Abstract
Paeoniflorin-6’-O-benzene sulfonate (CP-25) is a novel compound derived from paeoniflorin that has been demonstrated to have therapeutic effects in a rat model of rheumatoid arthritis (RA). However, the underlying mechanism has not been elucidated to date. We explored this mechanism in the present study by treating rats with adjuvant arthritis (AA) with CP-25. We found that the membrane EP4 protein level was downregulated; whereas, GRK2 was upregulated, in fibroblast-like synoviocyte (FLS)s of AA rats. Prostaglandin (PGE)2 stimulated FLS proliferation and enhanced the membrane EP4 receptor protein level; the latter was reversed by the administration of an EP4 receptor agonist, whereas the membrane GRK2 protein level gradually increased. The changes in the EP4 receptor and GRK2 expression were enhanced by TNF-α, and the former was accompanied by an alteration in the cyclic (c)AMP level. The EP4 receptor agonist stimulation increased the association between GRK2 and the EP4 receptor. GRK2 knockdown abrogated the abnormalities in FLS proliferation. The CP-25 treatment (100 mg/kg) suppressed joint inflammation with an efficacy that was similar to that of methotrexate. This finding was associated with EP4 upregulation and GRK2 downregulation in FLSs. Thus, GRK2 plays an important role in the abnormal FLS proliferation observed in AA possibly by promoting EP4 receptor desensitization and decreasing the cAMP level. Our results demonstrate that CP-25 has therapeutic potential for the treatment of human RA via GRK2 regulation.
Keywords: adjuvant-induced arthritis, paeoniflorin-6’-O-benzene sulfonate, fibroblast-like synoviocytes, G protein-coupled receptor kinase 2, prostaglandin E2
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by the abnormal proliferation of fibroblast-like synoviocytes (FLSs) and the infiltration of immune cells into synovial tissue [1, 2], resulting in the production of factors, such as prostaglandin (PG)E2, tumor necrosis factor (TNF)-α, and interleukin (IL)-6 that drive inflammation and pannus formation, leading to cartilage destruction and bone erosion [3–5]. Both the incidence and prevalence of RA are increasing. Anti-rheumatic drugs, non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticosteroids, and other agents used for RA treatment have adverse effects; thus, the rate of disability from RA remains high. Therefore, there is an urgent need for more effective therapeutic strategies.
PGE2 mediates inflammation through one of four PGE2 (EP) (EP1–4) receptors. The EP2 and EP4 receptors are coexpressed in FLSs in RA patients [6]. PGE2 is detected in serum and synovial fluid from these patients and plays an important role in disease pathogenesis [6, 7]. NSAIDs improve RA symptoms, such as morning stiffness and joint pain, by inhibiting cyclooxygenase activity and suppressing the production of prostanoids, including PGE2 [8–10]. Neutralizing antibodies against PGE2 reversed paw edema and interleukin (IL)-6 production in an animal model of polyarthritis, whereas EP4 receptor-deficient animals exhibited decreased incidence and severity of collagen-induced arthritis (CIA) in mice [11]. These findings suggest that PGE2 promotes disease progression at least partially by binding the EP4 receptor. The role of PGE2 in the regulation of immune responses is complex; previous studies have shown that the EP4 receptor exerted anti-inflammatory effects in the brain by blocking lipopolysaccharide-induced pro-inflammatory gene expression, whereas peripheral EP4 receptor activation protected the brain from systemic inflammation [12]. The PGE2 analog PGE1 improved arthritis in a CIA model [13]. The intracapsular administration of an EP4 receptor agonist inhibited inflammatory reactions in arthritis [14]. Finally, endogenous PGE2 produced in the rheumatoid synovium was found to negatively regulate aberrant rheumatoid synovial tissue overgrowth and osteoclast activity through EP4 [15]. Thus, the role of PGE2 and its receptors in RA is controversial.
EPs are G protein-coupled receptors (GPCRs) that are regulated by G proteins, G protein-coupled receptor kinases (GRKs), and β-arrestins. GRK activation of GPCRs results in receptor desensitization [16]. GRK2, which was formerly known as β-adrenergic receptor kinase-1, is a member of the GRK family of Ser/Thr protein kinases that phosphorylates and rapidly desensitizes GPCRs. Our previous studies showed that in the synovium of rats with adjuvant-induced arthritis (AA), EP2 and EP4 receptor expression and the cyclic (c)AMP level were decreased, suggesting that abnormal proliferation in FLSs is associated with G protein/adenylate cyclase/cAMP signaling in arthritis [17, 18]. Furthermore, in a CIA model, GRK2 was upregulated in the synovium during inflammation; this effect was reversed by paeoniflorin (Pae), which also inhibited FLS proliferation in vitro [19]. However, the precise mechanism by which the EP receptors are regulated by GRK2 and the relationship between the EP receptors and GRK2 in FLSs in RA patients remain to be elucidated.
Total glucosides of peony (TGP) are the first anti-inflammatory immune regulatory drug approved for RA treatment in China. Paeoniflorin-6’-O-benzene sulfonate (CP-25) is a monomer derived from a structural modification of Pae, which is the active ingredient of TGP [20]. CP-25 has shown more potent anti-inflammatory and immune regulatory activities than Pae in an RA model [20, 21]. In vitro studies suggest that CP-25 not only inhibits the growth and cytokine secretion capacity of FLSs, but also regulates dendritic cell function via PGE2 and TNF-α signaling [22, 23], although the detailed mechanism of action of CP-25 is not fully understood.
The current study investigated whether GRK2 is regulated by PGE2 to modulate EP receptor signaling during RA progression and whether the novel compound CP-25 targets GRK2 during this process.
Materials and methods
Animals
Male Sprague-Dawley rats (180 ± 20 g) were obtained from the Experimental Animal Center of Anhui Medical University (Hefei, China) and housed in a room with a relative humidity of 50% ± 10% and temperature of 22 °C ± 2 °C under pathogen-free conditions with freely available food and water. All experiments were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Pharmacology, Anhui Medical University.
Drugs and reagents
CP-25 was provided by the Chemistry Laboratory of the Institute of Clinical Pharmacology of Anhui Medical University, and its purity was >98.8% [20]. Methotrexate (MTX) was purchased from Shanghai Pharmaceutical Co. (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was obtained from HyClone (Logan, UT, USA). PGE2 was obtained from Cayman Chemical (Ann Arbor, MI, USA). The enzyme-linked immunosorbent assay (ELISA) kits for TNF-α and PGE2 were obtained from RayBiotech (Norcross, GA, USA) and R&D Systems (Minneapolis, MN, USA), respectively. Cell Counting Kit (CCK)-8 was obtained from Sigma-Aldrich (St. Louis, MO, USA). The anti-EP4 (sc-55596) and anti-GRK2 (sc-562) antibodies were obtained from the Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rat GRK2 short interfering (si)RNA was purchased from Shanghai GenePharma Co. (Shanghai, China).
AA induction and treatment
The rat AA model was established with an intradermal injection of 0.1 mL complete Freund’s adjuvant (CFA) emulsion prepared by resuspending heat-killed Mycobacterium butyricum in liquid paraffin at 10 mg/mL into the right hind metatarsal footpad. The rats were randomly divided into the following six groups: normal, AA, CP-25 (25, 50, and 100 mg/kg) and MTX (0.5 mg/kg) (n = 10/group). After the onset of arthritis on day 17, the rats were given CP-25 (once per day) for 14 days and MTX (every 3 days for a total of five times) by intragastric administration. The normal and AA rats were given equal volumes of 0.5% sodium carboxymethylcellulose.
Assessment of polyarthritis index
From day 17 after immunization, the polyarthritis index of each limb was determined every 2 days by two independent investigators with no knowledge of the treatment protocol using a macroscopic scoring system ranging from 0 to 4, yielding total scores of 0–16 per animal [24].
Histopathological examination
The rats were killed on day 32 after immunization. The left ankle joints were removed, fixed in 10% formalin, and decalcified in 5% HNO3 before embedding in paraffin. The sections were cut and stained with hematoxylin and eosin and examined microscopically by two independent investigators to assess synovial proliferation, cell infiltration, pannus formation, and cartilage erosion (on a scale of 0–4) as previously described [25].
FLS cultures
FLS cultures were established by the tissue transplantation method. Briefly, synovial tissue from the knee joints of the rats was cut into small pieces, washed extensively, and cultured in 100-mm dishes containing DMEM with 20% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5% CO2. After 7 days of incubation, the tissue was removed, and the confluent adherent cells were trypsinized and divided into a 1:3 ratio. Spindle-shaped cells from passages 3 to 8 constituting a homogeneous population of synoviocytes were used for the experiments.
Analysis of FLS proliferation
FLSs were resuspended at a density of 1 × 105 cells/mL in 96-well flat-bottomed culture plates. The cultures were incubated at 37 °C in 5% CO2 for 48 h. At 4 h before the end of the incubation period, 10 μL CCK-8 reagent was added to each well. The absorbance at 490 nm was measured with a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Measurement of cytokine levels
The serum concentrations of TNF-α and PGE2 were measured using ELISA kits according to the manufacturers’ instructions.
cAMP detection
FLSs were seeded at a density of 2 × 105 cells/well in six-well plates. TNF-α (20 ng/mL) was added to each well, followed by overnight culture. Then, the cells were incubated with the EP4 agonist CAY10598 (1 μM) for various time periods, followed by preincubation with PGE2 (10 μM) for 30 min. Subsequently, at various time points, 20 μL of 50 mM ethanoic acid was added to the well to terminate the reaction. The adherent cells were collected by scraping and disrupted by sonication. The cell lysates were collected by centrifugation, and the cAMP concentration in the FLS was measured with an ELISA kit according to the manufacturer’s instructions.
Confocal microscopy
FLSs were cultured in 35-mm glass-bottom dishes and fixed with 4% paraformaldehyde for 30 min at 4 °C. The cells were permeabilized with 0.1% bovine serum albumin and 0.1% saponin for 30 min at 4 °C and then incubated overnight at 4 °C with monoclonal anti-EP4 receptor and anti-GRK2 antibodies in a wet chamber. After washing with phosphate-buffered saline (PBS), the cells were incubated for 1 h at room temperature with Cy3-conjugated rabbit anti-mouse and fluorescein isothiocyanate-conjugated goat anti-rabbit antibodies. Images of the fluorescent signal were acquired under a TCS SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Flow cytometry
EP4 receptor expression on the surface of FLSs was detected by flow cytometry with a monoclonal anti-EP4 receptor antibody. Briefly, 1 × 106 treated FLSs were washed twice with PBS and labeled for 1 h at 37 °C with the antibody. After repeated washing, the cells were incubated with phycoerythrin (PE)-conjugated rabbit anti-mouse IgG for 30 min at room temperature. The cells were washed and resuspended in PBS, and the signal intensity was detected by fluorescence-activated cell sorting (FC500; Beckman Coulter, Brea, CA, USA). The basal cell fluorescence intensity was determined in cells labeled with the secondary antibody alone. The data were analyzed using FlowJo 7.6 software (Tree Star, Ashland, OR, USA).
The intracellular GRK2 expression in FLSs was detected with a monoclonal anti-GRK2 antibody. Briefly, the cells were washed, permeabilized and labeled with an antibody for 60 min at 37 °C. The cells were washed with cold PBS and incubated for 30 min at room temperature with PE-conjugated goat anti-rabbit IgG. The subsequent analysis was performed as described above.
Western blot analysis
FLSs were lysed and centrifuged at 14 000 r/min for 15 min at 4 °C. GRK2 and the EP4 receptor in the supernatant containing cytosolic proteins were detected by Western blotting. Some of the supernatant was centrifuged at 100 000 r/min for 60 min at 4 °C. The precipitate containing the membrane proteins was resuspended in cell lysis buffer comprising 20 mM HEPES and 1 mM phenylmethylsulfonyl fluoride and used to detect the EP4 receptor. The protein concentration was determined using the Lowry method. Aliquots of each sample containing equal amounts of protein were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and β-actin was used as a loading control. The proteins were transferred onto a polyvinylidene difluoride membrane (TransBlot Turbo transfer system; Bio-Rad, Hercules, CA, USA), which was blocked in 0.05% Tween 20-PBS with 5% skim milk for 2 h at 37 °C. The blot was incubated overnight at 4 °C with a mouse monoclonal antibody against the EP4 receptor (1:400) and a rabbit polyclonal antibody against GRK2 (1:500). After incubation for 2 h at 37 °C with a horseradish peroxidase-conjugated secondary antibody (1:5000), the immunoreactivity was detected by enhanced chemiluminescence. The optical density of the protein bands was determined from images and normalized to the level of β-actin. The experiment was performed in duplicate.
Coimmunoprecipitation assay
To examine the association between GRK2 and the EP4 receptor in FLSs after EP4 receptor agonist stimulation, the total cell lysate was immunoprecipitated using an anti-EP4 receptor antibody and analyzed by Western blotting with an anti-GRK2 or -EP4 receptor antibody. Briefly, 1000 μg of the total protein per lysate were incubated overnight at 4 °C with 10 μL of an anti-EP4 antibody on a rotating shaker, and 20 μL of protein A/G PLUS-agarose beads (Santa Cruz, CA, USA) were added to each tube. After 2 h on a rotating shaker at 4 °C, the tubes were centrifuged at 14 000 × g for 5 s, and the pellets were washed three times with lysis buffer before boiling in 4X loading buffer for 5 min for the Western blot analysis. The blots were probed with specific antibodies against the EP4 receptor and GRK2. The GRK2 level was corrected by EP4 receptor densitometry. The experiment was performed in duplicate.
Small interfering (si)RNA-mediated knockdown of gene expression
GRK2 expression was silenced in FLSs using the following three siRNAs: GRK2-rat-585 (sense: 5′-CCAUACAUUGAGGAGAUUUTT-3′, anti-sense: 5′-AAAUCUCCUCAAUGUAUGGTT-3′), GRK2-rat-673: (sense: 5′-GGAAGAAUGUAGAGCUCAATT-3′, anti-sense: 5′-UUGAGCUCUACAUUCUUCCTT-3′), and GRK2-rat-796 (sense: 5′-CCAUGAAGUGUCUGGACAATT-3′, anti-sense: 5′-UUGUCCAGACACUUCAUGGTT-3′). The cells were transfected at 70% confluence in six-well plates using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. After 48 h, the GRK2 knockdown was confirmed by Western blotting, and the FLSs were used for analyses of proliferation, EP4 receptor expression, and cAMP levels.
Statistical analysis
The data are expressed as the mean ± standard deviation. Comparisons between two and multiple groups were carried out with Student’s t-tests and analyses of variance, followed by Tukey’s post hoc test, respectively. P < 0.05 was considered statistically significant.
Results
EP4 receptor signaling is suppressed in FLSs from AA rats
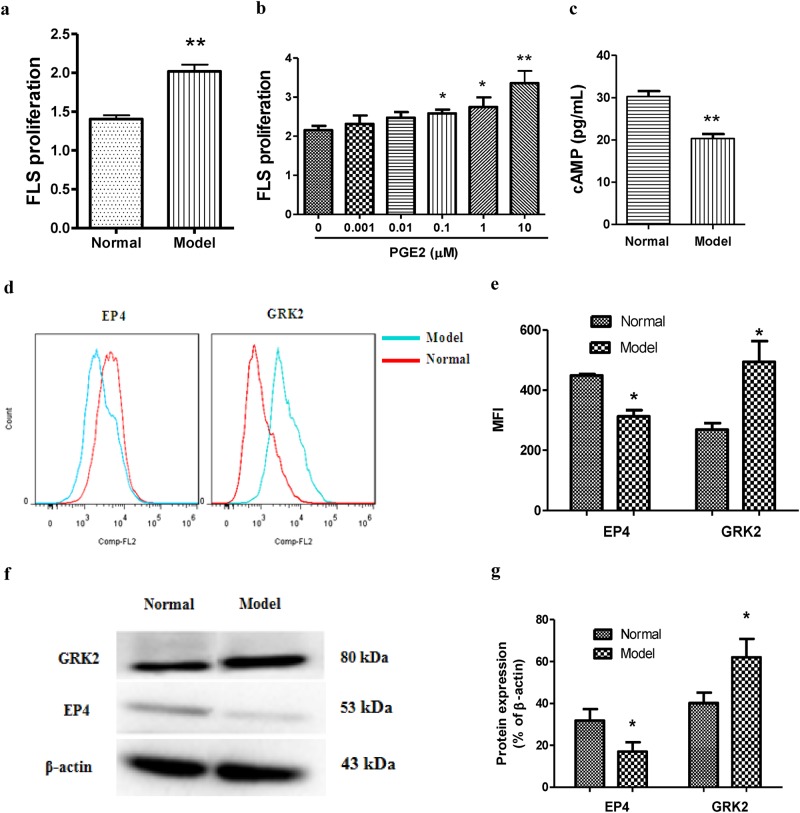
RA is characterized by abnormal FLS proliferation, and inflammatory mediators and cytokines, such as PGE2 and TNF-α, play key roles during this process. We observed that FLS proliferation was increased in the AA rat compared to that in the normal rats (Fig. 1a). PGE2 stimulated FLS proliferation in a concentration-dependent manner, and the optimum concentration was 10 μM (Fig. 1b). To determine whether abnormal FLS proliferation was caused by dysregulation of EP signaling, we assessed adenylyl cyclase activity in FLSs and found that the cAMP level (Fig. 1c) and membrane EP4 protein level (Fig. 1d–g) were decreased in the FLSs in the AA rats compared to those in the control rats as determined by the Western blotting and flow cytometry analyses, suggesting that the progression of inflammation in AA was associated with EP4 receptor desensitization.
Fig. 1.
FLS abnormal proliferation and EP4 receptor desensitization are more obvious in FLS from AA rats than those from control rats. a FLS was suspended in DMEM at a concentration of 2 × 105 cells/mL, and proliferation was examined using a CCK assay. b FLSs from normal rats were treated with different concentrations of PGE2 for 48 h, and proliferation was measured by a CCK-8 assay in vitro. c The level of cAMP in the FLS was assayed according to the manufacturer’s instructions. d–e The protein levels of GRK2 and the EP4 receptor were detected by flow cytometry. f–g The protein levels of GRK2 and the EP4 receptor were detected by Western blotting. *P < 0.05, **P < 0.01 vs. normal ( ± s, n = 3)
GRK2 plays an important role in agonist-stimulated GPCR desensitization. To determine whether EP4 receptor desensitization is regulated by GRK2, we examined the GRK2 levels in FLSs by Western blotting and flow cytometry and found that the protein was upregulated in the AA rats relative to that in the normal rats (Fig. 1d–g).
PGE2 pretreatment enhances agonist-induced EP4 receptor desensitization
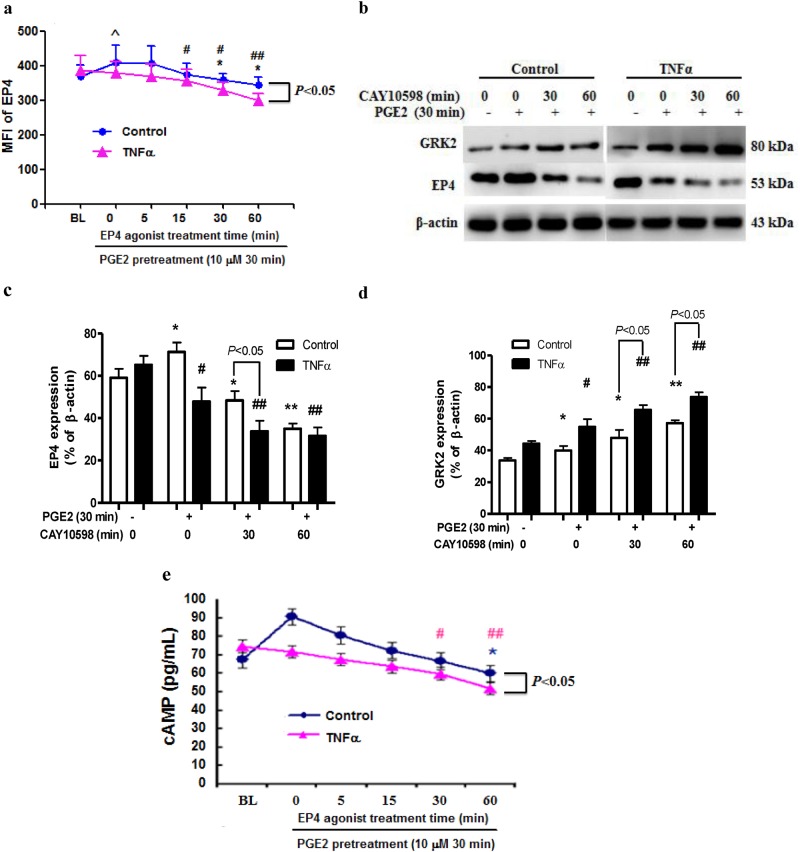
The protein level of the EP4 receptor was increased after the 30-min PGE2 stimulation (Fig. 2a). The treatment with the EP4 receptor agonist CAY10598 reduced the surface EP4 expression in the FLSs following the PGE2 pretreatment as determined by flow cytometry. The Western blotting also revealed that the CAY10598-induced EP4 receptor desensitization in the FLSs was enhanced by the PGE2 pretreatment (Fig. 2b, c). The EP4 receptor desensitization was associated with an increased membrane GRK2 protein level (Fig. 2b), indicating that GRK2 regulatory molecules contribute to GPCR internalization.
Fig. 2.
PGE2 pretreatment enhances agonist-induced EP4 receptor desensitization. a The change in the EP4 receptor in membrane FLSs treated with an EP4 agonist. ^P < 0.05 vs. BL in the control group; *P < 0.05 vs. 0 min in the control group; #P < 0.05, ##P < 0.01 vs. BL in the TNF-α group ( ± s, n = 3). BL: basal EP4 receptor expression in the control and TNF-α treated cells without any agonist stimulation. b The change in the EP4 receptor and GRK2 was detected by Western blotting in membrane of FLSs treated with an EP4 receptor agonist. Densitometric analysis of the EP4 receptor c and GRK2 d immunoblots in bar chart form. *P < 0.05, **P < 0.01 vs. PGE2 (−) in the control group, #P < 0.05, ##P < 0.01 vs. PGE2 (−) in the TNF-α group ( ± s, n = 3). e The level of cAMP in FLSs treated with an EP4 receptor agonist at different intervals. *P < 0.05 vs. BL in the control group, #P < 0.05, ##P < 0.01 vs. BL in the TNF-α group ( ± s, n = 3). BL: basal cAMP level in the control and TNF-α treated cells without any agonist stimulation
Pro-inflammatory cytokines (TNF-α and IL-1β and -6) play a significant role in RA. We examined whether inflammation promotes the agonist-dependent EP4 receptor desensitization of FLS and found that compared to the control group, the downregulation of the EP4 receptor and upregulation of GRK2 were enhanced in the presence of TNF-α (Fig. 2b–d). Moreover, the cAMP level was altered in conjunction with the changes in the EP4 membrane protein level (Fig. 2e). These results indicate that under inflammatory conditions, EP4 receptor activation leads to its desensitization, resulting in a decrease in the cAMP level [26].
Effects of EP4 receptor stimulation on the association between GRK2 and the EP4 receptor
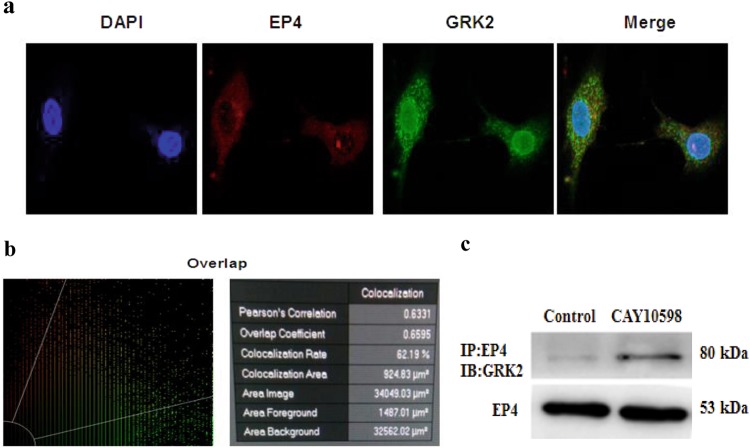
We examined the interaction between GRK2 and the EP4 receptor by immunofluorescence labeling and laser scanning confocal microscopy. The EP4 receptor and GRK2 were coexpressed on the membrane of FLSs (Fig. 3a) with an overlap coefficient of 0.6595 (Fig. 3b). This finding suggested that an interaction exists between the two proteins. This interaction was further examined with a coimmunoprecipitation assay following receptor activation. FLSs pretreated with PGE2 were stimulated with CAY10598 for 1 h; the EP4 receptor was immunoprecipitated from FLS extracts, and the presence of GRK2 was detected by Western blotting. The results demonstrated little interaction between GRK2 and the EP4 receptor in FLSs under normal conditions, but EP4 receptor activation induced their association (Fig. 3c).
Fig. 3.
Effects of EP4 receptor agonist stimulation on the association between GRK2 and the EP4 receptor. a Confocal imaging of GRK2 and the EP4 receptor in FLSs. b The co-localization between two proteins of the EP4 receptor and GRK2 in the FLS. c CAY10598-stimulated FLS lysates were immunoprecipitated using an anti-EP4 receptor antibody and subjected to Western blotting to visualize GRK2 to evaluate its association with the EP4 receptor
GRK2 knockdown abrogates abnormal FLS proliferation in response to PGE2
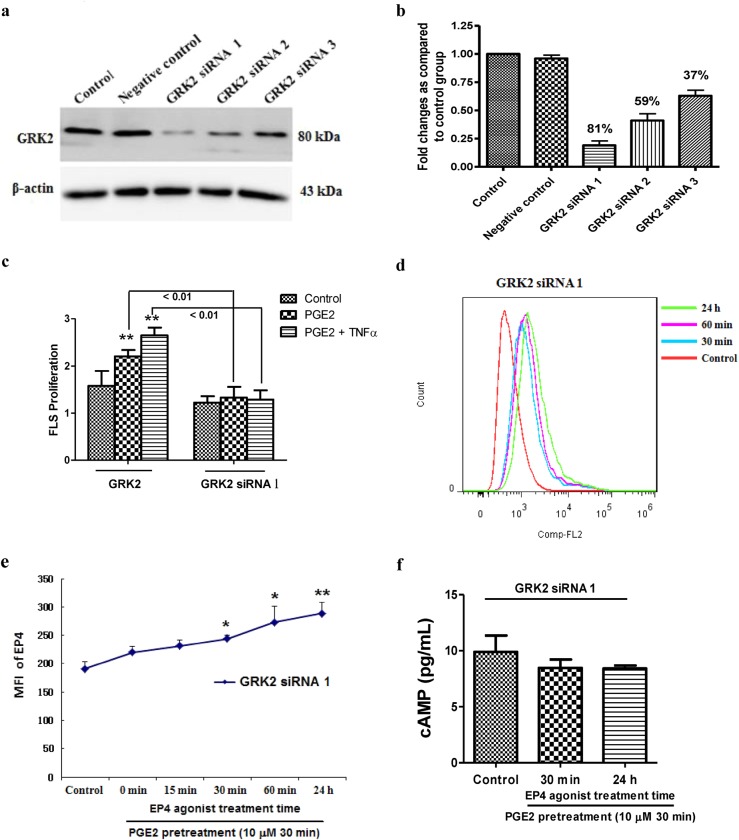
To further assess the role of GRK2 in mediating PGE2-induced EP4 receptor desensitization and abnormal FLS proliferation, GRK2 expression was silenced in FLSs, and EP4 receptor surface expression and the effect on FLS proliferation were examined. Of the three siRNAs tested, GRK2-rat-796 siRNA caused the greatest decrease in GRK2 protein expression in the FLSs (81%; Fig. 4a, b); therefore, GRK2-rat-796 siRNA was used in the subsequent experiments.
Fig. 4.
Effects of GRK2 knockdown on FLS function and EP4 receptor signaling. a, b Verification of effective sequence of siRNA for FLS GRK2 gene silencing (GRK2-rat-796: GRK2 siRNA 1, GRK2-rat-673: GRK2 siRNA 2, GRK2-rat-585: GRK2 siRNA 3). c Effects of GRK2 knockdown on FLS proliferation. **P < 0.01 vs. control ( ± s, n = 3). d, e The change in the EP4 receptor on the cell membrane in response to the EP4 receptor agonist treatment in FLSs transfected with GRK2 siRNA 1 was detected by flow cytometry. *P < 0.05, **P < 0.01 vs. control ( ± s, n = 3). f The level of cAMP in GRK2 silencing FLSs stimulated by an EP4 receptor agonist ( ± s, n = 3)
The GRK2 knockdown inhibited FLS proliferation in response to the stimulation by PGE2 or PGE2 combined with TNF-α (Fig. 4c). Furthermore, the GRK2 knockdown abolished the influences of the EP4 agonist on FLS surface EP4 receptor expression (Fig. 4d, e) and the suppressive effect of the EP4 receptor agonist on the cAMP level in FLSs stimulated by PGE2 (Fig. 4f). These results demonstrate that GRK2 is a key mediator of abnormal FLS proliferation, which may occur through enhancement of EP4 receptor desensitization and reduction in cAMP level.
CP-25 suppresses pro-inflammatory cytokine production and FLS proliferation in AA
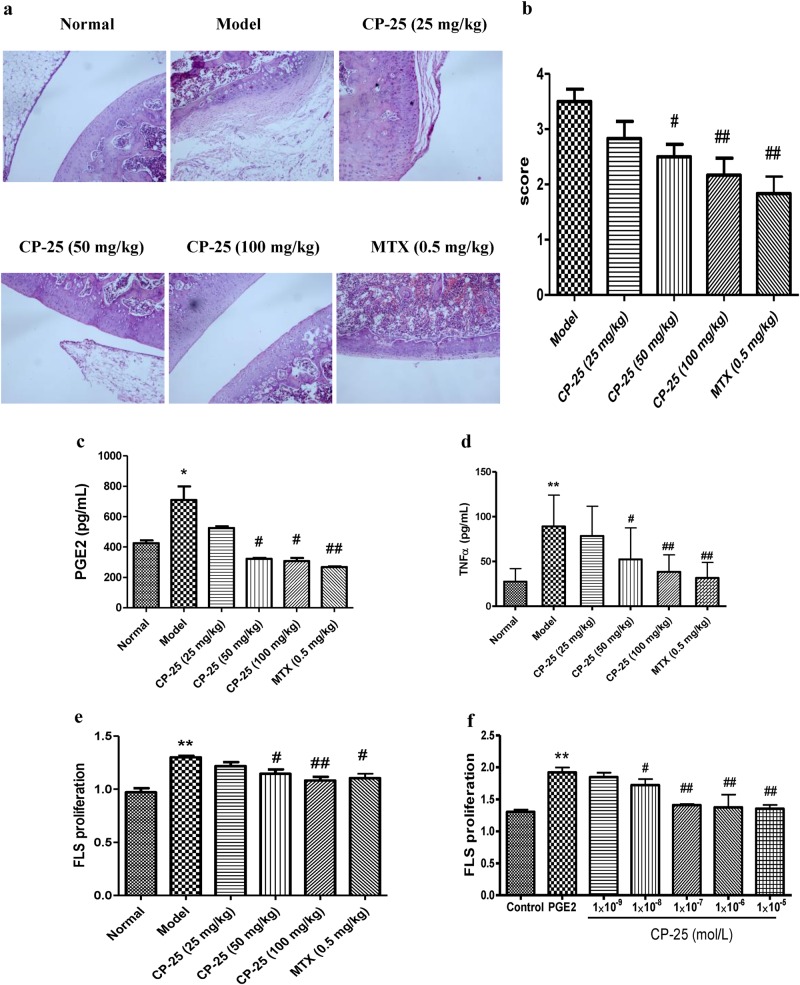
To assess the therapeutic effects of CP-25, we established a rat model of RA by injecting CFA. Arthritis gradually developed, and the polyarthritis index was increased on day 17 relative to that in the normal group. At the onset of arthritis on day 17, the rats were intragastrically administered CP-25 for 14 days, which decreased the polyarthritis index compared to that in the AA rats (Table 1). The histopathological examination revealed that in the model rats, synovial hyperplasia was increased, which was accompanied by inflammatory cell infiltration, pannus formation, articular cartilage, and bone erosion, and these effects were alleviated by the CP-25 (50 and 100 mg/kg) and MTX treatment (Fig. 5a, b).
Table 1.
Effects of CP-25 on arthritis index in AA rats
| Groups | Dose (mg/kg) | d17 | d20 | d23 | d26 | d29 | d32 |
|---|---|---|---|---|---|---|---|
| Model | — | 10.22 ± 0.94 | 11.44 ± 0.97 | 11.67 ± 1.08 | 10.56 ± 0.97 | 9.67 ± 0.99 | 8.86 ± 1.02 |
| CP-25 | 25 | 10.70 ± 1.25 | 10.90 ± 0.95 | 9.70 ± 0.68 | 8.70 ± 0.70 | 6.90 ± 0.57* | 4.90 ± 0.66** |
| 50 | 10.10 ± 1.33 | 10.10 ± 1.00 | 9.90 ± 1.12 | 7.40 ± 0.76 | 5.70 ± 0.67** | 4.10 ± 0.72** | |
| 100 | 10.60 ± 1.15 | 10.60 ± 0.91 | 8.30 ± 0.73 | 6.50 ± 0.73 | 5.10 ± 0.79** | 3.40 ± 0.69** | |
| MTX | 0.5 | 10.70 ± 1.23 | 10.60 ± 1.08 | 7.90 ± 0.85 | 5.70 ± 0.77** | 3.60 ± 0.64** | 2.00 ± 0.45** |
*P < 0.05, **P < 0.01 vs. model group ( ± s, n = 8)
Fig. 5.
Effects of CP-25 on AA rats. a The effects of CP-25 on the joint histopathology of AA rats. b A bar graph of the joints histological score is shown. #P < 0.05, ##P < 0.01 vs. model group ( ± s, n = 6). c–d Effects of CP-25 on PGE2 and TNF-α in AA rats. e Effects of CP-25 on FLS proliferation in AA rats. *P < 0.05, **P < 0.01 vs. normal group; #P < 0.05, ##P < 0.01 vs. model group ( ± s, n = 6–8). f Effects of CP-25 on FLS proliferation under stimulated PGE2 in vitro. **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. PGE2 ( ± s, n = 3)
The increase in FLS proliferation in the AA rats compared to that in the control rats was accompanied by higher levels of inflammatory cytokines and mediators. Compared to the AA rats, the CP-25-treatment decreased the serum levels of PGE2 and TNF-α (Fig. 5c, d) and suppressed FLS proliferation (Fig. 5e). Similar results were obtained in vitro upon the treatment of FLSs with CP-25 following stimulation with PGE2 (Fig. 5f).
CP-25 modulates GRK2 and EP4 receptor signaling in rats with AA
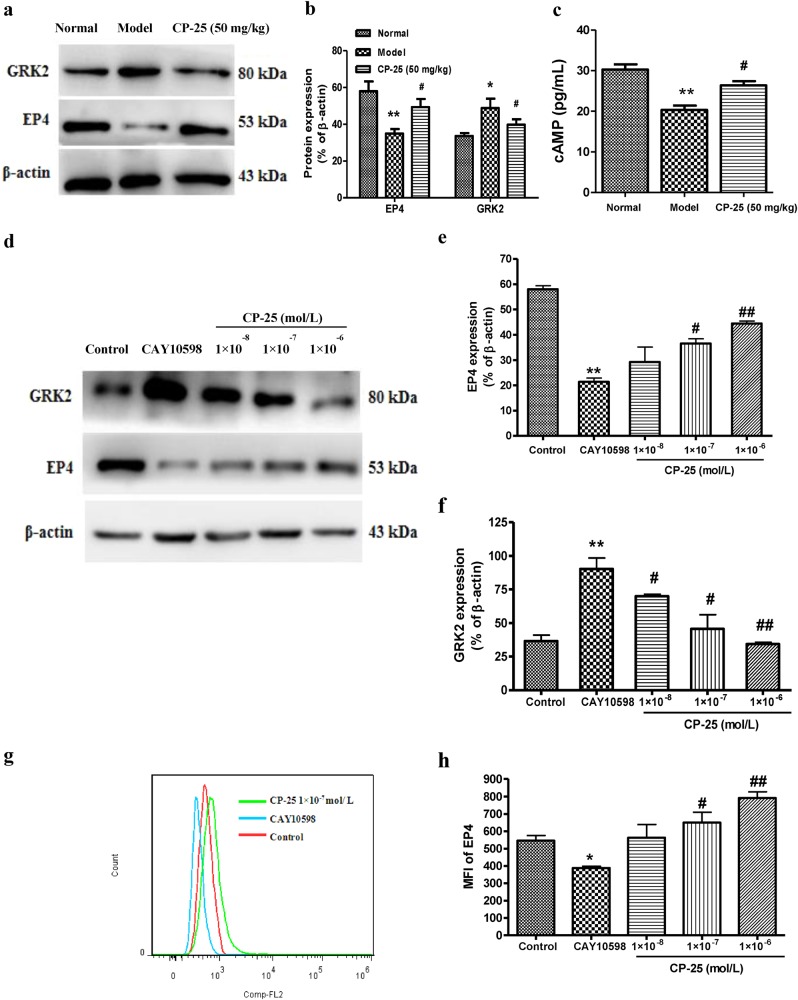
To clarify the mechanism underlying the protective effect of CP-25, we investigated the GRK2 and EP4 receptor signaling pathways in rats with AA. Compared to the normal rats, GRK2 was upregulated, whereas the EP4 receptor was downregulated in the FLSs of the AA rats (Fig. 6a, b). The treatment with CP-25 inhibited GRK2 and increased the EP4 receptor and cAMP levels (Fig. 6a–c), suggesting that CP-25 induces a protective against RA via the regulation of EP4.
Fig. 6.
CP-25 inhibits arthritis progression in AA rats by modulating EP4 receptor signaling. a–b Effects of CP-25 on the expression of GRK2 and the EP4 receptor in FLSs in vivo. c Effects of CP-25 on the level of cAMP. *P < 0.05, **P < 0.01 vs. normal, #P < 0.05 vs. model ( ± s, n = 4). d–h Effects of CP-25 on the membrane EP4 receptor and GRK2 protein level on FLSs treated with an EP4 agonist in vitro. The EP4 receptor and GRK2 were identified by Western blotting d–f. The EP4 receptor was identified by flow cytometry g–h. *P < 0.05, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. CAY10598 ( ± s, n = 4)
To confirm this possibility, we analyzed the membrane EP4 receptor protein level by Western blotting in cultured FLSs and found that it was downregulated following the 24-h CAY10598 treatment; this effect was reversed by CP-25 (1 × 10−7 and 1 × 10−6 mol/L) (Fig. 6d), which is consistent with the results of the flow cytometry analysis (Fig. 6g, h). Furthermore, CP-25 (1 × 10−7 and 1 × 10−6 mol/L) inhibited the GRK2 membrane protein level in the FLSs (Fig. 6d–f).
Discussion
A major hallmark of RA is progressive damage to the articular cartilage and bone. FLSs contribute to the degradation of cartilage and bone through abnormal proliferation and synthesis and secretion of pro-inflammatory cytokines (TNF-α and IL-1). PGE2 is present at high concentrations in the serum and synovial fluid of patients with RA [6, 7], and its deficiency has been shown to have a protective effect in RA models [11, 27]. The widespread use of NSAIDs for RA treatment highlights the importance of prostanoids as drivers of inflammation in this disease [28]. Paradoxically, PGE2 and EP4 receptor agonists have also been shown to have anti-inflammatory effects in various animal models [12–15], suggesting that PGE2 acting through the EP4 receptor plays both pro- and anti-inflammatory roles in vivo that contribute to the pathogenesis of RA. Thus, whether drugs should be developed to activate or block the receptor depends on the state of receptor desensitization. We recently proposed the soft regulation of inflammatory immune response (SRIIR) as a new direction for the development of drugs for the treatment of diseases related to inflammatory immune responses, such as RA [29]. SRIIR drugs modulate rather than completely suppress cellular activities and gene and protein expression to achieve physiological levels and thereby re-establish cellular homeostasis, which could reduce adverse reactions [29].
The results of the present study showed that PGE2 stimulated FLS proliferation in a concentration-dependent manner, which is consistent with the data obtained in preclinical models of RA, indicating that PGE2 exacerbates disease symptoms. However, which PGE2 (EP1–4) receptor contributes to RA progression remains unclear. The EP4 receptor, which has a long serine/threonine-rich C-terminal domain, shows rapid agonist-induced desensitization [30]. However, whether the EP4 receptor is phosphorylated in RA, which could lead to receptor desensitization and RA progression, is unknown. Our Western blotting and flow cytometry analyses revealed that the membrane EP4 receptor and intracellular cAMP levels were reduced in FLSs from AA rats compared to those in the control rats. The PGE2 pretreatment increased the EP4 receptor membrane protein level, which returned to the basal level upon the application of an EP4 receptor agonist; concomitant changes in the cAMP level were observed. Under inflammatory conditions, the membrane EP4 receptor protein level was decreased following the pretreatment with PGE2, suggesting that EP4 receptor desensitization resulted in a decrease in the cAMP level. Thus, the EP4 receptor/cAMP signaling plays a role in the abnormal FLS proliferation observed in RA.
The EP4 receptor is a GPCR with seven transmembrane domains, including a long serine and threonine-rich carboxyl-terminal domain. The agonist-induced receptor desensitization of GPCR has been shown to be mediated by receptor phosphorylation through either PKA or GRK [31]. The phosphorylation of PKA sites in the third intracellular loop and the C-terminal domain has been discussed as a possible mechanism of EP4 receptor desensitization [30]. However, some research has shown that the phosphorylation of the carboxyl-terminal EP4 receptor domain possibly by GRKs but not by PKA may be involved in the agonist-induced desensitization of the EP4 receptor [32, 33]. GRKs are serine/threonine protein kinases that phosphorylate intracellular serine/threonine residues of activated receptors, and β-arrestins uncouple phosphorylated GPCRs from heterotrimeric G protein-complexes, leading to receptor desensitization [34–36]. GRKs and β-arrestin proximally regulate β-adrenergic receptor desensitization, but knowledge regarding the mechanism by which GRKs regulate EP receptors is limited. Previous studies have shown that the agonist-induced desensitization of the EP4 receptor was mediated by GRKs and that residues essential for the desensitization of the EP4 receptor were located between 370 and 383 of serine [32, 33]. GRK2 is upregulated in experimental arthritis and regulates cellular responses during inflammation [19, 25, 37]. Here, we found that GRK2 expression was upregulated in FLSs in AA rats. To determine whether the increased expression of GRK2 is related to the decreased expression of the EP4 receptor, we used PGE2 to stimulate FLSs and found that the membrane GRK2 level was upregulated; the stimulation with the EP4 agonist and the pro-inflammatory cytokine TNF-α further enhanced this effect.
A laser scanning confocal microscopy analysis and the results of the coimmunoprecipitation assay showed that GRK2 interacts with the EP4 receptor in FLSs. Moreover, the abnormal FLS proliferation induced by PGE2 was almost completely abrogated and the membrane EP4 receptor level in the FLSs was restored by the GRK2 silencing following the stimulation with the EP4 receptor agonist CAY10598. These findings indicate that GRK2 plays an important role in the abnormal proliferation of FLSs in RA possibly by mediating EP4 receptor desensitization and decreasing the cAMP level. Thus, therapeutic strategies targeting PGE2/EP4 receptor signaling represent a promising approach for RA treatment.
The inflammation implicated in RA pathogenesis is associated with GPCR activation by pro-inflammatory agonists, such as cytokines (TNF-α and IL-1) and prostaglandins (PGE2). CP-25, which is a new ester derivative of Pae, inhibited the serum PGE2 and TNF-α levels while decreasing the polyarthritis index and improving the histopathological score in a rat RA model. The efficacy of CP-25 is similar to that of MTX, which is the main anti-rheumatic drug treatment for patients with active RA [38]. CP-25 inhibited and enhanced the activation of M1 and M2 macrophages, respectively, by modulating the balance between pro- and anti-inflammatory cytokines and, consequently, IL-17 production. In addition, CP-25 suppressed the receptor activator of nuclear factor kappa B ligand and matrix metalloproteinase 9 expression, which enhanced its anti-osteoclastic effects [20]. The present study showed that CP-25 suppressed FLS proliferation (both in vitro and in vivo) and PGE2 and TNF-α secretion in AA rats, which was accompanied by the up- and down-regulation of the membrane EP4 and GRK2 protein levels, respectively, in FLSs. Thus, CP-25 blocks AA progression in rats by restoring normal immune function and inhibiting abnormal FLS proliferation via modulation of GRK2/EP4 receptor expression. Interesting, we previously showed that EP4 receptor expression was increased in splenic T cells from AA rats and that this observation was reversed by CP-25 treatment [39], implying that EP4 receptor expression varies between FLS and T cells in AA.
In summary, here, we demonstrated for the first time that GRK2 expression, agonist-stimulated EP4 receptor desensitization, and the resultant perturbation of FLS proliferation and cytokine (TNF-α) and PGE2 secretion promote RA progression. CP-25 reversed these effects, thus blocking AA development and progression. Our results demonstrate that CP-25 is a promising new drug for the soft regulation of the inflammatory immune responses contributing to RA progression.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81330081, 31200675, 81573443, 81302845, 81603362, 81673444, and 81603121), the Anhui Province Natural Science Foundation in the University (KJ2015A317), Anhui Province Natural Science Fund (outstanding youth) (170808J10), and Key Projects of Outstanding Young Talent Support Program in Colleges and Universities (gxyqZD2016043).
Author contributions
XYJ designed and performed the experiments and wrote the manuscript. YC participated in the design of the study and performed the experiments and statistical analysis. FW, XD, XJS, SX, and XZY performed the experiments. Y.-J.W. carried out the flow cytometry assays and helped revise the manuscript. HXW and CW participated in the design of the study and helped revise the manuscript. W.W. conceived the study and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 3.Lefèvre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15:1414–20. doi: 10.1038/nm.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillen J, Geyer C, Heitzmann M, Beckmann D, Krause A, Winkler I, et al. Structural cartilage damage attracts circulating rheumatoid arthritis synovial fibroblasts into affectedjoints. Arthritis Res Ther. 2017;19:40. doi: 10.1186/s13075-017-1245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprintedaggressors. Nat Rev Rheumatol. 2013;9:24–33. doi: 10.1038/nrrheum.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojima F, Naraba H, Sasaki Y, Beppu M, Aoki H, Kawai S. Prostaglandin E2 is an enhancer of interleukin-1beta-induced expression of membrane-associated prostaglandin E synthase in rheumatoid synovial fibroblasts. Arthritis Rheum. 2003;48:2819–28. doi: 10.1002/art.11261. [DOI] [PubMed] [Google Scholar]
- 7.Largo R, Díez-Ortego I, Sanchez-Pernaute O, López-Armada MJ, Alvarez-Soria MA, Egido J, et al. EP2/EP4 signalling inhibits monocyte chemoattractant protein-1 production induced by interleukin 1beta in synovial fibroblasts. Ann Rheum Dis. 2004;63:1197–204. doi: 10.1136/ard.2003.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JA, Warner TD. Cyclo-oxygenase-2: pharmacology, physiology, iochemistry and relevance to NSAID therapy. Br J Pharmacol. 1999;128:1121–32. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards JE, McQuay HJ, Moore RA. Efficacy and safety of valdecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. Pain. 2004;111:286–96. doi: 10.1016/j.pain.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med. 2008;74:1423–9. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 11.Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–91. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Johansson J, Woodling NS, Wang Q, Montine TJ, Andreasson K. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184:7207–18. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriuchi-Murakami E, Yamada H, Ishii O, Kikukawa T, Igarashi R. Treatment of established collagen induced arthritis with prostaglandin E1 incorporated in lipid microspheres. J Rheumatol. 2000;27:2389–96. [PubMed] [Google Scholar]
- 14.Omote K, Kawamata T, Nakayama Y, Yamamoto H, Kawamata M, Namiki A. Effects of a novel selective agonist for prostaglandin receptor subtype EP4 on hyperalgesia and inflammation in monoarthritic model. Anesthesiology. 2002;97:170–6. doi: 10.1097/00000542-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Shibata-Nozaki T, Ito H, Mitomi H, Akaogi J, Komagata T, Kanaji T, et al. Endogenous prostaglandin E2 inhibits aberrant overgrowth of rheumatoid synovial tissue and the development of osteoclast activity through EP4 receptor. Arthritis Rheum. 2011;63:2595–605. doi: 10.1002/art.30428. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 17.Chen Q, Wei W. Effects and mechanisms of glucosides of chaenomeles speciosa on collagen-induced arthritis in rats. Int Immunopharmacol. 2003;3:593–608. doi: 10.1016/S1567-5769(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 18.Xu HM, Wei W, Jia XY, Chang Y, Zhang L. Effects and mechanisms of total glucosides of paeony on adjuvant arthritis in rats. J Ethnopharmacol. 2007;109:442–8. doi: 10.1016/j.jep.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Chen JY, Wu HX, Chen Y, Zhang LL, Wang QT, Sun WY, et al. Paeoniflorin inhibits proliferation of fibroblast-like synoviocytes through suppressing G-protein-coupled receptor kinase 2. Planta Med. 2012;78:665–71. doi: 10.1055/s-0031-1298327. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Jia X, Wei F, Wang C, Sun X, Xu S, et al. CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage. Sci Rep. 2016;6:26239. doi: 10.1038/srep26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JY, Wang Y, Wu HX, Yan SX, Chang Y, Wei WA modified compound from paeoniflorin, CP-25, suppressed immune responses and synovium inflammation in collagen-induced arthritis mice. Front Pharmacol. Published: 07 June 2018 10.3389/fphar.2018.00563 [DOI] [PMC free article] [PubMed]
- 22.Jia X, Wei F, Sun X, Chang Y, Xu S, Yang X, et al. CP-25 attenuates the inflammatory response of fibroblast-like synoviocytes co-cultured with BAFF-activated CD4(+) T cells. J Ethnopharmacol. 2016;189:194–201. doi: 10.1016/j.jep.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Sheng K, Chen J, Wu Y, Zhang F, Chang Y, et al. Regulation of PGE2 signaling pathways and TNF-alpha signaling pathways on the function of bone marrow-derived dendritic cells and the effects of CP-25. Eur J Pharmacol. 2015;769:8–21. doi: 10.1016/j.ejphar.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Chang Y, Wu Y, Wang D, Wei W, Qin Q, Xie G, et al. Therapeutic effects of TACI-Ig on rats with adjuvant-induced arthritis via attenuating inflammatory responses. Rheumatology. 2011;50:862–70. doi: 10.1093/rheumatology/keq404. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Chen J, Song S, Yuan P, Liu L, Zhang Y, et al. β2-adrenoceptor signaling reduction in dendritic cells is involved in the inflammatory response in adjuvant-induced arthritic rats. Sci Rep. 2016;6:24548. doi: 10.1038/srep24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Long H, Wu Z, Jiang X, Ma L. EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol Biol Cell. 2008;19:2973–83. doi: 10.1091/mbc.e07-10-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110(5):651–8. doi: 10.1172/JCI0215528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33:155–67. doi: 10.1016/S0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 29.Wei W. Soft regulation of inflammatory immune responses. Chin Pharmacol Bull. 2016;32:297–303. [Google Scholar]
- 30.Nishigaki N, Negishi M, Ichikawa A. Two Gs-coupled prostaglandin E receptor subtypes, EP2 and EP4, differ in desensitization and sensitivity to the metabolic inactivation of the agonist. Mol Pharmacol. 1996;50:1031–7. [PubMed] [Google Scholar]
- 31.Neuschäfer-Rube F, Hänecke K, Püschel GP. The C-terminal domain of the human EP4 receptor confers agonist-induced receptor desensitization in a receptor hybrid with the rat EP3beta receptor. FEBS Lett. 1997;415:119–24. doi: 10.1016/S0014-5793(97)01105-8. [DOI] [PubMed] [Google Scholar]
- 32.Neuschäfer-Rube F, Oppermann M, Möller U, Böer U, Püschel GP. Agonist-induced phosphorylation by G protein-coupled receptor kinases of the EP4 receptor carboxyl-terminal domain in an EP3/EP4 prostaglandin E(2) receptor hybrid. Mol Pharmacol. 1999;56:419–28. doi: 10.1124/mol.56.2.419. [DOI] [PubMed] [Google Scholar]
- 33.Bastepe M, Ashby B. Identification of a region of the C-terminal domain involved in short-term desensitization of the prostaglandin EP4 receptor. Br J Pharmacol. 1999;126:365–71. doi: 10.1038/sj.bjp.0702291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36:457–69. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beautrait A, Michalski KR, Lopez TS, Mannix KM, McDonald DJ, Cutter AR, et al. Mapping the putative G protein-coupled receptor (GPCR) docking site on GPCR kinase 2: insights from intact cell phosphorylation and recruitment assays. J Biol Chem. 2014;289:25262–75. doi: 10.1074/jbc.M114.593178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian X, Kang DS, Benovic JL. β-arrestins and G protein-coupled receptor trafficking. Handb Exp Pharmacol. 2014;219:173–86. doi: 10.1007/978-3-642-41199-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorton D, Bellinger DL, Schaller JA, Shewmaker E, Osredkar T, Lubahn C. Altered sympathetic-to-immune cell signaling via β-adrenergic receptors in adjuvant arthritis. Clin Dev Immunol. 2013; 2013:764395. [DOI] [PMC free article] [PubMed]
- 38.Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Han CC, Cui D, Luo TT, Li Y, Zhang Y, et al. Immunomodulatory effects of CP-25 on splenic T cells of rats with adjuvant arthritis. Inflammation. 2018;41:1049–63. doi: 10.1007/s10753-018-0757-z. [DOI] [PubMed] [Google Scholar]