Abstract
Olfactory bulb, as one of sensory organs opening to the outside, is susceptible to toxic environment and easy to deteriorate. Recent studies in Parkinson’s disease (PD) patients and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys have shown that abnormal α-synuclein is accumulated in the olfactory glomeruli, suggesting that the lesions of PD are not only confined to the substantia nigra (SN) but also located in the olfactory bulb. Thus, olfactory bulb might be the region of onset in PD pathogenesis and a targeted region for diagnosis and treatment of PD. However, the relationship between olfactory bulb and pathogenesis of PD remains unclear. In the present study, we investigated the inflammatory pathological alterations in olfactory bulb and the underlying mechanisms in chronic MPTP mice. Mice were treated with MPTP/P, i.e., MPTP (25 mg/kg, s.c.) plus probenecid (250 mg/kg, i.p.) every 4 days, for ten times. The mice displayed typical parkinsonian syndrome. Then we examined their olfactory function and the pathologic changes in olfactory bulb. The mice showed obvious olfactory dysfunction in a buried pellet test. Immunohistochemical studies revealed that tyrosine hydroxylase (TH) protein levels were significantly decreased, whereas abnormal α-synuclein was significantly increased in the olfactory bulbs. Furthermore, the olfactory bulbs in MPTP/P-treated mice showed significantly increased levels of interleukin-1β (IL-1β), caspase-1, glial fibrillary acidic protein (GFAP), Toll receptor 4 (TLR4), phosphorylation of p65, as well as activated molecules of NOD-like receptor protein 3 (NLRP3) that were associated with neuroinflammation. Our results demonstrate that MPTP/P-caused olfactory bulb damage might be related to NLRP3-mediated inflammation.
Keywords: Parkinson’s disease, olfactory bulb, α-synuclein, TLR4, NF-κB, NLRP3, neuroinflammation, MPTP, probenecid
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder with a wide variety of symptoms, including motor dysfunction and nonmotor symptoms [1]. The primary pathological alterations are the degeneration of dopaminergic neurons [2] and the aggregation of abnormal α-synuclein [3]; thus, PD is also called a proteinopathy [4]. Motor dysfunction includes bradykinesia, muscular rigidity and resting tremors [5], and nonmotor symptoms include hyposmia, constipation and other features [1]. The pathogenesis of PD remains elusive, but investigators have come to a consensus on the contribution of genetic and environmental factors to the mechanisms of PD [1]. Because nonmotor symptoms precede the behavioral disorders [1], it is necessary to investigate the mechanism underlying the nonmotor symptoms.
In addition to the loss of neurons in the SN, pathological changes also emerged in the olfactory bulb [6]. Given studies of PD patients and MPTP-induced monkeys, researchers have demonstrated that α-synuclein aggregation first appears in the olfactory bulb and vagus and then spreads to the central nervous system over decades [7]. Additionally, α-synuclein monomers and oligomers can transfer from olfactory bulb to other brain regions through neural pathways [8]. When MPTP and 6-hydroxydopamine (6-OHDA) were injected into the olfactory bulb in rabbits, dopamine (DA) neurons in the SN and striatum were lost, verifying the ability of external toxicants to accelerate PD pathogenesis and the dopaminergic projection relationship between the SN and olfactory bulb [9]. However, the mechanisms underlying olfactory bulb degeneration remain unknown.
Neuroinflammation is a common neuropathological finding in degenerative diseases. Studies have indicated that chronic mild inflammation contributes to degeneration of dopaminergic neurons in PD [10, 11] and can increase the production of reactive oxygen species (ROS), ultimately leading to neuronal death [12]. Many factors trigger neuroinflammation, such as ion channels, microRNAs, autophagy, and monocyte activation [13]. Therefore, it is essential to focus on the inflammatory process in the pathogenesis of PD. The NLRP3 inflammasome, closely related to IL-1β production, is a multiprotein complex that plays a key role in inflammation [14]. Hyposmia is an early symptom of PD, and neuroinflammation or neuron loss also appears in the olfactory bulb, but the molecular mechanism underlying these symptoms is still unknown. In this study, we investigated inflammation-related pathological alterations and the associated mechanisms in the olfactory bulb induced by chronic MPTP administration in mice.
Materials and methods
Materials
MPTP hydrochloride and probenecid were purchased from Sigma-Aldrich, USA. Antibodies were obtained from Santa Cruz, Abcam, Thermo and Wako and they are described in Table 1.
Table 1.
The information of the antibodies used in the present work
| Antibody | Source | Dilutions | Company |
|---|---|---|---|
| NF-κB/p65 | Mouse | 1:500 | Santa Cruz Biotechnology |
| TH | Rabbit | 1:500 | Santa Cruz Biotechnology |
| IL-1β | Rabbit | 1:500 | Santa Cruz Biotechnology |
| NLPR3 | Rabbit | 1:1000 | Abcam |
| Caspase-1 | Mouse | 1:1000 | Abcam |
| TLR4 | Rabbit | 1:500 | Santa Cruz Biotechnology |
| α-Synuclein(C20) nitrated | Mouse | 1:500 | Santa Cruz Biotechnology |
| Tyr125/Tyr133 α-synuclein | Mouse | 1:1000 | Thermo Fisher Scientific |
| p-p65 | Rabbit | 1:1000 | Cell Signaling Technology |
| β-actin | Mouse | 1:1000 | Sigma |
| GFAP | Mouse | 1:1000 | Wako |
Animals
Male C57BL/6 mice (8 weeks, 22–25 g) were ordered from Vital River Laboratory Animal Technology Co. Ltd, Beijing, China. The mice were raised in an experimental animal room and bred with an adequate supply of food and water. The room was managed by specific personnel to guarantee a 12 h light/dark cycle with a constant temperature of approximately 22 ± 1 °C.
Experimental procedures
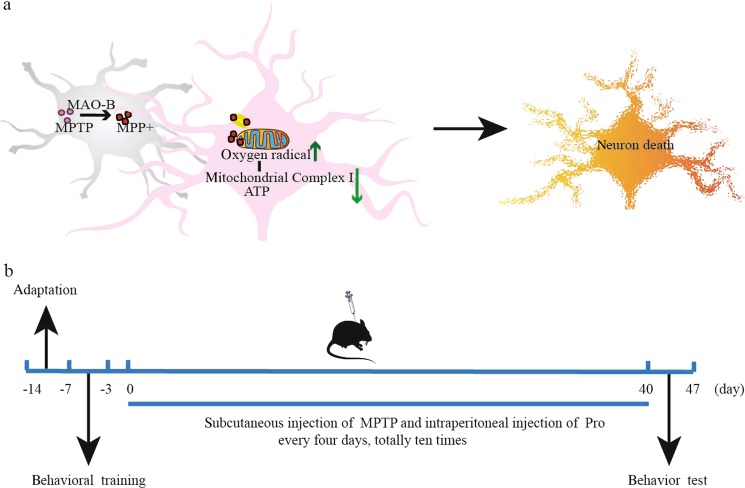
Mice were randomly divided into two groups after 7 days of adaptation: control and model groups. Probenecid was dissolved in 0.1 mol/L NaOH, and the pH adjusted to 7.4 with Tris-HCl (pH 6.8). MPTP-HCl was dissolved in 0.9% saline away from light and used immediately. Model mice were administered MPTP/P every 4 days, for a total of ten doses (Fig. 1b). The mice were given an intraperitoneal injection of probenecid (250 mg/kg) and a subcutaneous injection of MPTP-HCl (25 mg/kg) after 1 h. Control group mice were treated with equal volumes of 0.9% saline via subcutaneous injection.
Fig. 1.
Schematic showing the experimental design. a The neurotoxicity of MPTP triggers parkinsonism symptoms. b Schematic of the experimental procedure
Buried pellet test
This test was performed as described by Yan Liu et al. [15]. In a preliminary experiment, a buried pellet test was adopted to detect the smell sensations of mice. All mice were fasted for 16–18 h with normal access to drinking water before the test. Every mouse adapted itself to a clean storage cage (25 cm × 15 cm × 13 cm) for 5 min and then was transferred to a test box (46 cm × 23.5 cm × 20 cm) and allowed to adapt for 2 min. A piece of ordinary laboratory food (approximately 1.0 g) was buried at a depth of 1 cm below the bedding and placed on the walls of the box randomly. Each mouse was placed in the middle of the test box, and then, the time needed to find the food was recorded (the time was limited to 5 min).
Tissue preparation
Chloral hydrate was applied to deeply anesthetize the mice (400 mg/kg, i.p.). We quickly isolated the olfactory bulb and placed the tissues into a −80 °C freezer for biochemical analysis. For morphological observation, we perfused some of the anesthetized mice with 0.1 mol/L phosphate-buffered saline (PBS) and 4% paraformaldehyde (pH 7.4, dissolved with PBS) for histologic analysis. The olfactory bulb was kept in 4% paraformaldehyde for 24 h to fix tissues and then dehydrated in different ethanol concentrations (75%–85%–90%–95%–100%). Then, the olfactory bulb was embedded in paraffin, cut into slices (4 μm) and placed on glass slides.
Ultrastructural transmission electron microscopy (TEM)
Tissues were steeped in 4% paraformaldehyde and then cut into cubic sections approximately 1 mm3. After being fixed with 2.5% glutaraldehyde for 2 h, the sections were washed with 0.1 mol/L PBS and dehydrated on a gradient. Then, we embedded the sections inside of Epon resin and cut them into ultrathin slices. Finally, the sliced sections were doubly stained with uranyl acetate and lead citrate and observed with a transmission electron microscope (H-7650; Hitachi, Tokyo, Japan).
Immunohistochemistry (IHC) and immunofluorescence (IF)
Paraffin sections were placed successively into dimethylbenzene (100% I/II, 15 min) and different ethanol concentrations (100% I/II-90%–80%–70%–60%, 5 min) for dewaxing. Then, the sections were washed with double-distilled water for 10 min and PBS for 10 min. Next, sections were boiled in citrate buffer solution (0.01 mol/L) for antigen retrieval. When the sections cooled to room temperature, they were washed three times for 10 min in PBS and then permeabilized in 0.5% Triton X-100 for 10 min. Endogenous peroxidase activity was eliminated by treatment with 3% H2O2 for 10 min. After incubation with 5% bovine serum albumin (BSA) (dissolved in PBS) for 30 min to block nonspecific antigens, the sections were incubated with anti-TH (1:200), anti-nitrated Tyr125/Tyr133 α-synuclein (1:1000), or anti-GFAP (1:250) antibody overnight at 4 °C. For immunohistochemistry, sections were incubated with corresponding secondary antibodies for 2 h after being washed with PBST. After rinsing with PBST, the sections were stained with 3,3′-diamino-benzidine (DAB) and covered with slips and neutral glue. Finally, the slices were observed and imaged with an Olympus BA51 photomicroscope (Tokyo, Japan). For immunofluorescence, sections were incubated with Alexa Fluor-conjugated secondary antibodies and DAPI away from light for 2 h and then observed with a laser scanning confocal microscope (Leica TCS SP2).
Western blot analysis
Olfactory bulb tissues were homogenized with RIPA buffer containing protease inhibitor cocktail (Sigma, USA) and phosphatase inhibitors (Invitrogen, USA) and then centrifuged, and the supernatant was extracted. A BCA (bicinchoninic acid) kit was used for quantitative analysis of protein concentration. The olfactory protein extraction mixed with loading buffer was denatured in boiling water for 15 min. Equivalent amounts of protein from different groups were loaded and separated on SDS-PAGE gels and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). Then, the membranes were blocked with 3% BSA (dissolved in TBST buffer) and incubated overnight at 4 °C with the following primary antibodies: anti-β-actin (1:5000), anti-α-synuclein (C20) (1:500), anti-n-α-synuclein (1:1000), anti-TLR4 (1:250), anti-Nuclear factor kappa-light-chain-enhancer (NF-κB) (1:500), anti-phosphorylated-NF-κB (1:500), anti-NLRP3 (1:1000), anti-caspase-1 (1:1000), and anti-IL-1β (1:250). After being washed with TBST three times, the membranes were incubated with the corresponding secondary antibodies for 2 h, and then visualized with an enhanced chemiluminescence (ECL) plus system (Molecular Device, LMAX). The protein bands were quantitatively analyzed using Quantity One software (Toyobo, Japan).
Statistical analysis
GraphPad Prism 6.0 software (GraphPad, San Diego, CA, USA) has been widely adopted for statistical analysis. Data were analyzed via t-test analysis using an unpaired t-test and were displayed as the means ± standard error (SEM). Significance was considered at P < 0.05.
Results
Olfactory dysfunction was observed in mice injected with MPTP/Pro
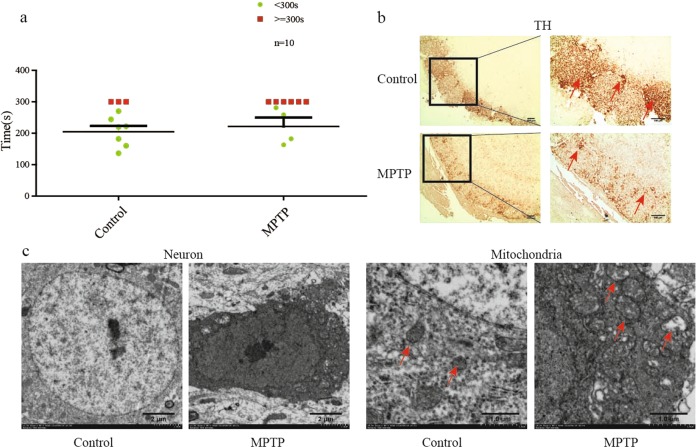
All mice performed the buried pellet test, and the number of mice missing food in the model group was twice that in the control group (Fig. 2a). TH is a key enzyme in dopamine production, and its level was significantly decreased in MPTP/Pro-treated mice based on IHC analysis (Fig. 2b). From ultrastructural observation, we found consolidation of neurons and damaged mitochondria (Fig. 2c). We confirmed cytomembrane disruption and chromatin condensation in MPTP-induced olfactory bulbs. Additionally, mitochondria had fewer cristae than observed in the normal group.
Fig. 2.
Mice treated with MPTP/Pro demonstrated olfactory dysfunction. a Mice in the group injected with MPTP/Pro exhibited remarkable olfactory dysfunction compared with control mice in behavior testing. b The TH protein level was decreased in MPTP/Pro-treated mice. c Damaged neurons and mitochondria appeared in the olfactory bulb based on transmission electron microscopy (TEM) observation. All data are expressed as the mean ± SEM. #P < 0.05 compared with the control group
Abnormal α-synuclein protein aggregation in olfactory bulbs from MPTP/Pro mice
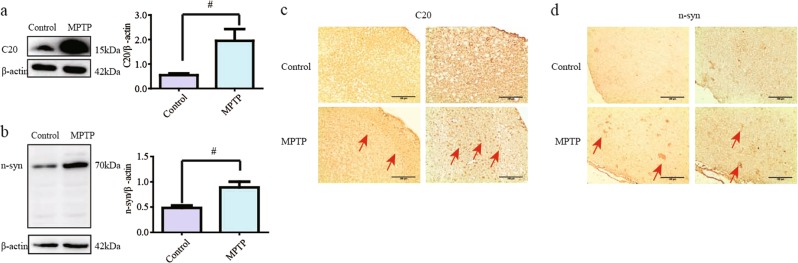
The α-synuclein protein level was assessed by staining with anti-α-synuclein (C20) antibody and was found to be increased in MPTP/p group mice (Fig. 3a, P < 0.05). The nitrated α-synuclein level was also significantly augmented after exposure to MPTP (Fig. 3b, P < 0.05). Similar alterations were demonstrated by the IHC results (Fig. 3c, d). Both C20- and nitrated α-synuclein-positive staining were mostly located at the edge of the olfactory bulb, specifically, in glomerular cells.
Fig. 3.
Abnormal α-synuclein appeared in the olfactory bulbs of mice treated with MPTP/Pro. a, b α-Synuclein protein levels were determined by immunoblotting with C20 and n-synuclein antibodies and were found to be enhanced in mice injected with MPTP/Pro compared with control group mice. c, d α-Synuclein was detected with C20 and n-synuclein antibodies in the olfactory bulb via immunohistochemistry. All data are expressed as the mean ± SEM. #P < 0.05 compared with the control group
Active inflammation-related protein expression was increased in the olfactory bulb medium after treatment with MPTP/Pro
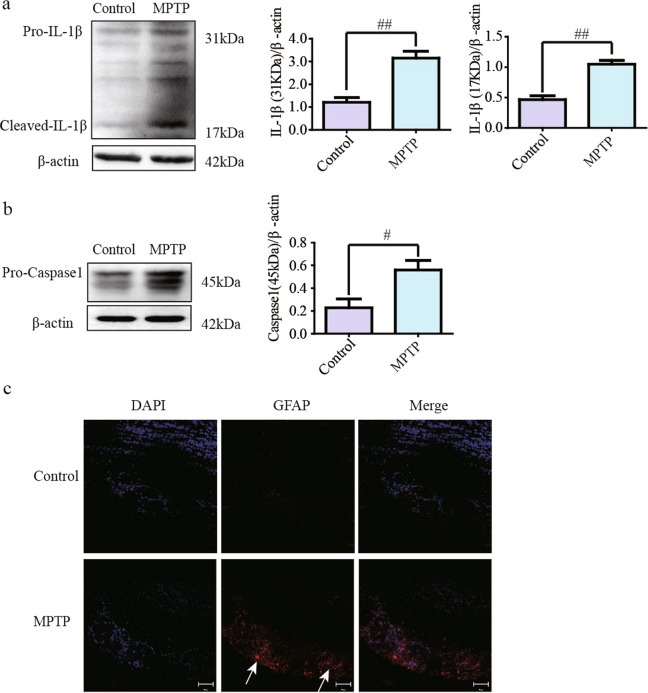
Results of western blotting showed that the protein levels of pro-IL-1β and cleaved-IL-1β were higher in olfactory bulbs from the MPTP/Pro group than those from the untreated group (Fig. 4a, P < 0.01). Caspase-1 cleaves pro-IL-1β, and thus, we detected the level of Caspase-1 via western blotting. The results showed that pro-Caspase-1 expression was increased in MPTP/Pro mice (Fig. 4b, P < 0.05), and the protein could be cleaved into active Caspase-1 to promote IL-1β generation. Moreover, sections were stained with GFAP antibody and imaged under a confocal microscope. The number of positively stained cells and the staining intensity of GFAP were much higher in the MPTP group than in the control group (Fig. 4c).
Fig. 4.
Inflammation-related protein levels in olfactory bulb medium. a The protein levels of pro-IL-1β and cleaved-IL-1β improved in mice injected with MPTP/Pro. b The pro-Caspase-1 protein level in the model group was higher than in the control group. c The fluorescence intensity of GFAP was much higher in olfactory glomeruli of the model group. All data are expressed as the mean ± SEM. #P < 0.05 compared with the control group
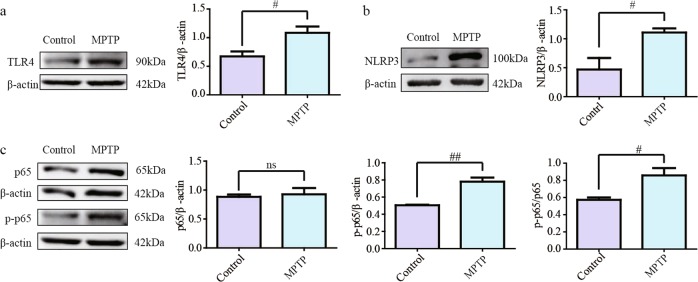
NF-κB-related signaling was activated in the olfactory bulb of MPTP/Pro-injected mice
TLR4 is closely associated with inflammation occurring in the central nervous system (CNS) of PD patients [16], and thus, we detected the protein level of TLR4. Western blotting results showed that the TLR4 level was remarkably increased in olfactory bulbs after treatment with MPTP (Fig. 5a, P < 0.05). NLRP3, an inflammasome that regulates Caspase-1 activation, was induced by MPTP (Fig. 5b, P < 0.05). NF-κB is a downstream signaling molecule of TLR4. Its subunit p65 relocates to the nucleus and is phosphorylated to promote the expression of target genes. The results demonstrated that the level of phosphorylated p65 was increased after mice were exposed to MPTP/Pro (Fig. 5c, P < 0.05 for p-p65 and P < 0.01 for p-p65/p65).
Fig. 5.
Alterations in TLR4-related inflammation protein molecule expression in the olfactory bulb. a, b The protein levels of TLR4 and NLRP3 improved in MPTP/Pro mice. c The activated NF-κB protein level was increased in the model group compared with the control group. All data are expressed as the mean ± SEM. #P < 0.05 compared with the control group
Discussion
As a complicated neurodegenerative disease, PD is gradually observed with high incidence in younger patients. However, there is not a clear definition of PD [1] because of its extensive pathogenesis. All the pathologic changes can gradually damage dopaminergic neurons and trigger a series of nonmotor and motor clinical symptoms. Motor symptoms appear in the advanced stage of PD, when approximately 60% dopaminergic neuron loss has occurred [17], and these symptoms are markers for clinical diagnosis [1]. In the early stage of PD, a variety of nonmotor symptoms related to the CNS and peripheral nervous system (PNS), such as anxiety [18], depression [19], and sleep disorders [20], may influence patients earlier and reduce quality of life. Additionally, olfactory dysfunction occurs in a vast majority of patients [21] and may be associated with the spread of α-synuclein in PD progression [22, 23]. Braak H and colleagues proposed that the olfactory bulb is one of the primary regions of Lewy pathology, which spreads to other brain regions [3]. In addition, the Lewy bodies in the olfactory bulb have been confirmed to have high sensitivity and specificity for early diagnosis of PD [24]. Therefore, it is necessary to focus on the pathology changes in the olfactory bulb to determine the PD mechanism.
In our study, mice were given subcutaneous injections of MPTP to mimic the lesions observed in PD patients. Previous studies have reported that environmental toxicants can induce PD and MPTP is an appropriate chemical substance used to construct a PD animal model [25]. MPTP penetrates the BBB (blood brain barrier) of mice and metabolizes to MPP+, the active ingredient of toxicity in astrocytes. Then, MPP+ is assimilated by dopaminergic neurons and has a toxic effect on mitochondria, causing oxidative stress and neuron death [26]. The analysis of olfactory bulb pathologic changes showed a decreased protein level of TH (Fig. 6), which essentially reflects the same change in the midbrain of PD patients.
Fig. 6.

Summary of our experiments. External environmental stimulation, such as with MPTP, triggers olfactory dysfunction, abnormal α-synuclein aggregation, and inflammation. The NLRP3 inflammasome pathway is involved in the pathological alterations in the olfactory bulb induced by MPTP/Pro
Additionally, toxic α-synuclein, including nitrated a-synuclein and abnormal monomeric a-synuclein, were detected by western blotting and IHC in MPTP-induced mice. Alpha-synuclein is the key protein in PD pathological changes. Normal α-synuclein, including monomers [27], exists in healthy people, while abnormal α-synuclein rich in Lewy bodies [28] appears in different brain regions and other areas of the peripheral nervous system in PD patients [1]. The toxic α-synuclein forms may be oligomers and fibrils, aggregated from unfolded monomers [29]. Furthermore, abnormal modifications of α-synuclein, such as nitrated α-synuclein, can also lead to neurotoxicity [30]. There are many toxic effects of abnormal α-synuclein, including nuclear dysfunction, mitochondrial dysfunction, and synaptic dysfunction. As a result of α-synuclein toxicity, neurons rupture and release the toxic α-synuclein, which triggers inflammation in the olfactory bulb. Furthermore, α-synuclein could spread via prion-like propagation [31] based on its construction and other agents. It is reasonable to propose that α-synuclein could propagate from the olfactory bulb to the midbrain and other critical brain regions.
Multiple factors, including oxidative stress and environmental toxicity, contribute to neuroinflammation. In our study, we verified the appearance of increased IL-1β and GFAP in the olfactory bulb, which suggested that MPTP caused inflammation. Neuroinflammation in PD is a phenomenon associated with activated astrocytes and microglia, and it might be the prevalent cause of neuronal damage [32]. In an epidemiological investigation, investigators have found that nonsteroidal anti-inflammatory drugs (NSAIDs) can decrease the morbidity rate of PD in an epidemiological investigation [33], which reminded us of the importance of neuroinflammation. TLR4/NF-κB is a classical inflammatory pathway and is involved in neuroinflammation in the SN [16]. TLR4, one of pathogen recognition receptors, influences the transcription factor NF-κB and plays a key role in chronic diseases [34]. Moreover, various molecules participate in the network of inflammation mediated by TLR4 [34]. TLR4, MyD88, Toll-interleukin-1 receptor-domain-containing adaptor protein (TIRAP), TIR-domain-containing adapter-inducing interferon-β (TRIF) and translocation-associated membrane protein (TRAM) receive extracellular signals jointly [35, 36]. Then, NF-κB in the cytoplasm accepts the signal transduction and translocates to the nucleus, stimulating inflammation through four different signaling pathways [37–41]. NLRP3, a type of inflammasome, can mediate host immune responses [42] and is involved in the neuroinflammation in the SN and thymus induced by MPTP [16, 43]. NLRP3 contributes to the cleavage of procaspase-1 into active caspase-1 and then increases the active IL-1β level [44, 45]. The results of our study indicate that the toxicity occurred not only in the SN [16] but also in the olfactory bulb. TLR4 recognizes extracellular signs of inflammation and then transmits the signal to NF-κB. Nuclear translocation of active NF-κB increases and stimulates the release of pro-IL-1β. Furthermore, NLRP3 activated by MPTP contributes to the increased IL-1β level (Fig. 6), which triggers obvious neuroinflammation and ultimately DA neuron damage.
However, it is uncertain whether we should put an emphasis on NLRP3 inflammasome activation in PD or other neurodegenerative diseases. After all, many factors can stimulate the NLRP3 inflammasome. Investigators have found that prion-protein-like α-synuclein fibrils can activate NLRP3 [46, 47] and increase its mRNA level [48]. As an endogenous disease-related signal, aggregated α-synuclein stimulates microglia to release pro-inflammatory cytokines [49, 50] and has an influence on the NLRP3 inflammasome [51]. Through cellular endocytic pathways [52], aggregated α-synuclein is encapsulated into intracellular lysosomes and triggers cathepsin B [53], which induces NLRP3 inflammasome activation [48]. Regarding cellular location, the NLRP3 inflammasome is typically detected in monocytes and microglia in the CNS [54]. Previous studies have demonstrated that NLRP3 inflammasome activation is involved in microglia and human monocyte activation in vitro [48, 55]. Interestingly, in recent years, researchers have found that the NLRP3 inflammasome also appears abnormally in neurons and triggers neuroinflammation. A paper published in NPJ Parkinson’s Disease showed that NLRP3 expression increased in DA neurons and induced inflammation in mesencephalon tissues from PD patients and neuronal cultures [56]. This suggests to us that the NLRP3-inflammasome-mediated inflammatory response has an intimate relationship with α-synuclein [48] and is significant in the pathology of PD.
Therefore, we can draw the conclusion that the NLRP3 inflammasome plays a key role in the inflammation that occurs during PD development. Researchers have completed some significant experiments related to NLRP3 and demonstrated that NLRP3 inhibition has positive effects on remission of PD. For example, one study indicated that absence of NLRP3 slowed symptom progression in PD induced by rotenone in mice [57]. In a PD model induced by acute treatment with MPTP, low expression of NLRP3 also benefited a nigral cell model [58]. A new study showed that NLRP3 activation is a common pathway in microglia of the substantia nigra in PD patients and rodent PD models and was triggered in a delayed but forceful way in vitro [59]. They also designed different administration methods to verify the remission of neuroinflammation, nigrostriatal dopaminergic degeneration and α-synuclein pathology induced by NLRP3 inhibition [59]. Additionally, a new interesting study showed that inhibition of hepatic NLRP3 still protected dopaminergic neurons in the brain [60]. All these studies suggest that further research on the connection between NLRP3 inhibition and pathological alterations in the olfactory bulb is needed. Considering the importance of TLR4, we also designed an experiment to verify the role of TLR4 in PD progression.
In summary, pathologic changes, including abnormal α-synuclein, TH protein expression and inflammation, were detected via a series of analytical methods in the olfactory bulb after treatment with MPTP. As one of organs directly connected to the outside, the olfactory bulb is sensitive to environmental toxicity and is a significant brain region in parkinsonian pathology. This reminds us of the prion-like propagation of α-synuclein and the hypothesis of Braak. The olfactory bulb may be sensitive to etiological factors and be the initiation region in PD. Then, the α-synuclein lesions spread from the margin to key sites in the brain, particularly the neurodegenerative regions [61]. Additionally, we might develop a new nasal delivery system for anti-inflammatory agents that target NLRP3 to delay PD progression. In conclusion, further studies are needed to directly demonstrate that α-synuclein can spread from the olfactory bulb to the SN and to determine the feasibility of assessing olfactory dysfunction for early diagnosis of PD.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81573640, 81773925, and U1402221), the Beijing Natural Science Foundation (7161011), and Key Laboratory of Molecular Pharmacology and Drug Evaluation (Yantai University), Ministry of Education (P201701).
Author contributions
Y-hY and N-hC designed the research; YC, Q-sZ, Q-hS, SW, and H-bW performed the research; Q-hS, SW, and H-bW contributed analytic tools; YC and Q-sZ analyzed data; YC wrote the paper. All authors reviewed the manuscript.
Competing interests
The authors declare no competing financial interests
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu-he Yuan, Phone: +86 10 63165182, Email: yuanyuhe@imm.ac.cn.
Nai-hong Chen, Phone: +86 10 63165177, Email: chennh@imm.ac.cn.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Trist BG, Davies KM, Cottam V, Genoud S, Ortega R, Roudeau S, et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol. 2017;134:113–27. doi: 10.1007/s00401-017-1726-6. [DOI] [PubMed] [Google Scholar]
- 5.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mundinano IC, Hernandez M, Dicaudo C, Ordonez C, Marcilla I, Tunon MT, et al. Reduced cholinergic olfactory centrifugal inputs in patients with neurodegenerative disorders and MPTP-treated monkeys. Acta Neuropathol. 2013;126:411–25. doi: 10.1007/s00401-013-1144-3. [DOI] [PubMed] [Google Scholar]
- 7.Rey NL, Wesson DW, Brundin P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis. 2018;109:226–48. doi: 10.1016/j.nbd.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rey NL, Petit GH, Bousset L, Melki R, Brundin P. Transfer of human alpha-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol. 2013;126:555–73. doi: 10.1007/s00401-013-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoglinger GU, Alvarez-Fischer D, Arias-Carrion O, Djufri M, Windolph A, Keber U, et al. A new dopaminergic nigro-olfactory projection. Acta Neuropathol. 2015;130:333–48. doi: 10.1007/s00401-015-1451-y. [DOI] [PubMed] [Google Scholar]
- 10.Kanaan NM, Kordower JH, Collier TJ. Age-related changes in glial cells of dopamine midbrain subregions in rhesus monkeys. Neurobiol Aging. 2010;31:937–52. doi: 10.1016/j.neurobiolaging.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiwari PC, Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialog-Clin Neurosci. 2017;19:71–80. doi: 10.31887/DCNS.2017.19.1/rpal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo JD, Zhao X, Li Y, Li GR, Liu XL. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review) Int J Mol Med. 2018;41:1817–25. doi: 10.3892/ijmm.2018.3406. [DOI] [PubMed] [Google Scholar]
- 13.Niranjan R. Recent advances in the mechanisms of neuroinflammation and their roles in neurodegeneration. Neurochem Int. 2018;120:13–20. doi: 10.1016/j.neuint.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Sun JD, Song LK, Li J, Chu SF, Yuan YH, et al. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav Brain Res. 2015;294:149–61. doi: 10.1016/j.bbr.2015.07.058. [DOI] [PubMed] [Google Scholar]
- 16.Zhang QS, Heng Y, Chen Y, Luo P, Wen L, Zhang Z, et al. A novel bibenzyl compound (20C) protects mice from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid toxicity by regulating the alpha-synuclein-related inflammatory response. J Pharmacol Exp Ther. 2017;363:284–92. doi: 10.1124/jpet.117.244020. [DOI] [PubMed] [Google Scholar]
- 17.Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark AJ, Ritz B, Prescott E, Rod NH. Psychosocial risk factors, pre-motor symptoms and first-time hospitalization with Parkinson’s disease: a prospective cohort study. Eur J Neurol. 2013;20:1113–20. doi: 10.1111/ene.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, et al. Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case-control study. Movement disorders: official journal of the Movement Disorder. Society. 2000;15:669–77. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Olanow CW, Schapira AH, Roth T. Waking up to sleep episodes in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder. Society. 2000;15:212–5. doi: 10.1002/1531-8257(200003)15:2<212::aid-mds1002>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Bohnen NI, Studenski SA, Constantine GM, Moore RY. Diagnostic performance of clinical motor and non-motor tests of Parkinson disease: a matched case-control study. Eur J Neurol. 2008;15:685–91. doi: 10.1111/j.1468-1331.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- 22.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:509. doi: 10.1038/nrn.2017.91. [DOI] [PubMed] [Google Scholar]
- 23.Silveira-Moriyama L, Holton JL, Kingsbury A, Ayling H, Petrie A, Sterlacci W, et al. Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett. 2009;453:77–80. doi: 10.1016/j.neulet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Beach TG, White CL, 3rd, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117:169–74. doi: 10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 26.Barker Roger. Environmental toxins and Parkinson's disease. Trends in Neurosciences. 1989;12(5):182. doi: 10.1016/0166-2236(89)90066-0. [DOI] [PubMed] [Google Scholar]
- 27.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–15. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 28.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 29.Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, et al. Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun. 2015;6:7314. doi: 10.1038/ncomms8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci: Off J Soc Neurosci. 2008;28:7687–98. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melki R. Alpha-synuclein and the prion hypothesis in Parkinson's disease. Revue Neurologique. 2018;174(9):644–652. doi: 10.1016/j.neurol.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–83. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 33.Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy A, Srivastava M, Saqib U, Liu D, Faisal SM, Sugathan S, et al. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol. 2016;40:79–89. doi: 10.1016/j.intimp.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Bowie A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–14. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 37.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 38.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol. 2015;11:339–46. doi: 10.1038/nchembio.1788. [DOI] [PubMed] [Google Scholar]
- 40.Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, et al. NOS1-derived nitric oxide promotes NF-kappaB transcriptional activity through inhibition of suppressor of cytokine signaling-1. J Exp Med. 2015;212:1725–38. doi: 10.1084/jem.20140654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 42.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen L, Zhang QS, Heng Y, Chen Y, Wang S, Yuan YH, et al. NLRP3 inflammasome activation in the thymus of MPTP-induced Parkinsonian mouse model. Toxicol Lett. 2018;288:1–8. doi: 10.1016/j.toxlet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–80. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 46.Shao QH, Zhang XL, Yang PF, Yuan YH, Chen NH. Amyloidogenic proteins associated with neurodegenerative diseases activate the NLRP3 inflammasome. Int Immunopharmacol. 2017;49:155–60. doi: 10.1016/j.intimp.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 47.Hafner-Bratkovic I, Bencina M, Fitzgerald KA, Golenbock D, Jerala R. NLRP3 inflammasome activation in macrophage cell lines by prion protein fibrils as the source of IL-1beta and neuronal toxicity. Cell Mol life Sci: CMLS. 2012;69:4215–28. doi: 10.1007/s00018-012-1140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, et al. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS ONE. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. [DOI] [PubMed] [Google Scholar]
- 50.Litteljohn D, Mangano E, Clarke M, Bobyn J, Moloney K, Hayley S. Inflammatory mechanisms of neurodegeneration in toxin-based models of Parkinson’s disease. Parkinson’s Dis. 2010;2011:713517. doi: 10.4061/2011/713517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masters SL, O’Neill LA. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med. 2011;17:276–82. doi: 10.1016/j.molmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci: Off J Soc Neurosci. 2005;25:6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:616–9. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 54.Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 55.Shi F, Kouadir M, Yang Y. NALP3 inflammasome activation in protein misfolding diseases. Life Sci. 2015;135:9–14. doi: 10.1016/j.lfs.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 56.von Herrmann KM, Salas LA, Martinez EM, Young AL, Howard JM, Feldman MS, et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson’s disease. NPJ Parkinson’s Dis. 2018;4:24. doi: 10.1038/s41531-018-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez EM, Young AL, Patankar YR, Berwin BL, Wang L, von Herrmann KM, et al. Editor’sHighlight: Nlrp3 Is Required for Inflammatory Changes and Nigral Cell Loss Resulting From Chronic Intragastric Rotenone Exposure in Mice. Toxicol Sci: Off J Soc Toxicol. 2017;159:64–75. doi: 10.1093/toxsci/kfx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 59.Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018; 10(465). pii: eaah4066. http://stm.sciencemag.org/content/10/465/eaah4066.full [DOI] [PMC free article] [PubMed]
- 60.Qiao C, Zhang Q, Jiang Q, Zhang T, Chen M, Fan Y, et al. Inhibition of the hepatic Nlrp3 protects dopaminergic neurons via attenuating systemic inflammation in a MPTP/p mouse model of Parkinson’s disease. J Neuroinflamm. 2018;15:193. doi: 10.1186/s12974-018-1236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omais S, Jaafar C, Ghanem N. “Till Death Do Us Part”: A potential irreversible link between aberrant cell cycle control and neurodegeneration in the adult olfactory bulb. Front Neurosci. 2018;12:144. doi: 10.3389/fnins.2018.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]