Abstract
Introduction:
The prospective trial PCM301 randomized 413 men with low-risk prostate cancer to partial gland ablation with vascular-targeted photodynamic therapy (n=207) or active surveillance (n=206). Two-year outcomes were reported previously. Here we report four-year rates of intervention with radical therapy and further assess efficacy with biopsy results.
Materials & Methods:
Prostate biopsies were mandated at 12 and 24 months. Thereafter, patients were monitored for radical therapy, with periodic biopsies performed according to standard-of-care in each institution. Efficacy of ablation was assessed by biopsy results overall and ‘in-field’ (treated lobe, or lobe with index cancer).
Results:
Conversion to radical therapy was less likely in the ablation cohort than the surveillance cohort: 7% versus 32% at two years, 15% versus 44% at three years, and 24% versus 53% at four years (HR 0.31, 95% CI=0.21–0.46). Triggers for radical therapy were similar in the two arms. Cancer progression rates overall and by grade were significantly lower in the ablation cohort (HR 0.42, CI = 0.29–0.59). End-of-study biopsy results were negative throughout the prostate in 50% after ablation versus 14% after surveillance (risk difference 36%, CI 28%, 44%) and Gleason 7 or higher cancer was less likely (ablation, 16%; surveillance, 41%). In-field biopsies contained Gleason 7 cancer in 10% after ablation versus 34% after surveillance.
Conclusions:
In this randomized trial of partial ablation for low-risk prostate cancer, photodynamic therapy significantly reduced the subsequent finding of higher-grade cancer on biopsy. Consequently, fewer patients converted to radical therapy, a clinically meaningful benefit that lowers treatment-related morbidity.
Keywords: Low risk prostate cancer, active surveillance, partial gland ablation, focal therapy, vascular-targeted phototherapy
Introduction
Given the low probability of mortality from low risk PCa, therapeutic decisions reflect a balance between cancer control and quality-of-life. Current guidelines recommend AS as the preferred treatment option.1,2 In practice, however, treatment algorithms are complex, influenced by the substantial rates of reclassification or progression to higher-grade or larger volume cancer over time, and by patient choice. Low-risk PCa does not always remain indolent. In published cohorts of men on AS, some 25–60% convert to RT (eg, RP, radiation therapy) within 5–10 years, exposing them to substantial treatment-related morbidity.3–8 Hence, there remains an unmet need for more effective control of low- risk cancers with treatments that pose minimal risks to urinary, sexual, or bowel function. PGA with VTP is one such therapeutic approach.
The first multicenter, phase 3, prospective randomized trial (CLIN1001 PCM301) evaluating PGA as treatment for localized PCa was recently published.9 VTP employs padeliporfin di-potassium (TOOKAD®), a stable, bacteriochlorophyll-derived photo-sensitizer.10 When excited by near-infrared light (753nm), TOOKAD generates superoxide and hydroxyl radicals, which initiate a cascade of events leading to rapid vascular occlusion and subsequent coagulative necrosis of the targeted prostate tissue.11 VTP is performed under anesthesia in an outpatient operating room, where intravenously injected TOOKAD is excited within the prostate with laser light delivered via transperineally placed light diffusers, targeting the lobe containing the largest cancer volume (hemi-gland ablation).
PCM301 randomized 413 men with low-risk PCa to VTP or AS. Patients with low-risk PCa (Gleason score ≤6 (GG12 1), clinical stage T2a or less and PSA ≤ 10 ng/ml), having 2 or 3 positive cores with MCCL ≤5mm or 1 positive core with MCCL between 3 and 5mm, and prostate volume 25–70 cc were eligible for inclusion. Treatment arm was not blinded for participants and investigators, but primary efficacy outcomes were assessed without knowledge of treatment.9 Twelve core systematic biopsies were mandated at 12 and 24 months after randomization with 6 evenly spaced cores taken from each lobe. Demographics, patient characteristics, and exclusion criteria are detailed in the original publication.9 PCM301 met both its co-primary and secondary endpoints. At 2 years, in the ITT analysis, the VTP cohort was less likely to have cancer in the end-of-study biopsy (p <0.001) or to have progression in grade or volume of cancer (p<0.001). Consequently, conversion to RT at two years was lower in the VTP arm (p <0.001). These positive trial results led the European Medicines Agency to approve VTP for treatment of unilateral low-risk, but not very low-risk, localized cancer in 2017.
In PCM301, the principal benefit of PGA with VTP appeared to be a substantial delay in RT compared to AS, with consequent reduction in treatment-related morbidity. Since the primary endpoints of PCM301, biopsy results, were assessed and reported at 24 months, few patients who converted to RT after that time point were captured in the initial report.9 Hence, we sought to assess the durability of PGA over the intermediate term by analyzing the rate of conversion to RT up to 4 years from randomization and to conduct additional, post-hoc analyses of biopsy outcomes as indicators of treatment efficacy.
Materials and Methods
The initial PCM301 protocol (registered at ClinicalTrials.gov, NCT01310894) and extended follow-up with data collection up to 8 years after randomization (protocol addendum PCM301 5FU) were approved by the IRB centrally and at the participating institutions at study initiation. All patients provided written informed consent. To assess the intermediate results of the planned extended follow-up, the dataset was frozen on August 30, 2017. After 24 months, study participants initially randomized to VTP or AS were managed by their physician following a ‘local standard-of-care’ principle, with management decisions, including the need for biopsy, at the discretion of individual physicians and patients in each center. Comprehensive data, including PSA levels, biopsy results, adverse events, additional treatments, and patient-reported quality of life were mandated at least annually using the approved clinical research forms submitted by the investigators.
Overall, 266 patients (64%) were followed for 4 or more years, including 147 (71%) in the VTP arm and 119 (58%) in the AS arm. Results at 4 years were calculated for cumulative risk of conversion to RT, and for metastasis-free, cancer-specific, and overall survival rates. Biopsy results were available for analysis of indications for RT in 118 (96%) of the 123 patients who converted to RT within 48 months. The types of RT used included RP in 80%, radiation therapy in 14%, whole gland cryotherapy or HIFU in 5%, and was unknown in 1%.
Additionally, post-hoc analyses of annual biopsy results during the first 24 months were conducted to assess rates of progression in grade to GG >1, the location and grade of positive biopsy results both ‘in-field’ (within the VTP treated lobe or for AS, within the lobe containing the largest, index cancer) and ‘out-of-field’ (in the contralateral lobe in both cohorts) and by spatial location (apex, mid-gland, or base).
Statistical analyses were done with SAS version 9.3. All randomized participants were included in the efficacy analyses according to assigned treatment (ITT). Time-to-progression was compared between the two treatment groups by the log-rank test and quantified using a Cox proportional hazards regression model to derive hazard ratios. Treatment group, age, number of positive cores, prostate volume, and disease status at baseline were used as covariates. End-of-study biopsy results were analyzed at 24 months (for patients who did not have a 24-month biopsy, the 12-month biopsy was considered end-of-study). Chi-square tests and risk ratios were used to compare proportions of participants in the two treatment groups according to biopsy outcome. Time to initiation of RT was estimated by the Kaplan-Meier method. Comparison between the two treatment groups was done by log-rank test and quantified with a univariable Cox proportional hazards regression model to derive hazard ratios at 4 years. Sensitivity analyses were conducted by first censoring at the time of RT subjects in both arms who converted by choice, without evidence of progression, and second, by censoring in addition those who converted after progression by PSA or volume criteria without progression in grade.
Results
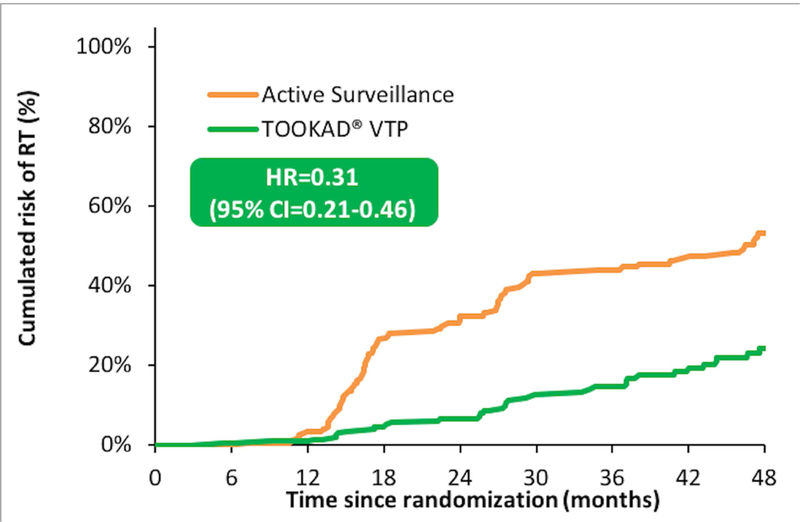
The lower rate of conversion to RT after VTP compared with AS at 2 years (7% vs 32%) was maintained at 3 (15% vs 44%) and 4 years (24% vs 53%) (HR=0.31, 95% CI=0.21–0.46; p<0.001) (Fig. 1) (Table 1). Triggers for conversion to RT were similar in the VTP and AS arms: increase in grade to GG 2 or higher (61% of 36 conversions in the VTP cohort vs 49% of 87 in the AS cohort), increase in cancer volume without change in grade (11% of VTP vs 26% of AS), PSA failure (0% vs 2%), and patient choice (28% vs 24%) (Table 2). Sensitivity analyses confirmed the substantially lower rate of conversion to RT in the VTP cohort when comparing only patients with objective evidence of progression (HR=0.29, 95% CI=0.18–0.45; p<0.001) and those with progression in grade (HR=0.38, 95% CI=0.23–0.64; p<0.001). (Supplementary Fig. 1S) The proportion of study participants in each arm who converted to RT by choice, without objective evidence of progression, was somewhat higher in the AS arm: 10 (5%) of 206 in the VTP cohort versus 19 (14%) of 207 in the AS cohort (RR=0.53, 95% CI=0.25–1.11; p=0.09). Four-year metastasis-free (99% vs 99%), cancer-specific (100% vs 100%) and overall survival (98% vs 99%) rates were similar between cohorts.
Fig. 1.
Probability of conversion to radical therapy over time in each arm (Kaplan-Meier estimate).
Table 1.
Cumulative probability of conversion to radical therapy by protocol year after randomization, comparing the vascular-targeted photodynamic therapy cohort to the active surveillance cohort, by intent-to-treat.
| Trial population (N=413) | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | ||
| AS | N at risk at period start | 207 | 189 | 118 | 77 |
| N with RT in the period | 7 | 54 | 16 | 10 | |
| Cumulative % with RT at end of period | 4% | 32% | 44% | 53% | |
| VTP | N at risk at period start | 206 | 195 | 177 | 127 |
| N with RT in the period | 2 | 11 | 12 | 11 | |
| Cumulative % with RT at end of period | 1% | 7% | 15% | 24% | |
| Absolute risk difference at end of period | 3% | 26% | 29% | 29% | |
AS, active surveillance; RT, radical therapy; VTP, vascular targeted photodynamic therapy.
Table 2:
Reasons for conversion to radical therapy by 48 months from randomization.
| VTP n (%) |
AS n(%) |
|
|---|---|---|
| Patients who converted to RT | 36 | 87 |
| Increase in grade to GG >1 | 22 (61%) | 43 (49%) |
| Increased volume of GG 1 without GG increase | 4 (11%) | 23 (26%) |
| PSA failure* | 0 (0%) | 2 (2%) |
| Patient choice | 10 (28%) | 19 (22%) |
PSA>10ng/mL in 3 consecutive measures
RT, radical therapy; VTP, vascular targeted photodynamic therapy; AS, active surveillance; GG, Gleason grade group; PSA, prostate-specific antigen
Rates of progression by any of the predefined criteria (Figure 2), and of progression in grade (Figure 3), were separately analysed post-hoc for the whole gland and for the in-field lobe and are reported at 2 years since annual biopsies were not mandated for all participants beyond that point. Progression rates were significantly lower in the VTP than the AS cohort for overall progression (HR=0.35; 95%CI = 0.25–0.48; p=0.001) and progression in grade (HR=0.42; 95%CI = 0.29–0.59; p=0.001). Reductions were even more pronounced when focusing on biopsy results in the in-field lobe, both for overall progression (HR=0.21; 95%CI = 0.14–0.31; p<0.001) and for progression in grade (HR=0.25; 95%CI = 0.16–0.40; p<0.001).
Fig. 2.

Progression rates by intent to treat comparing vascular-targeted photodynamic therapy (VTP) to active surveillance (AS) cohorts. (A.) Whole gland progression, defined by meeting any protocol definition of progression, and (B.) In-field progression, defined by biopsy results within the VTP treated lobe or for AS, within the lobe containing the index, or largest, cancer at baseline.
Figure 3.

Progression in grade to Grade Group 2 or higher comparing vascular-targeted photodynamic therapy (VTP) to active surveillance (AS) cohorts. (A.) Progression in grade by biopsy anywhere within the prostate, and (B.) In-field progression in grade, within the VTP treated lobe, or, for AS, within the lobe containing the index, or largest, cancer at baseline.
End of study biopsies were negative throughout the prostate in 50% of the VTP cohort versus 14% of the AS cohort (risk difference 36%, CI 28% to 44%; p<0.001) (Table 3). And higher-grade cancer (GG >1) was less likely on biopsy after VTP (16%) than AS (41%) (risk difference −25%; CI −33% to −16%; p<0.001). Comparing in-field biopsy results in the VTP and AS cohorts, cancer was found in 25% after VTP versus 65% in the AS cohort, and high-grade cancer (GG >1) in 10% after VTP versus 34% in the AS cohort. There were no significant differences between the two arms for rates of positive out-of-field biopsies overall or for GG >1. (Table 3)
Table 3.
Post-hoc analysis of biopsy results within each arm during first 24 months when annual biopsies were mandated (intent-to-treat analysis).
| VTP (N=206) n (%) |
AS (N=207) n (%) |
VTP vs. AS Risk difference; 95% CI |
|
|---|---|---|---|
| Negative biopsy | 104 (50%) | 30 (14%) | 36% (28% to 44%), p<0.001 |
| ‘In-field’ positive biopsy • GG 1 • GG 2 • GG 3 • GG 4 |
51 (25%) • 30 (15%) • 18 (8.7%) • 1 (0.5%) • 2 (1.0%) |
134 (65%) • 64 (31%) • 57 (28%) • 8 (3.9%) • 5 (2.4%) |
−40% (−49% to −31%), p<0.001 |
|
‘Out-of-field’ positive biopsy • GG 1 • GG 2 • GG 3 • GG 4 |
39 (19%) • 27 (13%) • 10 (4.9%) • 1 (0.5%) • 1 (0.5%) |
25 (12%) • 11 (5.3%) • 11 (5.3%) • 2 (1.0%) • 1 (0.5%) |
7% (0% to 14%), p=0.054 |
| No biopsy | 12 (5.8%) | 18 (9%) | |
|
Overall GG >1
‘In-field’ GG >1 ‘Out-of-field’ GG >1 |
33 (16%) 21 (10%) 12 (5.8%) |
84 (41%)
70 (34%) 14 (6.8%) |
−25% (−33% to −16%), p<0.001 −24% (−31% to −16%), p<0.001 −1% (−5% to 4%), p=0.7 |
GG = Gleason Grade Group; ‘In-field’ = within the VTP treated lobe, or, for AS, within the lobe containing the largest, index cancer at baseline; ‘Out-of-field’ = in the contralateral lobe; CI, 95% confidence intervals.
Spatially, biopsy results were analyzed in 85 consecutive patients in the VTP arm with available data. Prior to treatment, 39 (46%), 40 (47%), and 43 (51%) patients had positive biopsy results in the apex, mid-gland, and base, respectively. Location data were missing in 15 (18%) patients. There were no significant differences in the negative biopsy rates at 1 year by location in the VTP-treated lobe: 36 (92%) in the apex, 36 (90%) in the mid-gland, and 36 (84%) in the base.
Discussion
With the safety and efficacy of hemi-gland ablation with VTP established in phase 1–2 trials,13–16 PCM301 was designed to compare outcomes against AS, the standard-of-care for men with low-risk cancers.9 Of particular interest was the ability of VTP to eliminate biopsy-detectable cancer within the prostate and to prevent progression or reclassification in cancer grade and extent, which typically leads patients to refuse AS initially or to convert to RT subsequently.17 In the initial report of the results of PCM301, VTP substantially reduced progression overall and by each predefined criterion compared to AS at 24 months.9 In that time frame, conversions to RT in either arm were driven largely by the new finding of GG 2 or higher on biopsy and were substantially lower in the VTP cohort than in the AS group. Morbidity was low, and sexual, urinary, and bowel functions were preserved at 24 months, despite subsequent contralateral VTP treatment in 32%, ipsilateral retreatment in 11% and both contralateral treatment and retreatment in 2% of the VTP cohort.
While conversion to RT was a secondary endpoint of PCM301, the impact of VTP on the rate of conversion at 24 months was impressive, 7% versus 32% in the AS arm. Avoidance of RT and the resulting effects on function and quality of life appeared to be a major clinical benefit of VTP for men with low-risk, intermediate-volume PCa. Consequently, in analyzing longer-term outcomes, we were particularly interested in the durability of this apparent benefit and the degree to which conversions were based on objective indicators of disease progression rather than patient and physician choice alone. In fact, the reduced rate of conversion to RT at 2 years was maintained through 4 years (Fig. 1, Table 1) and was largely the result of the lower rate of progression to higher-grade cancer in the VTP cohort (Table 2). Compared with AS, VTP significantly reduced overall biopsy progression to GG 2 or higher, with even stronger reduction in the in-field lobe (Fig. 2 and 3). End-of-study biopsies at 24 months identified GG 2 or higher cancer in only 16% of the VTP cohort compared with 41% of the AS group (Table 3). In-field progression to GG 2 or higher was identified in only 10% after VTP compared with 34% in AS, supporting the ablative efficacy of VTP treatment without increasing progression in untreated, out-of-field areas (Table 3). Finally, conversion to negative biopsy results after VTP was equally likely throughout the prostate (apex, mid-gland, and base).
There are important implications of this study. In men on AS for low-risk prostate cancer, progression to or the finding of GG 2 or higher on biopsy commonly triggers definitive treatment with RP or radiation therapy, since such progression in grade is associated with higher rates of metastases and death from cancer.3 Converting to RT risks adverse effects on sexual, urinary and bowel function.1 In men on AS, progression to GG 2 or higher is strongly associated with conversion to RT, making presence of GG 2 or higher a useful predictive biomarker for cancer progression and subsequent treatment-related morbidity.3,6,7,12
Nevertheless, there remain concerns about PGA for low-risk prostate cancer with any technology. One issue is whether current biopsy and imaging techniques can sufficiently localize the index cancer, even when the therapeutic target is one entire lobe. In the present study, VTP was not completely effective in eliminating cancer within the targeted lobe. Cancer was present on biopsy in 25% of patients after VTP; however, only 10% had GG 2 or higher cancers within the treated lobe (Table 3).
There are other limitations to this study. Cancers were not characterized at diagnosis as thoroughly as might be done in a clinical trial of PGA today. Neither saturation biopsies nor confirmatory biopsies nor multi-parametric MRI were employed to further characterize the cancer found on the initial diagnostic biopsy. These factors may help explain the observed 53% rate of conversion to RT in the AS arm at 4 years. Although higher than some contemporary series, this rate of RT is not inconsistent with other large AS studies, which report RT rates within 5–10 years of 25–60%.3–6,18 Other explanations include the exclusion of very low-risk cancers from PCM301, the high compliance rate with the protocol-mandated annual biopsies, and the stringent biopsy criteria used to trigger recommendation for RT (eg, number of positive cores and MCCL are not used as triggers for RT in most AS studies). Nevertheless, techniques used herein for the initial diagnostic and subsequent monitoring biopsies (12-core systematic) accord with the standard of care in North America today. With randomization of patients at baseline, these factors affected both arms similarly, magnifying the importance of the differences observed with VTP treatment.
Other limitations include the lack of protocol-mandated biopsies after 24 months, which limits documentation of the objective progression rates in both arms beyond 2 years. We do, however, have biopsy results to document the reason for conversion in 96% of the 123 patients who had RT. Patient-reported functional outcomes (erectile dysfunction, urinary, and bowel symptoms) are not yet uniformly available beyond 24 months from initial randomization. Progression to clinical stage T3 cancer was more common in the AS cohort, but events were too few to assess long-term effectiveness of hemi-ablation with VTP in reducing clinical cancer events. More data will become available with further follow-up of the PCM301 trial and the post-marketing studies underway in Europe.
The present study provides the longest reported Level 1 evidence of safety and efficacy of PGA for PCa published thus far. Based on data from multiple phase 1–3 clinical trials, including PCM301, PGA, using a strategy targeting one full lobe (hemi-gland ablation) with VTP, followed by ipsilateral retreatment for persistent cancer, and contralateral ablation if significant cancer is found on subsequent biopsy, is safe with few adverse effects on quality of life.9,13–16 Furthermore, this strategy substantially reduces the likelihood of progression to higher Gleason grade and/or larger volume cancer on subsequent biopsy, markedly reducing the rate of intervention with RT with its attendant morbidity.9 As such, PGA with VTP provides a clinically meaningful benefit to selected men with low-risk (but not very low-risk) prostate cancer.
Conclusion
PCM301 is the only prospective multi-institutional randomized trial comparing PGA and AS for low-risk prostate cancer. With 4 years follow-up PGA with VTP decreased the risk of overall progression and progression in grade, and, consequently, reduced the rate of conversion to RT, a clinically-meaningful outcome that lowers treatment-related morbidity compared with AS, while improving cancer control.
Supplementary Material
Acknowledgement:
The authors recognize the many contributions to the trial by the following PCM301 investigators: Sébastien Vincendeau, MD, Eric Barret, MD, Antony Cicco, MD, François Kleinclauss, MD, Henk Gvan der Poel, MD, Christian G. Stief, MD, Jens Rassweiler, MD, Georg Salomon, MD, Eduardo Solsona, MD, Antonio Alcaraz, MD, Teuvo T. Tammela, MD, Derek J Rosario, MD, Francisco Gomez-Veiga, MD, Göran Ahlgren, MD, Fawzi Benzaghou, MD, Bertrand Gaillac, MD, Billy Amzal, PhD, Frans M.J. Debruyne, MD, PhD, Gaëlle Fromont, MD, Christian Gratzke, MD.
Funding Sources: National Institutes of Health/National Cancer Institute Grant R01 CA205058–01 (ISG); The Sidney Kimmel Center for Prostate and Urologic Cancers, David H. Koch Fund, and the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 (JAC, PTS) and the SPORE in Prostate Cancer P50 CA092629 (PTS); the Thompson Family Foundation (AS); United Kingdom National Institute of Health Research (NIHR) Senior Investigator and research support from the University College London Hospital and University College London NIHR Biomedical Research Centre (ME); STEBA Biotech (ARA)
Abbreviations
- AEs
adverse events
- CHMP
Committee for Medicinal Products for Human Use
- EMA
European Medicines Agency
- GG
Gleason Grade Group
- GS
Gleason Score
- HIFU
high intensity focused ultrasound
- IRB
Institutional Review Board
- ITT
intent-to-treat
- MCCL
maximum cancer core length
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PGA
partial gland ablation
- PSA
prostate-specific antigen
- RT
radical therapy
- US
United States
- VTP
vascular-targeted photodynamic
Footnotes
DISCLAIMER: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
References
- 1.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J Urol 2018. [DOI] [PubMed] [Google Scholar]
- 2.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol 2017. [DOI] [PubMed] [Google Scholar]
- 3.Musunuru HB, Yamamoto T, Klotz L, Ghanem G, Mamedov A, Sethukavalan P, Jethava V, Jain S, Zhang L, Vesprini D, Loblaw A. Active Surveillance for Intermediate Risk Prostate Cancer: Survival Outcomes in the Sunnybrook Experience. J Urol 2016;196:1651–8. [DOI] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, Oxley J, Robinson M, Staffurth J, Walsh E, Bollina P, Catto J, Doble A, Doherty A, Gillatt D, Kockelbergh R, Kynaston H, Paul A, Powell P, Prescott S, Rosario DJ, Rowe E, Neal DE, Protec TSG. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Mamawala M, Epstein JI, Landis P, Wolf S, Trock BJ, Carter HB. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379–85. PMCID: PMC4863946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term Results of Active Surveillance in the Goteborg Randomized, Population-based Prostate Cancer Screening Trial. Eur Urol 2016;70:760–6. [DOI] [PubMed] [Google Scholar]
- 7.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126–31. [DOI] [PubMed] [Google Scholar]
- 8.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, Bjartell A, van der Schoot DK, Cornel EB, Conti GN, Boeve ER, Staerman F, Vis-Maters JJ, Vergunst H, Jaspars JJ, Strolin P, van Muilekom E, Schroder FH, Bangma CH, Roobol MJ. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597–603. [DOI] [PubMed] [Google Scholar]
- 9.Azzouzi AR, Vincendeau S, Barret E, Cicco A, Kleinclauss F, van der Poel HG, Stief CG, Rassweiler J, Salomon G, Solsona E, Alcaraz A, Tammela TT, Rosario DJ, Gomez-Veiga F, Ahlgren G, Benzaghou F, Gaillac B, Amzal B, Debruyne FM, Fromont G, Gratzke C, Emberton M, Group PCMS. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 2017;18:181–91. [DOI] [PubMed] [Google Scholar]
- 10.Brandis A, Mazor O, Neumark E, Rosenbach-Belkin V, Salomon Y, Scherz A. Novel water-soluble bacteriochlorophyll derivatives for vascular-targeted photodynamic therapy: synthesis, solubility, phototoxicity and the effect of serum proteins. Photochem Photobiol 2005;81:983–93. [DOI] [PubMed] [Google Scholar]
- 11.Kimm SY, Tarin TV, Monette S, Srimathveeravalli G, Gerber D, Durack JC, Solomon SB, Scardino PT, Scherz A, Coleman J. Nonthermal Ablation by Using Intravascular Oxygen Radical Generation with WST11: Dynamic Tissue Effects and Implications for Focal Therapy. Radiology 2016;281:109–18. PMCID: PMC5047127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, Parwani AV, Reuter VE, Fine SW, Eastham JA, Wiklund P, Han M, Reddy CA, Ciezki JP, Nyberg T, Klein EA. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol 2016;69:428–35. PMCID: PMC5002992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzouzi AR, Barret E, Moore CM, Villers A, Allen C, Scherz A, Muir G, de Wildt M, Barber NJ, Lebdai S, Emberton M. TOOKAD((R)) Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int 2013;112:766–74. [DOI] [PubMed] [Google Scholar]
- 14.Moore CM, Azzouzi AR, Barret E, Villers A, Muir GH, Barber NJ, Bott S, Trachtenberg J, Arumainayagam N, Gaillac B, Allen C, Schertz A, Emberton M. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int 2015;116:888–96. [DOI] [PubMed] [Google Scholar]
- 15.Taneja SS, Bennett J, Coleman J, Grubb R, Andriole G, Reiter RE, Marks L, Azzouzi AR, Emberton M. Final Results of a Phase I/II Multicenter Trial of WST11 Vascular Targeted Photodynamic Therapy for Hemi-Ablation of the Prostate in Men with Unilateral Low Risk Prostate Cancer Performed in the United States. J Urol 2016;196:1096–104. PMCID: PMC5483996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzouzi AR, Barret E, Bennet J, Moore C, Taneja S, Muir G, Villers A, Coleman J, Allen C, Scherz A, Emberton M. TOOKAD(R) Soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol 2015;33:945–53. PMCID: PMC4480329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam R, Carter HB, Landis P, Epstein JI, Mamawala M. Conditional probability of reclassification in an active surveillance program for prostate cancer. J Urol 2015;193:1950–5. PMCID: PMC4696016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klotz L Defining ‘progression’ and triggers for curative intervention during active surveillance. Curr Opin Urol 2015;25:258–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.