Abstract
The present study examined the effect of meal intake on physiological and psychological indices during self-selected high intensity interval exercise (HIIE). Seventeen active men and women (age = 26.4 ± 5.8 yr) completed ramp cycle ergometry to determine maximal oxygen uptake and peak power output. On two subsequent days, they performed a session of self-selected HIIE consisting of ten 1 min bouts separated by 1 min recovery in the fed or fasted state, whose order was randomized. Meal intake consisted of a banana and a Zone™ bar containing 315 kcal, which were ingested 2 h pre-exercise, and the fasted state required no food for > 12 h pre-exercise. Participants ingested an identical meal the evening before each session. Heart rate (HR), oxygen uptake (VO2), blood glucose and blood lactate concentration, rating of perceived exertion (RPE), affect, and enjoyment were measured during exercise. Irrespective of fed state, both bouts elicited intensities equal to 94% HRmax which represents HIIE. Our results showed no difference in HR (174.0 ± 13.5 vs. 173.2 ± 12.9 b/min in fed and fasted state, p = 0.17), VO2 (2.43 ± 0.54 vs. 2.40 ± 0.52 L/min in fed and fasted state, p = 0.14), RPE (p = 0.44), affect (p = 0.79), or enjoyment (103 ± 14 vs. 101 ± 13, p = 0.77) between the fed and fasted state. Despite its high reliance on carbohydrate, performance and perception of low-volume HIIE are not altered by ingestion of a meal before exercise.
Keywords: Interval training, Overnight fast, Oxygen uptake, Blood lactate concentration, Affect
INTRODUCTION
It is evident that carbohydrate (CHO) intake before endurance exercise increases blood glucose concentration (BG) and modifies substrate oxidation [1]. In active men and women, CHO consumption (up to 312 g ingested 4 h before exercise) led to significant increases in cycling performance which was consequent with greater blood glucose availability and enhanced CHO oxidation [1]. Previous findings [2–3] denote that for moderate to high intensity exercise of at least 1 h duration, CHO should be ingested prior to optimize performance. However, the efficacy of CHO ingested within 1 h of exercise is mixed, as there are reports of positive [4], negative [5], or minimal effects [6] of pre-exercise CHO on performance. Overall, these findings emphasize the importance of CHO availability during prolonged moderate to intense exercise, but suggest that nutrient timing may or may not alter changes in performance in response to CHO ingestion.
In addition to moderate intensity endurance exercise, CHO availability may also be critical during more intense intermittent exercise which due to its maximal or supramaximal intensity is more dependent on glycogen utilization to provide ATP for muscle contraction. For example, McCartney et al. [7] demonstrated a 40% decline in muscle glycogen concentration in response to four supramaximal bouts of cycling consisting of only 2 min of exercise. In male athletes, Little et al. [8] reported an ergogenic effect of low and high glycemic index CHO-rich meals (containing 1.5 g/kg bw of CHO) ingested 2 h before completion of intermittent exercise compared to fasting. Similarly, Galloway et al. [9] reported that 32 g of CHO ingested 30 min pre-exercise enhanced time to exhaustion at 90 percent peak power output (PPO) versus placebo. Nevertheless, no effect of a small meal (160 kcal and 20 g of CHO) ingested 15 or 60 min before exercise was shown in cyclists who performed 18 modified Wingate tests [10].
Previous data show that modifying BG via food intake alters mood. For example, Benton and Owens [11] concluded that increased BG (although within the normal range) is associated with lower tension. In addition, negative affect occurs in persons with insulin-dependent diabetes [12] as well as adults in a hypoglycemic state [13]. A few studies [14–15] have examined changes in perceptual responses during intermittent performance when CHO availability was manipulated, yet despite significant increases in performance, change in fatigue and mood acquired from the Profile of Mood States, rather than affect, was the primary perceptual-related variable. Recent studies [16–17] show that in-task affective valence is typically more negative in response to HIIE versus MICE which is due to the enhanced hyperventilation and blood lactate accumulation. Despite this result, post-exercise enjoyment has been reported to be higher in response to HIIE versus MICE [16], which may broaden its application in many adults.
Overall, there is a paucity of data examining the effects of CHO intake on performance of HIIE and resultant perceptual responses, and existing findings are equivocal which merits further study. The efficacy of chronic HIIE to improve VO2max and glycemic control is well-documented in various populations [18–20]. It is apparent that optimizing adaptation to exercise training is paramount to achieving athletic success as well as improving health and fitness [21], and meal intake around exercise is an important element to foster this goal. Consequently, this study investigated the effect of meal intake on HIIE performance, and in addition, included indices of perceptual responses which can be altered by pre-exercise dietary manipulations [22] and may be related to long-term adherence [23].
MATERIALS AND METHODS
Study Design
Participants performed three sessions of exercise separated by a minimum of 48 h, with time of day maintained within participants between 8:00–10:00 am. Initially, they performed incremental exercise to determine maximal oxygen uptake (VO2max) and peak power output (PPO), and during two subsequent sessions, performed a session of HIIE in the fed or fasted state, whose order was randomized. Physiological and perceptual responses were measured during exercise. Prior to all sessions, they were required to be well-rested and hydrated and refrain from intense exercise for 48 h. Participants were not aware of the study hypothesis and were not blinded to the content of the meal.
Subjects
Seventeen habitually active men and women were recruited through convenience sampling; their physical characteristics are shown in Table 1. A questionnaire [24] was used to confirm that they completed > 150 min/wk of physical activity including resistance training, non-competitive sport, surfing, group fitness classes, or aerobic exercise in the last year. All participants completed a medical history questionnaire to ensure absence of known cardiorespiratory or muscular contraindications or any medication use which may modify study outcomes. They provided written informed consent before participation in the study, whose procedures were approved by the University Institutional Review Board. The experiments reported in the manuscript were performed in accordance with the ethical standards of the Helsinki Declaration.
TABLE 1.
Participant demographic characteristics and maximal gas exchange data.
| Parameter | Mean ± SD | Range |
|---|---|---|
| Age (yr) | 26.4 ± 5.8 | 20–46 |
| Gender (M:F) | 10:7 | NA |
| BMI (kg/m2) | 24.4 ± 2.1 | 21.6–28.6 |
| PA (h/wk) | 4.7 ± 1.4 | 3.0–7.0 |
| VO2max (mL/kg/min) | 38.6 ± 4.5 | 30.1–45.0 |
| VO2max (L/min) | 2.8 ± 0.6 | 2.0–4.0 |
| PPO (W) | 281.7 ± 59.6 | 198–400 |
| HRmax (b/min) | 185.8 ± 7.4 | 171–200 |
| BLa (mM) | 11.2 ± 1.4 | 8.9–14.2 |
BMI = body mass index; PA = physical activity; PPO = peak power output; HR = heart rate; BLa = blood lactate concentration.
Procedures
During the initial session, participants were seated for 5 min after which blood was acquired from a sterile fingertip using a lancet (Owen Mumford, Inc., Marietta, GA) and portable monitor (Lactate Plus, Nova Biomedical, Waltham, MA) which were used to measure blood lactate concentration (BLa). They subsequently completed a brief survey confirming adherence to our pre-test guidelines which included being well-rested and hydrated and not sick. Height and body mass were determined to calculate body mass index (kg/m2). A plastic strap was placed on the trunk to measure heart rate via telemetry (Polar, Woodbury, NY), and participants initiated ramp exercise on an electronically-braked cycle ergometer (Velotron Dynafit Pro, Racermate, Seattle, WA). Exercise began with a 2 min warm up at power output equal to 40–70 W followed by 20–35 W/min increases until volitional exhaustion (pedal cadence < 50 rev/min). During the bout, pulmonary gas exchange data were obtained every 15 s using a metabolic cart (Parvomedics True One, Sandy, UT) which was calibrated before testing according to the manufacturer. Verbal encouragement was provided throughout the test. VO2max was identified as the average of the two highest 15 s values from the last 45 s of exercise, and attainment of VO2max was confirmed by incidence of a plateau in VO2 (< 150 mL/min) as well as RERmax > 1.15 and HRmax within 10 b/min of 220 – age [25]. The power output consequent with volitional exhaustion was identified as PPO. Three minutes after this bout, another blood sample was obtained from the fingertip to determine maximal BLa. Subsequently, the participant was familiarized with using the handlebar controller to freely modify workload during cycling as required in the next two sessions. Settings for the seat and bar height were recorded and repeated within subjects for all subsequent trials.
High intensity interval exercise sessions
These sessions were preceded by the following dietary conditions: on one day, they came into the lab after an overnight fast (> 12 h) or alternatively, after ingesting a small meal at home 2 h prior (1 medium banana and 1 chocolate peanut butter or fudge graham Zone™ bar), with total energy composition equal to 315 kcal and 63/18/19% carbohydrate (49 g), fat (6 g), and protein (16 g), respectively. Before the first session, participants recorded their dietary intake in the 24 h prior on a written log, which was photocopied and returned to them to follow for the last session. In addition, participants were provided a gift card to Jersey Mike’s™ and required to ingest a sandwich and bag of chips of their choice for dinner the night before each session (energy composition ranging from 740–1,060 kcal), which was eaten between 6:00–8:00 pm.
Participants were initially seated for 5 min, then blood samples were obtained from the fingertip to measure BLa and blood glucose concentration (BG) (Bayer Contour Next, Mishawaka, IN), and they completed a brief survey confirming adherence to the pre-test guidelines. A 4 min warm-up was completed at 10% PPO, during which time they were re-familiarized with use of the handlebar controller. Participants completed ten 1 min bouts of HIIE separated by 1 min recovery. During exercise, participants were informed to perform as much work as possible and attain a minimum RPE equal to 7 on the Borg CR10 scale [26] which elicits workloads of approximately 95% PPO [27]. They were instructed to use the handlebar controller to freely modify work rate in fixed increments (10% PPO) as used in a recent study [27]. Participants were informed as to the elapsed duration of each interval in 15 s increments, yet were blinded to the change in HR, power output, or VO2.
Measures
HR was continuously measured during exercise with telemetry (Polar, Woodbury, NY), and pulmonary gas exchange data were measured using a metabolic cart (Parvomedics True One, Sandy, UT). Mean HR was determined as the average value for the entire exercise session not including the warm-up or recovery, and peak HR was identified as the mean of the highest values attained from all ten intervals. Pre-exercise and for bouts 2, 4, 6, 8, and 10, values of HR and VO2 for each HIIE regime were calculated from the last two 15 s data points and first two values in recovery. Self-selected power output (in W) was continuously recorded during HIIE and expressed as a mean (including warm-up and recovery) and peak value for each session. Blood lactate concentration was also measured immediately after bout 5 and 3 min post-exercise; in addition, blood glucose concentration was measured 3 min post-exercise.
Before each session, participants were read specific instructions according to what RPE and affect represented. They were asked to respond to each scale in terms of how they felt at that moment. The meaning of the CR-10 scale [26] was communicated by instructing participants to report perceptions of their exertion in terms of their breathing, heart rate, and level of fatigue. For affect [28], they were read the following text: While participating in exercise, it is common to experience changes in mood. Some individuals find exercise pleasurable; whereas, others find it to be unpleasant. Additionally, feeling may fluctuate across time. That is, one might feel good and bad a number of times during exercise. Rating of perceived exertion and affect were recorded before the warm-up and immediately at the end of bouts 2, 4, 6, 8, and 10. Affect (11-point scale, rating from +5 very good to -5 very bad) was recorded at the same time points as RPE. Ten minutes post-exercise, participants’ level of enjoyment was measured through the PACES scale [29] which required them to answer 18 items on a 1–7 scale.
Statistical Analyses
Data are expressed as mean ± standard deviation (SD) and were analyzed using SPSS Version 24.0 (Chicago, IL). The Shapiro-Wilks test was used to test normality of all variables. Two-way analysis of variance with repeated measures (ANOVA) was used to measure differences in VO2, BLa, BG, affect, RPE, and HR across time (2–6 levels) and fed state (2 levels). The Greenhouse-Geisser correction was used to account for the sphericity assumption of unequal variances across groups. Tukey’s post hoc analysis was used to determine significant differences between means when a significant F ratio was obtained. Differences in mean and peak HR, power output, and PACES between fed state were determined by a dependent t-test. Sample size was comparable to previous studies comparing oxygen uptake, affect, and enjoyment between various HIIE regimes [27, 30]. G Power [31] was used to confirm that a sample size of 11 is adequate to detect a change in affect equal to 1.0 unit, VO2 equal to 0.20 L/min, and PACES equal to 10 units between conditions. Statistical significance was equal to p ≤ 0.05.
RESULTS
Eleven of 17 participants preferred performing HIIE in the fed versus fasted state. The mean HR from the HIIE intervals was equal to 175 ± 12 b/min, which is equivalent to 94% HRmax and represents intensities coincident with interval training.
Changes in oxygen uptake and heart rate
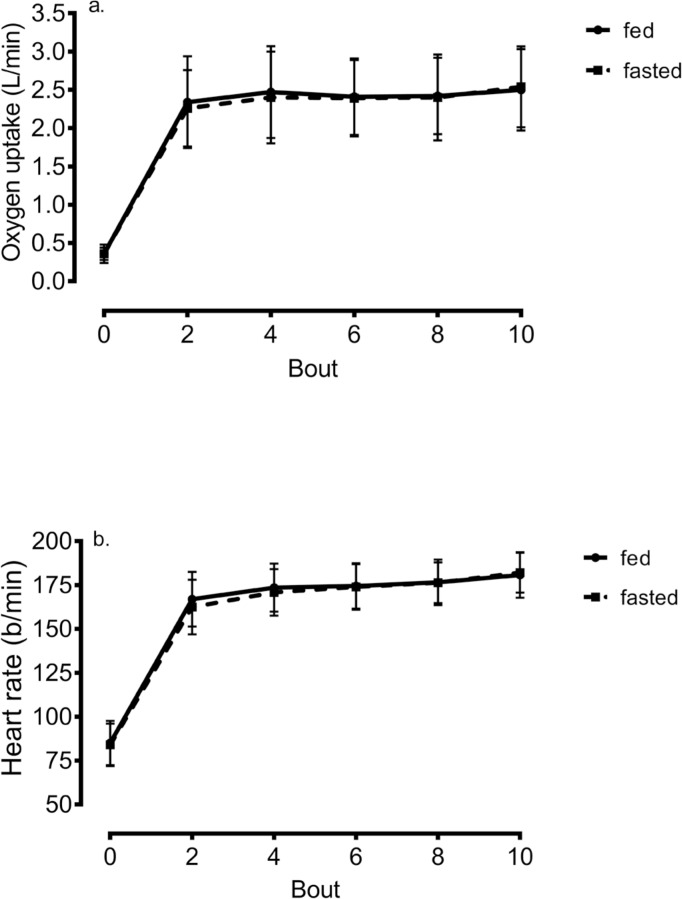
Compared to pre-exercise (VO2 = 0.36 ± 0.10 L/min), oxygen uptake increased (p < 0.001) more than six-fold with initiation of HIIE and peaked at values equal to 2.51 ± 0.53 and 2.54 ± 0.53 L/min in the fed and fasted state, representing 89% VO2max. However, there was no main effect of time (p = 0.14) or meal X time interaction (p = 0.38). Results show that HR significantly increased over time (p < 0.001) yet there was no main effect of meal (p = 0.17) or meal X time interaction (p = 0.08). Peak HR from end-exercise was equal to 181 ± 12 b/min. No difference was shown in mean (p= 0.25) or peak HR (p = 0.82) between the fed and fasted state. These findings are shown in Figure 1.
FIG. 1.
Change in a) oxygen uptake and b) heart rate during self-paced HIIE in the fed and fasted state. * = p < 0.05 compared to pre-exercise measure within condition.
Changes in blood lactate and blood glucose concentration
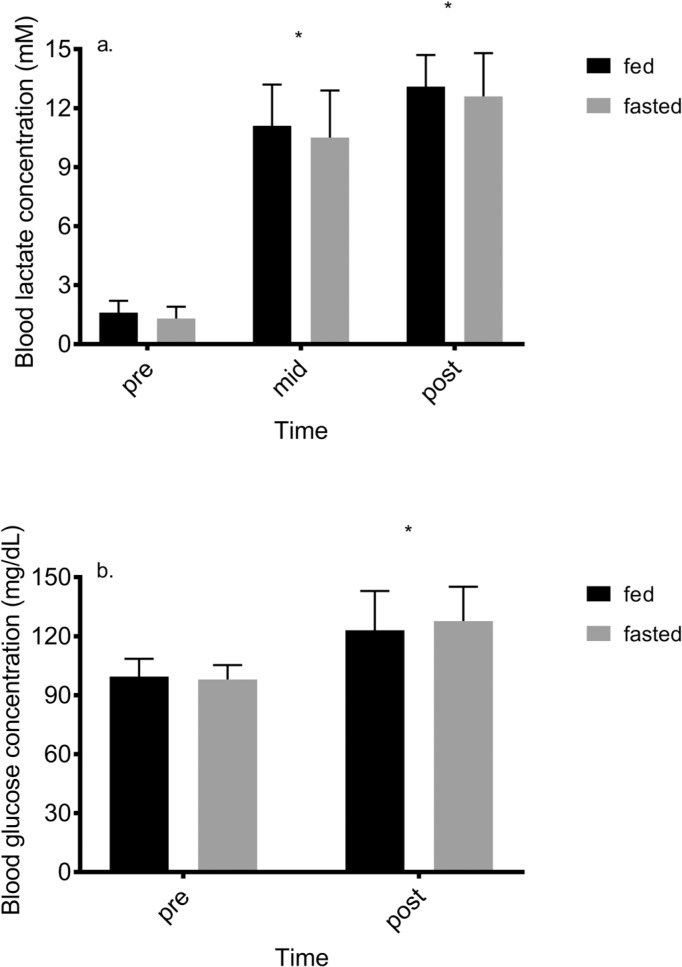
Compared to pre-exercise, BLa increased over time (p < 0.001) from 1.5 ± 0.6 mM to 12.7 ± 1.8 mM at end-exercise, but there was no effect of meal (p = 0.11) or meal X time interaction (p = 0.79). Blood glucose increased following exercise (p < 0.001) but there was no main effect of meal (p = 0.39) or meal X time interaction (p = 0.11). These results are shown in Figure 2.
FIG. 2.
Change in a) blood lactate concentration and b) blood glucose concentration during self-paced HIIE in the fed and fasted state. * = p < 0.05 compared to pre-exercise measure within condition.
Changes in energy expenditure and power output
There was no difference (p = 0.18) in calorie expenditure between HIIE in the fed (225 ± 48 kcal) and fasted state (221 ± 48 kcal). Similarly, there was no difference (p = 0.48) in mean power output (115 ± 29 W vs. 116 ± 28 W) or peak power output (239 ± 62 W vs. 245 ± 64 W, p = 0.33) between the fed and fasted state.
Changes in perceptual responses
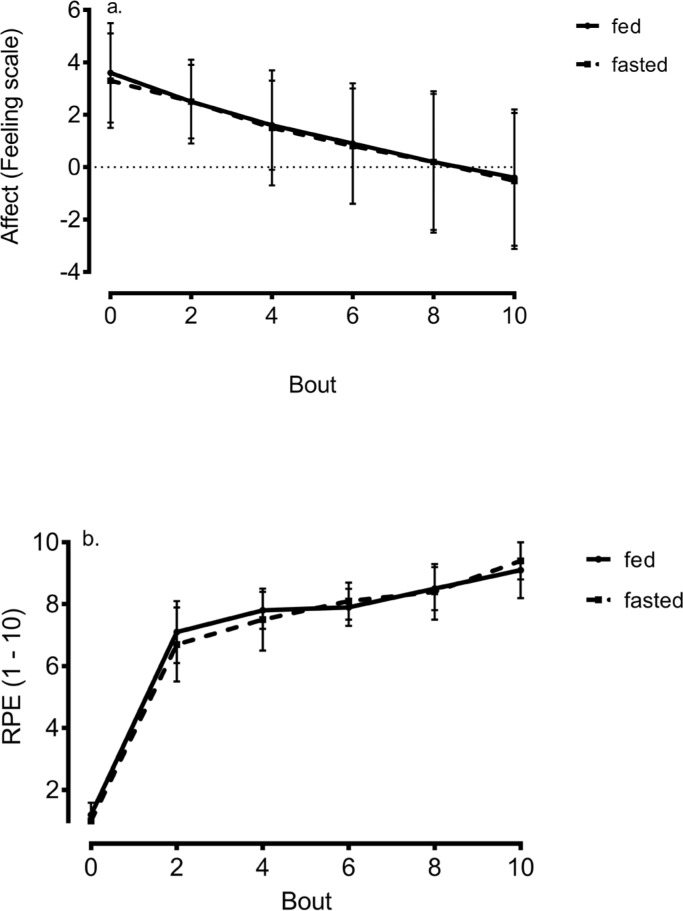
These data are shown in Figure 3. Affect gradually declined during exercise (p < 0.001) compared to pre-exercise in the fed and fasted state (3.59 ± 1.90 and 3.28 ± 1.82). Our results show an aversive response in end-exercise affect which was equal to -0.35 ± 2.60 and -0.53 ± 2.60 in response to HIIE in the fed and fasted state. There was no main effect of meal (p = 0.79) or meal X time interaction (p = 0.85). RPE increased during exercise (p < 0.001) and peaked at values equal to 9.1 ± 0.9 and 9.3 ± 0.6 in the fed and fasted state, yet there was no effect of meal (p = 0.44) or meal X time interaction (p = 0.32). Our data show no difference in PACES (p = 0.77) between HIIE performed in the fed (103 ± 14) versus fasted state (101 ± 13).
FIG. 3.
Change in a) affect and b) rating of perceived exertion during self-paced HIIE in the fed and fasted state.
DISCUSSION
Many years of research document the importance of CHO availability for improving endurance performance [32], yet it is equivocal whether intermittent exercise such as HIIE, which depends heavily on CHO utilization yet is shorter duration, can benefit from intake of CHO. Our data demonstrate no change in physiological or perceptual indices when HIIE is performed in the fasted compared to fed state, which suggests that eating a small CHO-rich meal 2 hours before low-volume interval-based exercise has no benefit.
Previous studies [8, 33] showing a benefit of CHO intake on intermittent performance used a 90 min intermittent running protocol on a treadmill which simulated soccer [8] or the Loughborough intermittent shuttle test (LIST) [33, as described in reference 34]), which is a 1.5 h exercise bout consisting of jogging, running, and sprinting. These bouts cause substantial glycogen degradation and in the case of the latter, it has an energy expenditure of 1300 kcal which is six-fold higher than our value observed for low-volume HIIE (see Results). In addition, in the Backhouse et al. [33] study, trials were preceded by a glycogen-depleting exercise session that was followed by a low-CHO meal which further reduce CHO availability and in turn may augment any potential benefit of pre-exercise CHO intake. In contrast, in our study, we required participants to abstain from intense activity for 48 h before each session and to ingest a relatively high-CHO meal the evening prior, which should elicit relatively adequate levels of muscle glycogen. A previous study showed significant (20% ) glycogen utilization in response to completion of two 20 s “all-out” sprints at 7.5% body weight [35], which is slightly lower than the magnitude of glycogen degradation characteristic of our HIIE protocol (30–40% , Dr. Martin Gibala, personal communication). Despite this, no measure of performance (power output or oxygen uptake) was altered in response to our intervention, which is likely due to the lower volume of work required, the relatively small meal ingested pre-exercise, and the intake of a high-CHO meal the night before. Nevertheless, Galloway et al. [9] reported significantly longer time to exhaustion when 32 g of CHO was ingested in a drink 30 min pre-exercise compared to 120 min prior or a placebo treatment. This is surprising considering the notion that CHO is not limiting to bouts of this relatively brief duration [7]. They explained this finding through an elevation in pre-exercise blood glucose, which promotes CHO oxidation and blunts lipid oxidation, as well as a potential to augment liver glycogen stores which then could be utilized during the subsequent bout.
There is a paucity of data concerning perceptual responses to intermittent exercise with manipulation of fed state. Little et al. [8] revealed a lower RPE when a low glycemic index meal was ingested before intermittent running compared to placebo. Backhouse et al. [33] exhibited higher perceived activation towards the end of the LIST with CHO ingestion versus placebo, although there was no change in RPE or affect despite non-significant trends emerging for higher pleasure and lower exertion at the end of exercise. Although Galloway et al. [9] reported no significant difference in any index of mood between CHO and placebo trials, their data show higher vigor and attenuated fatigue with CHO ingested 30 min pre-exercise versus all other trials. Our data show no effect of fed state on RPE, affect, or enjoyment which is likely related to the lack of differences in the power output and oxygen uptake response to HIIE. Although we required participants to continuously regulate work rate during HIIE, they were “clamped” at an RPE > 7, which may have led to the lack of changes in perceptual responses. Whether similar findings would be revealed during repeated Wingate tests or other sprint interval training regimes such as reduced exertion high intensity training [35] is unknown and merits further study. Examining perceptual responses to exercise is important, as the suitability of sprint interval training for inactive populations has been debated [36–37] despite its documented efficacy and feasibility [35, 38] in these individuals.
Our data (Figure 2) show exercise-induced increases in BLa and BG, yet there was no effect of fed state on these outcomes. Our post-exercise BLa values equal to 12.6–13.1 mM support previous studies [9, 39] and demonstrate the high intensity of our HIIE regime. In previous studies [8–9] showing improved exercise performance with CHO, no difference in BLa was shown. Although speculative, this would imply that glycolytic flux or alternatively, lactate oxidation and clearance, are significantly altered when CHO is ingested prior to HIIE. In addition, the fact that pre-exercise BG was not different across conditions is likely due to the 2 h duration between meal ingestion and this measure. When 32 g of CHO were ingested 2 h before intense exercise at 90% PPO, there was no change in BG from baseline to pre-exercise, unlike when CHO was ingested only 30 min prior which showed a significant rise in BG [9]. When a high glycemic index (GI) meal containing 38 kJ/kg of CHO was ingested 2 h prior to exercise, BG was significantly elevated versus baseline for 90 min post-ingestion, which did not occur with ingestion of a low GI meal of similar energy composition [8]. Although our meal was high in CHO, a banana has a relatively low GI equal to 60, and the protein bar contains s 60% of calories from fat and protein, which reduces its glycemic load. Consequently, meal intake 2 h pre-exercise as well as the low glycemic index of the meal likely led to no difference in pre-exercise BG between the fed and fasted state. It is plausible that this similar BG response between conditions led to a similar metabolic and perceptual state before exercise which resulted in no difference in any outcome between the fed and fasted state.
Our data apply to young, active people participating in low-volume HIIE, so results cannot be generalized to other populations such as sedentary individuals, the elderly, or athletes. Our meal was ingested 2 h pre-exercise at home rather than in the laboratory, so we are not certain that it was ingested at this exact time. However, we asked participants to verify this using a brief survey and verbal confirmation. In addition, the meal was ingested 2 h pre-trial to avoid rebound hypoglycemia which can occur if CHO is ingested within 60 min of exercise [40]. However, some results suggest that there is no benefit of CHO when ingested 75–120 min pre-trial [9], although other data refute this claim [1]. In addition, the meal contained only 50 g of CHO as well as protein and fat, so it is unclear if the relatively small amount of CHO and mixed macronutrient composition were optimal to enhance HIIE performance. However, the study is strengthened by close supervision of physical activity before each trial as well as standardization of meal intake on the evening prior to each session. In addition, our mixed meal better represents the recommended constituents of a pre-exercise meal (CHO for energy and protein for muscle repair) rather than solely CHO which may be undesirable for many exercisers or cause gastrointestinal distress [41].
In overweight women [42], data showed that 6 wk of either fed or fasted HIIT led to significant and similar improvements in body composition as well as increases in citrate synthase and b-hydroxyacyl-CoA dehydrogenase activity. More recently, Terada et al. [43] showed that performing 12 sessions of sprint interval training in the fasted state led to superior improvements in exercise performance in cyclists compared to CHO supplementation. In addition, performing a single Wingate test preceded by glucose ingestion abolished the increase in SIRT1 which plays an important role in lipid oxidation, mitochondrial biogenesis, and glucose control [44]. Despite these somewhat mixed findings regarding potential benefits of performing interval training in the fasted state, our data show no effect of ingestion of a CHO-rich meal 2 h pre-exercise on physiological or perceptual responses to low-volume HIIE requiring only 10 min of work. However, 65% of participants preferred exercise in the fed state which may be related to individual perceptions of exercise and whether they typically intake food before exercise. Further study examining larger amounts and different types of CHO ingested closer to the HIIE session are needed to provide dietitians and clinicians with clearer guidelines regarding efficacy of meal intake before intermittent exercise.
CONCLUSIONS
Many exercisers wonder whether food needs to be ingested before exercise to enhance their performance or potential for experiencing various adaptations to exercise training including weight loss or gains in fitness. Intermittent exercise such as interval training relies heavily on CHO utilization to fuel these bouts, although its brief duration may not require ingestion of CHO before exercise. Our data, albeit preliminary and acquired in a small sample, show no effect of meal intake on changes in various physiological or perceptual measures during low-volume interval exercise, suggesting little benefit of ingesting a carbohydrate-rich meal a few hours before this bout to enhance performance.
Acknowledgements
The authors acknowledge all participants for dedicating their time in completing this study as well as many undergraduate students who assisted us in data collection.
Conflict of interest declaration
The authors declare that there is no conflict of interest in completion of this study.
REFERENCES
- 1.Sherman WM, Brodowicz G, Wright DA, Allen WK, Simonsen J, Dernbach A. Effects of 4 h preexercise feedings on cycling performance. Med Sci Sports Exerc. 1989;21(5):598–604. [PubMed] [Google Scholar]
- 2.Febbraio M, Chiu A, Angus DJ, Arkinstall MJ, Hawley JA. Effects of carbohydrate ingestion before and during exercise on glucose kinetics and performance. J Appl Physiol. 2000;89(6):2220–2226. doi: 10.1152/jappl.2000.89.6.2220. [DOI] [PubMed] [Google Scholar]
- 3.Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. 2011;29(S1):S17–S27. doi: 10.1080/02640414.2011.585473. [DOI] [PubMed] [Google Scholar]
- 4.Gleeson M, Maughan RJ, Greenhaff PL. Comparison of the effects of pre-exercise feedings of glucose, glycerol, and placebo on endurance and fuel homeostasis in man. Eur J Appl Physiol. 1986;55:643–653. doi: 10.1007/BF00423211. [DOI] [PubMed] [Google Scholar]
- 5.Foster C, Costill DL, Fink WJ. Effects of preexercise feedings on endurance performance. Med Sci Sports. 1979;11(1):1–5. [PubMed] [Google Scholar]
- 6.Chryssanthopoulos C, Hennessy LC, Williams C. The influence of pre-exercise glucose ingestion on endurance running capacity. Br J Sports Med. 1994;28:105–109. doi: 10.1136/bjsm.28.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCartney N, Spriet LL, Heigenhauser GJ, Kowalchuk JM, Sutton JR, Jones N. Muscle power and metabolism in maximal intermittent exercise. J Appl Physiol. 1986;60(4):1184–1189. doi: 10.1152/jappl.1986.60.4.1164. [DOI] [PubMed] [Google Scholar]
- 8.Little JP, Chilibeck PD, Ciona D, Forbes S, Rees H, Vandenberg A, Zello GA. Effect of low- and high-glycemic index meals on metabolism and performance during high-intensity, intermittent exercise. Int J Sports Nutr Exerc Metab. 2011;20:447–456. doi: 10.1123/ijsnem.20.6.447. [DOI] [PubMed] [Google Scholar]
- 9.Galloway SDR, Lott MJE, Toulouse LC. Preexercise carbohydrate feeding and high-intensity exercise capacity: Effects of timing of intake and carbohydrate concentration. Int J Sports Nutr Exerc Metab. 2014;24:258–266. doi: 10.1123/ijsnem.2013-0119. [DOI] [PubMed] [Google Scholar]
- 10.Pritchett K, Bishop P, Pritchett R, Kovacs M, Davis JK, Casaru C, Green M. Effects of time of pre-exercise nutrient intake on glucose responses and intermittent cycling performance. South Afr J Sports Med. 2008;20(3):86–90. [Google Scholar]
- 11.Benton D, Owens D. Is raised blood glucose associated with the relief of tension? J Psychosom Res. 1993;37:723–735. doi: 10.1016/0022-3999(93)90101-k. [DOI] [PubMed] [Google Scholar]
- 12.Gonder-Frederick LA, Cox DJ, Bobbitt SA, Pennebaker JW. Mood changes associated with blood glucose fluctuations in insulin-dependent diabetes mellitus. Health Psychol. 1989;8(1):45–59. doi: 10.1037//0278-6133.8.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Gold AE, MacLeod KM, Frier BM, Deary IJ. Changes in mood during acute hypoglycemia in healthy subjects. J Pers Soc Psychol. 1995;68(3):498–504. doi: 10.1037//0022-3514.68.3.498. [DOI] [PubMed] [Google Scholar]
- 14.Welsh RS, Davis JM, Burke JR, Williams HG. Carbohydrates and physical/mental performance during intermittent exercise to fatigue. Med Sci Sports Exerc. 2002;34(4):723–731. doi: 10.1097/00005768-200204000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Winnick JJ, Davis JM, Welsh RS, Carmichael MD, Murphy EA, Blackmon JA. Carbohydrate feedings during team sport exercise preserve physical and CNS function. Med Sci Sports Exerc. 2005;37(2):306–315. doi: 10.1249/01.mss.0000152803.35130.a4. [DOI] [PubMed] [Google Scholar]
- 16.Backhouse SH, Bishop NC, Biddle SJ, Williams C. Effect of carbohydrate and prolonged exercise on affect and perceived exertion. Med Sci Sports Exerc. 2005;37(10):1768–1773. doi: 10.1249/01.mss.0000181837.77380.80. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira BRR, Slama FA, Deslandes AC, Furtado ES, Santos TM. Continuous and high-intensity interval training: Which promotes higher pleasure? PLoS One. 2013;8:e79965. doi: 10.1371/journal.pone.0079965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milanovic Z, Sporis G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45(10):1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 19.Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, Davies MJ. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015;16(11):942–961. doi: 10.1111/obr.12317. [DOI] [PubMed] [Google Scholar]
- 20.Astorino TA, Edmunds RM, Clark A, King L, Gallant RA, Namm S, Fischer A, Wood KM. High intensity interval training increases cardiac output and VO2max. Med Sci Sports Exerc. 2017;49(2):265–273. doi: 10.1249/MSS.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 21.Camera DM, Smiles WJ, Hawley JA. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol Med. 2016;98:131–143. doi: 10.1016/j.freeradbiomed.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Backhouse SH, Bishop NC, Biddle SDH, Williams CA. Effect of carbohydrate and prolonged exercise on affect and perceived exertion. Med Sci Sports Exerc. 2005;37(10):1768–1773. doi: 10.1249/01.mss.0000181837.77380.80. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes RE, Kates A. Can the affective response to exercise predict future motives and physical activity behavior? A systematic review of published evidence. Ann Behav Med. 2015;49(5):715–731. doi: 10.1007/s12160-015-9704-5. [DOI] [PubMed] [Google Scholar]
- 24.The International Physical Activity Questionnaire 2005. Available at http://www.ipaq.ki.se/http://www.ipaq.ki.se.
- 25.Astorino TA, White AC, Dalleck LC. Supramaximal testing to confirm attainment of VO2max in sedentary men and women. Int J Sports Med. 2008;32:1–6. doi: 10.1055/s-0028-1104588. [DOI] [PubMed] [Google Scholar]
- 26.Borg G. Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 27.Kellogg E, Cantacessi C, McNamer O, Holmes H, von Bargen R, Ramirez R, Gallagher D, Vargas S, Santia B, Rodriguez K, Astorino TA. J Str Cond Res. 2018. Comparison of psychological and physiological responses to imposed vs. self-selected high-intensity interval training. (in press) [DOI] [PubMed] [Google Scholar]
- 28.Hardy CJ, Rejeski WJ. Not what, but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol. 1989;11:304–317. [Google Scholar]
- 29.Kendzierski D, DeCarlo KJ. Physical activity enjoyment scale: Two validation studies. J Sport Exerc Psychol. 1991;13(1):50–64. [Google Scholar]
- 30.Tucker WJ, Sawyer BJ, Jarrett CL, Bhammar DM, Gaesser GA. Physiological responses to high-intensity interval exercise differing in interval duration. J Strength Cond Res. 2015;29(12):3326–3335. doi: 10.1519/JSC.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 31.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavioral Res Meth. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 32.Coggan AR, Coyle EF. Metabolism and performance following carbohydrate ingestion late in exercise. Med Sci Sports Exerc. 1989;21(1):59–65. doi: 10.1249/00005768-198902000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Backhouse SH, Ali A, Biddle SJ, Williams C. Carbohydrate ingestion during prolonged high-intensity intermittent exercise: impact on affect and perceived exertion. Scand J Med Sci Sports. 2007;17(5):605–610. doi: 10.1111/j.1600-0838.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 34.Nicholas CW, Nuttall FE, Williams C. The loughborough intermittent shuttle test: a field test that simulates the activity pattern of soccer. J Sports Sci. 2000;18:97–104. doi: 10.1080/026404100365162. [DOI] [PubMed] [Google Scholar]
- 35.Metcalfe RS, Tardif N, Thompson D, Vollaard NB. Changes in aerobic capacity and glycaemic control in response to reduced-exertion high-intensity interval training (REHIT) are not different between sedentary men and women. Appl Physiol Nutr Metab. 2016;41(11):1117–1124. doi: 10.1139/apnm-2016-0253. [DOI] [PubMed] [Google Scholar]
- 36.Hardcastle SJ, Ray H, Beale L, Hagger MS. Why sprint interval training is inappropriate for a largely sedentary population. Front Psychol. 2014;5:1505. doi: 10.3389/fpsyg.2014.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Astorino TA, Thum JS. Response: Commentary: Why sprint interval training is inappropriate for a largely sedentary population. Front Psychol. 2016;7:746. doi: 10.3389/fpsyg.2016.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillen JB, Percival ME, Skelly LE, Martin BJ, Tan RB, Tarnopolsky MA, Gibala MJ. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS One. 2014;9(11):0111489. doi: 10.1371/journal.pone.0111489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astorino TA, DeRevere J, Anderson T, Kellogg E, Holstrom P, Ring S, Ghaseb N. Eur J Appl Physiol. 2018. Change in VO2max and time trial performance in response to high-intensity interval training prescribed using ventilatory threshold. (in press) [DOI] [PubMed] [Google Scholar]
- 40.Williams CA, Lamb DR. Does a high-carbohydrate breakfast improve performance? Sports Sci Exchange. 2008;21(2):108. [Google Scholar]
- 41.Position of the American Dietetic Association, dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J Am Diet Assoc. 2009;109:509–527. doi: 10.1016/j.jada.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity. 2013;21(11):2249–2255. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- 43.Terada T, Toghi Eshghi SR, Liubaoerjijin Y, Kennedy M, Myette-Côté E, Fletcher K, Boule NG. J Sports Med Phys Fitness. 2018. Overnight fasting compromises exercise intensity and volume during sprint interval training but improves high-intensity aerobic endurance. (in press) [DOI] [PubMed] [Google Scholar]
- 44.Guerra B, Guadalupe-Grau A, Fuentes T, Ponce-González JG, Morales-Alamo D, Olmedillas H, Guillén-Salgado J, Santana A, Calbet JA. SIRT1, AMP-activated protein kinase phosphorylation and downstream kinases in response to a single bout of sprint exercise: influence of glucose ingestion. Eur J Appl Physiol. 2010;109(4):731–743. doi: 10.1007/s00421-010-1413-y. [DOI] [PubMed] [Google Scholar]