Abstract
CrossFit is high-intensity interval training involving routines called ‘workouts of the day’ (WOD). The aim of the present study is to analyse biochemical parameters and physical performance after two modalities of CrossFit WODs, and to evaluate 48-hour recovery. Twelve trained CrossFit practitioners (age: 30.4 ± 5.37 years; VO2max: 47.8 ± 3.63 ml/min/kg; 1RM Power Clean: 93.2 ± 7.62 kg) participated in the study. A crossover design was applied, and participants completed two modalities of WODs on separate days: WOD1 (as many rounds as possible) and WOD2 (rounds for time). Blood lactate, ratings of perceived exertion and heart rate were measured to determine the intensity of training sessions. Biochemical parameters and physical performance were evaluated before, immediately after, 24 hours after and 48 hours after exercise. There were significant differences in intensity between WOD1 and WOD2 (lactate: 13.3±1.87 vs. 18.38±2.02 mmol/L, heart rate mean: 127.6±11.1 vs. 159.8±12.1 bpm), and blood glucose concentrations were significantly higher after WOD2 (135.4 ± 19.6 vs. 167.4±19.6 mg/dL). After exercise, WOD1 and WOD2 caused significant increases of hepatic transaminases, creatine phosphokinase and blood glucose, as well as a large decrease in the physical performance evaluated by the plank test. All these values returned to baseline by 48 hours after exercise. Both WODs caused metabolic and muscular stress, as well as a decrease in physical performance. All the levels recovered at 48 hours, so the stress caused by CrossFit WODs did not induce a pathological state.
Keywords: High-intensity training, Fatigue, Muscle damage, Heart rate, Blood analysis, Functional performance
INTRODUCTION
CrossFit can be defined as high-intensity functional training [1]. It is characterised by the performance of training sessions called ‘workouts of the day’ (WOD), which consist of a wide variety of exercises performed repeatedly, quickly, and with limited or no recovery time between rounds. In general, typical workouts consist of Olympic and power lifting, gymnastic, strongman, plyometric and calisthenic exercises [2]. These workouts can be performed for a best time (‘rounds for time’, or RFT) or to achieve the highest number of repetitions without checking the time (‘as many rounds as possible’, or AMRAP) [3].
CrossFit, like other high-intensity training modalities, has elicited improvements in body composition, cardiovascular responses and physical fitness [4]. However, other studies have concluded that this modality of high-intensity functional training creates a high risk of musculoskeletal injury [5] and overreaching [6,7], particularly if performed consistently. In addition, several cases of exercise-induced rhabdomyolysis have been described after CrossFit workouts [8].
CrossFit workouts require the performance of exercises at a high level of technique and power without long recovery periods, leading to overload situations and considerable fatigue [5]. However, although fatigue caused by CrossFit workouts could lead to injury in subsequent sessions, there is very little research that has studied acute responses or recovery from this modality of training session. Drum et al. [9] point out that a CrossFit workout is more strenuous, causing greater fatigue and muscle pain, than a training session based on the American College of Sport Medicine recommendations. Previous research has elicited an acute blood oxidative stress response and an increase in indirect blood markers of muscle damage (interleukin-6 and CK) after a CrossFit bout [10,11]. Moreover, high blood lactate concentrations (ranging between 11 and 15 mmol/L) have been recorded after different CrossFit workouts [12,13]. The magnitude of the physiological responses could depend on the CrossFit workout performed, since the exercise routines differ in intensity, duration, number of exercises and inclusion of rest periods. WODs have different psycho-physiological demands; some of them (e.g., “Fran” and “15.5”) can be identified as high-intensity whereas others (e.g., “CrossFit triplet”) can be considered moderate [1]. A questionnaire completed by 101 CrossFit participants revealed that they consider the hardest workouts to be ‘Fran’, ‘Murph’, ‘Fight Gone Bad’, ‘Helen’ and ‘Filthy’ [9]. In this sense, Fernandez-Fernandez et al. [3] observed that ‘Fran’ resulted in significantly longer time spent above 1 on the respiratory exchange ratio than the ‘Cindy’ routine. Another study concluded that a gymnastic WOD, consisting of completing the highest number of sets of five pull-ups, 10 push-ups and 15 air squats in 20 minutes, caused greater levels of blood lactate than a WOD with eight sets of skipping rope jumps x 20 s/10 s rest [13]. However, training sessions of different duration (shorter: ~ 4 min and longer: 17 min) could induce similar cardiovascular responses with a high metabolic impact [14].
On the other hand, along with these physiological and metabolic responses, significant reductions were produced in jump height performance and average power after a CrossFit routine consisting of gymnastic exercises [13]. However, Tibana et al. [6] reported a decrease in anti-inflammatory cytokines without decrements in muscle power after 24 hours of CrossFit workout, although rest intervals between sets and exercises were allowed during the training. For all that, and given the training paradigm of WODs, it is difficult to evaluate physical performance in CrossFit using traditional measures of aerobic and anaerobic power and capacity [15,16].
Assessing the physiological stress and fatigue produced by a CrossFit workout, as well as detecting whether the individual is recovered before starting a new training session, is necessary to avoid injuries and overtraining situations. Despite the aforementioned studies, there is a lack of information on the kinetics of biochemical parameters and their recovery over time after different modalities of WODs. Therefore, the aims of the present study are to analyse biochemical parameters and physical performance after two modalities of CrossFit WODs (AMRAP and RFT) and to evaluate recovery over time (24 and 48 hours after both WODs).
MATERIALS AND METHODS
Participants
Twelve trained men participated voluntarily in the study and they were recruited from a CrossFit training centre. Characteristics of participants are shown in Table I. All of them fulfilled the following criteria for inclusion: no cardiovascular, metabolic or neurological diseases, no injuries in the last three months, at least one year of experience in CrossFit training with two days of training a week, and a relative strength greater than 1.2 (with respect to bodyweight) in the maximum repetition (1RM) of Power Clean. It was also required that participants did not perform any type of strenuous physical exercise or ingest any type of stimulating substance or alcohol in the 48 hours prior to the session and maintained their habitual lifestyle and normal dietary intake during the study. Before the study, all participants were informed about the protocol and potential risks and signed informed written consent. This study was developed following the guidelines of the Declaration of Helsinki and was conducted following approval from the Committee of Biomedical Ethics of the University of Extremadura.
TABLE I.
Characteristics of participants.
| Variable | Mean ± SD |
|---|---|
| Age (years) | 30.4 ±5.37 |
| Weight (kg) | 75.92 ± 7.50 |
| Height (m) | 1.74 ± 0.04 |
| Body Mass Index | 25.02 ± 2.23 |
| Σ Skinfolds (mm) | 46.3 ± 15.96 |
| Lean Body Mass (kg) | 68.15 ± 6.91 |
| Fat Body Mass (kg) VO2max (ml/min/kg) HRmax (bpm) |
6.13 ± 1.96 47.8 ± 3.63 178 ± 15.28 |
| 1RM Power Clean (kg) | 93.2 ± 7.62 |
Experimental design
All participants performed two different modalities of CrossFit WODs in a randomised and balanced order, separated by 72 hours. Both sessions were performed at the same time of day (± 1h) with similar conditions of temperature and humidity (20–23°C and 40–45.0% respectively). Two weeks before the intervention, the researchers attended the CrossFit training centre to recruit the participants. The protocol of the investigation was explained to all the participants. The movement technique of the participants was checked and it was verified that they were familiar with the exercises foreseen in the WODs (Burpees, Toes to Bar, Wall Ball and Power Clean). After that, the maximum load lifted (1RM) in the Power Clean was recorded, following the protocol developed by Faigenbaum et al. [17].
Both WODs were carried out at the facilities of the CrossFit training centre with the equipment that the participants used regularly in their training sessions. Before the WODs, a warm-up was performed consisting of five minutes of low intensity running followed by five minutes of joint mobility. WOD1 (AMRAP) consisting of as many rounds as possible of Burpees and Toes to Bar increasing repetitions (1–1, 2–2, 3–3…) in five minutes. Each round had to be properly executed according to minimum exercise standards to continue onto the next round. The number of repetitions performed in WOD1 was 91.36 ± 13.89. WOD2 (RFT) consisting of three rounds of 20 repetitions of Wall Ball (9 kg) and 20 repetitions of Power Clean (a load of 40% 1RM) in the shortest possible time. All repetitions of the first exercise had to be completed before beginning the next exercise. No rests were scheduled or required between rounds. The total time to perform WOD2 was 534 ± 58.96 s.
Outcome measures
Anthropometric measurements. Measurements were taken at the first visit of participants to the laboratory. Body mass and height were measured using a portable stadiometer (Seca 213, Germany) and body mass index (BMI) was calculated from the ratio of weight/height2 (kg/m2). Subcutaneous fat skinfolds (triceps, subscapular, abdomen, suprailiac, thigh and leg) were measured on the left side of the body using a skinfold caliper (Harpenden, West Sussex, UK), following the recommendations of the International Society for the Advancement of Kinanthropometry [18]. These measurements were always made by the same researcher.
1RM Power Clean test. The test was carried out in the CrossFit training centre two weeks before the intervention. Prior to testing, all participants performed a 5-minute warm-up session which included dynamic movement activities for the joints. The maximum repetition of Power Clean was recorded as the maximum load that could be lifted using proper exercise technique for one repetition. Before attempting a 1 RM, participants performed a progressive series of five submaximal sets of 1 to 2 reps with moderate to heavy loads (50–90% of the estimated 1 RM). If a weight was lifted with proper form during a 1 RM trial, the subsequent 1 RM weight attempt was increased by approximately 2.5 to 7 kg and the participant attempted another 1 RM trial following 3 minutes of rest. Each 1 RM was determined within 3 to 5 trials.
Maximal incremental test. The test was carried out after the anthropometric assessment made at the first visit to the laboratory (72 h prior to the training sessions). A maximal incremental test on a cycle ergometer (Ergoselect 100, Ergoline GmbH, Germany) was performed. After a 5 min warm-up at 50 W and 1 min of rest, participants started cycling at 75 W and the work rate was increased 25 W every 2 min until exhaustion. During the test, heart rate was recorded using a pulsometer (Polar Z9, Kempele, Finland) and maximum oxygen uptake (VO2max) was measured with a gas analyser (Metalyzer 3b, CORTEX Biophysik GmbH, Germany). The measurement finished when participants were unable to maintain the minimal required pedalling frequency (i.e. 60 rpm), with a plateau in VO2 despite an increase in work rate and a respiratory exchange ratio above 1.15.
Blood lactate. At the end of each WOD, lactate concentration was measured with a fast and reliable portable analyser (Lactate Scout+, SensLab GmbH, Germany) that uses an enzymatic-amperometric detection method and only requires 0.5 μL of blood.
Ratings of perceived exertion (RPE). RPE was assessed using the OMNI-Resistance Exercise Scale (OMNI-RES), a validated RPE for resistance exercise [19]. The OMNI-RES consisted of 10 reporting options between 1 (extremely easy) and 10 (extremely hard). Thirty minutes after finishing the protocols, a copy of this scale was given to the participants, who rated how hard the whole training session had been.
Heart rate. During training sessions, participants were monitored using a pulsometer (Polar Z9, Kempele, Finland). Beats per minute (bpm) were recorded throughout the session. Maximum heart rate (HRmax), mean heart rate (HRmean) and time spent in each intensity zone (calculated according to% HRmax recorded in the initial maximal incremental test) were defined after each WOD.
Physical performance tests. Countermovement jumps (CMJs) and plank tests were performed before warm-up and after exercise (5 min after the end of the training sessions) and again 24 and 48 hours after both WODs. First, the CMJ was performed on a portable contact platform (Chronojump, Boscosystem, Spain) where jump height data were instantaneously recorded using the free software distributed by the manufacturer (Software Chronojump V1.8.0, Boscosystem, Spain). Participants performed two maximal CMJs with 30 seconds’ rest between them, and the average jump height was recorded. Participants began in a stationary upright position. On command, participants flexed their knees and jumped as high as possible while keeping their hands on their waist before landing with both feet. Secondly, the plank test was performed to evaluate the endurance of the core stabilising muscles. Participants started with the upper body supported off the ground by the elbows and forearms, and the legs straight with the weight taken by the toes. The hip was lifted off the floor, creating a straight line from head to toe. The test was over when the individual was not able to hold the back straight and the hip was lowered. The score was the total time completed.
Biochemical parameters. Blood samples were taken before and after exercise, and 24 and 48 hours after both WODs. Participants attended the research laboratory after a minimum of eight hours’ overnight fasting for measurements. Participants could have breakfast after the blood draw, at least two hours before the beginning of the training session. Blood samples (5 ml) were taken from the antecubital vein by one experienced nurse and included the determination of blood urea nitrogen (BUN), total bilirubin (TBIL), transaminase GOT, transaminase GPT, lactate dehydrogenase (LDH), creatine phosphokinase (CPK) and glucose (GLU). One hundred uL of blood was collected in heparinised microwells and centrifuged for five minutes at 6000 rpm (MC6 centrifuge, Sarstedt, Nümbrecht, Germany). The analysis of samples was performed with an automatic dry-chemistry analyser system (Spotchem EZ SP-4430; Arkray, Inc., Kyoto, Japan). The calibration was checked daily through indicated reagent cards, according to the manufacturer’s recommendations.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM, Armonk, New York, USA). The Shapiro-Wilk test was applied in order to verify a normal distribution of data, and Levene’s test was used to assess the homogeneity of variance. An ANOVA with repeated measures and a Bonferroni post hoc test were conducted to analyse differences between WODs over time. A one way ANOVA was performed to analyse differences in blood lactate, RPE, HRmax and HRmean between WODs. The effect size (d) [20] was estimated for all variables using partial eta squared (η2). The magnitude of the difference was considered small (0.02), medium (0.1), or large (0.25). The significance level was set at p ≤ 0.05, with a confidence level of 95%. Means and standard deviations (SD) were used as descriptive statistics.
RESULTS
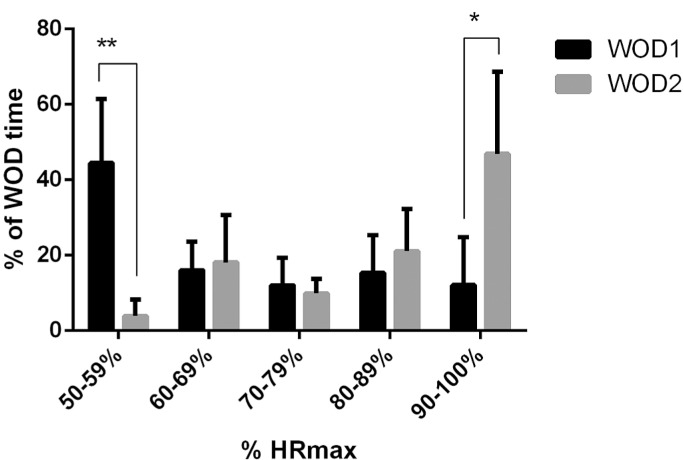
Table II shows the differences between WODs in the internal load parameters (blood lactate, RPE and HR). Compared to WOD1, values of blood lactate and HRmean are significantly greater in WOD2. Similar results, showing the percentage of WOD time that the participant spent in each intensity zone during both WODs, are observed in Figure 1. In WOD1, participants were 44.5 ± 16.9% of the WOD time in the intensity zone of 50–59% HRmax. During WOD2, participants spent most of the time (46.9 ± 21.8%) in the intensity zone of 90–100% HRmax.
TABLE II.
Parameters of internal load in both WODs.
| Variable | WOD1 | WOD2 | p | η2 |
|---|---|---|---|---|
| Lactate (mmol) | 13.3±1.87 | 18.38±2.02* | 0.003 | 0.680 |
| RPE (0-10) | 7.2±1.3 | 8.2±0.4 | 0.143 | 0.248 |
| HRmax (bpm) | 177.8±11.2 | 184.2±8.6 | 0.341 | 0.341 |
| HRmean (bpm) | 127.6±11.1 | 159.8±12.1* | 0.002 | 0.705 |
Significant difference between WOD1 and WOD2.
η2: Effect size: Partial Eta Square
FIG. 1.
Time spent in each intensity zone during WODs.
The time course of biochemical parameters and physical performance values after both WODs are shown in Table III. GOT (+58%), GPT (+109%), CPK (+21%) and glucose (+37%) concentrations presented a statistically significant increase after WOD1 compared to baseline, and there was a significant decrease (-34%) in the plank test results. At 24 hours, the difference from baseline remained significant only in the values of CPK and the plank test. 48 hours after finishing WOD1, all values had returned to baseline. Regarding WOD2, significant increases of TBIL (+24%), GOT (+ 58%), GPT (+122%), CPK (+22%) and glucose (+71%) were observed after the training. With the exception of blood glucose, these values remained significantly elevated over the next 24 hours, although they returned to baseline by 48 hours after exercise. Additionally, performance in the plank test decreased significantly after WOD2 (-45%) and remained decreased at 24 hours after exercise (-23%). A significant difference in glucose concentrations was observed between WODs after the sessions (135.4 ± 19.6 vs. 167.4 ± 19.6), but no other difference was observed between WODs at any point in time.
TABLE III.
Time-course analysis of biochemical parameters and physical performance after both WODs.
| Baseline (A) | Post (B) | Δ (A-B) | 24h. (C) | Δ (A-C) | 48h. (D) | Δ (A-D) | Effect size | ANOVA, p-values | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | % | Mean ± SD | % | Mean ± SD | % | ƞ2 | time | time x WOD | ||
| BUN (mg/dL) | WOD 1 | 17.6 ± 4.3 | 17.8 ± 4.0 | 1.1 | 17.2 ± 5.0 | -2.2 | 16.8 ± 3.8 | -4.5 | 0.237 | 0.734 | 0.387 |
| WOD 2 | 20.8 ± 5.4 | 20.5 ± 4.4 | -1.4 | 18.2 ± 1.6 | -12.5 | 20.6 ± 3.6 | -0.9 | 0.453 | |||
| TBIL (mg/dL) | WOD 1 | 0.58 ± 0.2 | 0.68 ± 0.3 | +17.2 | 0.54 ± 0.2 | -6.8 | 0.56 ± 0.19 | -3.44 | 0.389 | 0.287 | 0.179 |
| WOD 2 | 0.50 ± 0.2 | 0.62 ± 0.1* | +24.0 | 0.70 ± 0.2* | +40.0 | 0.60 ± 0.2 | +20.0 | 0.048 | |||
| GOT (IU/L) | WOD 1 | 33.6 ± 14.3 | 53.2 ± 11.5* | +58.6 | 37.4 ± 8.6 | +11.3 | 27.6 ± 8.8 | -17.8 | 0.372 | 0.000 | 0.196 |
| WOD 2 | 29.4 ± 6.0 | 46.6 ± 7.2* | +58.5 | 36.4 ± 11.0* | +23.8 | 28.8 ± 8.6 | -2.0 | 0.000 | |||
| GPT (IU/L) | WOD 1 | 31.6 ± 14.7 | 66.4 ± 13.8* | +109 | 34 ± 11.4 | +9.4 | 29.2 ± 13.5 | -7.5 | 0.089 | 0.000 | 0.721 |
| WOD 2 | 28.6 ± 8.8 | 63.6 ± 11.8* | +122 | 33.8 ± 9.0* | +18 | 29 ± 9.2 | +1.4 | 0.000 | |||
| LDH (IU/L) | WOD 1 | 276.4 ± 57.1 | 308.6 ± 48.6 | +11.5 | 324 ± 61.6 | +17.3 | 229 ± 30.5 | +17.0 | 0.256 | 0.546 | 0.356 |
| WOD 2 | 260.2 ± 26.8 | 248.6 ± 26.9 | -4.4 | 288.6 ± 61.5 | +10.9 | 252.4 ± 27.9 | -3.0 | 0.181 | |||
| CPK (IU/L) | WOD 1 | 566.4 ± 159.1 | 689.6 ± 281.9* | +21.7 | 864.0 ± 369.5* | +52.6 | 485.4 ± 180.4 | -14.3 | 0.340 | 0.023 | 0.233 |
| WOD 2 | 406.8 ± 201 | 492.2 ± 203.8* | +22.7 | 673.8 ± 444.1* | +65.6 | 410.6 ± 221.3 | +1.0 | 0.000 | |||
| GLU (mg/dL) | WOD 1 | 98.2 ± 11.1 | 135.4 ± 19.6* | +37.4 | 95.6 ± 5.7 | -2.6 | 95.4 ± 9.8 | -2.8 | 0.451 | 0.021 | 0.033 |
| WOD 2 | 97.6 ± 10.3 | 167.4 ± 19.6*† | +71.5 | 85.8 ± 15.2 | -12.0 | 94.7 ± 9.8 | -3.0 | 0.000 | |||
| CMJ (cm) | WOD 1 | 42.4 ± 6.3 | 45.3 ± 4.7 | +6.8 | 40.3 ± 5 | -4.9 | 41.5 ± 5.1 | -2.1 | 0.124 | 0.108 | 0.628 |
| WOD 2 | 42.2 ± 7.7 | 44.1 ± 6 | +4.5 | 41.1 ± 6 | -2.6 | 41.7 ± 5.8 | -1.1 | 0.232 | |||
| Plank (s) | WOD 1 | 180 ± 19.3 | 117.4 ± 20.5* | -34.70% | 130 ± 27.5* | -27.7 | 166.8 ± 20.6 | -7.2 | 0.26 | 0.016 | 0.349 |
| WOD 2 | 177.8 ± 13.6 | 96.4 ± 17.7* | -45% | 135 ± 22.2* | -23.7 | 161.2 ± 15.5 | -9.0 | 0.010 | |||
Indicates differences with respect to baseline (post hoc t-test with Bonferroni correction).
Indicates differences between groups.
DISCUSSION
The aims of the present study were to analyse biochemical parameters and physical performance after two modalities of CrossFit WODs (AMRAP and RFT) and to evaluate 48-hour recovery. Both WODs caused changes in the biochemical parameters (e.g. GOT, GPT, CPK, glucose) and in physical performance (plank time), but these variations returned to baseline within 48 hours after finishing the training sessions. Nonetheless, the WOD that focused on performing the proposed rounds in the shortest possible time showed significantly higher values of HRmean, lactate and glucose than the AMRAP WOD after training sessions.
These results confirm that CrossFit is a fitness activity of moderate-high intensity. Other studies have shown that major physiological and metabolic responses (heart rate > 90–95% HRmax and blood lactate > 14 mmol/L) and perceptual levels (RPE > 8) were achieved after a CrossFit WOD [1,3]. In this sense, the high initial levels of LDH and CPK (above the normal reference values) could indicate that the participants in the study had a certain level of fatigue and muscle damage [21] caused by the usual practice of high intensity CrossFit sessions.
Both WODs elicited significant changes in the biochemical parameters after the training sessions, as a result of the effort performed. These results are supported by other investigations that studied oxidative stress [10] or inflammatory [6] and cardiometabolic [22] responses after different modalities of CrossFit WODs. However, to the best of our knowledge, the present study is the first to analyse the time-course analysis (from before to 48 h after exercise) of biomarkers of fatigue, protein catabolism, muscular damage, hepatic enzymes and metabolism. The concentration of hepatic transaminases (GOT and GPT) was analysed to assess the liver overload during the effort. In both WODs, there was a significant increase after exercise; these levels remained high at 24 hours only after WOD2 (higher intensity than WOD1). The accelerated metabolic demands of the muscle exercise cannot be met without a robust response from the liver [23], so it is logical to observe an increase in liver enzymes. There are even studies that claim that intensive muscular exercise (e.g. weightlifting) can increase the liver function for at least seven days after the exercise [24]. That is not what happened in our study, where the values of hepatic transaminases returned to baseline at 48 hours after exercise. An increase in total bilirubin, which remained elevated until 24 hours after exercise, was observed only after WOD2. Bilirubin can be elevated because of haemolysis, which is typical of high-intensity exercise, although these values also returned to baseline at 48 hours, indicating that there was no continuous intravascular haemolysis [21]. In addition, after both CrossFit WODs, an increase in blood concentrations of CPK and LDH (without achieving statistical significance in this last parameter) was observed, which peaked at 24 hours and returned to baseline at 48 hours after exercise. These parameters can provide information about metabolism and muscle damage, and their values increase during intense muscular exercise [25]. This rise is in line with the results of previous studies, in which similar increases in muscle damage markers were observed after performing high-intensity resistance training [26,27]. Therefore, the results of the present study would suggest that muscle damage has occurred after CrossFit sessions, although without reaching the extreme values of CPK that occur associated with exercise-induced rhabdomyolysis caused by a CrossFit session [8]. With regards to blood glucose, which is the main metabolic substrate used at the muscular level during intense exercise, a significant increase was observed after both WODs. Similar results were observed by Tibana et al. [6] after two different AMRAP WODs including power exercises and gymnastic movements over 10–12 minutes. In addition, previous studies have shown that high-intensity training (HIT) causes increases in blood glucose as a result of increased metabolic demand [28]. Subsequently, as might be expected in healthy people, blood glucose returned to baseline at 24 hours.
Regarding physical performance, significant decreases were observed in the time spent in the plank test, both immediately after and 24 hours after finishing both WODs. The core muscles are the centre of most kinetic chains in the body and have a significant effect on the transfer forces to the extremities [29], so both training sessions that included functional body exercises certainly caused fatigue in this muscle zone. This fatigue had a negative effect on the plank test results, since the stability and activation of the central muscles of the body are essential to the performance of this test [30]. The test values returned to baseline at 48 hours, which would indicate that the participants were already recovered. However, no significant decrease was observed in the performance of CMJ. The type of exercise and the movement velocity during the WODs seem to play an important role in the muscle fatigue of knees. In this sense, Maté-Muñoz et al. [22] observed a decrease in performance in the CMJ after the ‘Cindy’ WOD (five pull-ups, 10 push-ups and 15 air squats in 20 min), but they did not find any significant difference after another WOD consisting of double skip rope (eight sets of 20 s with 10 s of rest between sets). On the other hand, the measurement of CMJ was made before the warm-up of the training session, so the participants may not have obtained their best mark in the initial test.
Finally, WOD1 showed lower levels of blood lactate, RPE, HRmax and HRmean than WOD 2. Moreover, participants trained longer at moderate intensity (50–59% HRmax), but they spent more time at higher intensity (90–100% HRmax) during WOD2. This difference of intensity between sessions caused significantly higher blood glucose concentrations after WOD2, because the metabolism of carbohydrates was more involved and a greater amount of glucose was required at the muscular level [28]. The differences between sessions could be explained by the total duration of WOD2 being greater than that of WOD1. In addition, participants trained with external loads during WOD2, while in WOD1 the exercises were always carried out with body weight. Similar results were observed by Tibana et al. [31], who found higher metabolic, cardiovascular, and RPE responses after a protocol that had a weightlifting exercise (snatch) than after performing a protocol of similar duration only of cardiovascular exercises (rowing and burpees).
As limitations of the study, it was impossible to match the training load between both modalities of WODs (AMRAP and RFT). In addition, the study was conducted only with trained people and the results could vary depending on the training status, especially if it is considered that the sample size was not too large. In this sense, CrossFit is characterized by the variety of sessions and types of exercise, which is a motivating factor for its practitioners. However, the prescription of the training sessions should be based on rigorous monitoring of the training and proper balanced programming of WODS, based not only on the external load of the training (time, repetitions, kg, etc.), but also on the internal load (biological stress caused by the external load).
CONCLUSIONS
In conclusion, the intensity of effort exhibited during WOD2 (RFT) was greater than that exhibited during WOD1 (AMRAP). However, the performance of both CrossFit sessions caused significant changes in liver transaminases, markers of muscle damage and metabolism, and a decrease in physical performance. All the levels recovered and returned to baseline at 48 hours; therefore, the metabolic and muscular stress caused by both WODs was not so high as to induce a pathological state. These results could help trainers and CrossFit practitioners to control and plan their training properly so as to avoid situations of overreaching and chronic fatigue. Given the wide variety of WODs, exercise types and activities in CrossFit, further investigation is necessary for an in-depth understanding of the physiological, biochemical and performance changes that this fitness modality can cause.
Acknowledgment
This study has been supported by the Government of Extremadura with funding from the European Regional Development Fund under grant (Ref: GR18003).
Conflict of interest declaration
All authors declare no conflicts of interest.
REFERENCES
- 1.Claudino JG, Gabbett TJ, Bourgeois F, Souza HS, Miranda RC, Mezêncio B, et al. CrossFitCrossFit Overview: Systematic Review and Meta-analysis. Sports Med Open. 2018;4(1):11. doi: 10.1186/s40798-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BS, Dullabh M, Forbes G, Brandkamp JL, Shaw I. Analysis of physiological determinants during a single bout of CrossfitCrossFit. Int J Perform Anal Sport. 2015;15(3):809–15. [Google Scholar]
- 3.Fernandez-Fernandez J, Sabido-Solana R, Moya D, Manuel Sarabia J, Moya M. Acute physiological responses during CrossfitCrossFit workouts. Eur J Hum Mov. 2015;35:114–24. [Google Scholar]
- 4.Meyer J, Morrison J, Zuniga J. The Benefits and Risks of CrossFitCrossFit: A Systematic Review. Workplace Health Saf. 2017;65(12):612–8. doi: 10.1177/2165079916685568. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron MF, Nindl BC, Deuster PA, Baumgartner N, Kane SF, Kraemer WJ, et al. Consortium for Health and Military Performance and American College of Sports Medicine consensus paper on extreme conditioning programs in military personnel. Curr Sports Med Rep. 2011;10(6):383–9. doi: 10.1249/JSR.0b013e318237bf8a. [DOI] [PubMed] [Google Scholar]
- 6.Tibana RA, de Almeida LM, Frade de Sousa NM, Nascimento DaC, Neto IV, de Almeida JA, et al. Two Consecutive Days of CrossfitCrossFit Training Affects Pro and Anti-inflammatory Cytokines and Osteoprotegerin without Impairments in Muscle Power. Front Physiol. 2016;7:260. doi: 10.3389/fphys.2016.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake N, Smeed J, Carper M, Crawford D. Effects of short-term crossfitCrossFit training: A magnitud based-approach. J Exerc Physiol Online [Internet] 2017;20(2):111–33. [Google Scholar]
- 8.Meyer M, Sundaram S, Schafhalter-Zoppoth I. Exertional and CrossFitCrossFit-Induced Rhabdomyolysis. Clin J Sport Med. 2018;28(6):e92–e4. doi: 10.1097/JSM.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 9.Drum SN, Bellovary BN, Jensen RL, Moore MT, Donath L. Perceived demands and postexercise physical dysfunction in CrossFitCrossFit® compared to an ACSM based training session. J Sports Med Phys Fitness. 2017;57(5):604–9. doi: 10.23736/S0022-4707.16.06243-5. [DOI] [PubMed] [Google Scholar]
- 10.Kliszczewicz B, Quindry CJ, Blessing LD, Oliver DG, Esco RM, Taylor JK. Acute Exercise and Oxidative Stress: CrossFitCrossFit(™) vs. Treadmill Bout. J Hum Kinet. 2015;47:81–90. doi: 10.1515/hukin-2015-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heavens KR, Szivak TK, Hooper DR, Dunn-Lewis C, Comstock BA, Flanagan SD, et al. The effects of high intensity short rest resistance exercise on muscle damage markers in men and women. J Strength Cond Res. 2014;28(4):1041–9. doi: 10.1097/JSC.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 12.Perciavalle V, Marchetta NS, Giustiniani S, Borbone C, Petralia MC, Buscemi A, et al. Attentive processes, blood lactate and CrossFitCrossFit. Phys Sportsmed. 2016;44(4):403–6. doi: 10.1080/00913847.2016.1222852. [DOI] [PubMed] [Google Scholar]
- 13.Maté-Muñoz JL, Lougedo JH, Barba M, García-Fernández P, Garnacho-Castaño MV, Domínguez R. Muscular fatigue in response to different modalities of CrossFitCrossFit sessions. PLoS One. 2017;12(7):e0181855. doi: 10.1371/journal.pone.0181855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tibana R, De Sousa N, Prestes J, Voltarelli F. Lactate, heart rate and rating of perceived exertion responses to shorter and longer duration CrossfitCrossFit training sessions. J Funct Morphol Kinesiol [Internet] 2018;3(4):60. doi: 10.3390/jfmk3040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher SJ, Neyedly TJ, Horvey KJ, Benko CR. Do physiological measures predict selected CrossFitCrossFit(®) benchmark performance? Open Access J Sports Med. 2015;6:241–7. doi: 10.2147/OAJSM.S88265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellar D, Hatchett A, Judge LW, Breaux ME, Marcus L. The relationship of aerobic capacity, anaerobic peak power and experience to performance in CrossFitCrossFit exercise. Biol Sport. 2015;32(4):315–20. doi: 10.5604/20831862.1174771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faigenbaum AD, McFarland JE, Herman RE, Naclerio F, Ratamess NA, Kang J, et al. Reliability of the one-repetition-maximum power clean test in adolescent athletes. J Strength Cond Res. 2012;26(2):432–7. doi: 10.1519/JSC.0b013e318220db2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart A, Marfell-Jones M, Olds T, de Ridder H. International standards for anthropometric asessment. London: International Society for the Advancement of Kinanthropometry. 2011. p. 137. [Google Scholar]
- 19.Robertson RJ, Goss FL, Rutkowski J, Lenz B, Dixon C, Timmer J, et al. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc. 2003;35(2):333–41. doi: 10.1249/01.MSS.0000048831.15016.2A. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Banfi G, Colombini A, Lombardi G, Lubkowska A. Metabolic markers in sports medicine. Adv Clin Chem. 2012;56:1–54. doi: 10.1016/b978-0-12-394317-0.00015-7. [DOI] [PubMed] [Google Scholar]
- 22.Maté-Muñoz JL, Lougedo JH, Barba M, Cañuelo-Márquez AM, Guodemar-Pérez J, García-Fernández P, et al. Cardiometabolic and Muscular Fatigue Responses to Different CrossFitCrossFit® Workouts. J Sports Sci Med. 2018;17(4):668–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Trefts E, Williams AS, Wasserman DH. Exercise and the Regulation of Hepatic Metabolism. Prog Mol Biol Transl Sci. 2015;135:203–25. doi: 10.1016/bs.pmbts.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettersson J, Hindorf U, Persson P, Bengtsson T, Malmqvist U, Werkström V, et al. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol. 2008;65(2):253–9. doi: 10.1111/j.1365-2125.2007.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brancaccio P, Maffulli N, Buonauro R, Limongelli FM. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27(1):1–18. vii. doi: 10.1016/j.csm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Spada TC, Silva JMRD, Francisco LS, Marçal LJ, Antonangelo L, Zanetta DMT, et al. High intensity resistance training causes muscle damage and increases biomarkers of acute kidney injury in healthy individuals. PLoS One. 2018;13(11):e0205791. doi: 10.1371/journal.pone.0205791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartolomei S, Sadres E, Church DD, Arroyo E, Gordon JA, Varanoske AN, et al. Comparison of the recovery response from high-intensity and high-volume resistance exercise in trained men. Eur J Appl Physiol. 2017;117(7):1287–98. doi: 10.1007/s00421-017-3598-9. [DOI] [PubMed] [Google Scholar]
- 28.Adams OP. The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab Syndr Obes. 2013;6:113–22. doi: 10.2147/DMSO.S29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinkle J, Nesser TW, Demchak TJ, McMannus DM. Effect of core strength on the measure of power in the extremities. J Strength Cond Res. 2012;26(2):373–80. doi: 10.1519/JSC.0b013e31822600e5. [DOI] [PubMed] [Google Scholar]
- 30.Schellenberg KL, Lang JM, Chan KM, Burnham RS. A clinical tool for office assessment of lumbar spine stabilization endurance: prone and supine bridge maneuvers. Am J Phys Med Rehabil. 2007;86(5):380–6. doi: 10.1097/PHM.0b013e318032156a. [DOI] [PubMed] [Google Scholar]
- 31.Tibana RA, Almeida LM, DE Sousa Neto IV, DE Sousa NMF, DE Almeida JA, De Salles BF, et al. Extreme Conditioning Program Induced Acute Hypotensive Effects are Independent of the Exercise Session Intensity. Int J Exerc Sci. 2017;10(8):1165–73. [PMC free article] [PubMed] [Google Scholar]