Abstract
Hispidulin (4’,5,7-trihydroxy-6-methoxyflavone) is a phenolic flavonoid isolated from the medicinal plant S. involucrata, which exhibits anti-neoplastic activity against several types of cancer. However, the mechanism underlying its anti-cancer activity against hepatocellular carcinoma (HCC) has not been fully elucidated. In this study, we investigated whether and how hispidulin-induced apoptosis of human HCC cells in vitro and in vivo. We showed that hispidulin (10, 20 μmol/L) dose-dependently inhibited cell growth and promoted apoptosis through mitochondrial apoptosis pathway in human HCC SMMC7721 cells and Huh7 cells. More importantly, we revealed that its pro-apoptotic effects depended on endoplasmic reticulum stress (ERS) and unfolded protein response (UPR), as pretreatment with salubrinal, a selective ERS inhibitor, or shRNA targeting a UPR protein CHOP effectively abrogated hispidulin-induced cell apoptosis. Furthermore, we showed that hispidulin-induced apoptosis was mediated by activation of AMPK/mTOR signaling pathway as pretreatment with Compound C, an AMPK inhibitor, or AMPK-targeting siRNA reversed the pro-apoptotic effect of hispidulin. In HCC xenograft nude mice, administration of hispidulin (25, 50 mg/kg every day, ip, for 27 days) dose-dependently suppressed the tumor growth, accompanied by inducing ERS and apoptosis in tumor tissue. Taken together, our results demonstrate that hispidulin induces ERS-mediated apoptosis in HCC cells via activating the AMPK/mTOR pathway. This study provides new insights into the anti-tumor activity of hispidulin in HCC.
Keywords: hispidulin, hepatocellular carcinoma, mitochondrial apoptosis, endoplasmic reticulum stress, CHOP, AMPK/mTOR, Z-LEHD-FMK, salubrinal, Compound C
Introduction
Hepatocellular carcinoma (HCC) is a major public health problem worldwide, but it is especially problematic in China due to hepatitis B virus infection [1, 2]. Epidemiological statistics show that ~782,000 newly diagnosed cases of HCC and 745,000 deaths occurred worldwide in 2012 [3]. Surgical resection and traditional chemotherapy are typical treatment options for patients with HCC. However, the overall prognosis of patients with HCC is poor, and only a minority of HCC patients are eligible for surgical resection due to late stage diagnosis [4]. One of the greatest obstacles in HCC treatment is the occurrence of multi-drug resistance in response to chemotherapeutic drugs, such as 5-FU [5] and sorafenib [6, 7]. Hence, the discovery of new potential therapeutic targets and treatment strategies has been a research hotspot for HCC.
Apoptosis, or programmed cell death, plays a crucial role in controlling cellular homeostasis and has been considered as a major target for chemotherapeutic agents to eradicate cancer cells [8]. The endoplasmic reticulum (ER), a eukaryotic organelle, is responsible for protein biosynthesis and folding and calcium storage regulation, and ER dysfunction may be triggered by a number of environmental, physiological, or pathological stimuli, such as oxidative stress, disordered calcium metabolism, elevated protein synthesis, and nutritional imbalance [9]. ER stress causes an accumulation of unfolded or misfolded proteins. In response to ER stress, cells activate UPR as an attempt to correct ER stress and restore normal function. Three ER transmembrane sensors, protein kinase RNA-like ER kinase (PERK), inositol-requiring 1α (IRE1α), and activating transcription factor-6 (ATF6) from the binding immunoglobulin protein (BiP), comprise the three arms of UPR. However, under conditions of prolonged stress, UPR commits the cells to apoptosis [10]. Recently, accumulating studies have highlighted the critical role of ER in cancerous cell apoptosis [11]. Given the ability of prolonged or severe ER stress to initiate cell apoptosis via UPR [10], inducing ER stress has been considered as a novel mechanism for anti-cancer agents.
Natural products have been shown to provide health benefits to humans [12]. Hispidulin (4′,5,7-trihydroxy-6-methoxyflavone, C16H12O6, molecular weight 300.3 g/mol) is a phenolic flavonoid isolated mainly from S. involucrata, a medicinal plant traditionally used in oriental medicine [13]. In recent years, accumulating evidence has demonstrated the pleiotropic effects of hispidulin, including anti-inflammatory, antioxidant, anti-thrombotic, anti-epileptic, neuroprotective, and anti-osteoporotic activities [14–19]. More importantly, many in vivo and in vitro studies have demonstrated that hispidulin exerts anti-tumor effects against a variety of solid tumors and hematological malignancies [20–22]. Previous studies also evidenced the anti-cancer activities of hispidulin in renal cell carcinoma, acute myeloid leukemia, gallbladder cancer, colorectal cancer, and hepatocellular carcinoma [23–28]. In the context of HCC, it has been identified that hispidulin exerts anti-proliferative and pro-apoptotic effects on HepG2 cells [29, 30]. However, evidence that ERS and apoptosis are associated with the anti-cancer effect of hispidulin remains elusive in HCC.
In this paper, we report that the activation of ERS signaling by hispidulin at least partly contributed to its pro-apoptotic effect against HCC cells. Moreover, the pro-apoptotic effect of hispidulin is associated with mitochondrial apoptosis and modulation of the AMPK/mTOR signaling pathway.
Methods and materials
Cell culture and treatment
Human SMMC7721 and Huh7 cells were obtained from the Shanghai Cell Bank, China, and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen, USA). Cells were grown at 37 °C in a 5% CO2 (v/v) with humidified atmosphere.
Determination of cell viability
Cell viability was determined by Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China). Briefly, cells were treated withdifferent concentrations of hispidulin for 24 h or 48 h. Following treatment, the optical density of viable cells was measured at 450 nm in a spectrophotometer (Tecan Group Ltd, Männedorf, Switzerland). Cell viability assays were performed in triplicate.
Colony formation assay
Approximately 500 HCC cells were plated in each well of a six-well plate. Cultures were maintained for 14 days without fresh medium feeding at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Cells were fixed with 4% paraformaldehyde for 15 min, stained with crystal violet for 30 min, and photographed using a digital camera (Nikon DXM-1200, Tokyo, Japan). Cell colonies with over 50 cells were counted. Colony formation assays were performed in triplicate.
Hoechst 33258 staining
Nuclear staining was performed using a Hoechst 33258 Staining Kit (Beyotime, China). Briefly, Hoechst 33258 (2 µg/mL, Beyotime, China) was added to count the total cells and dead cells. After incubation for 30 min at 37 °C, the stained cells were examined using a fluorescence microscope.
Flow cytometry analysis of apoptosis
Cell apoptosis was determined using a FITC Annexin V apoptosis kit (BD Pharmingen, Franklin Lakes, NJ, USA). Briefly, the cells were harvested at a density of 5 × 105 cells/mL and incubated with Annexin V-FITC and propidium (PI) in the dark for 15 min before detection with flow cytometry (Beckman Coulter Inc, Miami, FL, USA). Flow cytometry analysis was performed in triplicate.
Measurement of mitochondrial membrane potential
Fluorochrome dye JC-1 was used to evaluate the changes in the mitochondrial membrane potential (MMP) as described previously [31]. Briefly, Huh7 or SMMC7721 cells were incubated with 10 or 20 µM hispidulin for 48 h. Following incubation, the cells were stained with JC-1 for 20 min. Cells were then harvested and rinsed with JC-1 staining buffer (1×) twice. The stained cells were imaged immediately at ×200 using a fluorescence microscope (OLYMPUS, Japan).
Caspase-9 and caspase-12 activity assay
The activity of caspase-9 and caspase-12 was measured using an ELISA kit following the manufacturer’s instructions (Abcam, Shanghai, China). The activity of caspase-9 or caspase-12 was assessed by measuring the fluorescence intensity. All caspase activity assays were performed in triplicate.
Western blotting
Proteins were isolated from the control and hispidulin-treated cells as previously described [25]. Approximately 40 µg of total protein samples were electrophoresed on SDS-PAGE before they were transferred to a PVDF membrane. The membranes were then blocked with 5% (w/v) non-fat milk, washed with Tris-buffered saline-Tween solution (TBST), and incubated overnight at 4 °C with primary antibodies according to the manufacturer’s instructions. The primary antibodies against cleaved caspase-3, cleaved PARP, cleaved caspase-9, cleaved caspase-12, mitochondrial cyto C, cytosol cyto C, PUMA, CHOP, GRP78, p-PERK, ATF4, p-eIF2a, Bcl-2, Bax, p-AMPK, AMPK, p-mTOR, mTOR, and Ki-67 were purchased from Abcam (Shanghai, China); β-actin and COX-IV were used as internal controls and purchased from Beyotime (Shanghai, China). The secondary antibodies used in this study were goat anti-rabbit IgG-HRP and anti-mouse IgG-HRP (Beyotime, Shanghai, China). After washing with TBST, a secondary antibody was added and incubated for 2 h. The blots were washed, and the signal was detected using a chemiluminescent substrate (KPL., Guildford, UK). BandScan software (Glyko, Novato, CA) was used to quantify the blot density. All Western blotting assays were performed in triplicate.
Interference vector construction and transfection
The shRNA oligo nucleotide-targeting CHOP was synthesized according to a previously published sequence [32]. AMPK α1/α2 siRNA was purchased from Santa Cruz (Santa Cruz, CA). Cells were cultured overnight and then transfected with shRNA (siRNA) or scramble shRNA (siRNA) using Lipofectamine 3000 (Invitrogen). At 48 h post transfection, the knockdown was verified by Western blot analysis.
Xenograft model
Eight-week-old male athymic BALB/c nu/nu mice were kept under pathogen-free conditions. Animal experiments for this study were approved by the Institutional Animal Care and Use Committee at Qingdao University. Huh7 cells (1 × 107 cells/mL) were injected into the left flanks of the mice. Fifteen days after injection, the mice were randomly divided into three groups (eight mice per group) to receive intraperitoneal (IP) injections as follows: (A) vehicle (0.9% sodium chloride and 1% DMSO), (B) hispidulin (25 mg/kg/day for 27 days), and (C) hispidulin (50 mg/kg/day for 27 days). Mouse body weight and tumor volumes were measured every 3 days. IHC staining and TUNEL assay were performed on cryostat sections (4 μm/section) of Huh7 cells xenograft tumors as described [33].
Statistical analysis
The data are presented as mean ± SD (standard deviation) and represent the results of three separate experiments. Statistical comparisons between cell lines were analyzed by one-way ANOVA followed by Dunnett’s t test. Experimental data were analyzed by GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). A P < 0.05 was considered to be statistically significant, and a P < 0.01 indicated notable statistical significance.
Results
Hispidulin effectively suppresses cell growth and promotes apoptosis
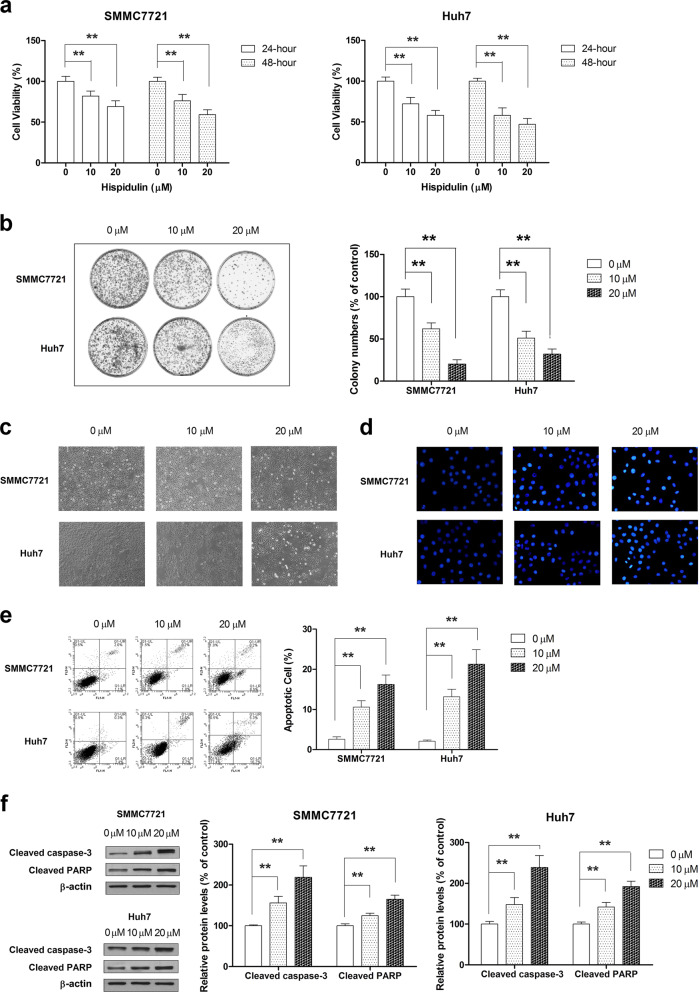
To investigate the effect of hispidulin on cell proliferation, we performed CCK-8 assays to evaluate the cell viability of SMMC7721 and Huh7 cells in response to hispidulin. Hispidulin effectively reduced the cell viability in a dose- and time-dependent manner, relative to controls (Fig. 1a). We also examined the effect of hispidulin on the clone-forming ability of HCC cells. Hispidulin treatment caused a significant and dose-dependent decrease in the colony formation ability of SMMC7721 and Huh7 cells (Fig. 1b). Furthermore, microscopic images (Fig. 1c) indicated that hispidulin exposure significantly increased the number of drifting cells and reduced the rate of cellular attachment relative to controls. To test whether apoptosis is the primary contributor of the growth inhibitory effect of hispidulin, Hoechst 33258 and Annexin V/PI staining were used to identify apoptosis. Hoechst 33258 and Annexin V/PI staining results confirmed that hispidulin at 10 µM and 20 µM dosages significantly increased the percentage of apoptotic cells (Fig. 1d, e) (P < 0.01). Furthermore, we found that hispidulin effectively increased the cleavage of caspase-3 and PARP in SMMC7721 and Huh7 cells (Fig. 1f). Overall, these results indicated that the growth inhibitory effects of hispidulin on HCC cells are mediated by apoptosis.
Fig. 1.
Hispidulin promotes cell death in HCC cells. a Cell viability of SMMC7721 and Huh7 cells was measured using a CCK-8 assay after hispidulin (10 and 20 μM) treatment for 24 or 48 h. b Colony formation assay of SMMC7721 and Huh7 cells after hispidulin treatment at dosages of 10 and 20 μM. c Cell morphology was observed under an inverted phase contrast microscope after SMMC7721 and Huh7 cell exposure to hispidulin at dosages of 10 and 20 μM for 48 h. d Hoechst 33258 staining analysis of the apoptotic cell population after SMMC7721 and Huh7 cells were exposed to hispidulin at doses of 10 and 20 μM for 48 h. Apoptotic cells were observed under a fluorescence microscope (excitation wavelength: 488 nm). e SMMC7721 and Huh7 cells were treated with hispidulin (10 and 20 μM) for 48 h, and flow cytometry was used to quantify the apoptotic cells. f Immunoblot analysis of cleaved caspase-3 and cleaved PARP in SMMC7721 and Huh7 cells after exposure to hispidulin 10 and 20 μM for 48 h. **P < 0.01
Hispidulin induces cell apoptosis in SMMC7721 and Huh7 cells via the intrinsic pathways
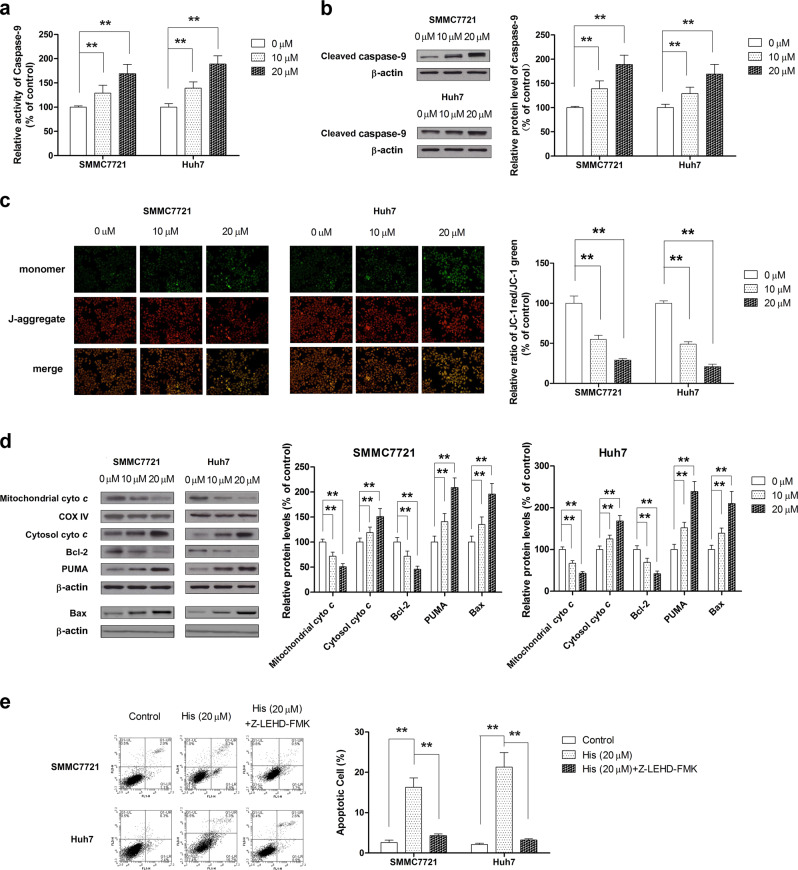
Our previous study identified that intrinsic pathways are involved in the pro-apoptotic effect of hispidulin in HCC cells [30]. To confirm whether hispidulin triggered cell apoptosis through intrinsic pathways, we first examined the activity and protein expression of caspase-9 in SMMC7721 and Huh7 cells after exposure to hispidulin (10 µM and 20 µM). The results indicated that hispidulin increased the activity and cleavage of caspase-9 in a dose-dependent manner (Fig. 2a, b) (P < 0.01). In addition, we examined whether hispidulin treatment affected the MMP, which plays a pivotal role in triggering apoptosis. As shown in Fig. 2c, hispidulin treatment caused a disruption of MMP. Moreover, hispidulin significantly increased the expression of cytosolic cytochrome C, PUMA, and Bax and decreased mitochondrial cytochrome C and Bcl-2 expression (Fig. 2d). From these results, we can confirm that hispidulin triggers apoptosis via the intrinsic mitochondrial pathway of apoptosis (P < 0.01). We further confirmed these findings by demonstrating that pretreatment with a caspase-9 inhibitor (Z-LEHD-FMK) for 6 h significantly inhibited the pro-apoptotic effect of hispidulin on HCC cells (Fig. 2e) (P < 0.01). Taken together, these results suggest that hispidulin-induced apoptosis of SMMC7721 and Huh7 cells via intrinsic pathways.
Fig. 2.
Hispidulin activates the intrinsic mitochondrial apoptotic pathway in HCC cells. a SMMC7721 and Huh7 cells were treated with hispidulin (10 and 20 μM) for 48 h, and the activity of caspase-9 was measured using a caspase-9 ELISA kit. b SMMC7721 and Huh7 cells were treated with hispidulin (10 and 20 μM) for 48 h and analyzed for protein expression of cleaved caspase-9 by immunoblotting. c SMMC7721 and Huh7 cells were incubated with hispidulin (10 μM and 20 μM) for 48 h, and JC-1 staining was performed to detect the changes in the mitochondrial membrane potential (MMP). d SMMC7721 and Huh7 cells were treated with hispidulin (10 and 20 μM) for 48 h, and immunoblot analysis was performed to analyze the protein expression of mitochondrial cyto C, cytosolic cyto C, Bcl-2, Bax, and PUMA. COX-IV and β-actin were used as loading controls. e SMMC7721 and Huh7 cells were pretreated with caspase-9 inhibitor Z-LEHD-FMK for 6 h, incubated with hispidulin (20 μM) for 48 h and analyzed by flow cytometry to quantify the apoptotic cells ratio. **P < 0.01
Hispidulin induces ER stress and the UPR pathway
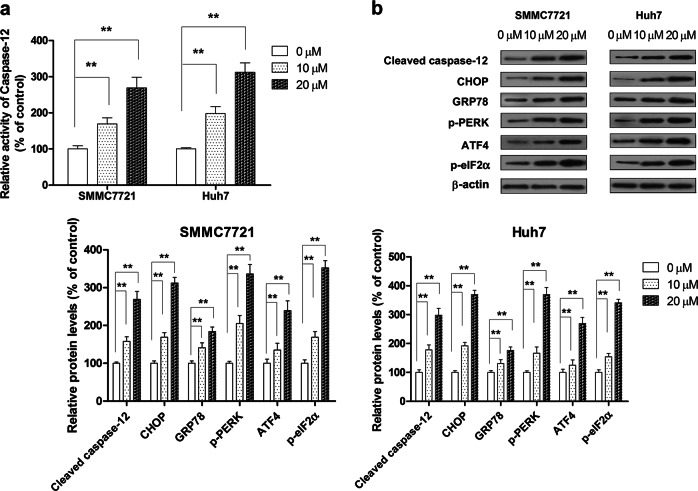
To confirm whether hispidulin treatment activates ERS in HCC cells, we analyzed the activity and expression of ERS-related proteins in response to hispidulin. Hispidulin significantly elevated the activity of caspase-12, which plays a crucial role in cell apoptosis mediated by the ERS signaling pathway (Fig. 3a) (P < 0.01). UPR is activated in response to ER stress. Upon UPR activation, the UPR sensor PERK dissociates from GRP78, which promotes PERK oligomerization and autophosphorylation. PERK then phosphorylates the translation initiation factor eIF2α (eukaryotic initiation factor 2α), which triggers the activation of the transcription factor ATF4. ATF4 induces the expression of proteins involved in cell apoptosis, including CHOP and caspase-12 [34]. The expression of ER stress marker GRP78 was significantly elevated in the hispidulin (10 and 20 µM) treatment group relative to the control group (P < 0.01). Moreover, the phosphorylation of PERK and eIF2α and the expression of ATF4, CHOP, and cleaved caspase-12 were markedly increased in a dose-dependent manner in hispidulin-treated cells compared with controls (Fig. 3b) (P < 0.01). Together, these results show that hispidulin-induced ERS in SMMC7721 and Huh7 cells at least partly via the PERK sensor and its downstream pathways.
Fig. 3.
The pro-apoptotic effect of hispidulin in HCC cells is mediated by the ERS-related apoptotic pathway. a SMMC7721 and Huh7 cells were treated with hispidulin (10 and 20 μM) for 48 h, and the activity of caspase-12 was measured using a caspase-12 ELISA kit. b Immunoblot analysis shows the expression of ERS-related proteins, including cleaved caspase-12, CHOP, GRP78, p-PERK, ATF4, and p-eIF2α. **P < 0.01
Hispidulin exhibits pro-apoptotic effects mainly through the activation of ERS and downstream signaling pathway
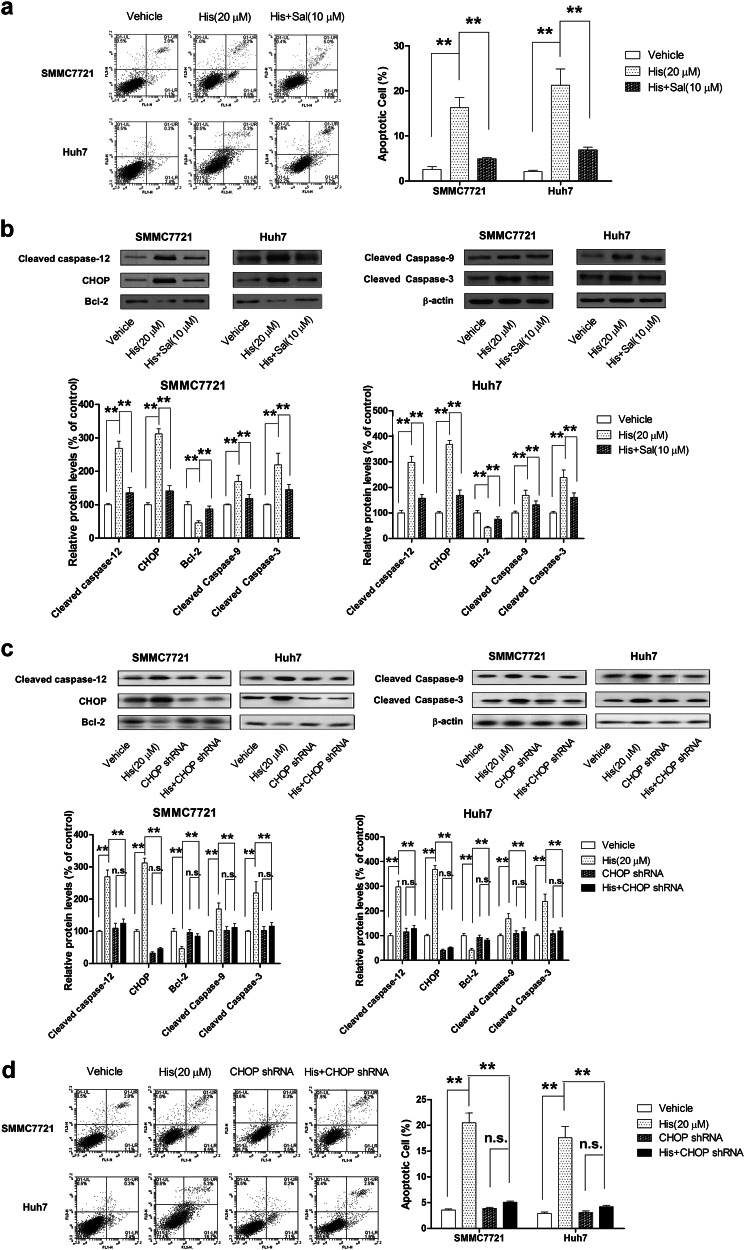
To confirm whether hispidulin-induced SMMC7721 and Huh7 cell apoptosis through the ERS-related apoptotic pathway, the apoptotic cell population and related protein expression were analyzed in the presence of salubrinal (Sal/10 µM), a selective inhibitor of eIF2α dephosphorylation. Hispidulin (20 µM) induced HCC cell apoptosis, and pretreatment of cells with Sal for 6 h significantly abrogated this effect (Fig. 4a). In addition, Sal reversed the hispidulin-induced increase in the levels of cleaved caspase-12, CHOP, cleaved caspase-9, and cleaved caspase-3 and decrease in the expression of anti-apoptotic protein Bcl-2 (Fig. 4b). To further identify the role of ERS in hispidulin-induced apoptosis in HCC cells, a shRNA strategy was applied to stably knockdown CHOP. CHOP shRNA effectively downregulated the expression of CHOP in HCC cells (Fig. 4c). We found that hispidulin-induced apoptosis (Fig. 4d) and changes in protein expression (Fig. 4c) were attenuated in CHOP-silenced HCC cells. These findings confirmed that ER stress activation mediated the pro-apoptotic effects of hispidulin in HCC cells.
Fig. 4.
Blocking the ERS signaling pathway reverses hispidulin-induced apoptosis in HCC cells. a SMMC7721 and Huh7 cells were pretreated with eIF2α selective dephosphorylation inhibitor (Sal 10 μM) for 6 h and incubated with hispidulin (20 μM) for 48 h, and then, the apoptotic cell ratio was measured by flow cytometry. b SMMC7721 and Huh7 cells were pretreated with ERS inhibitor (Sal) for 6 h and then incubated with hispidulin (20 μM) for 48 h, and immunoblot analysis of the expression of cleaved caspase-12, CHOP, Bcl-2, cleaved caspase-9, and cleaved caspase-3 in HCC cells was performed. c Immunoblot analysis of cleaved caspase-12, CHOP, Bcl-2, cleaved caspase-9, and cleaved caspase-3 in HCC or CHOP shRNA transfection HCC cells after exposure to hispidulin (20 μM) for 48 h. d Flow cytometry analysis of apoptotic cells in SMMC7721 and Huh7 cells or CHOP shRNA transfection cells after treatment with hispidulin (20 μM). **P < 0.01; n.s. not significant
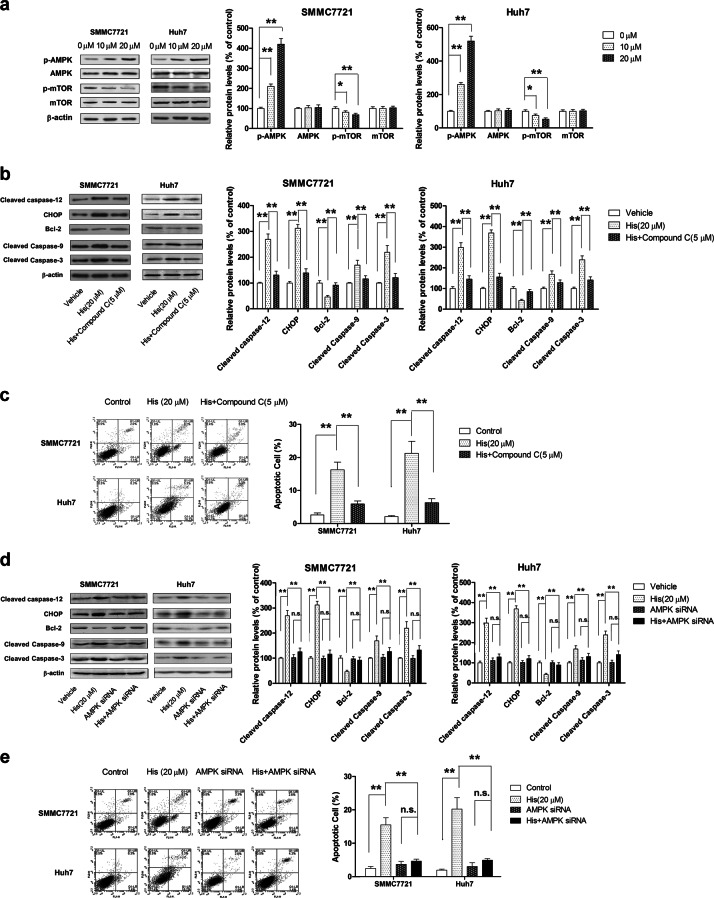
Hispidulin-induced apoptosis is mediated by activation of the AMPK signaling pathway
Several studies have demonstrated that the AMPK pathway is implicated in ERS-related cell apoptosis [35]. In line with these findings, our results showed that hispidulin treatment increased phosphorylation of AMPK and decreased the phosphorylation level of mTOR in HCC cells in a dose-dependent manner. Total AMPK and mTOR were used as pan controls (Fig. 5a). We next used AMPK inhibitor Compound C (5 µM) or AMPK siRNA to block the AMPK pathway and examine the effects of hispidulin on apoptosis and the expression of related proteins. Pretreatment with AMPK inhibitor Compound C significantly attenuated hispidulin-induced apoptosis and abolished the effects of hispidulin on the expression of related proteins, including cleaved caspase-12, CHOP, Bcl-2, cleaved caspase-9, and cleaved caspase-3. No significant changes were observed when cells were treated with Compound C alone (data not shown) (Fig. 5b, c). Consistent with these findings, AMPK siRNA also significantly abolished the pro-apoptotic effects of hispidulin (Fig. 5d, e). These results collectively demonstrated that hispidulin exerts pro-apoptotic functions via the AMPK/mTOR signaling pathway.
Fig. 5.
Hispidulin triggers ERS-related apoptosis through the activation of the AMPK/mTOR pathway. a Immunoblot analysis of the phosphorylation level and total protein expression of AMPK and mTOR in SMMC7721 and Huh7 cells after treatment with hispidulin (10 and 20 μM) for 48 h. b SMMC7721 and Huh7 cells were pretreated with AMPK inhibitor Compound C (5 μM) for 6 h and then incubated with hispidulin (20 μM) for 48 h. Immunoblot analysis of the expression of cleaved caspase-12, CHOP, Bcl-2, cleaved caspase-9, and cleaved caspase-3. c Flow cytometry analysis of apoptotic cells in SMMC7721 and Huh7 cells after pretreatment with AMPK inhibitor Compound C (5 μM) for 6 h and incubation with hispidulin (20 μM) for 48 h. d Immunoblot analysis of cleaved caspase-12, CHOP, Bcl-2, cleaved caspase-9, and cleaved caspase-3 in AMPK siRNA-transfected SMMC7721 and Huh7 cells after treatment with hispidulin (20 μM) for 48 h. e Flow cytometry analysis of apoptotic cells in AMPK siRNA-transfected SMMC7721 and Huh7 cells after treatment with hispidulin (20 μM) for 48 h. *P < 0.05. **P < 0.01; n.s. not significant
Hispidulin induces ERS-related apoptosis in vivo
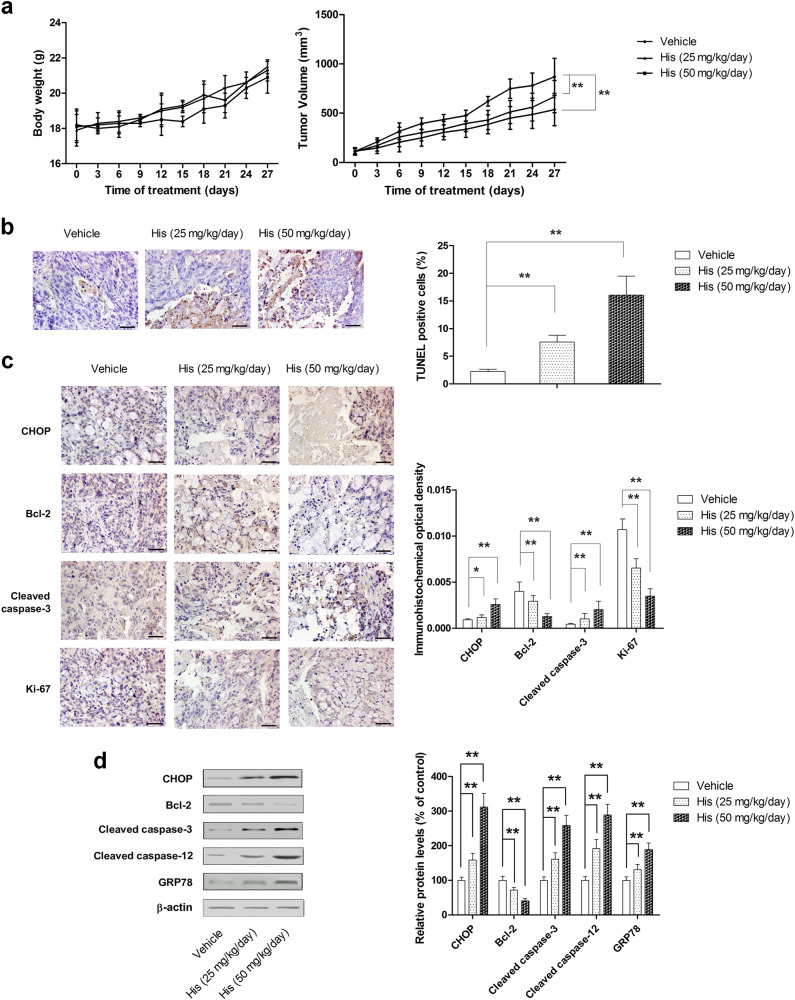
On the basis of our in vitro results, an HCC xenograft mouse model was used to test the in vivo therapeutic effects of hispidulin. Dosages of hispidulin used were 25 mg/kg/day and 50 mg/kg/day. Hispidulin significantly suppressed tumor growth at both doses relative to the vehicle group (P < 0.01) (Fig. 6a); however, 25 mg/kg/day and 50 mg/kg/day hispidulin did not significantly affect the body weight of mice compared with the vehicle group (P > 0.05). Moreover, in animals treated with hispidulin, a significant increase in TUNEL-positive cells in tumor issues was observed (Fig. 6b), which indicated the pro-apoptotic effect of hispidulin. We also examined the expression of CHOP, Bcl-2, cleaved caspase-3, and Ki-67 in tumor tissues using immunohistochemistry. In line with our in vitro findings, the results showed that hispidulin significantly upregulated the expression of CHOP and cleaved caspase-3 while downregulating the level of Bcl-2 and Ki-67 (Fig. 6c). Furthermore, hispidulin effectively increased the protein expression of CHOP, GRP78, cleaved caspase-3, and cleaved caspase-12 and decreased the expression of Bcl-2 in tumor tissues (Fig. 6d). These results confirmed that hispidulin-induced ERS and apoptosis in vivo.
Fig. 6.
Hispidulin exhibits anti-tumor activity in vivo through the activation of the ERS signaling pathway. a Measurement of the body weight and tumor volume every 3 days in a HCC xenograft mice model after treatment with different dosages of hispidulin (25 and 50 mg/kg/day). b TUNEL assay analysis of apoptotic cell cryostat sections (4 μm sections) of HCC xenograft tumors after treatment with hispidulin. c Immunohistochemistry staining was performed on the cryostat sections (4 μm sections) of HCC xenograft tumors to detect the expression of CHOP, Bcl-2, cleaved caspase-3, and Ki-67 after treatment with hispidulin. d The protein expression of CHOP, Bcl-2, GRP78, cleaved caspase-3, and cleaved caspase-12 in tumor tissues was detected by immunoblot analysis. Data are presented as the mean ± SD, n = 8. *P < 0.05; **P < 0.01
Discussion
Compared with conventional chemotherapeutic drugs, natural compounds may exert potent anti-tumor effects without causing many adverse effects. Therefore, the anti-tumor activity of a number of bioactive chemical structures from natural sources has been studied. Hispidulin is a flavonoid compound, and its anti-neoplastic activity has been documented [21, 22, 36]. The role of hispidulin as a chemopreventive agent was first reported in 1992 [37]. In 2010, Way et al. reported that hispidulin-induced apoptosis in ovarian cancer and glioblastoma multiforme cells in vitro [22, 38]. The pro-apoptotic effect of hispidulin has also been evidenced in hepatoma cells by different groups [29, 39]. Moreover, our previous studies also showed that hispidulin suppressed cell growth and induced apoptosis in vitro and in vivo in clear cell renal cell carcinoma and gallbladder cancer. In the present study, our results showed that hispidulin caused the induction of apoptosis and a reduction in cell viability in HCC cells. Mechanistically, hispidulin-induced ER stress and UPR by activating the AMPK pathway, leading to intrinsic apoptosis in HCC cells.
The ER is a eukaryotic organelle that is responsible for regulating calcium homeostasis, lipid biosynthesis, protein synthesis, folding, and quality control [40]. ERS, which is triggered by numerous environmental, physiological, and pathological insults that disrupt the protein folding environment in the ER, leads to protein misfolding and accumulation [41]. The role of ERS in the development and progression of cancer is controversial. Endoplasmic reticulum stress generally plays a protective role in tumor development by activating the adaptive stress response elements and attenuating the apoptotic pathways. Accumulating evidence demonstrated that ERS is linked to tumor initiation [42], quiescence and aggressiveness [43], epithelial-to-mesenchymal transition (EMT) [44], angiogenesis, and metabolic processes [45]. Furthermore, clinical evidence and in vitro studies suggested a strong link between ERS and drug resistance. Various studies have shown that GRP78 protects against drug-induced apoptosis [46]. In contrast, some studies have shown that the therapeutic induction of ERS-induced apoptosis might be beneficial for killing cancer cells. An early study showed that Icariin protects rat H9c2 cells from apoptosis by inhibiting ERS signaling [47]. In EC109 cells, the ERS pathway is involved in adenosine-induced apoptosis [48]. Moreover, N-Myc downstream-regulated gene 2 (NDRG2) acts as a PERK co-factor to facilitate the PERK branch of UPR- and ERS-induced cell apoptosis in human hepatoma SK-Hep-1 and HepG2 cells [49]. Zhou et al. found that rosoloactone induces apoptosis in human cervical cancer cells through endoplasmic reticulum stress and mitochondrial damage [50]. In the present study, our results revealed that hispidulin-induced ER stress and the inhibition of ER stress by a specific inhibitor or by using CHOP shRNA to inhibit UPR significantly attenuated hispidulin-induced apoptosis, suggesting that ER stress induced by hispidulin is an upstream signaling event that triggers mitochondrial apoptosis in HCC cells.
AMPK is an energy sensor involved in regulating energy balance at both cellular and organismal levels [51]. AMPK is activated when it is phosphorylated at Thr172 (P-AMPKα-Thr172) either by the tumor suppressor kinase LKB1 or an alternate pathway involving the Ca2+/calmodulin-dependent kinase, CAMKK2 [52]. Accumulating evidence suggests that the activation of AMPK signaling may facilitate growth inhibition and cell killing [53]. A number of studies have shown that small molecules and compounds induce ER stress, leading to apoptosis mainly through the activation of the CaMKKβ-AMPK-mTOR signaling pathway [54]. For instance, thapsigargin causes ERS and sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation [55]. Our previous studies indicated that hispidulin exhibits anti-cancer activity by activating the AMPK signaling pathway in a variety of tumor cells, including glioblastoma, ovarian cancer cells, colorectal cancer, and hepatocellular carcinoma [21, 22, 27, 28]. However, little is known as to whether hispidulin triggers the ERS pathway through AMPK-mTOR signaling. In this study, hispidulin effectively increased the phosphorylation of AMPK, leading to mTOR inhibition. Treatment of cells with AMPK siRNA or an inhibitor (Compound C) significantly abrogated the effects of hispidulin on CHOP expression and apoptotic-related protein expression, providing evidence that hispidulin triggers ERS by modulating AMPK signaling (Fig. 7).
Fig. 7.
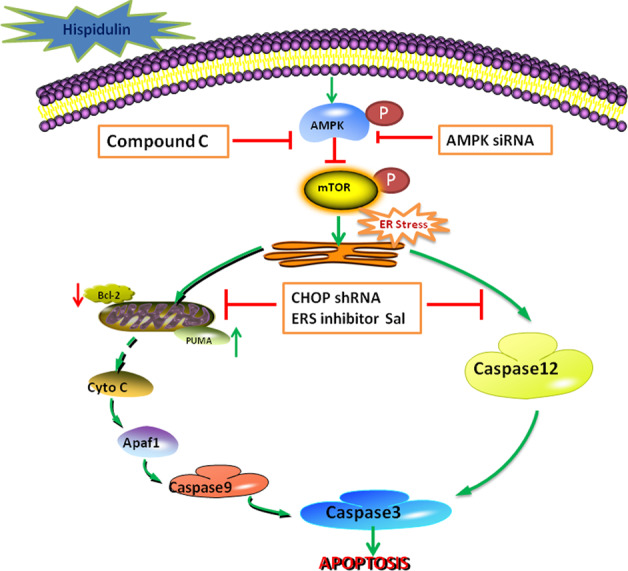
Schematic model illustrating the potential pathway associated with hispidulin-induced apoptosis in HCC cells
In summary, our results showed that hispidulin triggers ERS-mediated apoptosis by modulating the AMPK/mTOR signaling. These findings suggest that ERS may serve as a potential therapeutic target for the prevention and treatment of cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 81603337 and 81274126) and Project of Clinical Medicine + x, Medical College, Qingdao University (grant number 2017M38).
Author contribution
MH, HG, and JX designed the study. YPY, QY, MQG, and KLL performed the study. XHC and YTH contributed new reagents and analytic tools. ZWH analyzed the data; and HG wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1038/s41401-021-00730-4
Change history
9/7/2021
A Correction to this paper has been published: 10.1038/s41401-021-00730-4
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse Sean F., McGlynn Katherine A., Reichman Marsha E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. Journal of Clinical Oncology. 2009;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Yao M, Dong Z, Zhang Y, Yao D. Circulating specific biomarkers in diagnosis of hepatocellular carcinoma and its metastasis monitoring. Tumour Biol. 2014;35:9–20. doi: 10.1007/s13277-013-1141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Q, Sui Z, Xu W, Quan X, Sun J, Li X, et al. Ubenimex suppresses Pim-3 kinase expression by targeting CD13 to reverse MDR in HCC cells. Oncotarget. 2017;8:72652–65. doi: 10.18632/oncotarget.20194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Jiejie, Zhai Bo, Sun Weihua, Hu Fengli, Cheng Hao, Xu Jun. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multi-drug resistance to sorafenib in hepatocellular carcinoma cells. PLOS ONE. 2017;12(9):e0185088. doi: 10.1371/journal.pone.0185088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13:1589–98. doi: 10.1158/1535-7163.MCT-13-1043. [DOI] [PubMed] [Google Scholar]
- 8.Lin S, Fujii M, Hou DX. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch Biochem Biophys. 2003;418:99–107. doi: 10.1016/j.abb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–33. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 10.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 11.Ding W, Zhang X, Huang H, Ding N, Zhang S, Hutchinson SZ, et al. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS ONE. 2014;9:e94545. doi: 10.1371/journal.pone.0094545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey A, Edrada-Ebel R, Quinn R. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–29. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 13.Xu YJ, Zhao DX, Fu CX, Cheng LQ, Wang NF, Han LJ, et al. Determination of flavonoid compounds from Saussurea involucrata by liquid chromatography electrospray ionisation mass spectrometry. Nat Prod Res. 2009;23:1689–98. doi: 10.1080/14786410802187742. [DOI] [PubMed] [Google Scholar]
- 14.Bourdillat B, Delautier D, Labat C, Benveniste J, Potier P, Brink C. Mechanism of action of hispidulin, a natural flavone, on human platelets. Prog Clin Biol Res. 1988;280:211–4. [PubMed] [Google Scholar]
- 15.Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, et al. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br J Pharmacol. 2004;142:811–20. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu X, Chen J, Wang P, Zhou H, Li S, Zhang M. The effects of hispidulin on bupivacaine-induced neurotoxicity: role of AMPK signaling pathway. Cell Biochem Biophys. 2014;70:241–9. doi: 10.1007/s12013-014-9888-5. [DOI] [PubMed] [Google Scholar]
- 17.Tan RX, Lu H, Wolfender JL, Yu TT, Zheng WF, Yang L, et al. Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med. 1999;65:64–7. doi: 10.1055/s-1999-13965. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen T, et al. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol. 2008;120:1–6. doi: 10.1016/j.jep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Zhou R, Wang Z, Ma C. Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochem Biophys. 2014;69:311–7. doi: 10.1007/s12013-013-9800-8. [DOI] [PubMed] [Google Scholar]
- 20.Yu CY, Su KY, Lee PL, Jhan JY, Tsao PH, Chan DC, et al. Potential therapeutic role of hispidulin in gastric cancer through induction of apoptosis via NAG-1 signaling. Evid Based Complement Altern Med. 2013;2013:518301. doi: 10.1155/2013/518301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Liu W, He X, Fei Z. Hispidulin enhances the anti-tumor effects of temozolomide in glioblastoma by activating AMPK. Cell Biochem Biophys. 2015;71:701–6. doi: 10.1007/s12013-014-0252-6. [DOI] [PubMed] [Google Scholar]
- 22.Yang JM, Hung CM, Fu CN, Lee JC, Huang CH, Yang MH, et al. Hispidulin sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by AMPK activation leading to Mcl-1 block in translation. J Agric Food Chem. 2010;58:10020–6. doi: 10.1021/jf102304g. [DOI] [PubMed] [Google Scholar]
- 23.Gao H, Gao M, Peng J, Han M, Liu K, Han Y. Hispidulin mediates apoptosis in human renal cell carcinoma by inducing ceramide accumulation. Acta Pharmacol Sin. 2017;38:1618–31. doi: 10.1038/aps.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Gao M, Gao H, Han M, Liu K, Peng J, Han Y. Hispidulin suppresses tumor growth and metastasis in renal cell carcinoma by modulating ceramide-sphingosine 1-phosphate rheostat. Am J Cancer Res. 2017;7:1501–14. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Gao H, Liu Y, Li K, Wu T, Peng J, Jing F. Hispidulin induces mitochondrial apoptosis in acute myeloid leukemia cells by targeting extracellular matrix metalloproteinase inducer. Am J Transl Res. 2016;8:1115–32. [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H, Xie J, Peng J, Han Y, Jiang Q, Han M, et al. Hispidulin inhibits proliferation and enhances chemosensitivity of gallbladder cancer cells by targeting HIF-1α. Exp Cell Res. 2015;332:236–46. doi: 10.1016/j.yexcr.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Liu Kaili, Gao Hui, Wang Qiaoyun, Wang Longyuan, Zhang Bin, Han Zhiwu, Chen Xuehong, Han Mei, Gao Mingquan. Hispidulin suppresses cell growth and metastasis by targeting PIM1 through JAK2/STAT3 signaling in colorectal cancer. Cancer Science. 2018;109(5):1369–1381. doi: 10.1111/cas.13575. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Han M, Gao H, Ju P, Gao MQ, Yuan YP, Chen XH, et al. Hispidulin inhibits hepatocellular carcinoma growth and metastasis through AMPK and ERK signaling mediated activation of PPARgamma. Biomed Pharmacother. 2018;103:272–83. doi: 10.1016/j.biopha.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Scoparo CT, Valdameri G, Worfel PR, Guterres FA, Martinez GR, Winnischofer SM, et al. Dual properties of hispidulin: antiproliferative effects on HepG2 cancer cells and selective inhibition of ABCG2 transport activity. Mol Cell Biochem. 2015;409:123–33. doi: 10.1007/s11010-015-2518-8. [DOI] [PubMed] [Google Scholar]
- 30.Gao H, Wang H, Peng J. Hispidulin induces apoptosis through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway in HepG2 cancer cells. Cell Biochem Biophys. 2014;69:27–34. doi: 10.1007/s12013-013-9762-x. [DOI] [PubMed] [Google Scholar]
- 31.Herrerias Tatiana, Oliveira AlexandreA, Belem MauriacutecioL, Oliveira BraacutesH, Carnieri EvaGS, Cadena SiacutelviaMSC, et al. Effects of natural flavones on membrane properties and citotoxicity of HeLa cells. Rev Bras De Farmacogn. 2010;20:403–8. doi: 10.1590/S0102-695X2010000300018. [DOI] [Google Scholar]
- 32.Wu K, Li N, Sun H, Xu T, Jin F, Nie J. Endoplasmic reticulum stress activation mediates Ginseng Rg3-induced anti-gallbladder cancer cell activity. Biochem Biophys Res Commun. 2015;466:369–75. doi: 10.1016/j.bbrc.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Yang X, Zhao N, Peng J, Gao H, Qiu X. Alpinumisoflavone induces apoptosis in esophageal squamous cell carcinoma by modulating miR-370/PIM1 signaling. Am J Cancer Res. 2016;6:2755–71. [PMC free article] [PubMed] [Google Scholar]
- 34.Avril T, Vauleon E, Chevet E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis. 2017;6:e373. doi: 10.1038/oncsis.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhuo XZ, Wu Y, Ni YJ, Liu JH, Gong M, Wang XH, et al. Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis. 2013;18:800–10. doi: 10.1007/s10495-013-0843-5. [DOI] [PubMed] [Google Scholar]
- 36.He L, Wu Y, Lin L, Wang J, Chen Y, Yi Z, et al. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011;102:219–25. doi: 10.1111/j.1349-7006.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu YL, Ho DK, Cassady JM, Cook VM, Baird WM. Isolation of potential cancer chemopreventive agents from Eriodictyon californicum. J Nat Prod. 1992;55:357–63. doi: 10.1021/np50081a012. [DOI] [PubMed] [Google Scholar]
- 38.Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, et al. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK) J Agric Food Chem. 2010;58:9511–7. doi: 10.1021/jf1019533. [DOI] [PubMed] [Google Scholar]
- 39.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, et al. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-beta signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–27. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 40.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–46. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 41.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–58. doi: 10.1016/S0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 42.Niederreiter L, Fritz T, Adolph T, Krismer A, Offner F, Tschurtschenthaler M, et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med. 2013;210:2041–56. doi: 10.1084/jem.20122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schewe D, Aguirre-Ghiso J. ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci USA. 2008;105:10519–24. doi: 10.1073/pnas.0800939105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Chen X, Gao Y, Wu J, Zeng F, Song F. XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell Signal. 2015;27:82–9. doi: 10.1016/j.cellsig.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Drogat B, Auguste P, Nguyen D, Bouchecareilh M, Pineau R, Nalbantoglu J, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 2007;67:6700–7. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 46.Lee E, Nichols P, Spicer D, Groshen S, Yu M, Lee A. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, Li H, Wang S, Liu M, Feng Y, Wang X. Icariin protects rat cardiac H9c2 cells from apoptosis by inhibiting endoplasmic reticulum stress. Int J Mol Sci. 2013;14:17845–60. doi: 10.3390/ijms140917845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, Wei B, Guo Y, Ye Y, Li G, Pu Z, et al. Apoptosis induced by adenosine involves endoplasmic reticulum stress in EC109 cells. Int J Mol Med. 2012;30:797–804. doi: 10.3892/ijmm.2012.1085. [DOI] [PubMed] [Google Scholar]
- 49.Zhang M, Liu X, Wang Q, Ru Y, Xiong X, Wu K, et al. NDRG2 acts as a PERK co-factor to facilitate PERK branch and ERS-induced cell death. FEBS Lett. 2017;591:3670–81. doi: 10.1002/1873-3468.12861. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Qin J, Ma L, Li H, Li L, Ning C, et al. Rosoloactone: a natural diterpenoid inducing apoptosis in human cervical cancer cells through endoplasmic reticulum stress and mitochondrial damage. Biomed Pharmacother. 2017;95:355–62. doi: 10.1016/j.biopha.2017.08.069. [DOI] [PubMed] [Google Scholar]
- 51.Hardie DG. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–52. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Pradelli LA, Beneteau M, Chauvin C, Jacquin MA, Marchetti S, Munoz-Pinedo C, et al. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene. 2010;29:1641–52. doi: 10.1038/onc.2009.448. [DOI] [PubMed] [Google Scholar]
- 54.Zhao F, Huang W, Zhang Z, Mao L, Han Y, Yan J, et al. Triptolide induces protective autophagy through activation of the CaMKKbeta-AMPK signaling pathway in prostate cancer cells. Oncotarget. 2016;7:5366–82. doi: 10.18632/oncotarget.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Z, Fan C, Yang Y, Di S, Hu W, Li T, et al. Thapsigargin sensitizes human esophageal cancer to TRAIL-induced apoptosis via AMPK activation. Sci Rep. 2016;6:35196. doi: 10.1038/srep35196. [DOI] [PMC free article] [PubMed] [Google Scholar]