Abstract
Anxiety disorders arise from disruptions among the highly interconnected circuits that normally serve to process the streams of potentially threatening stimuli. The resulting imbalance among these circuits can cause a fundamental misinterpretation of neural sensory information as threatening and can lead to the inappropriate emotional and behavioral responses observed in anxiety disorders. There is considerable preclinical evidence that the GABAergic system, in general, and its α2- and/or α5-subunit-containing GABA(A) receptor subtypes, in particular, are involved in the pathophysiology of anxiety disorders. However, the clinical efficacy of GABA-A α2-selective agonists for the treatment of anxiety disorders has not been unequivocally demonstrated. In this review, we present several human pharmacological studies that have been performed with the aim of identifying the pharmacologically active doses/exposure levels of several GABA-A subtype-selective novel compounds with potential anxiolytic effects. The pharmacological selectivity of novel α2-subtype-selective GABA(A) receptor partial agonists has been demonstrated by their distinct effect profiles on the neurophysiological and neuropsychological measurements that reflect the functions of multiple CNS domains compared with those of benzodiazepines, which are nonselective, full GABA(A) agonists. Normalizing the undesired pharmacodynamic side effects against the desired on-target effects on the saccadic peak velocity is a useful approach for presenting the pharmacological features of GABA(A)-ergic modulators. Moreover, combining the anxiogenic symptom provocation paradigm with validated neurophysiological and neuropsychological biomarkers may provide further construct validity for the clinical effects of novel anxiolytic agents. In addition, the observed drug effects on serum prolactin levels support the use of serum prolactin levels as a complementary neuroendocrine biomarker to further validate the pharmacodynamic measurements used during the clinical pharmacological study of novel anxiolytic agents.
Keywords: anxiety disorders, GABA-A receptors, clinical pharmacology
Anxiety is a commonly occurring, negative human emotional state and is characterized by subjective feelings of worry and fear. These subjective phenomena are usually accompanied by physical symptoms, such as increased heart rate, shakiness, fatigue, and muscle tension, as well as cognitive and behavioral manifestations. Anxiety can be adaptive if it occurs in response to a threat and prepares a person to cope with the environment. However, anxiety becomes pathological when it causes significant personal distress and impairs everyday functioning. To be diagnosed with an anxiety disorder, individuals must experience a certain number of symptoms that are disproportionate to either actual or imagined environmental threats for at least 6 months [1, 2].
Anxiety disorders are chronic, disabling conditions that impose enormous costs on both individuals and society [3–6]. These disorders are prevalent in Western countries and less common in Asian countries, such as China. According to a recent 3-year, multi-method study covering 30 European countries, 14% of the total population (i.e., 514 million people) were suffering from anxiety disorders [4]. The meta-analysis performed by Guo et al. obtained pooled current and lifetime prevalence values of 2.4% and 4.1%, respectively, for anxiety disorders [7]. In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM 5) [1], seven anxiety syndromes are classified, including panic disorder, agoraphobia, social anxiety disorder (SAD), generalized anxiety disorder (GAD), specific phobias, separation anxiety disorder, and selective mutism. The etiology of anxiety disorders is multifactorial and includes genetic factors, to a certain extent, for some syndromes. In addition, drug withdrawal, substance/medication (e.g., alcohol, caffeine, and benzodiazepines (BZDs)) abuse and dependence, occupational exposure to organic solvents, and life stressors have been associated with the etiology of anxiety disorders, while endocrine disorders, such as pheochromocytoma and hyperthyroidism, have been demonstrated to mimic anxiety disorders. However, despite advances in neuroscience and genetic research over the past two decades, there are still few genetic or other biomarkers that can reliably guide the diagnosis of psychiatric disorders. Thus, a DSM-informed psychiatric diagnosis is based primarily on self-reports of feelings and experiences by patients with diverse backgrounds and on the clinicians’ understanding of psychiatric terms or observations of behavior [8]. The phenomenologically based diagnostic classification and the multifactorial nature of anxiety disorders are expected to affect the efficacy of the anxiolytic drugs that have been discovered over the past decades.
Current treatment modalities for anxiety disorders can be categorized into psychological treatments, such as cognitive behavioral therapy, in combination with systematic exposure and relaxation techniques, and pharmacological interventions [2]. The pharmacological interventions can further be divided into chronic or maintenance treatments and short-term treatments that induce acute anxiolysis. Monoamine modulating drugs, such as the selective serotonin reuptake inhibitors (SSRIs) and serotonin-noradrenaline reuptake inhibitors (SNRIs), are considered to be the first-line drugs for anxiety disorders primarily because of their “broad-spectrum” anxiolytic efficacy for both short-term and long-term therapy and their relatively good tolerability in terms of side effects and treatment adherence [2]. However, because it is not unusual for treatment response to occur only after 6 weeks of treatment at a therapeutic dose, the delayed onset of action for SSRIs and SNRIs remains a major disadvantage. In fact, the dose-response relationships for most SSRIs have not been identified in patients with various mental disorders [9]. When patients do not respond to or are intolerant of SSRI/SNRI treatment, alternative classes of psychotropic drugs, such as other antidepressant drugs (e.g., tricyclic antidepressants, the irreversible monoamine oxidase inhibitor phenelzine), the anticonvulsant drug pregabalin, antipsychotic drugs (e.g., quetiapine), and the anti-histaminergic drug hydroxyzine, are considered. However, even after treatment with multiple anxiolytic drugs, up to 40% of patients with anxiety disorders either do not respond to treatment at all or only respond partially [10]. It is noteworthy that the comorbidity of mood disorders that is commonly observed in patients with anxiety disorders prevents the direct extrapolation of findings from anxiolytic drug trials to clinical practice and, therefore, interferes with the translation of pharmacological effects to clinical anxiolysis. This problem undoubtedly contributes to the high percentage of anxiety patients that manifest as treatment non-responders.
BZDs are prescribed in many patients with anxiety disorders for their rapid-onset effectiveness, especially for panic disorder, GAD, and SAD patients. The use of BZDs should, however, be minimalized and preferably be reserved for short-term treatments to mitigate the risks of troublesome sedation, cognitive impairment, and discontinuation symptoms after abrupt withdrawal [11], following both short-term and long-term treatment, and to avoid the development of tolerance and dependence with prolonged use. An obvious unmet clinical need in the pharmacological treatment of anxiety disorders raises the opportunity for identifying novel pharmacological approaches that demonstrate a rapid anxiolytic efficacy, which would be superior to existing treatments, and that lacks tolerance induction, abuse liability, and withdrawal symptoms.
The brain circuitry involved in anxiety and the role of GABA in the amygdala
On a neurobiological level, anxiety disorders arise from disruption among the highly interconnected circuits that normally serve to process the streams of potentially threatening stimuli detected by the human brain from the outside world. Perturbations anywhere among these circuits can cause an imbalance in the entire system, resulting in the fundamental misinterpretation of neural sensory information as threatening and leading to the inappropriate emotional and behavioral responses observed in anxiety disorders [12].
Briefly speaking, anxiety is linked to compromised interactions among several brain regions: the medial prefrontal cortex (mPFC) and the ventral hippocampus (vHPC) act in a coordinated fashion with the amygdala and the bed nucleus of the stria terminalis (BNST), forming a distributed network of interconnected structures that control anxiety in both rodents and humans [13].
The mPFC-amygdala coupling is inversely correlated with self-reported measures of anxiety or anxious temperament, indicating that the mPFC functions to actively regulate the amygdala and that an impaired connection between these two neural structures may lead to an inadequate response to threatening stimuli. In contrast, emerging evidence from functional magnetic resonance imaging supports the idea that the amygdala is the key active brain region during responses to negative emotional stimuli in healthy volunteers [14–16]. Patients with anxiety disorders are prone to increased activation in the amygdala compared with non-anxious controls in response to a threatening stimulus [17]. In addition, the successful treatment of anxiety disorders with cognitive behavioral therapy leads to the extinction of this observed hyperactivation in the amygdala [18]. These results suggest that the mPFC functions to regulate the amygdala function by actively suppressing activity; therefore, a deficiency in the top-down regulation of the mPFC and the hyperactivation of the amygdala have been implicated in the pathophysiology of anxiety-related disorders.
In the amygdala, two groups of nuclei should be noted: the basolateral amygdala complex (BLA) and the centromedial amygdala complex, in particular the central nucleus (CeA) [19, 20]. The BLA receives afferent information regarding potentially negative emotional signals from the thalamus and the sensory association cortex. The BLA activates the CeA either directly, through an excitatory glutamatergic pathway, or indirectly, by activating a relay of inhibitory GABAergic interneurons that lie between the BLA and the CeA and exert an inhibitory influence upon the latter [21, 22]. The CeA is the principal efferent pathway from the amygdala. Inhibitory GABAergic neurons project from the CeA to the hypothalamus and brainstem; the activation of these neurons leads to the somatic manifestations of anxiety [23]. Projections to other basal forebrain nuclei, such as the ventrotegmental area and the locus coeruleus, may be involved in the subjective effects that are related to anxiety, such as apprehension and dysphoria [24]. In addition, neurons from the BLA also activate cells in the adjacent BNST, which project to the same areas as the CeA and apparently play a similar role [20, 24].
Animal studies using the elevated plus maze or the open field suggest that the vHPC–mPFC pathway encodes aspects of the context relevant to anxiety. Neural synchrony between the vHPC and the mPFC in the θ-range (4–12 Hz) increases while mice explore the elevated plus maze or the open field compared to a familiar environment [25]. The experimental suppression of this pathway, using a local injection of gap junction blockers [13] or the optogenetic activation of the BLA-vHPC projection [26], has been shown to reduce anxiety during explorations of the elevated plus maze and the open field. In addition, evidence from human neuroimaging studies during negative affective states [27] indicated the co-activation of the amygdala and the insular cortex, and the hippocampus and the mPFC were identified as part of a separately co-activated network that interacts with the limbic network. In humans, similar to the results observed in rodents, the activity of the anterior hippocampus (the human analog of the rodent vHPC) was found to correlate with trait anxiety [28], suggesting the conservation of the brain structures regulating anxiety between rodents and humans.
Although the precise roles of each component in the network are unknown, one hypothesis is that contextual and sensory input from the mPFC and the vHPC is integrated by the BLA, which then drives the central nucleus of the amygdala and the BNST. These two structures, in turn, may activate downstream regions, contributing to anxiety-related symptoms.
The neurobiological process underlining anxiety disorders serves as the basis of the search for novel anxiolytic agents. Compounds that manipulate this potential pathway may provide new options for the treatment of anxiety disorders. Moreover, neuroimaging and neurophysiological measurements that address the corresponding processes may be used to assess human responses to drug-mediated target modulation.
The involvement of GABA system in the pathophysiology of anxiety and anxiety disorders
By providing the major source of inhibitory neurotransmission in the mPFC and the amygdala, GABA exerts a powerful influence on a range of fear- and anxiety-related behaviors, including fear extinction [29–33]. GABA(A) receptor agonists contribute to fear extinction, through the temporary inactivation of the infralimbic subregion or the BLA (but not the prelimbic cortex) [34, 35]. Infusions of GABA or GABA receptor agonists into the amygdala were found to reduce measures of fear and anxiety (possibly related to effects on memory reconsolidation) in several animal species [36, 37]. With GABA antagonists, however, the infusion of bicuculline was found to block chlordiazepoxide-induced anxiolytic-like activity in rats, and the injection of bicuculline methiodide into the anterior BLA of rats elicited anxiogenic-like effects in both the social interaction paradigm and the conflict paradigm, while the microinjection of bicuculline methiodide into the central nucleus of the amygdala elicited no changes in experimental anxiety [38].
In humans, administration of BZDs is translated into anxiolytic effects by attenuating the activation of the amygdala in response to negative emotional stimuli [39, 40]. In contrast, Nutt et al. [41] performed an interesting study, in which they injected the BZD antagonist flumazenil into 10 patients with panic disorder and 10 healthy control subjects. Subjective anxiety responses after flumazenil infusion were significantly higher in patients with panic disorder than in the controls, and panic attacks were successfully induced in 8 patients with panic disorder, while no panic attacks occurred in the controls. Although these findings have not been replicated [42], they are regarded as a potential signal for the possible shift of the “receptor set-point” in panic disorder [41]. Nikolaus et al. reviewed 14 nuclear neuroimaging (positron emission tomography [PET] and single-photon emission computed tomography) studies conducted in patients with anxiety disorders (160 patients [mostly GAD patients] vs. 172 healthy controls). They identified a wide-spread decline in GABA(A) receptor-binding sites and reduced binding extent throughout the whole mesolimbocortical system in patients suffering from anxiety disorders, suggesting the attenuation of physiological central depression. Disturbances in the downstream dopaminergic and serotonergic neurotransmission are thought to result, at least in part, from the diminished tone of GABAergic neurotransmission [43]. A decrease in the number of cortical GABA neurons and a reduction in GABA levels were reported in patients with major depressive disorder (MDD), using proton magnetic resonance spectroscopy [44]. Considering the frequent comorbidity of MDD with anxiety states, a shared underlying pathology that emphasizes the causal contribution of a GABAergic deficit has been proposed for both anxiety disorders and depression [45–47]. A similar reduction in the number of GABA(A) receptors has been observed in patients with panic anxiety and post-trauma stress disorder. It is noteworthy that the extent of the GABA(A)-receptor deficit is significantly correlated with the clinical severity of these two disorders [48–52], suggesting an “exposure-response” relationship and reinforcing the contribution of a GABAergic deficit to anxiety status.
In summary, all of the aforementioned research findings suggest that GABAergic neurotransmission in the mPFC-amygdala coupling is a promising target for the modulation of anxiety-related responses.
GABA(A) receptor structure, function, and implications in the pharmacotherapy of anxiety disorders
The discovery of the GABA(A) receptor in the 1970s, originally called the BZD receptor, was essential for elaborating the mechanism of action of BZDs. It was the recognition of BZD-sensitive GABA(A) receptor subtypes that widened the field of GABA pharmacology [53].
GABA(A) receptors belong to the class of ligand-gated ion channels [54]. The GABA(A) receptors are hetero-pentamers that traverse the neuronal membrane. To date, a large number of GABA(A) receptor subtypes have been identified: α1–6, β1–3, γ1–3, δ, ρ1–3, θ, and π [55]. These subunits construct a cylinder. The activation of a receptor by GABA leads to a conformational change in the protein subunits and results in the transient opening of a pore along the axis of the cylinder, allowing the flow of chloride ions from one side of the membrane to the other [56]. The pharmacological interaction between BZDs and GABA(A) receptors occurs at a different site, independent of the GABA-binding site on the GABA(A) receptor. GABA binds within the two interfaces between the α and β subunits on the GABA(A) receptor (Fig. 1). BZDs bind within the interface between the α and γ subunits (Fig. 1), thereby potentiating the GABA-related activation of the chloride conductance through allosteric modulation [57]. However, this BZD recognition site does not exist for all α and γ2 subunit combinations. Therefore, although GABA(A) receptors containing β, γ2, and one of the α1, α2, α3, or α5 subunits possess a binding site for classical BZDs, analogous receptors containing α4 or α6 subunits do not. The research by Seeburg et al. has attributed the BZD-sensitivity of α1, α2, α3, and α5 subunits to the histidine residue in a homologous position of their N-terminal extracellular region, which switches to an arginine residue in the BZD-insensitive α4 and α6 subunits [58].
Fig. 1.
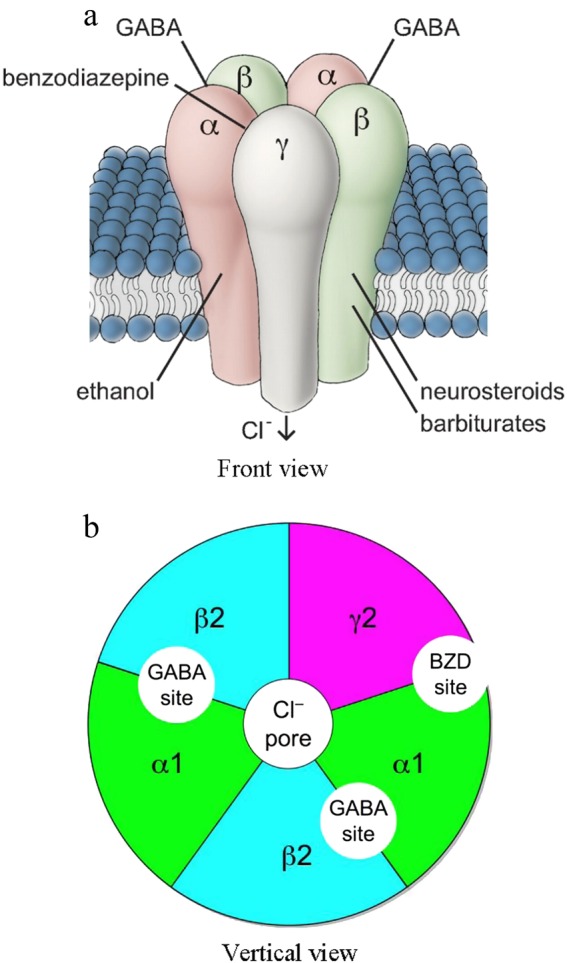
The scheme of a GABA-A receptor. Note: benzodiazepines bind to the interface between subunits α and γ, while endogenous GABA binds to the interface between subunits α and β. The figure is adapted from "Richter L, et al. (2012). Diazepam-bound GABAA receptor models identify new benzodiazepine binding-site ligands.Nat. Chem. Biol. 8(5):455–464. "and" Uusi-Oukari M and Korpi ER.Pharmacol Rev. 2010:62 (1):97–135. Regulation of GABA(A) receptor subunit expression by pharmacological agents
Given the evolutionary preservation of the GABAA/Gly receptor-like (GRL) gene sequences in vertebrates [59], the functions of GABA(A) receptor subtypes have been elucidated in many animal studies using various methods. The functions of GABA(A) receptor subtypes were initially investigated in gene knockout mice. However, a compensatory upregulation of other GABA(A) receptor subunit genes might be induced by gene knockouts and confound the functions of the knocked-out gene. Using new gene-targeting techniques that enable the introduction of specific point mutations and recognizing that a single amino-acid residue in the α subunit determines the sensitivity of a GABA(A) receptor to diazepam, point mutations replacing the histidine with an arginine in the α1, α2, α3, and α5 subunits was employed in in vivo animal studies to silence the agonism of BZDs on the α1,2,3,5-containing GABA(A) receptors [60]. This knock-in approach was used to investigate the pharmacological action of the manipulated receptor subunit. More recently, the developments of chemogenetics and optogenetics have contributed to our understanding of brain circuits and their functions. However, these techniques are not designed to identify signal transduction pathways or proteins, such as specific GABA(A) receptor subtypes, that are naturally involved in modulating the activity of specific cells. Therefore, these approaches should complement rather than substitute each other [61].
Based on various experimental mice models using conditional mutagenesis, α1-containing GABA(A) receptors have been linked to sedation, amnesia, and anticonvulsant effects [62–64], while spinal α2/α3 GABA(A) receptors were found to mediate analgesia [65–67], and α5-containing GABA(A) receptors, which are relatively specifically expressed in the hippocampus (the central domain for learning and memory), are associated with cognition [68–73]. The GABA(A) subtype responsible for the anxiolytic effects of BZDs are less clear. The involvement of α2 GABA(A) receptors in anxiolysis was anticipated, given their high expression within the human amygdala–prefrontal circuitry [74, 75]. Most studies have suggested that the α2, rather than the α3, subtype is related to BZD-induced anxiolysis [76, 77], while previous pharmacological studies, using either an α3-selective inverse agonist [78] or an α3-selective agonist [79], have implicated the α3 subtype. The conclusions of these articles have been challenged recently by the findings in triple α-subunit-mutant mice, in which only one functionally intact BZD-binding α-subunit remains to assess the subtype-specific effects of diazepam modulation [80]. Moreover, the α3-subtype selectivity of TP003, a so-called α3-specific agonist, was also questioned by the observed comparable in vitro efficacy on all subtypes induced by BZDs [81, 82]. Therefore, the role of α3-containing GABA-A receptors in the control and modulation of anxiety has not yet been defined. However, there is growing evidence for the contribution of α5-containing GABA(A) receptors. A recent study showed that α5-containing GABA(A) receptors expressed in a small neuronal population in the central amygdala can regulate anxiety behaviors [83]. Subsequently, this finding was translated into anxiolytic-like actions mediated by systemic α5 modulations [80]. It is noteworthy that the behavioral functions of the α5 subtypes are manifold. However, the undesired cognition-impairing effect of α5 modulation obscures, to some extent, the usefulness of an α5-subtype-selective positive modulator but casts light on the prospect of developing neuronal population-specific drug delivery techniques.
To date, none of the known compounds is truly specific for any one GABA(A) receptor subtype. However, for therapeutic purposes, relative selectivity, rather than absolute specificity, is often sufficient, and thus, these compounds represent promising therapeutic avenues. In this review, we present several human pharmacology studies that were performed to identify the pharmacologically active doses/exposure levels of several GABA(A) subtype-selective novel compounds with potential anxiolytic effects. Because of their relative pharmacological selectivity for the α2 GABA(A) receptor subtypes, these drugs were expected to elicit clinical anxiolysis, with fewer sedating effects than BZDs. An overview of the performance of the selected and validated pharmacodynamic (PD) measurements [84] has summarized the utility of these neurophysiological and neuropsychological biomarkers for use in the early clinical development of novel anxiolytic drugs by our group. Here we will evaluate the methods used in this overview and propose some methodological modulation to that overview [84]. Moreover, the difficulty in evaluating therapeutic anxiolytic drug effects in healthy volunteers has led to further explorations of neuroendocrine biomarkers and the integration of a stress-challenging procedure into the evaluations.
Novel α2-subtype-selective compounds for anxiolysis
In contrast to other areas of pharmacology, it has been particularly difficult for medicinal chemists to develop subtype-selective ligands in the field of GABA(A)ergic receptor modulators [85], primarily because of the high flexibility among GABA(A) receptors and the existence of multiple drug-binding sites. In addition, the distinct subunit composition among the GABA(A) receptor subtypes, the contributions of distinct subunit sequences to binding sites of different receptor subtypes, and the fact that even subunits that are not directly connected to a binding site are able to influence the affinity and efficacy of drugs contribute to the unique pharmacology of each GABA(A) receptor subtype [86].
The binding and efficacy profiles of candidate α2-subtype-selective drugs can be classified as either binding-selectivity or efficacy-selectivity. A potentially anxiolytic compound with binding-selectivity is expected to have a higher affinity for α2 than for other subtypes in vitro and, therefore, to have a specific receptor occupancy and central nervous system (CNS) distribution in vivo. Although the compound may have comparable efficacy when bound to any of the four BZD-sensitive GABA(A) receptor subtypes, its pharmacological selectivity is determined in vivo by preferential occupancy. An ideal efficacy-selective compound should have opposite pharmacological interactions at different subtypes. In other words, it should be an agonist at the α2-subtype, but an antagonist or inverse agonist at the α1 and α5 subtypes. Between these two extreme conditions, there could be multiple permutations, including a compound that behaves as a full agonist or a relatively high partial agonist at the α2-subtype but has weak or no activity at the α1 and α5 subtypes.
Based on these principles, a number of conceptual GABA(A) α2-subtype-selective compounds have been identified through in vitro studies using recombinant human GABA(A) receptors and advanced into clinical development. Because of their pharmacological selectivity, these compounds are expected to have favorable therapeutic effect with fewer sedating or cognition-impairing effects. Table 1 lists the in vitro pharmacological properties of these novel GABAergic compounds, for which the relative efficacy of maximal potentiation compared to that of a full GABAA agonist can be attributed to the partial agonism of the subtype-selective GABA-A compounds.
Table 1.
In vitro pharmacological properties of the GABAergic compounds
| Compound | α1 | α2 | α3 | α5 | ||||
|---|---|---|---|---|---|---|---|---|
| Kia (nM) | Efficacyb (%) | Ki (nM) | Efficacy (%) | Ki (nM) | Efficacy (%) | Ki (nM) | Efficacy (%) | |
| TPA023 | 0.27 | 0c | 0.31 | 11c | 0.19 | 21c | 0.41 | 5c |
| MK-0343 | 0.22 | 18b | 0.40 | 23b | 0.21 | 45b | 0.23 | 18b |
| SL65.1498 | 17 | 45d | 73 | 1154 | 80 | 83d | 215 | 48d |
| Zolpidem | 20 | 75e | 400 (d) | 78e | 400 (d) | 80e | 5000 (d) | 9e |
| AZD7325 | 0.5 | 0c | 0.3 | 18c | 1.3 | 15c | 230 | 8c |
| AZD6280 | 0.5 | 0c | 21 | 32c | 31 | 34c | 1680 | 7c |
| NS11821 | 1.6 | 4f | 9.7 | 17f | 3.8 | 40f | 2.5 | 41f |
aKi = constant of receptor subtype binding
bEfficacy relative to chlordiazepoxide, as assessed by whole-cell patch clamp recordings with human recombinant GABAA receptors [86, 94]
cRelative efficacy denoted for each compound represents the positive modulatory effect on membrane current to a GABA EC10-concentration at a 1 µM test concentration and normalized as a percentage of that produced by a supramaximal concentration (1 µM) of diazepam [81]
dRelative efficacy denotes maximal potentiation compared to diazepam [127]
eMean values of three experiments in Xenopus oocytes with human recombinant αβ2γ2 receptor; efficacy relative to diazepam [128, 129]
fRelative efficacy denotes maximal potentiation compared to diazepam [115]
However, the value of relative efficacy should be interpreted with caution because they represent the effect potentiation associated with the highest concentration examined in vitro, which is virtually unattainable and thus irrelevant for in vivo settings. In addition, the control compound used in these tests was either diazepam or chlordiazepoxide, but the maximum efficacy of these two standard BZDs differ widely from each other, which makes it difficult to compare these data across the subtype-selective compounds.
In addition, the underlying biological process associated with BZD dependency and addiction has been evaluated through a receptor subtype-specific approach. The mesolimbic dopamine (DA) system is critically involved in mediating the reward properties in response to drugs of abuse [87, 88]. The α1-containing GABA-A receptor subtype has been demonstrated to mediate BDZ-evoked synaptic plasticity and disinhibition (i.e., there is stronger hyperpolarization of the GABA neurons in the presence of BZDs, while the inhibition of the DA neurons is no longer observed in in vivo single-unit recordings [89]) in the ventral tegmental area (a key brain region involved in the mesolimbic DA pathway). In a self-administration study conducted in monkeys, the addictive property of L-838417, a positive BZD site modulator of α2-/α3- and α5-containing GABA-A receptors (GABAARs) but an antagonist at α1-containing GABA-A receptors, was compared with those of diazepam, midazolam, and zolpidem (an α1-selective GABA-A receptor agonist). The monkeys were first trained to self-administer (intravenous administration) the short-acting barbiturate methohexital and were then offered the abovementioned four classes of drugs that act at the BZD site [90, 91]. The monkeys self-administered all four compounds, suggesting that each of these compounds had reinforcing properties [90, 91]. However, the breakpoint (i.e., the number of lever presses that was made by the animal to obtain an injection of drug) was the highest for zolpidem, followed by midazolam, diazepam, and L-838417. These findings led to the conclusion that α1-containing GABAARs are sufficient, but not necessary, for mediating the addictive properties of BZDs.
Evaluation of human pharmacology
BZDs exert their CNS actions in a concentration-dependent manner [92]. The anxiolytic, muscle relaxant, amnesic, and hypnotic effects of BZDs generally appear consecutively, and the onset and duration of action correlate closely with the pharmacokinetic (PK) profiles of these compounds. Based on nonclinical investigations, using in vitro assays and animal models of anxiety, the human pharmacology of novel GABAergic agents is approached through clinical pharmacology studies investigating the PKs, receptor occupancy, and PDs of drugs in healthy volunteers. Direct links have been proposed between plasma drug concentrations and GABA receptor occupancy [93], as well as between plasma drug concentrations and the PD measurements [94–97]. This PK/PD relationship warrants the search for surrogate biomarkers that can be used in healthy volunteers treated with a single-dose administration of selective novel GABAergic compound(s).
More than 170 PD tests or test variants have been developed to assess the CNS effects of BZDs. De Visser et al. [92] analyzed the inter-study consistency, sensitivity, and pharmacological specificity of the frequently used biomarkers. Saccadic peak velocity (SPV) and the visual analog scale of alertness (VASalertness) were identified as the most sensitive parameters for BZDs. Both measurements showed consistently dose-dependent responses to a variety of BZDs. Based on these findings and on those of similar reviews for other drug classes [92, 98–102], the Centre for Human Drug Research (CHDR) has established a selection of computerized CNS-PD tests, called NeuroCart [103]. As the most essential PD tool in the CHDR, the NeuroCart test battery is a movable cart equipped with an encephalogram, eye movement test devices, two computers, and the necessary accessories for test administration and electronic data collection. The NeuroCart test battery has been implemented in various CHDR studies and has led to more than 78 publications. The components of this battery cover a variety of neurophysiological and/or neuropsychological domains (Table 2). Among this battery of tests, adaptive tracking, saccadic eye movements, and body sway have been shown to be sensitive to the sedating effects of sleep deprivation [104], as well as to the effects of BZDs and other GABAergic hypnotic drugs, in a dose-dependent manner [95, 97]. The responses of the NeuroCart measurements to various CNS-acting drugs can be explained as evidence of the blood-brain barrier penetration of the investigated drugs. In a variety of case studies, this battery also provided an early glimpse into the desired and/or adverse effects of compounds on the CNS. In short, these reports indicate that the human PD approach, using sensitive and CNS-domain-specific neuropsychological and neurophysiological measures, is useful for predicting the clinical effects of a drug on the CNS. Inter-species differences have also noted between humans and rodents or primates; although a low in vitro efficacy at the α1-containing GABA(A) receptors may not lead to an overtly sedative effect in experimental animals, it apparently causes sedation in humans at comparable exposure levels. The following questions remain to be answered: (1) is a reduction of SPV a promising surrogate marker for clinical anxiolysis? (2) Can we differentiate partial agonism from the full agonism of BZDs using this PD characterization? (3) Is a selective CNS-PD effect profile a characteristic of the family of GABA(A) α2-subtype receptor agonists?
Table 2.
Component tests of the NeuroCart battery and the related CNS domains
| NeuroCart test | Targeted function | Related CNS domains |
|---|---|---|
| Saccadic eye movement | Neurophysiologic function | Superior colliculus, substantia nigra, amygdala |
| Smooth pursuit | Neurophysiologic function | Midbrain |
| Adaptive tracking | Visuo-motor coordination | Neocortex, basal nuclei, brainstem, cerebellum |
| Body sway | Balance | Cerebellum, brainstem |
| Visual verbal learning test (VVLT) | Memory | Hippocampus |
| VAS Bond and Lader | Alertness, mood, calmness | Cortex, prefrontal cortex |
| VAS Bowdle | Feeling high, internal, and external perception | Cortex, prefrontal cortex, amygdala |
Development of novel subtype-selective GABAergic anxiolytic drugs
For more than two decades, no mechanistically novel anxiolytic agents have been approved and marketed for the treatment of anxiety disorders. This situation may be attributed to the lack of a solid understanding of the underlying pathophysiology of anxiety disorders, as well as the insufficiency of the development and application of valid animal models and their inability to reliably predict clinical anxiolytic effects in humans [105]. Moreover, anxiety is a fundamental characteristic for survival, which evolutionarily has been embedded deeply into the central and peripheral physiology [106]. The ensuing complexity of pharmacological networks refutes the identification of key targets for treatment of anxiety disorders. In addition, anxiety disorders actually represent a heterogeneous group of illnesses, and current diagnostic classifications lack a robust neurobiological basis for differentiating these anxiety-related phenomena. The changing diagnostic landscape and the uncertain boundaries between the various anxiety disorders and mood disorders have introduced further challenges for drug development [105]. Meanwhile, the search for novel pharmacotherapies for the treatment of various anxiety disorders has been driven by a growing medical need, based on a desire for clinically available drugs with improved efficacies and/or reduced side-effect profiles [107].
The pharmacotherapeutic pipeline of anxiolytic treatments in development can be outlined into three major trends: (1) the exploration of compounds that act on novel targets and that regulate the underlying neural circuits involved in anxiety disorders, for which the glutamate, various neuropeptide, and endocannabinoid systems show particular promise as targets for future drug development [108–110]; (2) the design of compounds with an established mechanism of action that are relevant to anxiety but have undergone pharmacological modifications, for example, the development of subtype-selective GABA-A-ergic partial agonists; likewise, the recently marketed multi-target serotoninergic compounds, such as vortioxetine, vilazodone, and agomelatine [105], have been shown to be effective as antidepressant agents, and their efficacy on anxiety disorders has been shown in a small population of patients; (3) the repositioning of registered drugs for other indications in the treatment of anxiety disorders, such as clinical trials investigating the effects of antipsychotic drugs on anxiety disorders and the approval of pregabalin by the European Medicines Agency for the treatment of GAD in 2006 [2, 105].
As the predominant inhibitory neurotransmitter system in the human brain, the GABAergic system has been implicated in the pathophysiology of anxiety disorders. Since the serendipitous discovery of BZDs in the 1950s, they have been widely used for anxiolysis, sedation, seizure suppression, and muscle relaxation. In patients with anxiety disorders, the hypnotic effect of BZDs could be useful for anxiety-related symptomatology, such as insomnia. However, for the management of daytime anxiety, such effects are undesirable. The sedative effects and their ensuing cognition impairment and the potential for tolerance development and abuse liability are the major obstacles against the wide and long-term use of BZDs in the treatment of anxiety disorders. Previous research conducted in GABA-A receptor subunit knock-in animals suggested these undesirable effects may be, to some extent, associated with the pharmacological activities of BZDs on the GABA(A) receptors containing α1 and α5 subunits [93–96]. As a result, novel GABA(A)-ergic α1- and α5-subtype sparing partial agonists, with either disproportional binding affinities or disproportional in vitro efficacies at the BZD-targeted GABA(A)-ergic receptor subtypes, are expected to separate the anxiolytic effects from the BZD-induced sedative and cognition-impairing effects.
Across the industry, the most common reason for developmental failure of drugs in phase 2 has been the lack of efficacy [111]. There are many areas of uncertainty regarding the translation of preclinical pharmacology data to humans. These questions cannot be readily answered unless we know whether the drug actually expressed the intended pharmacology by modulating its target(s). In the entire process of clinical drug development, the demonstration of pharmacological effects with clinically tolerable doses is termed a proof-of-mechanism (POM) study. Generally speaking, these types of studies should be comprised of three goals: (1) observing drug exposure at the target site of action; (2) detecting drug interactions with the intended drug target; and (3) exploring the effects of the drug on human biology using biomarker(s). Such an investigational approach may be especially useful for anxiety disorders because, in therapeutic exploratory studies in patients, it can be difficult to achieve a clinically meaningful end point, due to the nature of subjective assessments, the relatively large sample size, the high probability of placebo effect, and other ethical or practical issues [112, 113].
In recent years, a number of early-phase POM studies of six α2 subunit-selective GABA(A) agonists (i.e., TPA023, SL65.1498, MK-0343, AZD7325, AZD6280, and NS11821) evaluated the PKs and PDs of these novel compounds for at least two dose level, compared with those of an active control (lorazepam) at its therapeutic dose and a placebo control [93–96, 114–116]. Table 3 summarizes the clinical developmental status of these novel compounds. Most of the studies were single-dose, double-blind, randomized, cross-over phase I clinical trials in healthy volunteers. Using the NeuroCart test battery [103], a number of validated PD measurements were taken to address the effects of these drugs on psychomotor, neurophysiological, and neuroendocrine functions. Clear distinctions were observed between the effect profile of the non-subtype-selective full GABA(A) agonist and those of selective partial GABA(A) agonists. These studies demonstrated compound-specific effect profiles on neurophysiological function, postural balance, visuo-motor coordination, cognition, and subjective feelings. Moreover, the concept of pharmacological selectivity was demonstrated by the relatively dominant effects of these novel compounds on saccadic eye movements, which measure the GABA(A) α2-subtype receptor-related PD responses, in comparison with their minimal or lack of effects on postural stability, subjective alertness (i.e., measurements reflecting GABA(A) α1-subtype receptor modulation), and cognition (i.e., GABA(A) α5-subtype-specific effects). In contrast, the effect size of lorazepam-induced SPV reduction was generally similar to its effect size on the other non-SPV neurophysiologic biomarkers, indicating a comparable nonselective interaction with different GABA(A) receptor subtypes.
Table 3.
Summary of the clinical developmental status of novel α2,3 subtype-selective GABA(A)-ergic compounds (according to registration in the website of www.clinicaltrials.gov)
| Compound | Developmental phase | Causes of discontinuation |
|---|---|---|
| TPA023 |
Halted in phase II Showed significant differences from the placebo arm in the pooled analysis for three prematurely discontinued phase II studies |
Unexpected adverse findings in long-term animal toxicity studies |
| MK-0343 |
Halted in phase I No proof-of-concept study conducted |
Sedating effect at potential anxiolytic doses |
| SL65.1498 |
Halted in phase I No proof-of-concept study conducted |
Unknown |
| AZD7325 |
Halted in phase II Showed marginally significant superiority over placebo for primary end point (Hamilton Rating Scale for Anxiety Total Score) and secondary end point (Hospital Anxiety and Depression Scale for Anxiety Total Score) in GAD patients |
Rationalization of company portfolio |
| AZD6280 |
Halted in phase I No proof-of-concept study conducted |
Rationalization of company portfolio |
| NS11821 |
Halted in phase I No proof-of-concept study conducted |
Nonlinear pharmacokinetics |
Based on our previous experience with another α2-subtype-selective GABA(A) partial agonist, TPA023, the drug-induced SPV reduction observed in healthy volunteers could be translated as a clinical anxiolytic effect in patients with generalized anxiety disorder [93]. Therefore, similar effect sizes of the evaluated α2 GABA(A) subtype-selective agonists on SPV suggest potentially efficacious anxiolytic effects that are comparable to that of the clinically effective dose of the non-subtype-selective GABA(A) modulator, lorazepam. In addition, the flat concentration-effect curves of the α2-selective GABA(A)-ergic compounds on subjective alertness, visuo-motor coordination, postural balance, and cognition, indicate the relatively favorable clinical side-effect profiles of these drugs compared to those of traditional non-subtype-selective full GABA(A) agonists, such as lorazepam. Taken together, these results demonstrate an equipotent α2 GABA(A) effect in the absence of either α1 or α5 effects, provide support to further pursue the clinical development and can potentially guide future dose selection for studies in both healthy volunteers and patients with anxiety disorders.
However, because therapeutic efficacy studies are still lacking for most novel GABA(A)-ergic drugs, their dose equivalence to 2 mg lorazepam is still unknown. Therefore, the lack of effects on the abovementioned CNS domains cannot be directly interpreted as an improvement in adverse effects. To resolve this problem, these PD measurements have been incorporated into an SPV-normalized regression model. The PD-SPV regression models established on simultaneously measured PD end points actually reflect the relative effect profiles of the investigated drug across a wide range of plasma drug concentrations. The effect size on SPV was used as the normalizer because SPV has been shown to be associated with α2 GABA(A) receptor subtype modulation [117]. Interestingly, recent studies [118] have reported the quantitative correlation between disturbed performance in a saccadic eye movement paradigm and the severity of various anxiety disorders. These results suggest that the measurement of saccadic eye movements might also serve as a neuropathophysiological biomarker for the status or severity of anxiety. Moreover, two additional findings provide indications that saccadic eye movement may also be a predictive biomarker for clinical anxiolytic effects: (1) TPA023, a previously developed GABA(A) receptor α2-subtype-selective agonist that induced significant SPV reduction and minimal sway impairment and no memory change in single-dose study performed in healthy volunteers [93], has demonstrated a better-than-placebo anxiolytic effect in its phase 2 proof-of-efficacy studies [83]; and (2) another study performed by our group with both GABA(A)-ergic and non-GABA anxiolytic compounds (i.e., alprazolam and pregabalin) showed similar SPV-depressive effects with single doses of these drugs at their clinically recommended doses [119].
The previously published pooled data analysis of various GABA(A) modulators was performed to summarize the common pharmacological characteristics of these compounds based on their subtype selectivity [84]. Three α2-selective GABA(A) agonists (i.e., TPA023, TPACMP2 [i.e., MK-0343], and SL65.1498), one α1-selective GABA(A) agonist (zolpidem), and another full GABA(A) agonist (alprazolam) were examined through this approach. Pharmacological selectivity was assessed by determining the regression lines for the change of a PD end point (ΔPD) versus the change from baseline for SPV (ΔSPV). The absolute slopes of the ΔPD-ΔSPV relationships were consistently lower with the α2-selective GABA(A) agonists than with lorazepam, indicating that their effects on non-SPV PD measurements are less than their effects on SPV. The ΔSPV-ΔPD relations of lorazepam were comparable to those of alprazolam. In contrast, zolpidem, an α1-selective GABA(A) agonist, on average, showed relatively higher impairments in the α1-relevant PD parameters relative to the effect on SPV. These ΔPD-ΔSPV findings support the pharmacological selectivity of the α2-selective GABA(A) agonists, implying that the clinical anxiolytic effect of these drugs might be accompanied by a different (less adverse)) side-effect profile for psychomotor and cognitive functions.
Although none of the first three compounds that were evaluated with the PD approach have reached the market, the lessons we learned from their early clinical development are valuable. The development of SL65.1498 was discontinued due to unexpected amnestic effects during clinical trials. However, when the compound was investigated in phase I studies, memory tests were not included as a PD measure in the study in healthy volunteers [95]. MK-0343 also displayed an anxioselective profile in animal models but produced sedation in humans at 1 mg, with a low level of receptor occupancy (<10%) [120]. This result had not been predicted in the human pharmacology study because the highest dose used was only 0.75 mg [94]. The phase II studies of TPA023 were terminated prematurely, despite exhibiting anxioselective activity in GAD patients, due to preclinical toxicity (cataract formation) in long-term dosing studies [83].
Thereafter, the design of such POM studies has been improved for the selection of doses and PD measurements. The results of later studies were informative and affected the decision for the further clinical development of each specific novel compound: (1) because 10 mg AZD7235 was associated with 80%–90% receptor occupancy, the small effect sizes of 2 and 10 mg AZD7325 indicated insufficient receptor modulation of the compound at the investigated doses [114]; (2) for NS11821, the PD effects observed at the moderate-to-high dose levels in the first-in-human study helped to identify and select the pharmacologically active doses for future clinical trials [116]; (3) for AZD6280, the PD effect size on SPV was similar to that of lorazepam, suggesting potentially comparative clinical anxiolytic effect, while the ignorable effects of this compound on body sway, VASalertness, and cognitive tests were thought to predict a reduced profile of CNS side effects [115]. This clinical pharmacological profile was considered promising for further development, and future clinical doses were likely limited to the range of 10–40 mg. However, the clinical development of AZD6280 was halted due to the rationalization of the company portfolio.
Potential peripheral neuroendocrine biomarkers for the effects of selective and nonselective GABA receptor modulators were also explored [121]. The effects of two novel α2 subunit-selective GABA(A) receptor modulators, AZD7325 and AZD6280, on serum prolactin levels were evaluated in healthy male volunteers and compared with those of the nonselective GABA(A) modulator lorazepam. Following the administration of lorazepam at a 2 mg dose and AZD6280 at 10 and 40 mg doses, prolactin levels increased significantly compared with those of placebo (differences of 42.0%, 19.8%, and 32.8%, respectively), suggesting that the α2 receptor subtypes are involved in the GABAergic modulation of prolactin secretion, although possible roles for the α1 and α5 receptor subtypes cannot be excluded. The increases in prolactin levels after the administration of AZD7325 at 2 and 10 mg doses (differences of 7.6% and 10.5%, respectively) did not reach significance, suggesting that the doses of AZD7325 or the intrinsic efficacy at the α2 receptor subtype may have been too low. Compounds with distinguishable modulatory effects between α2 and α1 subunit-containing GABA-A receptors may differ significantly from nonselective BZDs in their effects on dopaminergic circuits [109]. Thus, the measurement of serum prolactin levels in healthy male volunteers can be used to evaluate the effects of α2 subunit-selective GABAergic drugs on the activity of the tuberoinfundibular dopaminergic pathway compared with those of lorazepam and placebo. A similar dose-dependency of the prolactin-enhancing effect was also shown with the BZD alprazolam [122, 123], indicating a causal relationship between the drug administration and the increase in the circulating prolactin level. The increase in prolactin levels suggests that the postulated stimulatory effect of GABA transmission (by suppressing the tuberoinfundibular dopaminergic neurons in the hypothalamus) exceeds the postulated inhibitory effect of GABA transmission (directly at the anterior pituitary gland). It is noteworthy that the magnitude of the effect of lorazepam on prolactin levels was rather small, especially compared to the much more potent prolactin-elevating effects of DA D2 receptor antagonists (haloperidol at a 3 mg dose increases prolactin levels by 130.9% [124]). Such a small effect size warrants the consideration of a relatively large sample size for this PD end point, even under a cross-over study design.
Furthermore, the fear-potentiated-startle (FPS) paradigm was used to simulate the responses to conditioned and unconditioned threats of electronical shock in healthy volunteers [125]. The former scenario represents fear, whereas the latter is associated with anxiety. FPS was combined with saccadic and smooth pursuit eye movement tests, visual analog scales measuring subjective effects, adaptive tracking, and body sway to evaluate anxiolytic drug effects on the FPS-stimulated neurophysiological and neuropsychological responses. The PD effects of two anxiolytic drugs (alprazolam and pregabalin) and one hypnotic drug (diphenhydramine) were characterized in the FPS study, and significant improvements in subjective calmness were observed with the two anxiolytic drugs. These findings corroborate the sensitivity and specificity of the CNS-PD measures for single therapeutic doses of GABAergic (alprazolam) and non-GABAergic (pregabalin) anxiolytic compounds. In fact, clinically available anxiolytic drugs, such as BZDs or SSRIs, do not consistently result in significant increases in subjective calmness in healthy volunteers in stress-free experimental settings. Therefore, the measurable effects on subjective calmness, as well as the test procedure modifications with FPS integration, may warrant the use of stress-challenged subjective measurements and neurophysiological tests for the simulation of clinical anxiolytic drug effects.
Conclusion
The GABAergic system has been implicated in the pathogenesis of various anxiety disorders. Clinically effective pharmacological treatments, such as BZDs, have been demonstrated to target the GABA(A) receptors, through which they exert acute anxiolytic effects in anxiety patients. However, the side effects of these nonselective GABA(A)-ergic compounds, such as sedation, postural imbalance, or potential abuse, limit their use in clinical practice. Based on the understanding of the mechanism of action for BZDs, the emergence of α2-subtype-selective GABA(A) modulators is expected to provide a novel pharmacological approach that alleviates anxiety symptoms but spares the common undesired side effects. Most of these compounds are still in early clinical development, in which POM studies are usually performed in healthy volunteers. The findings from our studies consistently present a similar pattern in the PD effect profiles of the α2-subtype-selective GABA(A) modulators compared to those of the nonselective full GABA(A) agonist, lorazepam. The future application of anxiogenic symptom provocation models that combine subjective measurements and/or neuroendocrine biomarker assays may provide further construct validity for the clinical anxiolytic effects of α2-subtype-selective GABA(A) modulators. In addition, these findings are expected to provide insights into the translation of the preclinical pharmacological properties of α2-subtype-selective GABA(A)-ergic compounds into clinical effects in patients with anxiety disorders through human pharmacology studies.
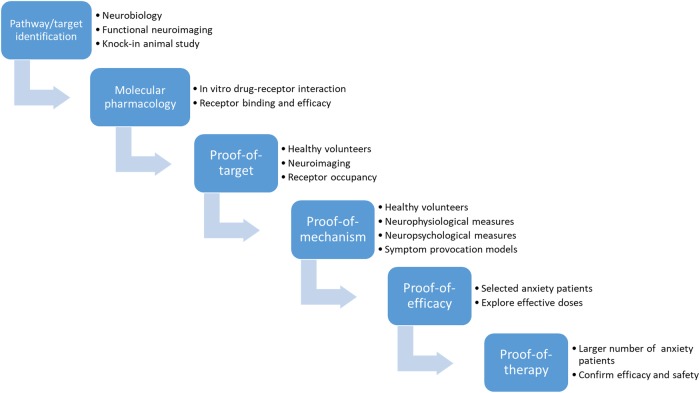
In summary, the development of novel GABAergic compounds can be structured, step by step, as shown in the preclinical-to-clinical translation process depicted in Fig. 2. First, the neurobiological investigation of anxiety and the clinical experience with BZDs revealed the GABAergic neurotransmission system as a potential pathway for new drug development. Further, knock-in animal studies suggested that the pharmacological selectivity of a ligand for a certain GABA(A) receptor subtype could be achieved, either through affinity differentiation (i.e., forming or not forming a receptor–ligand complex) or through efficacy differentiation (i.e., eliciting or not eliciting a biological response after binding to the receptor) [126]. Using 18F-flumazenil as the tracer, a PET study provided information on the dose-dependency or exposure-dependency of the in vivo GABA(A) receptor occupancy of the drug, thereby helping to determine the dose range to be administered in future clinical development. In a clinical pharmacology study, the compound was assessed for its pharmacological effects and PK exposures within the tolerated dose range, at which considerable receptor occupancy can be reached, based on the findings of the previous neuroimaging study. The observed effects indicated biological interactions between the ligand and the targeted receptors. More specifically, in the case of GABA(A)-ergic novel compounds, the subtype-specific PD biomarkers, in conjunction with the simultaneously measured plasma drug concentrations, allowed the determination of the effect amplitude and effect potency of GABA(A) receptor subtype modulation elicited by the investigated drug and the active control and demonstrated the PK/PD profile distinctions that one would expect between a full agonist and a partial agonist [115]. In addition, the relationship among these effects builds a bridge that connects the in vitro pharmacological activity to the in vivo physiological responses and supports the concept of pharmacological selectivity for α2-subtype-selective GABA-A agonists.
Fig. 2.
Schematic graph showing the developmental steps of GABAergic novel compounds from pathway/target identification to clinical research
Acknowledgements
The authors would like to thank the entire CHDR study team for their contributions to the studies cited in this article. This study was supported by National Natural Science Foundation of China 81671369.
Competing interests
The authors declare no competing interests.
Contributor Information
Xia Chen, Email: connie_6096@126.com.
Joop van Gerven, Email: jm.v.gerven@ccmo.nl.
Gabriel Jacobs, Email: GJacobs@chdr.nl.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition: DSM-5®. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403–39. doi: 10.1177/0269881114525674. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC. The global burden of anxiety and mood disorders: putting the European Study of the Epidemiology of Mental Disorders (ESEMeD) findings into perspective. J Clin Psychiatry. 2007;68(Suppl. 2):10–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–79. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, et al. Development of lifetime comorbidity in the World Health Organization world mental health surveys. Arch Gen Psychiatry. 2011;68:90–100. doi: 10.1001/archgenpsychiatry.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ormel J, Petukhova M, Chatterji S, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, et al. Disability and treatment of specific mental and physical disorders across the world. Br J Psychiatry. 2008;192:368–75. doi: 10.1192/bjp.bp.107.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X, Meng Z, Huang G, Fan J, Zhou W, Ling W, et al. Meta-analysis of the prevalence of anxiety disorders in mainland China from 2000 to 2015. Sci Rep. 2016;6:28033. 10.1038/srep28033. [DOI] [PMC free article] [PubMed]
- 8.Casey BJ, Craddock N, Cuthbert BN, Hyman SE, Lee FS, Ressler KJ. DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci. 2013;14:810–4. doi: 10.1038/nrn3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katakam Kiran Kumar, Sethi Naqash Javaid, Jakobsen Janus Christian, Gluud Christian. Great boast, small roast on effects of selective serotonin reuptake inhibitors: response to a critique of our systematic review. Acta Neuropsychiatrica. 2018;30(5):251–265. doi: 10.1017/neu.2017.38. [DOI] [PubMed] [Google Scholar]
- 10.Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry. 2006;11:805–14. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin DS, Montgomery SA, Nil R, Lader M. Discontinuation symptoms in depression and anxiety disorders. Int J Neuropsychopharmacol. 2007;10:73–84. doi: 10.1017/S1461145705006358. [DOI] [PubMed] [Google Scholar]
- 12.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/S1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 15.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 16.Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc Cogn Affect Neurosci. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etkin A, Wager TD. Functional neuroimaging of anxiety: a metaanalysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WH. Effects of cognitive–behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Etkin Amit. Behavioral Neurobiology of Anxiety and Its Treatment. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. Functional Neuroanatomy of Anxiety: A Neural Circuit Perspective; pp. 251–277. [DOI] [PubMed] [Google Scholar]
- 20.Davis M. Neural circuitry of anxiety and stress disorders. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia: Lippincott, Williams, & Wilkins; 2002. p. 729–43.
- 21.Pitkanen A, Savander V, LeDoux JE. Organization of intraamygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–23. doi: 10.1016/S0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 22.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–83. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jongen-Relo AL, Amaral DG. Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. Eur J Neurosci. 1998;10:2924–33. doi: 10.1111/j.1460-9568.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 24.Forster GL, Novick AM, Scholl JL, Watt MJ. The role of the amygdala in anxiety disorders. In: Ferry B, editor. The amygdala: a discrete multitasking manager. Rijeka: InTech; 2012. p. 61–102.
- 25.Adhikari Avishek, Topiwala Mihir A., Gordon Joshua A. Synchronized Activity between the Ventral Hippocampus and the Medial Prefrontal Cortex during Anxiety. Neuron. 2010;65(2):257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felix-Ortiz Ada C., Beyeler Anna, Seo Changwoo, Leppla Christopher A., Wildes Craig P., Tye Kay M. BLA to vHPC Inputs Modulate Anxiety-Related Behaviors. Neuron. 2013;79(4):658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kober H, Barret LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satpute AB, Mumford JA, Naliboff BD, Poldrack RA. Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion. 2012;12:58–68. doi: 10.1037/a0026517. [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35:1625–52. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–63. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtin J, Bienvenu TC, Einarsson EO, Herry C. Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience. 2013;240:219–42. doi: 10.1016/j.neuroscience.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Domschke K, Zwanzger P. GABAergic and endocannabinoid dysfunction in anxiety—future therapeutic targets? Curr Pharm Des. 2008;14:3508–17. doi: 10.2174/138161208786848784. [DOI] [PubMed] [Google Scholar]
- 34.Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36:23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmes NM, Parkes SL, Killcross AS, Westbrook RF. The basolateral amygdala is critical for learning about neutral stimuli in the presence of danger, and the perirhinal cortex is critical in the absence of danger. J Neurosci. 2013;33:13112–25. doi: 10.1523/JNEUROSCI.1998-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–6. doi: 10.1016/0091-3057(95)00153-N. [DOI] [PubMed] [Google Scholar]
- 37.Barbalho CA, Nunes-de-Souza RL, Canto-de-Souza A. Similar anxiolytic-like effects following intra-amygdala infusions of benzodiazepine receptor agonist and antagonist: evidence for the release of an endogenous benzodiazepine inverse agonist in mice exposed to elevated plus-maze test. Brain Res. 2009;1267:65–76. doi: 10.1016/j.brainres.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 38.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–6. doi: 10.1016/0091-3057(95)00153-N. [DOI] [PubMed] [Google Scholar]
- 39.Del-Ben CM, Ferreira CA, Sanchez TA, et al. Effects of diazepam on BOLD activation during the processing of aversive faces. J Psychopharmacol. 2012;26:443–51. doi: 10.1177/0269881110389092. [DOI] [PubMed] [Google Scholar]
- 40.Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dosedependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–8. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 41.Nutt DJ, Glue P, Lawson C, Wilson S. Flumazenil provocation of panic attacks. Evidence for altered benzodiazepine receptor sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47:917–25. doi: 10.1001/archpsyc.1990.01810220033004. [DOI] [PubMed] [Google Scholar]
- 42.Strohle A, Kellner M, Holsboer F, Wiedemann K. Behavioral, neuroendocrine, and cardiovascular response to flumazenil: no evidence for an altered benzodiazepine receptor sensitivity in panic disorder. Biol Psychiatry. 1999;45:321–6. doi: 10.1016/S0006-3223(98)00295-9. [DOI] [PubMed] [Google Scholar]
- 43.Nikolaus S, Hautzel H, Müller HW. Focus on GABA(A) receptor function. A comparative analysis of in vivo imaging studies in neuropsychiatric disorders. Nuklearmedizin. 2014;53:227–37. doi: 10.3413/Nukmed-0647-14-03. [DOI] [PubMed] [Google Scholar]
- 44.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 45.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 46.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 48.Bremner JD, Innis RB, Southwick SM, Staib L, Zoghbi S, Charney DS. Decreased benzodiazepine receptor binding in prefrontal cortex in combat-related post-traumatic stress disorder. Am J Psychiatry. 2000;157:1120–6. doi: 10.1176/appi.ajp.157.7.1120. [DOI] [PubMed] [Google Scholar]
- 49.Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–20. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- 50.Hasler G, Nugent AC, Carlson PJ, Carson RE, Geraci M, Drevets WC. Altered cerebral gamma-aminobutyric acid type A-benzodiazepine receptor binding in panic disorder determined by [11C]flumazenil positron emission tomography. Arch Gen Psychiatry. 2008;65:1166–75. doi: 10.1001/archpsyc.65.10.1166. [DOI] [PubMed] [Google Scholar]
- 51.Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol Bull. 2003;37:133–46. [PubMed] [Google Scholar]
- 52.Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. J Clin Psychiatry. 2004;65(Suppl 1):11–7. [PubMed] [Google Scholar]
- 53.Möhler H. The legacy of the benzodiazepine receptor: from flumazenil to enhancing cognition in Down syndrome and social interaction in autism. Adv Pharmacol. 2015;72:1–36. doi: 10.1016/bs.apha.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Chebib M, Johnston GA. The ‘ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–40. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- 55.Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–43. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABA(A) receptor pore-lining M2 segment. Nat Neurosci. 2001;4:477–85. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- 57.Sigel E, Luscher BP. A closer look at the high affinity benzodiazepine binding site on GABAA receptors. Curr Top Med Chem. 2011;11:241–6. doi: 10.2174/156802611794863562. [DOI] [PubMed] [Google Scholar]
- 58.Wieland HA, Lüddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–9. [PubMed] [Google Scholar]
- 59.Tsang SY, Ng SK, Xu Z, Xue H. The evolution of GABAA receptor-like genes. Mol Biol Evol. 2007;24:599–610. doi: 10.1093/molbev/msl188. [DOI] [PubMed] [Google Scholar]
- 60.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor a1 subtype. Nat Neurosci. 2000;3:587–92. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 61.Engin E, Benham RS, Rudolph U. An emerging circuit pharmacology of GABA(A) receptors. Trends Pharmacol Sci. 2018;39:710–32. doi: 10.1016/j.tips.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102:915–20. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific g-aminobutyric acid A receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 64.Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–4. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 65.Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor pointmutated mice. Pain. 2009;141:233–8. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 66.Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EOslash, Larsen JS, et al. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [30-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–81. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- 67.Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for a5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–9. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 68.Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, et al. RO4938581, a novel cognitive enhancer acting at GABAA a5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–23. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- 69.Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, et al. Identification of a novel, selective GABAA a5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–40. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- 70.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HTJ, Taverna FA, et al. a5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–20. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the a5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–80. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, et al. An inverse agonist selective for a5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–45. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- 73.Harris D, Clayton T, Cook J, Sahbaie P, Halliwell RF, Furtmüller R, et al. Selective influence on contextual memory: physiochemical properties associated with selectivity of benzodiazepine ligands at GABAA receptors containing the a5 subunit. J Med Chem. 2008;51:3788–803. doi: 10.1021/jm701433b. [DOI] [PubMed] [Google Scholar]
- 74.Bukalo O, Pinard CR, Holmes A. Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders. Br J Pharmacol. 2014;171:4690–718. doi: 10.1111/bph.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 76.Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 77.Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, et al. A schizophrenia-related sensorimotor deficit links a3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci U S A. 2005;102:17154–9. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, et al. Anxiogenic properties of an inverse agonist selective for a3 subunit-containing GABAA receptors. Br J Pharmacol. 2005;144:357–66. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dias R, Sheppard WFA, Fradley RL, Garrett EM, Stanley JL, Tye SJ, et al. Evidence for a significant role ofa3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–8. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson AJ, et al. A pharmacogenetic ‘restriction-of-function’ approach reveals evidence for anxiolytic-like actions mediated by α5-containing GABAA receptors in mice. Neuropsychopharmacology. 2016;41:2492–501. doi: 10.1038/npp.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christian EP, Snyder DH, Song W, Gurley DA, Smolka J, Maier DL, et al. EEG-β/γ spectral power elevation in rat: a translatable biomarker elicited by GABA(Aα2/3)-positive allosteric modulators at nonsedating anxiolytic doses. J Neurophysiol. 2015;113:116–31. doi: 10.1152/jn.00539.2013. [DOI] [PubMed] [Google Scholar]
- 82.de Lucas AG, Ahring PK, Larsen JS, Rivera-Arconada I, Lopez-Garcia JA, Mirza NR, et al. GABAA α5 subunit-containing receptors do not contribute to reversal of inflammatory-induced spinal sensitization as indicated by the unique selectivity profile of the GABAA receptor allosteric modulator NS16085. Biochem Pharmacol. 2015;93:370–9. doi: 10.1016/j.bcp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 83.Botta P, et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–1500. doi: 10.1038/nn.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, de Haas S, de Kam M, van Gerven J. An overview of the CNS-pharmacodynamic profiles of nonselective and selective GABA agonists. Adv Pharmacol Sci. 2012;2012:134523. doi: 10.1155/2012/134523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crestani F, Rudolph U. Behavioral functions of GABAA receptor subtypes--the Zurich experience. Adv Pharmacol. 2015;72:37–51. doi: 10.1016/bs.apha.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Sieghart W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv Pharmacol. 2015;72:53–96. doi: 10.1016/bs.apha.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 88.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 89.Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–74. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Aca Sci U S A. 2005;102:915–20. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rowlett JK, Lelas S. Comparison of zolpidem and midazolam self-administration under progressive-ratio schedules: consumer demand and labor supply analyses. Exp Clin Psychopharmacol. 2007;15:328–37. doi: 10.1037/1064-1297.15.4.328. [DOI] [PubMed] [Google Scholar]
- 92.de Visser SJ, van der Post JP, de Waal PP, Cornet F, Cohen AF, van Gerven JM. Biomarkers for the effects of benzodiazepines in healthy volunteers. Br J Clin Pharmacol. 2003;55:39–50. doi: 10.1046/j.1365-2125.2002.t01-10-01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atack JR. Subtype-selective GABA(A) receptor modulation yields a novel pharmacological profile: the design and development of TPA023. Adv Pharmacol. 2009;57:137–85. doi: 10.1016/S1054-3589(08)57004-9. [DOI] [PubMed] [Google Scholar]
- 94.de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoemaker RC, Rijnbeek B, et al. Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J Psychopharmacol. 2007;21:374–83. doi: 10.1177/0269881106072343. [DOI] [PubMed] [Google Scholar]
- 95.de Haas SL, de Visser SJ, van der Post JP, Schoemaker RC, van Dyck K, Murphy MG, et al. Pharmacodynamic and pharmacokinetic effects of MK-0343, a GABA(A) alpha2,3 subtype selective agonist, compared to lorazepam and placebo in healthy male volunteers. J Psychopharmacol. 2008;22:24–32. doi: 10.1177/0269881107082108. [DOI] [PubMed] [Google Scholar]
- 96.de Haas SL, Franson KL, Schmitt JA, Cohen AF, Fau JB, Dubruc C, et al. The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABA-A alpha2,3 selective agonist, in comparison with lorazepam in healthy volunteers. J Psychopharmacol. 2009;23:625–32. doi: 10.1177/0269881108092595. [DOI] [PubMed] [Google Scholar]
- 97.de Haas SL, Schoemaker RC, van Gerven JM, Hoever P, Cohen AF, Dingemanse J. Pharmacokinetics, pharmacodynamics and the pharmacokinetic/ pharmacodynamic relationship of zolpidem in healthy subjects. J Psychopharmacol. 2010;24:1619–29. doi: 10.1177/0269881109106898. [DOI] [PubMed] [Google Scholar]
- 98.De Visser SJ, Van der Post J, Pieters MSM, Cohen AF, Van Gerven JMA. Biomarkers for the effects of antipsychotic drugs in healthy volunteers. Br J Clin Pharmacol. 2001;51:119–32. doi: 10.1111/j.1365-2125.2001.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dumont GJH, De Visser SJ, Cohen AF, Van Gerven JMA. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy volunteers. Br J Clin Pharmacol. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dumont GJM, Verkes RJ. A review of acute effects of 3,4-methylene- dioxymethamphetamine in healthy volunteers. J Psychopharm. 2006;20:176–87. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- 101.Zuurman L, Ippel AE, Moin E, Van Gerven JMA. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 2009;67:5–21. doi: 10.1111/j.1365-2125.2008.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zoethout RWM, Delgado WL, Ippel AE, Dahan A, van Gerven JMA. Functional biomarkers for the acute effects of alcohol on the central nervous system in healthy volunteers. Br J Clin Pharmacol. 2011;71:331–50. doi: 10.1111/j.1365-2125.2010.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Groeneveld GJ, Hay JL, van Gerven JM. Measuring blood-brain barrier penetration using the NeuroCart, a CNS test battery. Drug Discov Today Technol. 2016;20:27–34. doi: 10.1016/j.ddtec.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 104.van Steveninck AL, van Berckel BN, Schoemaker RC, Breimer DD, van Gerven JM, Cohen AF. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 1999;13:10–7. doi: 10.1177/026988119901300102. [DOI] [PubMed] [Google Scholar]
- 105.Murrough JW, Yaqubi S, Sayed S, Charney DS. Emerging drugs for the treatment of anxiety. Expert Opin Emerg Drugs. 2015;20:393–406. doi: 10.1517/14728214.2015.1049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loonen AJ, Ivanova SA. Circuits regulating pleasure and happiness: the evolution of the amygdalar-hippocampal-habenular connectivity in vertebrates. Front Neurosci. 2016;10:539–55. doi: 10.3389/fnins.2016.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–87. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Micale V, Di Marzo V, Sulcova A, et al. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther. 2013;138:18–37. doi: 10.1016/j.pharmthera.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 109.Wu Gang, Feder Adriana, Wegener Gregers, Bailey Christopher, Saxena Shireen, Charney Dennis, Mathé Aleksander A. Central functions of neuropeptide Y in mood and anxiety disorders. Expert Opinion on Therapeutic Targets. 2011;15(11):1317–1331. doi: 10.1517/14728222.2011.628314. [DOI] [PubMed] [Google Scholar]
- 110.Wierońska Joanna M, Pilc Andrzej. Glutamate-based anxiolytic ligands in clinical trials. Expert Opinion on Investigational Drugs. 2013;22(8):1007–1022. doi: 10.1517/13543784.2013.803066. [DOI] [PubMed] [Google Scholar]
- 111.Allison M. Reinventing clinical trials. Nat Biotechnol. 2012;30:41–9. doi: 10.1038/nbt.2083. [DOI] [PubMed] [Google Scholar]
- 112.Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today. 2012;17:419–24. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 113.Dumont GJH, De Visser SJ, Cohen AF, Van Gerven JMA. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy volunteers. Br J Clin Pharmacol. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen X, Jacobs G, de Kam M, Jaeger J, Lappalainen J, Maruff P, et al. The central nervous system effects of the partial GABA-Aα2,3 -selective receptor modulator AZD7325 in comparison with lorazepam in healthy males. Br J Clin Pharmacol. 2014;78:1298–314. doi: 10.1111/bcp.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen X, Jacobs G, de Kam ML, Jaeger J, Lappalainen J, Maruff P, et al. AZD6280, a novel partial γ-aminobutyric acid A receptor modulator, demonstrates a pharmacodynamically selective effect profile in healthy male volunteers. J Clin Psychopharmacol. 2015;35:22–33. doi: 10.1097/JCP.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 116.Zuiker RG, Chen X, Østerberg O, Mirza NR, Muglia P, de Kam M, et al. NS11821, a partial subtype-selective GABAA agonist, elicits selective effects on the central nervous system in randomized controlled trial with healthy subjects. J Psychopharmacol. 2016;30:253–62. doi: 10.1177/0269881115620435. [DOI] [PubMed] [Google Scholar]
- 117.Bittencourt J, Velasques B, Teixeira S, Basile LF, Salles JI, Nardi AE, et al. Saccadic eye movement applications for psychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:1393–409. doi: 10.2147/NDT.S45931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen NT, Clarke PJ, Watson TL, Macleod C, Guastella AJ. Biased saccadic responses to emotional stimuli in anxiety: an antisaccade study. PLoS ONE. 2014;9:e86474. doi: 10.1371/journal.pone.0086474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atack JR. GABAA receptor α2/α3 subtype-selective modulators as potential nonsedating anxiolytics. Curr Top Behav Neurosci. 2010;2:331–60. doi: 10.1007/7854_2009_30. [DOI] [PubMed] [Google Scholar]
- 120.Mohler H. The rise of a new GABA pharmacology. Neuropharmacology. 2011;60:1042–9. doi: 10.1016/j.neuropharm.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 121.Te Beek ET, Chen X, Jacobs GE, Nahon KJ, De Kam ML, Lappalainen J, et al. The effects of the nonselective benzodiazepine lorazepam and the α2/α3 subunit‐selective GABAA receptor modulators AZD7325 and AZD6280 on plasma prolactin levels. Clin Pharmacol Drug Dev. 2015;4:49–154. doi: 10.1002/cpdd.165. [DOI] [PubMed] [Google Scholar]
- 122.Zemishlany Z, McQueeney R, Gabriel SM, Davidson M. Neuroendocrine and monoaminergic responses to acute administration of alprazolam in normal subjects. Neuropsychobiology. 1990;23:124–8. doi: 10.1159/000119437. [DOI] [PubMed] [Google Scholar]
- 123.Sevy S, Brown SL, Wetzler S, et al. Effects of alprazolam on increases in hormonal and anxiety levels induced by meta-chlorophenylpiperazine. Psychiatry Res. 1994;53:219–29. doi: 10.1016/0165-1781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 124.Liem-Moolenaar M, te Beek ET, de Kam ML, et al. Central nervous system effects of haloperidol on THC in healthy male volunteers. J Psychopharmacol. 2010;24:1697–708. doi: 10.1177/0269881109358200. [DOI] [PubMed] [Google Scholar]
- 125.Baas JPM, Mol N, Kenemans JL, Prinssen EP, Niklson I, Xia-Chen C, et al. Validating a human model for anxiety using startle potentiated by cue and context: the effects of alprazolam, pregabalin, and diphenhydramine. Psychopharmacology (Berl) 2009;205:73–84. doi: 10.1007/s00213-009-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABA(A) receptor subtypes. Nat Rev Drug Discov. 2011;10:685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Griebel G, Perrault G, Simiand J, Cohen C, Granger P, Decobert M, et al. SL651498: an anxioselective compound with functional selectivity for alpha2- and alpha3-containing gamma-aminobutyric acid (A) (GABA(A)) receptors. J Pharmacol Exp Ther. 2001;298:753–68. [PubMed] [Google Scholar]
- 128.Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–10. doi: 10.1016/S0014-2999(02)02191-X. [DOI] [PubMed] [Google Scholar]
- 129.Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–4. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]