide a moderate level of evidence that exposure to PBDEs is associated with decrements in IQ in humans. This conclusion was based upon the critical evaluation of a published SR of the human evidence. Lam et al. (2017) found a decrease of 3.7 IQ points in children per 10-fold increase in serum PBDE concentration.
Although both the animal and human evidence supported a relationship between PBDE exposure and effects on learning, estimated exposures to PBDEs in humans are quite different from those used in animal studies. For example, human intakes of PBDEs estimated from levels found in food and dust appear to be about 500,000-fold lower than the BMD5s associated with effects in animals (Lorber 2008). Comparisons of internal doses of BDE-47 also demonstrated large disparities in the exposure between humans and animals, but not as large as suggested by the intake data. For lipid adjusted BDE-47 plasma levels, the 95th percentile exposure in humans was about 170-fold lower (NCHS, 2007) than the level in rats treated with doses near the BMD5 (Darnerud et al. 2007; Koenig et al. 2012; Ta et al. 2011). Similarly, the highest BDE-47 level seen in any child in the CHAMACOS cohort was 36-fold lower than that seen in rat plasma level (Bradman et al. 2012). Another observation was that internal dose measurements were more similar between rodents and humans on a ng/g serum basis than on a ng/g lipid basis (NRC 2017). Thus, there is significant uncertainty associated with the internal dose correspondence between rodents and humans, and the most relevant dose metric is unclear.
Overall, data demonstrated that the SR process was valuable for evaluating the potential neurotoxic effects of PBDE exposure. As noted by previous NASEM committees, SR provides a framework for identifying, selecting, and evaluating evidence in a consistent and explicit manner, maximizing transparency in how the assessments were performed, and facilitating clear presentation of the basis for scientific judgments. Moreover, this SR identified a number of key limitations in study design, conduct or reporting for studies of neurotoxicity that led to high RoB ratings for many studies, with subsequent downgrading of the overall level of confidence in the body of evidence. Greater confidence will result if future studies improve reporting of their experimental design, procedures, and results; increase use of standardized neurobehavioral test batteries, animal models, and exposure regimens; and utilize more consistent methods for categorizing responses.
Supplementary Material
Acknowledgments
Funded by a contract to the National Academies of Sciences, Engineering, and Medicine from the US Environmental Protection Agency. The authors thank Jaime Blanck at the William H. Welch Medical Library at Johns Hopkins University for her assistance with planning and performing the literature searches to support the SRs. Selected tables, figures, and selected text from a 2017 report of the National Academies of Sciences, Engineering, and Medicine, Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals are reprinted with permission from the National Academies Press, Washington, DC.
Footnotes
Competing financial interests declaration
The authors declare they have no actual or potential competing financial interests.
References
- Biesemeier JA, Beck MJ, Silberberg H, Myers NR, Ariano JM, Radovsky A, Freshwater L, Sved DW, Jacobi S, Stump DG, Hardy ML, and Stedeford T.. 2011. An oral developmental neurotoxicity study of decabromodiphenyl ether (DecaBDE) in rats. Birth Defects Research Part B Developmental and Reproductive Toxicology 92:17–35. doi: 10.1002/bdrb.20280. [DOI] [PubMed] [Google Scholar]
- Blanco J, Mulero M, Heredia L, Pujol A, Domingo JL, and Sánchez DJ. 2013. Perinatal exposure to BDE-99 causes learning disorders and decreases serum thyroid hormone levels and BDNF gene expression in hippocampus in rat offspring. Toxicology 308:122–28. doi: 10.1016/j.tox.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Wall PM, Nakai JS, Yagminas A, Wade M, and Li N.. 2015. Behavioral and thyroid effects of in utero and lactational exposure of Sprague-Dawley rats to the polybrominated diphenyl ether mixture DE71. Neurotoxicology and Teratology 52:127–42. doi: 10.1016/j.ntt.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Sjodin A, Fenster L, Jones RS, Harley KG, Chevrier J, Holland NT, and Eskenazi B.. 2012. Factors associated with serum polybrominated diphenyl ether (PBDE) levels among school-age children in the CHAMACOS cohort. Environmental Science and Technology 46:7373–81. doi: 10.1021/es3003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell L, Glinianaia SV, Rankin J, Rose M, Fernandes A, Harrad S, and Pless-Mulolli T.. 2016. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environment International 92-93:680–94. doi: 10.1016/j.envint.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Buratovic S, Viberg H, Fredriksson A, and Eriksson P.. 2014. Developmental exposure to the polybrominated diphenyl ether PBDE 209: Neurobehavioural and neuro-protein analysis in adult male and female mice. Environmental Toxicology and Pharmacology 38:570–85. doi: 10.1016/j.etap.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Butryn DM, Gross MS, Chi LH, Schecter A, R Olson J, and Aga DS. 2015. “One-shot” analysis of polybrominated diphenyl ethers and their hydroxylated and methoxylated analogs in human breast milk and serum using gas chromatography-tandem mass spectrometry. Analytica Chimica Acta 892:140–47. doi: 10.1016/j.aca.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantón RF, Scholten DE, Marsh G, de Jong PC, and van Den Berg M.. 2008. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs). Toxicology and Applied Pharmacology 227:68–75. doi: 10.1016/j.taap.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Cantón RF, Sanderson JT, Letcher RJ, Bergman A, and van Den Berg M.. 2005. Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. Toxicological Sciences 88:447–55. doi: 10.1093/toxsci/kfi325. [DOI] [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, and Lichtensteiger W.. 2006. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology 220:104–16. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Chen A, Yolton K, Rauch SA, Webster GM, Hornung R, Sjödin A, Dietrich KN, and Lanphear BP. 2014a. Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: The HOME study. Environmental Health Perspectives 122:856–62. doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Lebetkin EH, Sanders JM, and Burka LT. 2006. Metabolism and disposition of 2,2′,4,4′,5-pentabro-modiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica 36:515–34. doi: 10.1080/00498250600674477. [DOI] [PubMed] [Google Scholar]
- Chen YH, Li ZH, Tan Y, Zhang CF, Chen JS, He F, Yu YH, and Chen DJ. 2014b. Prenatal exposure to decabrominated diphenyl ether impairs learning ability by altering neural stem cell viability, apoptosis, and differentiation in rat hippocampus. Human and Experimental Toxicology. February 24. Epub ahead of print. doi: 10.1177/0960327113509661. [DOI] [PubMed] [Google Scholar]
- Cheng J, Gu J, Ma J, Chen X, Zhang M, and Wang W.. 2009. Neurobehavioural effects, redox responses and tissue distribution in rat offspring developmental exposure to BDE-99. Chemosphere 75:963–68. doi: 10.1016/j.chemosphere.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, and Goodman SN. 2014. Random effects meta-analysis of inconsistent effects: A time for change. Annals of Internal Medicine 160:267–70. doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- Costa LG, Pellacani C, Dao K, Kavanagh TJ, and Roque PJ. 2015. The brominated flame retardant BDE-47 causes oxidative stress and apoptotic cell death in vitro and in vivo in mice. Neurotoxicology 48:68–76. doi: 10.1016/j.neuro.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowens KR, Simpson S, Thomas WK, and Carey GB.2015. Polybrominated diphenyl ether (PBDE)-induced suppression of phosphoenolpyruvate carboxykinase (PEPCK) decreases hepatic glyceroneogenesis and disrupts hepatic lipid homeostasis. Journal of Toxicology and Environmental Health A 78:1437–49. doi: 10.1080/15287394.2015.1098580. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, and Hallgren S.. 2007. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere 67:S386–S392. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, and Sinjari T.. 1996. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroxine and TSH blood levels in rats and mice. Organohalogen Compounds 29:316–19. PMID: 11482517. [DOI] [PubMed] [Google Scholar]
- de-Miranda AS, Kuriyama SN, da-Silva CS, do-Nascimento MS, Parente TE, and Paumgartten FJ. 2016. Thyroid hormone disruption and cognitive impairment in rats exposed to PBDE during postnatal development. Reproductive Toxicology 63:114–24. doi: 10.1016/j.reprotox.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, van Den Berg M, and Westerink RH. 2011. Neurotoxicity of brominated flame retardants: (In) direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environmental Health Perspectives 119:900–07. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, and Westerink RH. 2011. Neurotoxicity of brominated flame retardants: (in) direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environmental Health Perspectives 119 (7):900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll LL, Gibson AM, and Hieb A.. 2009. Chronic postnatal DE-71 exposure: Effects on learning, attention and thyroxine levels. Neurotoxicology and Teratology 31:76–84. doi: 10.1016/j.ntt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Driscoll LL, Kaplan J, Bucuvalas E, Allen H, Kraut J, and Fitzpatrick J.. 2012. Acute postnatal exposure to the pentaBDE commercial mixture DE-71 at 5 or 15 mg/kg/day does not produce learning or attention deficits in rats. Neurotoxicology and Teratology 34:20–26. doi: 10.1016/j.ntt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, and Driscoll LL. 2005. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicological Sciences 88:172–80. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority). 2011. Scientific opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA Journal 9: 2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (U.S. Environmental Protection Agency). 2010. An Exposure Assessment of Polybrominated Diphenyl Ethers. EPA/600/R-08/086F National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC: [online]. Available at: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=210404 [accessed May 10, 2018]. [Google Scholar]
- Eriksson P, Jakobsson E, and Fredriksson A.. 2001. Brominated flame retardants: A novel class of developmental neurotoxicants in our environment?. Environmental Health Perspectives 109:903–08. PMCID: PMC1240439. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faass O, Ceccatelli R, Schlumpf M, and Lichtensteiger W.. 2013. Developmental effects of perinatal exposure to pbde and pbc on gene expression in sexually dimorphic rat brain regions and female sexual behavior. General and Comparative Endocrinology 188:232–241. doi: 10.1016/j.ygcen.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Fayiga A, and Ipinmoroti M.. 2017. Detection of toxic flame retardants in aquatic and terrestrial environment: An emerging global concern. Electronic Journal of Biology 13:38–55. [Google Scholar]
- Fischer C, Fredriksson A, and Eriksson P.. 2008. Coexposure of neonatal mice to a flame retardant PBDE 99 (2, 2′,4, 4′,5-pentabromodiphenyl ether) and methyl mercury enhances developmental neurotoxic defects. Toxicological Sciences 101:275–85. doi: 10.1093/toxsci/kfm271. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Fairbrother A, Baecher-Steppan L, and Kerkvliet NI. 1994. Immunologic and endocrine effects of the flame retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology 86:49–61. PMID: 8134923. [DOI] [PubMed] [Google Scholar]
- Gassmann K, Schreiber T, Dingemans MM, Krause G, Roderigo C, Giersiefer S, Schuwald J, Moor M, Unfried K, Bergman K, Weterink RH, Rose CR, and Fritsche E.. 2014. BDE-47 and 6-oh-BDE-47 modulate calcium homeostasis in primary fetal human neural progenitor cells via ryanodine receptor-independent mechanisms. Archives of Toxicology 88:1537–48. doi: 10.1007/s00204-014-1217-7. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, and McDonald TA. 2004. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds 66:3820–25. [Google Scholar]
- Ghassabian A, El Marroun H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H, and White T.. 2014. Downstream effects of maternal hypothroxinemia in early pregnancy: Nonverbal IQ and brain morphology in school-age children. Journal of Clinical Endocrinology and Metabolism 99:2383–90. doi: 10.1210/jc.2013-4281. [DOI] [PubMed] [Google Scholar]
- Gill S, Hou Y, Li N, Pulido O, and Bowers W.. 2016. Developmental neurotoxicity of polybrominated diphenyl ethers mixture de71 in Sprague-Dawley rats. Journal of Toxicology and Environmental Health A 79:482–93. doi: 10.1080/15287394.2016.1182001. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Debeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, and Schunemann HJ. 2011. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- He P, Wang A, Niu Q, Guo L, Xia T, and Chen X.. 2011. Toxic effect of PBDE-47 on thyroid development, learning, and memory, and the interaction between PBDE-47 and PCB153 that enhances toxicity in rats. Toxicology and industrial Health 27:279–88. doi: 10.1177/0748233710387002. [DOI] [PubMed] [Google Scholar]
- He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, and Chen XM. 2009. Mechanisms underlying the developmental neurotoxic effect of PBDE-47 and the enhanced toxicity associated with its combination with PCB153 in rats. Neurotoxicology 30:1088–95. doi: 10.1016/j.neuro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- He YMB, Murphy RM, Yu MH, Hecker LM, Giesy JP, Wu RS, and Lam PK. 2008. Effects of 20 PBDE metabolites on steroidogenesis in the H295R cell line. Toxicology Letters 176:230–38. doi: 10.1016/j.toxlet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Gurevitch J, and Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–56. doi: 10.2307/177062. [DOI] [Google Scholar]
- Higgins JPT, and Green S, eds. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; [online]. Available: www.handbook.cochrane.org [accessed May 10, 2018] [Google Scholar]
- Ho KL, Yau MS, Murphy MB, Wan Y, Fong BM, Tam S, Giesy JP, Leung KS, and Lam MH. 2015. Urinary bromophenol glucuronide and sulfate conjugates: Potential human exposure molecular markers for polybrominated diphenyl ethers. Chemosphere 133:6–12. doi: 10.1016/j.chemosphere.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Horn S, and Heuer H.. 2010. Thyroid hormone action during brain development: More questions than answers. Molecular and Cellular Endocrinology 315:19–26. doi: 10.1016/j.mce.2009.09.008. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine). 2011. Finding What Works in Health Care: Standards for Systematic Review. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Karpeta A, Barc J, Ptak A, and Gregoraszczuk EL. 2013. The 2,2′,4,4′-tetrabromodiphenyl ether hydroxylated metabolites 5-OH-BDE-47 and 6-OH-BDE-47 stimulate estradiol secretion in the ovary by activating aromatase expression. Toxicology 305:65–70. doi: 10.1016/j.tox.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, and Altman DG. 2010. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology 8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, Lein PJ, and Pessah IN. 2011. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environmental Health Perspectives 119:519–26. doi: 10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Royland JE, Osorio C, Winnik WM, Oritz P, Pei L, Ramabhadran R, and Alzate O.. 2015. Developmental exposure to a commercial PBDE mixture: Effects on protein networks in the cerebellum and hippocampus of rats. Environmental Health Perspectives 123:428–36. doi: 10.1289/ehp.1408504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig CM, Lango J, Pessah IN, and Berman RF. 2012. Maternal transfer of BDE-47 to offspring and neurobehavioral development in C57BL/6J mice. Neurotoxicology and Teratology 34:571–80. doi: 10.1016/j.ntt.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse MJ 2011. On the meta-analysis of response ratios for studies with correlated and multi-group designs. Ecology 92:2049–55. PMID: 22164829. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear B, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, and Woodruff T.. 2017. Developmental PBDE exposure and IQ/ADHD in childhood: A systematic review and meta-analysis. Environmental Health Perspectives 125:086001. doi: 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal H, Hack A, Roth-Harer A, Grande SW, and Talsness CE. 2006. Effects of developmental exposure to 2,2⊠,4,4⊠,5-pentabromodiphenyl ether (pbde-99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environmental Health Perspectives 114 (2):194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares V, Belles M, and Domingo JL. 2015. Human exposure to PBDE and critical evaluation of health hazards. Archives of Toxicology 89:335–56. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Llansola M, Hernandez-Viadel M, Erceg S, Montoliu C, and Felipo V.. 2009. Increasing the function of the glutamate-nitric oxide-cyclic guanosine monophosphate pathway increases the ability to learn a Y-maze task. Journal of Neuroscience Research 87:2351–55. doi: 10.1002/jnr.22064. [DOI] [PubMed] [Google Scholar]
- Lorber M. 2008. Exposure of Americans to polybrominated diphenyl ethers. Journal of Exposure Science and Epidemiology 18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Van Zanden JJ, Luijks EA, Van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, and Brouwer A.. 2000. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicological Sciences 56:95–104. PMID: 10869457. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, and Brouwer A.. 2001. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybromi- nated bisphenol A compounds. Environmental Health Perspectives 109:399–407. PMCID: PMC1240281. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado-Feliciano M, and Bigsby RM. 2008. Hydroxylated metabolites of the polybrominated diphenyl ether mixture DE-71 are weak estrogen receptor-alpha ligands. Environmental Health Perspectives 116:1315–21. doi: 10.1289/ehp.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, and Ghassabian A.. 2015. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatrics 169:838–45. doi: 10.1001/jamapediatrics.2015.0498. [DOI] [PubMed] [Google Scholar]
- NCHS (National Center for Health Statistics). 2007. National Health And nutrition examination survey 2003–2004 [online]. Available: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L28PBE_C.htm [accessed September 7, 2018].
- NASEM (National Academies of Science, Engineering, and Medicine). 2017. Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- NTP (National Toxicology Program). 2015. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation, Division, National Toxicology Program, National Institute of Environmental Health Sciences; January 9, 2015 [online]. Available: http://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf [accessed September 21, 2015]. [Google Scholar]
- Ptak A, Ludewig G, Lehmler HJ, Wojtowicz AK, Robertson LW, and Gregoraszczuk EL. 2005. Comparison of the actions of 4-chlorobiphenyl and its hydroxylated metabolites on estradiol secretion by ovarian follicles in primary cells in culture. Reproductive Toxicology 20:57–64. doi: 10.1016/j.reprotox.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Ptak A, Ludewig G, Kapiszewska M, Magnowska Z, Lehmler HJ, Robertson LW, and Gregoraszczuk EL. 2006. Induction of cytochromes P450, caspase-3 and DNA damage by PCB3 and its hydroxylated metabolites in porcine ovary. Toxicology Letters 166:200–11. doi: 10.1016/j.toxlet.2006.07.304. [DOI] [PubMed] [Google Scholar]
- Qiu X, Mercado-Feliciano M, Bigsby RM, and Hites RA. 2007. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromodiphenyl ether mixture. Environmental Health Perspectives 115:1052–58. doi: 10.1289/ehp.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW 2009. Analyzing effect sizes: Random-effects models Pp. 295–315 in The Handbook of Synthesis Research and Meta-Analysis, 2nd Ed., Cooper H, Hedges LV, and Valentine JC, eds. New York: Russell Sage Foundation. [Google Scholar]
- Reverte I, Pujol A, Domingo JL, and Colomina MT. 2014. Thyroid hormones and fear learning but not anxiety are affected in adult apoE transgenic mice exposed postnatally to decabromodiphenyl ether (BDE-209). Physiology and Behavior 133:81–91. doi: 10.1016/j.physbeh.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Reverte I, Klein AB, Domingo JL, and Colomina MT. 2013. Long term effects of murine postnatal exposure to decabromodiphenyl ether (BDE-209) on learning and memory are dependent upon APOE polymorphism and age. Neurotoxicology and Teratology 40:17–27. doi: 10.1016/j.ntt.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Rice DC, Thompson WD, Reeve EA, Onos KD, Assadollahzadeh M, and Markowski VP. 2009. Behavioral changes in aging but not young mice after neonatal exposure to the polybrominated flame retardant decaBDE. Environmental Health Perspectives 117:1903–11. doi: 10.1289/ehp.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, and Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health assessments. Environmental Health Perspectives 122:711–18. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MA, Laessig RH, and Reed KD. 2003. Polybrominated diphenyl ethers (PBDEs): New pollutants-old diseases. Clinical Medicine and Research 1:281–90. PMCID: PMC1069057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Pei R, Letcher RJ, and Gunsch C.. 2009. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environmental Health Perspectives 117:197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, and Birnbaum LS. 2006. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicological Sciences 94:28–37. doi: 10.1093/toxsci/kfl091. [DOI] [PubMed] [Google Scholar]
- Ta TA, Koenig CM, Golub MS, Pessah IN, Qi L, Aronov PA, and Berman RF. 2011. Bioaccumulation and behavioral effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicology and Teratology 33:393–404. doi: 10.1016/j.ntt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuresson K, Höglund P, Hagmer L, Sjödin A, Bergman A, and Jakobsson K.. 2006. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environmental Health Perspectives 114:176–81. PMCID: PMC1367828. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P, Singh P, and Gandhi BS. 2013. Prophylactic efficacy of Bacopa monnieri on decabromodiphenyl ether (PBDE-209)-induced alterations in oxidative status and spatial memory in mice. Asian Journal of Pharmaceutical and Clinical Research 6:242–47. [Google Scholar]
- Verma P, Singh P, and Gandhi BS. 2014. Neuromodulatory role of Bacopa monnieri on oxidative stress induced by postnatal exposure to decabromodiphenyl ether (PBDE-209) in neonate and young female mice. Iranian Journal of Basic Medical Sciences 17:307–11. PMCID: PMC4046233. [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, and Eriksson P.. 2003. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicology and Applied Pharmacology 192:95–106. PMID: 14550744. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, and Eriksson P.. 2006. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicological Sciences 92:211–18. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. 2005. Bias and efficiency of meta-analytic variance estimators in the random-effects model. Journal Of Educational and Behavioral Statistics 30 (3):261–293. doi: 10.3102/10769986030003261. [DOI] [Google Scholar]
- Viechtbauer W. 2010. conducting meta-analysis in r with the metafor package. Journal Of Statistical Software 36 (3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- Vorhees CV, and Williams MT. 2006. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols 1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman SB, Wan Y, Chang H, Zhang X, Hecker M, Jones PD, and Giesy JP. 2011. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Marine Pollution Bulletin 63:179–88. doi: 10.1016/j.marpolbul.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, and Sutton P.. 2014. The Navigation Guide systematic review methodology: A rigorous and transparent method for translating environmental health science into better health outcomes. Environmental Health Perspectives 122:1007–14. doi: 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH, Kostyniak PJ, Pessah IN, Berman RF, and LaSalle JM. 2012. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Human Molecular Genetics 21:2399–411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li X, Nie J, and Niu Q.. 2013. Lactation exposure to BDE-153 damages learning and memory, disrupts spontaneous behavior and induces hippocampus neuron death in adult rats. Brain Research 1517:44–56. doi: 10.1016/j.brainres.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Zhao W, Cheng J, Gu J, Liu Y, Fujimura M, and Wang W.. 2014. Assessment of neurotoxic effects and brain region distribution in rat offspring prenatally co-exposed to low doses of BDE-99 and methylmercury. Chemosphere 112:170–76. doi: 10.1016/j.chemosphere.2014.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.