Abstract
Background
Extrapulmonary tuberculosis (EPTB) represents an increasing percentage of tuberculosis (TB) cases in Europe. However, strategies on TB prevention and successful treatment outcomes primarily target pulmonary TB. In this nationwide study, we present characteristics of EPTB, treatment outcomes, and predictors for unfavorable treatment outcomes.
Methods
All patients diagnosed with EPTB from 2009 to 2014 were included. Logistic regression analyses were used to identify risk factors for unfavorable outcome. The following definitions were used: unfavorable outcome: the sum of treatment failed, lost to follow-up, and not evaluated; patient delay: time from TB-related symptom onset until first hospital contact related to TB; doctor delay: time from first TB-related contact in the health care system to start of TB treatment.
Results
A total of 450 EPTB cases were notified, which represented 21.1% of all TB cases in Denmark. Immigrants accounted for 82.9%. Lymph nodes were the most common site of EPTB (55.4%) followed by pleural TB (13.4%). Patient delay was significantly longer among immigrants than Danes (60 vs 30 days; P < .01), whereas doctor delay was significantly longer among Danes (38.5 vs 28 days; P < .01). Treatment completion rates were high and reached 90.9% in 2014. Male gender (odds ratio [OR], 5.18; 95% confidence interval [CI], 1.79–15.04) and age 0–24 years (OR, 16.39; 95% CI, 2.02–132.64) were significantly associated with unfavorable outcome.
Conclusions
EPTB represented a significant number of all TB cases and was predominantly seen among younger immigrants in Denmark. To maintain high treatment completion rates, increased focus on male gender and young age is needed.
Keywords: epidemiology, extrapulmonary tuberculosis, low incidence, surveillance, treatment outcome
Extrapulmonary tuberculosis (EPTB) represents 20% of tuberculosis (TB) cases in the majority of countries in the European Union (EU) [1, 2], and even in resource-rich settings, the management of EPTB remains a challenge due to difficulties in diagnosis, delay in treatment and monitoring of treatment outcomes [3]. This is due to the varying localization, appearance, and pausibacillary nature and requirement of invasive sampling. Patients with extrapulmonary localization to the central nervous system, bone, or abdomen have a higher risk of sequela after completion of treatment, which may interfere with assessment of the outcome [4–8].
Overall, in the EU and the European Economic Area (EEA), TB notification rates have been decreasing since 2002 due to decreasing rates of pulmonary TB. Conversely, the rates for EPTB have remained stable, and consequently the proportion of EPTB has been increasing from 16.4% in 2002 to 22.8% in 2016 in the EU/EEA [1, 2]. Similar results have been reported in the United States [9].
Pulmonary TB, which is the main source of Mycobacterium tuberculosis (Mtb) transmission, still accounts for the majority of TB cases. However, EPTB contributes considerably to morbidity, lifelong sequelae, and mortality [4–8]. Routine contact tracing is generally only recommended in patients with smear-positive pulmonary TB [10, 11]. Yet, a recent study from the United Kingdom performed in a high–TB incidence setting found that EPTB cases were an indicator of household active and latent TB [12].
EPTB does not receive the same attention as pulmonary TB, and treatment outcomes for EPTB are often not referred to in TB control programs. Studies on treatment outcome in EPTB in low-incidence countries are scarce, and the treatment completion rates are reported with great discrepancies: from 56.9% to 89.2% [1, 8, 13–15]. The most recent Danish study from 1992 reported a treatment completion rate for culture-positive EPTB of 67.6% [14]. Because reporting of TB treatment outcome is voluntary in Denmark, reports are often incomplete, and accurate information regarding treatment outcome in EPTB is missing. As a result, there is a need to address EPTB specifically, as this disease manifestation represents a significant burden of morbidity and mortality and has an impact on the health care system.
This study was conducted to elucidate EPTB treatment completion rates and to explore predictors for unfavorable treatment outcome in Denmark from 2009 to 2014.
METHODS
We conducted a nationwide retrospective cohort study in Denmark from January 1, 2009, to December 31, 2014.
Study Population
As part of a larger study, all TB cases notified were identified. All patients notified with EPTB were included in this study, whereas outcomes for patients with pulmonary TB are reported elsewhere. EPTB was defined according to the European Centre for Disease Prevention and Control (ECDC) classification: any bacteriologically confirmed or clinically diagnosed case of TB involving organs or anatomical sites other than the lungs, the tracheobronchial tree, or the larynx [16].
Patients were excluded if they were diagnosed with and treated for latent TB infection or infection with the Bacillus Calmette-Guérin (BCG) strain of Mycobacterium bovis due to intravesical BCG instillation only. If TB was diagnosed and treatment completed outside of Denmark, cases were excluded.
Patients were followed from time of first contact with the hospital due to EPTB until 1 year after TB treatment was completed.
Additional EPTB cases were included if a relapse/new episode of EPTB was identified in the patient record, and this case was not notified. A new episode/relapse was defined according to World Health Organization (WHO)/ECDC guidelines, and cases were only included once during a 12-month period [2, 17].
Data Source
Notification Data
TB notification has been mandatory in Denmark since 1905 and centralized since 1920 [18].
TB cases were diagnosed according to WHO definitions [17].
Microbiological Data
The International Reference Laboratory of Mycobacteriology at Statens Serum Institut performs mycobacteria diagnostics on all suspected TB cases in Denmark. This laboratory provided all bacteriological data.
Danish National Patient Registry
The Danish National Patient Registry (DNPR) contains data on all admissions to Danish public hospitals since 1977 and data on outpatient contacts since 1994 [19].
Data were obtained on all patients notified with TB in Denmark during the study period and on all patients who were assigned a TB diagnosis during the study period. Data from DNPR were used to calculate Charlson Comorbidity Index (CCI) scores [20] and to determine history of mental illness and previous TB diagnosis based on the patients' discharge diagnoses.
Data Linkage
To cross-link the registers, we used the unique Danish civil registration number (CRN), which is assigned to all residents of Denmark at birth or after residing legally in Denmark for 3 months. Patients who do not meet the criteria for obtaining a CRN are assigned a temporary CRN at first point of contact with the health care system. To accommodate for alterations in temporary CRNs, probabilistic linkage was done.
Hospital Records
For all patients identified by the notification system, medical records were reviewed for sociodemographics, clinical characteristics, and TB treatment. TB treatment outcome was obtained from hospital records, as reporting is voluntary and data from the Department of Infectious Disease Epidemiology & Prevention are incomplete [21]. TB treatment outcome was classified according to WHO definitions (Supplementary Table 1) [17].
Patient delay was calculated as time from TB-related symptom (constitutional symptoms: fever, weight loss, night sweats, and symptoms related to site of infection) onset until first hospital contact related to TB, whereas doctor delay was calculated as time from first TB-related contact with the health care system to start of TB treatment.
TB location was classified according to the origin of the sample submitted for culture and information from medical records. Cases that included >1 disease site were classified according to their major site. TB location was further divided into superficial and deep disease; skin and/or peripheral lymph node TB was considered superficial disease.
Alcohol abuse was quantified according to the Danish Health Authorities' recommendations (>14 units per week of alcohol for women and >21 units for men) [22].
Nonadherence was defined as 2 or more instances of nonattendance for clinical appointments and/or nonattendance described in the patient record.
Immigrant status was defined as patients born abroad or those born in Denmark for whom 1 or both parents had been born abroad, including in Greenland.
All hospital records were reviewed by I.K.H., and a preset format was used. In case of unclear interpretation, data were re-examined by a TB specialist: I.S.J.
Statistical Analysis
All EPTB cases were included in the data analysis describing patient characteristics. Categorical data were described by total numbers and percentages; the denominator for calculated percentages was the number of cases with known information. Data comparisons were made using the chi-square test or Fisher exact test if 20% of the expected cell values were ≤5. Continuous variables were described as medians and interquartile ranges and compared using the Wilcoxon rank-sum test. A P value of <.05 (5%) was considered statistically significant.
In the data analysis describing predictors for unfavorable treatment outcome, multidrug-resistant (MDR; n = 4), isoniazid-resistant EPTB treated with second-line drugs (n = 10) and patients who died (n = 15) or transferred out (n = 24) were excluded. Finally, 1 case was excluded due to missing medical record. Cases were excluded if the patient died or was transferred outside of Denmark during the observation period to ensure that all patients in the study population had a chance to complete treatment successfully. Treatment outcome was categorized into 2 groups: treatment completed or unfavorable outcome (the sum of treatment failed, treatment interrupted, and not evaluated). Characteristics of cases with unfavorable outcomes were compared with cases with treatment completion using univariable logistic regression. Variables for multivariable analysis were selected if they showed a univariable association with the unfavorable outcome (P < .05).
Incidence rates were calculated using the midyear estimates from Statistics Denmark [23]. The population was defined as contributing to 1 person-year per resident per year in the incidence rate analyses. The crude annual incidence rates were calculated as the number of incident cases per 100 000 person-years. Incidence rate ratios were assessed using Poisson regression.
Data from hospital records were entered into a Microsoft Excel 2010 version 16.29.1 (Microsoft, Redmond, Washington, USA) workbook. All statistical analysis was performed using Stata, version 15.2 (StataCorp Inc., College Station, TX, USA).
Ethics
The study was approved by the Danish Data Protection Agency (Jnr. 15/34961) and the Danish Health Authority (Jnr 3-3013-1213/1).
RESULTS
Population
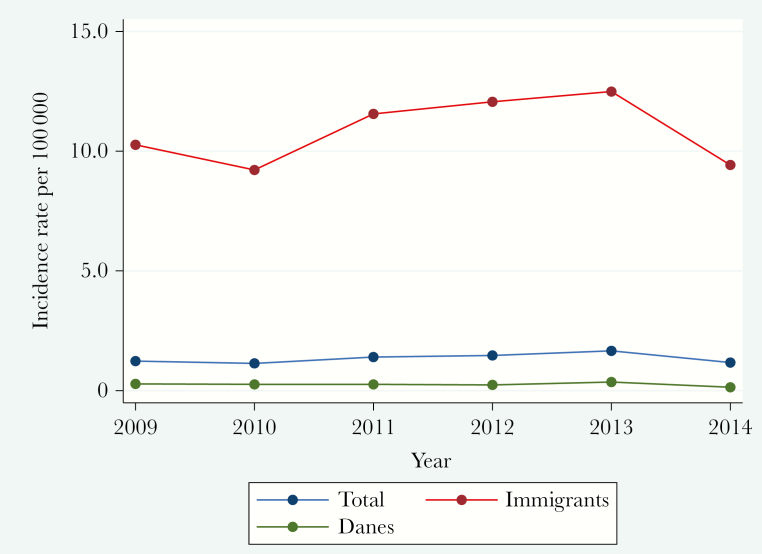
Between January 1, 2009, and December 31, 2014, a total of 2150 TB cases were notified, 36 cases were excluded (latent TB [n = 6], infection with BCG strain of Mycobacterium bovis due to intravesical BCG instillation [n = 4], double registration [n = 12], diagnosed and treated before 2009 or after 2015 [n = 6], misdiagnosed and TB treatment terminated [n = 7], diagnosed and treated outside of Denmark [n = 1]), and an additional 17 cases were included due to relapse of TB that was not reported; this resulted in a total of 2131 cases. EPTB accounted for 450 (21.1%) cases, who were all included in this study. The distribution between PTB and EPTB did not change significantly from 2009 to 2014 (20.7%–20.8%), nor did the incidence rate of EPTB (1.23–1.17 per 100 000). When the study population was divided into immigrants (10.27–9.42 per 100 000) and Danes (0.28–0.14 per 100 000), there was still no significant change in incidence rates of EPTB during the study period (Figure 1).
Figure 1. .
Extrapulmonary tuberculosis incidence rate in Denmark, 2009–2014.
Sociodemographic and Clinical Characteristics
The general characteristics of the population are summarized in Table 1.
Table 1. .
Characteristics of Patients With Extrapulmonary Tuberculosis in Denmark, 2009–2014
| Characteristics | Danes | Immigrants | |||||
|---|---|---|---|---|---|---|---|
| n | Na | % | n | Na | % | P | |
| Patients | 77 | 17.1 | 373 | 82.9 | |||
| Sex | |||||||
| Male | 46 | 77 | 59.7 | 183 | 373 | 49.1 | .09 |
| Age, y | |||||||
| Median (IQR) | 47 | 77 | (32–62) | 33 | 373 | (26–44) | <.01 |
| 0–24 | 12 | 77 | 15.6 | 70 | 373 | 18.8 | <.01 |
| 25–44 | 26 | 77 | 33.8 | 210 | 373 | 56.3 | |
| 45–64 | 22 | 77 | 28.5 | 82 | 373 | 22.0 | |
| ≥65 | 17 | 77 | 22.1 | 11 | 373 | 2.9 | |
| Predisposing factors | |||||||
| Alcohol | 17 | 76 | 22.4 | 16 | 341 | 4.7 | <.01 |
| Tobacco | 34 | 76 | 44.7 | 84 | 336 | 25.0 | <.01 |
| Cannabis | 4 | 75 | 5.3 | 9 | 2.7 | 337 | .23 |
| History of illegal drug use | 3 | 77 | 3.9 | 7 | 368 | 1.9 | .28 |
| Homelessness | 2 | 77 | 2.6 | 9 | 371 | 2.4 | .93 |
| History of incarceration | 0 | 77 | 0 | 3 | 368 | 0.8 | .43 |
| Previous TB | 8 | 69 | 10.4 | 31 | 357 | 8.7 | .64 |
| Charlson comorbidity score | <.01 | ||||||
| 0 | 37 | 77 | 48.0 | 288 | 356 | 80.9 | |
| 1 | 18 | 77 | 23.4 | 39 | 356 | 11.0 | |
| ≥2 | 22 | 77 | 28.6 | 29 | 356 | 8.1 | |
| Identification by contact tracing | 9 | 77 | 11.7 | 6 | 368 | 1.6 | <.01 |
| Delay | |||||||
| Patient delay, median (IQR), d | 30 | 61 | (9–120) | 60 | 336 | (26–120) | <.01 |
| Doctors' delay, median (IQR), d | 38.5 | 70 | (14–82) | 28 | 358 | (12–63) | <.01 |
| Total delay, median (IQR), d | 90 | 58 | (53–180) | 98 | 328 | (46–184) | <.01 |
| Symptoms at first contact | |||||||
| Weight loss | 27 | 76 | 35.5 | 139 | 365 | 38.1 | .68 |
| Night sweat | 23 | 72 | 31.9 | 115 | 349 | 33.0 | .87 |
| Fever | 24 | 76 | 31.6 | 141 | 366 | 38.5 | .26 |
| Pain TB site | 25 | 76 | 32.9 | 164 | 367 | 44.7 | .06 |
| Anemiab | 23 | 40 | 57.5 | 72 | 167 | 43.1 | .10 |
Abbreviations: IQR, interquartile range; TB, tuberculosis.
aInformation was not available on all included patients.
bWomen: Hgb < 7.5 mmol/L. Men: Hgb < 8.0 mmol/L.
The population consisted of 373 (82.9%) immigrants and 77 (17.1%) Danes (Table 1). Among immigrants, 353 (78.4%) were foreign born; of these, 99 (28.1%) patients were diagnosed with EPTB within 2 years of arrival. The majority of immigrants originated from Asia (n = 220; 59.0%); of these, Southeast Asia accounted for 44.1% (n = 97). Cases from Africa represented 27.3% (n = 102), and 55.9% (n = 57) originated from Somalia. Europeans (n = 35; 9.4%) and Greenlanders (n = 16; 4.3%) comprised the remaining cases.
The median age of the study population (interquartile range [IQR]) was 35 (27–48) years. Danes were significantly older than immigrants (47 vs 33 years; P < .01). Significantly more men were homeless, smokers, and had abused alcohol when compared with women (P < .01). The Danish cases were older and had higher CCI scores, and abuse of alcohol and tobacco was significantly more frequent among Danes when compared with immigrants (P < .01).
A total of 348 (75.3%) cases had an HIV test performed at any time during TB treatment. Nine tested positive for HIV. During the study period, the number of cases tested for HIV increased significantly, from 52.9% in 2009 to 95.5% in 2014.
The most common symptom at first contact was pain at the TB site and anemia. Patient delay was significantly longer among immigrants, whereas doctor delay was significantly longer among Danes (Table 1).
Site and Microbiological Characteristics of EPTB
The most common site of EPTB infection was lymph node (n = 246; 55.4%), followed by pleural TB (n = 62; 13.4%); this was applicable among all age groups and across immigration status (Table 2). There was a significant disparity in the distribution of EPTB within age groups. TB meningitis was most frequent among the 0–24-year age group (odds ratio [OR], 6.27; P < .05), whereas lymph node TB was more common in the age group 24–44 years (OR, 2.55; P < .05). Pleural TB was most frequent among those age 45–64 years (OR, 1.96; P < .05). Finally, urogenital TB was most common in the age group >64 years (OR, 4.39; P = .05).
Table 2. .
Disease Site and Microbiological Characteristics of Patients With Extrapulmonary Tuberculosis in Denmark, 2009–2014
| Characteristics | Danes | Immigrants | |||||
|---|---|---|---|---|---|---|---|
| n | Na | % | n | Na | % | P | |
| Site of disease | <.01 | ||||||
| Pleural | 23 | 77 | 29.9 | 39 | 373 | 10.5 | |
| Lymphatic | 30 | 77 | 39.0 | 216 | 373 | 57.9 | |
| Spondylodiscitis | 2 | 77 | 2.6 | 32 | 373 | 8.6 | |
| Abdominal | 3 | 77 | 3.9 | 25 | 373 | 6.7 | |
| Genitourinary | 3 | 77 | 3.9 | 12 | 373 | 3.2 | |
| Meningitis | 3 | 77 | 3.9 | 12 | 373 | 3.2 | |
| Skin | 4 | 77 | 5.2 | 8 | 373 | 2.4 | |
| Bone and joint | 3 | 77 | 3.9 | 10 | 373 | 2.7 | |
| Intracerebral | 0 | 77 | 0 | 2 | 373 | 0.5 | |
| Ocular | 4 | 77 | 5.2 | 9 | 373 | 2.4 | |
| Otherb | 0 | 77 | 0 | 5 | 373 | 1.3 | |
| Superficial diseasec | 34 | 77 | 44.2 | 220 | 373 | 59.0 | .02 |
| Deep tissue disease | 43 | 77 | 55.8 | 153 | 373 | 41.0 | |
| Concomitant site | |||||||
| Any | 0 | 77 | 0 | 18 | 373 | 4.8 | .05 |
| >1 | 0 | 77 | 0 | 1 | 373 | 0.3 | .65 |
| Diagnosed supported by | |||||||
| Culture positive | 46 | 66 | 69.7 | 282 | 341 | 82.7 | .02 |
| Smear positive | 11 | 66 | 16.7 | 79 | 340 | 23.2 | .24 |
| Nucleic amplification | |||||||
| Positive | 33 | 53 | 62.3 | 181 | 299 | 60.5 | .81 |
| Tuberculin skin test | 7 | 9 | 77.8 | 19 | 23 | 82.6 | .75 |
| IGRA | 30 | 44 | 68.2 | 180 | 216 | 83.3 | <.01 |
| Drug resistance | |||||||
| INH mono-resistance | 1 | 37 | 2.7 | 21 | 254 | 8.3 | .23 |
| MDR (INH and RIF resistance) | 0 | 37 | 0 | 4 | 254 | 1.6 | .44 |
| XDR TB | 0 | 37 | 0 | 0 | 254 | 0 | 0 |
Abbreviations: IGRA, interferon gamma release assay; INH, isoniazid; MDR, multidrug-resistant; RIF, rifampicin; TB, tuberculosis; XDR, extensively drug-resistant TB resistant to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs.
aInformation was not available on all included patients.
bGenitourinary tract, cerebral other than meningitis, pericarditis, abscess.
cSuperficial site of disease: lymph node or/and skin.
Only 18 (3.5%) cases had a concomitant site of infection and fulfilled the criteria for disseminated TB; 1 of these was co-infected with HIV, and all cases were immigrants.
In total, 55 (12.2%) patients with EPTB were previously treated. Among these, 5 patients relapsed within 12 months. Two patients were diagnosed and initiated TB treatment outside of Denmark. In 406 cases, a clinical specimen was sampled for microbiologic diagnostics: 90 (20.1%) cases were smear positive, 328 (73.2%) cases were Mycobacterium tuberculosis culture positive, and 87 (26.6%) cases were smear and culture positive. In 291 (88.7%) cases, drug susceptibility testing was done (Table 2). Isoniazid monoresistance was identified in 22 (4.9%) cases, MDR-TB in 4 (0.9%) cases, and none of the cases had XDR-TB (Table 2). A nucleic amplification test was performed in 352 cases, of which 214 (60.6%) were positive. A total of 103 (22.9%) cases were diagnosed on clinical criteria only.
Treatment
A total of 444 cases started TB treatment. The remaining 6 patients (1.3%) died before treatment was initiated. The median treatment duration (IQR) was 6 (6–7) months. Among the drug-susceptible EPTB cases, 216 (51.1%) received the standard treatment of 2 (3 months) (the national guideline was modified in 2010; the intensive phase was decreased from 3 to 2 months) months of intensive-phase treatment including 4 drugs (rifampicin, isoniazid, pyrazinamide, ethambutol) and 4 months of continuation-phase treatment including 2 drugs (rifampicin, isoniazid).
TB Treatment Outcome
Treatment completion increased from 79.4% to 90.9% during the study period (Figure 2).
Figure 2. .
Extrapulmonary tuberculosis treatment outcome in Denmark, 2009–2014.a aNone of the patients had the following outcome: treatment failed, not evaluated, or still on treatment.
The highest risk for unfavorable treatment outcome was in the age group 0–24 years, and all patients were between 15 and 24 years old. Among these, the majority were male (66.7%), and 88.9% were immigrants. None of these patients smoked or abused cannabis, alcohol, or narcotics or were homeless. All were lost to follow-up and did not complete TB treatment. The median time of treatment (IQR) was 2 (1.6–3.5) months. One of the patients returned to the hospital 1.5 years later with pulmonary TB and completed TB treatment.
In total, 15 patients died. These patients were significantly older (mean age, 58 vs 37 years) and had higher CCI scores (median CCI, 0 vs 3) when compared with the remaining cases. Of those who died, Danes represented 60% of the patients. Nine patients initiated treatment. The median treatment duration (IQR) was 1.1 (1–3.5) months. The majority of the patients who died had pleural TB (40%). Two patients were diagnosed with TB meningitis, and another 2 patients had lymphatic TB. None of the patients had a concomitant site of infection.
Table 3 shows the final multivariable model fitted on 395 cases. A significantly increased risk of an unfavorable outcome was found among males and among patients <25 years of age.
Table 3. .
Odds Ratio for Unfavorable Outcome vs Treatment Completion Among EPTB Patients in Denmark, 2009–2014
| Characteristics at Time of EPTB Diagnosis | No. of Cases (%) | Factors Associated With Unfavorable Treatment Outcome, Univariate Logistic Regression | Factors Associated With Unfavorable Treatment Outcome, Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|
| Unfavorable Outcome | Treatment Completion | OR | 95% CI | OR | 95% CI | |
| Total | 20 (5.1) | 376 (94.9) | ||||
| Sex | ||||||
| Female | 4 (2.0) | 195 (98.0) | RF | |||
| Male | 16 (8.2) | 181 (91.8) | 4.33 | 1.42–13.23 | 5.18 | 1.79–15.04 |
| Age, y | ||||||
| 0–24 | 9 (12.3) | 64 (87.7) | 13.36 | 1.65–108.32 | 16.39 | 2.02–132.64 |
| 25–44 | 9 (4.4) | 195 (95.6) | 4.41 | 0.55–35.39 | 4.44 | 0.55–35.64 |
| 45–64 | 1 (1.0) | 95 (99.0) | RF | RF | ||
| ≥65 | 1 (4.4) | 22 (95.6) | 4.32 | 0.26–72.01 | 5.73 | 0.33–100.3 |
| Country of origin | ||||||
| Denmark | 3 (4.5) | 64 (95.5) | RF | |||
| Other | 17 (5.2) | 313 (94.8) | 1.17 | 0.33–4.11 | ||
| Predisposing factors | ||||||
| Alcohol | 2 (7.4) | 25 (92.6) | 1.87 | 0.40–8.73 | ||
| Tobacco | 7 (6.7) | 98 (93.3) | 1.98 | 0.72–5.49 | ||
| Cannabis | 2 (16.7) | 10 (83.3) | 4.83 | 0.96–24.4 | ||
| History of illegal drug use | 1 (12.5) | 7 (87.5) | 2.75 | 0.32–23.58 | ||
| Homelessness | 2 (20.0) | 8 (80.0) | 5.08 | 1.00–25.74 | ||
| History of incarceration | 0 (0.0) | 1 (100.0) | NA | |||
| Previously TB | 3 (8.6) | 32 (91.4) | 2.09 | 0.57–7.70 | ||
| History of mental illness | 2 (11.8) | 15 (88.2) | 2.67 | 0.57–12.58 | ||
| Charlson comorbidity score | ||||||
| 0 | 17 (5.8) | 275 (94.2) | ||||
| 1 | 0 (0.0) | 52 (100.0) | ||||
| ≥2 | 1 (2.4) | 40 (97.6) | 0.05–3.12 | 0.38 | ||
| Concomitant site | ||||||
| Any | 0 (0.0) | 14 (100.0) | NA | |||
| Superficial site of diseasec | 11 (4.8) | 218 (95.2) | RF | |||
| Deep site of disease | 9 (5.4) | 158 (94.6) | 1.12 | 0.45–2.78 | ||
| Diagnosis supported by: | ||||||
| Culture positive | 16 (5.0) | 307 (95.0) | 0.90 | 0.29–2.79 | ||
| Identification by contact tracing | 0 (0.0) | 13 (100.0) | NA | |||
| Clinical symptoms | ||||||
| Symptom duration >30 d | 11 (5.1) | 205 (94.9) | 1.18 | 0.42–3.27 | ||
| Time to diagnosis >30 d | 8 (4.2) | 181 (95.8) | 0.81 | 0.31–2.10 | ||
| Weight loss | 8 (5.6) | 136 (94.4) | 1.14 | 0.45–2.87 | ||
| Fever | 10 (7.1) | 131 (92.9) | 1.85 | 0.75–4.56 | ||
| Night sweet | 9 (7.3) | 114 (92.3) | 1.70 | 0.68–4.23 | ||
| Adherenceb | 3 (0.8) | 357 (99.2) | ||||
| Nonadherence | 16 (48.5) | 17 (51.5) | 112 | 29.7–422.38 | ||
Abbreviations: CI, confidence interval; EPTB, extrapulmonary tuberculosis; OR, odds ratio; RF, reference; TB, tuberculosis.
aA total of 395 cases were included in the multivariable analysis.
bNonadherence: if described in patient records and/or ≥2 episodes of nonattendance for clinical appointments.
cSuperficial site of disease: lymph node or/and skin.
Discussion
In this nationwide study, we found that EPTB mainly affects young healthy immigrants from high-incidence countries. Lymph node TB was the most frequent site of EPTB. In spite of significant patient delay, the overall treatment completion rate was high and increased during the study period (P = .06). Unfavorable outcome was significantly associated with male gender and age 0–24 years.
In Denmark, the incidence of TB increased marginally from 2009 to 2012 (6.0–6.9 per 100 000) and declined thereafter [24]. The increase in TB was caused by an increased incidence in TB among immigrants [25]. The overall incidence of EPTB peaked in 2013 and decreased thereafter; however, this was not significant. The proportion of EPTB followed the same trend. Hence, we did not find a significant change in incidence or proportion of EPTB in Denmark during our study period. The proportion of PTB was persistently high, and the smear positivity among PTB cases was high at 54.6% during the study period, which is a predictor for insufficient TB control in Denmark [21].
The localization of EPTB has been related to age, gender, and ethnicity. We found that lymph node TB was the most common site of EPTB, followed by pleural TB. In addition, certain types of EPTB were associated with specific age groups. This is in line with earlier studies from low-incidence countries [7, 15, 26–31]. Earlier studies from high-incidence countries have indicated that EPTB is associated with female sex [32, 33]. We were not able to demonstrate this, as 50.9% were males. However, this is in accordance with studies from other low-incidence countries [8, 34, 35].
Overall, TB treatment completion rates were high and did increase during the study period, reaching 90.9% in 2014. We recently found an overall treatment completion rate of 86.6% in 2014 in pulmonary TB [21]. This difference could be explained by higher adherence and less substance abuse and homelessness in the EPTB group. A Finnish study found a treatment completion rate of 56.9% in patients with EPTB; however, these patients were older (median age, 70.1 years) and had more comorbidities, and immigrants only represented 10.9% [8]. A study from Texas reported an EPTB treatment completion rate of 82.5%. The study population differed in terms of higher prevalence of HIV and drug use. Location of EPTB close to the central nervous system and peritoneum was more frequent [5]. This emphasizes that even between low-incidence countries, TB treatment outcomes can be challenging to compare without knowledge regarding risk factors and comorbidity.
All patients classified as having an unfavorable outcome were lost to follow-up. In line with an earlier study, men were identified to be at significantly higher risk for unfavorable treatment outcomes [8]. This could be partly explained by a significantly higher frequency of use of cannabis, alcohol abuse, and being homeless, all factors that contribute to poor adherence among men with EPTB. The risk of an unfavorable outcome was significantly associated with the age group 0–24 years. All these patients were lost to follow-up. We did not find any known risk factors for being lost to follow-up: for example, abuse of alcohol or drugs or homelessness. However, these patients were predominantly immigrants and might have experienced language difficulties that led to misunderstandings regarding treatment and follow-up.
Treatment outcome was evaluated at the time of treatment completion and categorized as successful if there were no signs of failure according to the current definition by the WHO [17]. Signs of failure were typically assessed by clinical appearance, advanced imaging techniques, inflammation markers, and Hgb. New tissue samples were often not collected, as this would involve an invasive procedure; consequently, these patients could not be classified as having experienced treatment failure. In line with earlier studies, we suggest that treatment outcomes should be assessed 12 months after treatment completion, and in case of relapse, the treatment outcome should be categorized as treatment failure [36, 37].
Immigrants had a significantly longer patient delay than Danes. The Danish health care system provides free and equal health care to all legal residents in Denmark; hence the patient delay among immigrants cannot be explained by inequality in access to health care. However, immigrants might experience language barriers that result in misunderstandings and misinterpretations when approaching the health care system. This emphasizes the need for professional translators. In addition, cultural differences can cause delay in seeking professional care, as TB still is stigmatizing in many high-incidence countries [38, 39]. Doctor delay was significantly higher among Danes when compared with immigrants. This might be explained by less suspicion of EPTB among Danes because of a low incidence of EPTB in this group. The fact that Danes were older and had coexisting illness could have made the EPTB diagnosis difficult and contributed to delay. In a recent study, we found that doctor delay in pulmonary TB was much lower for both Danes and immigrants (9 and 5 days, respectively) [21]. EPTB can be challenging to diagnose, as the disease can affect virtually all organs and the clinical presentation can be nonspecific. As a result, these patients can present to doctors with minimal experience with TB. In addition, the EPTB site can be relatively inaccessible, causing sampling of a specimen to be challenging.
This is the first study to provide a complete review of EPTB treatment outcomes during a 6-year period in Denmark. Additionally, this study provides clinical data as well as comprehensive outcome information on a large group of Danish EPTB patients. However, the study has limitations: The population was identified by notification data; hence patients who were not notified were not included. This could have introduced selection bias. Yet, a recent study from Denmark has assessed the underreporting of TB to 7.5% in the same period. The non-notified cases were all culture-negative and did not differ significantly in treatment outcomes and risk factors from the notified cases [40]. All clinical information was from hospital records; hence there was no direct patient contact. The quantity and quality of information solely depended on the hospital records. Treatment was prolonged in patients who did not respond satisfactorily, and new samples were not collected because of the risk associated with an invasive procedure. This has potentially led to an underestimation of treatment failure. Patients who were lost to follow-up may still have completed treatment. However, this concern is limited, as the CRN allowed us to trace patients who appeared at another hospital or emigrated during the study period. CCI could not be assessed for patients with temporary CRNs, because they were not registered in the DNPR. These patients accounted for 3.8% (n = 17) of the entire population. Finally, the number of patients with an unfavorable outcome was relatively small, thereby decreasing the power of the study and potentially preventing us from identifying predictors associated with an unfavorable outcome.
Conclusions
EPTB represents a significant number of TB cases in Denmark. Doctor delay was significantly longer among Danish patients, which emphasizes that EPTB should be considered a differential diagnosis in Danish patients, especially in the older age group. Even with a treatment completion rate as high as 90.9% in 2014, a continuous effort is needed to prevent unfavorable outcomes and mortality, especially among males and those aged <25 years, which as male sex and age <25 years were identified as risk factors for an unfavorable outcome.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. I.K.H. and I.S.J. are responsible for the conception and design. I.K.H. is responsible for the analysis, interpretation, and the writing. I.K.H., I.S.J., P.H.A., T.L., C.W., and S.B. are responsible for the review and revision of the manuscript. All authors have read and approved the final manuscript.
Financial support. This work has not received any financial support.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sandgren A, Hollo V, van der Werf MJ. Extrapulmonary tuberculosis in the European Union and European Economic Area, 2002 to 2011. Euro Surveill 2013; 18, pii=20431. [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2018 – 2016 data. Stockholm: European Centre for Disease Prevention and Control, 2018; 2018 [Google Scholar]
- 3. Solovic I, Jonsson J, Korzeniewska-Kosela M, et al. . Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro Surveill 2013; 18, pii=20432. [PubMed] [Google Scholar]
- 4. Johansen IS, Nielsen SL, Hove M, et al. . Characteristics and clinical outcome of bone and joint tuberculosis from 1994 to 2011: a retrospective register-based study in Denmark. Clin Infect Dis 2015; 61:554–62. [DOI] [PubMed] [Google Scholar]
- 5. Pusch T, Pasipanodya JG, Hall RG 2nd, Gumbo T. Therapy duration and long-term outcomes in extra-pulmonary tuberculosis. BMC Infect Dis 2014; 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen AS, Andersen AB, Thomsen VO, et al. . Tuberculous meningitis in Denmark: a review of 50 cases. BMC Infect Dis 2011; 11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez OY, Adams G, Teeter LD, et al. . Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis 2003; 7:1178–85. [PubMed] [Google Scholar]
- 8. Vasankari T, Holmström P, Ollgren J, et al. . Treatment outcome of extra-pulmonary tuberculosis in Finland: a cohort study. BMC Public Health 2010; 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peto HM, Pratt RH, Harrington TA, et al. . Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 2009; 49:1350–7. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Recommendations for Investigating Contacts of Persons With Infectious Tuberculosis in Low- and Middle-Income Countries. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 11. The European Centre for Disease Prevention and Control and The European Respiratory Society. European Union Standards for Tuberculosis Care 2017 Update. Solna, Sweden: European Centre for Disease Prevention and Control (ECDC). 2017. [Google Scholar]
- 12. Humphreys A, Abbara A, Williams S, et al. . Screening contacts of patients with extrapulmonary TB for latent TB infection. Thorax 2018; 73:277–8. [DOI] [PubMed] [Google Scholar]
- 13. Cegolon L, Maguire H, Mastrangelo G, et al. . Predictors of failure to complete tuberculosis treatment in London, 2003–2006. Int J Tuberc Lung Dis 2010; 14:1411–7. [PubMed] [Google Scholar]
- 14. Lillebaek T, Poulsen S, Kok-Jensen A. Tuberculosis treatment in Denmark: treatment outcome for all Danish patients in 1992. Int J Tuberc Lung Dis 1999; 3:603–12. [PubMed] [Google Scholar]
- 15. Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax 2009; 64:1090–5. [DOI] [PubMed] [Google Scholar]
- 16. European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2018 – 2016 Data. Stockholm: European Centre for Disease Prevention and Control; 2018. [Google Scholar]
- 17. World Health Organization. Definitions and Reporting Framework for Tuberculosis – 2013 Revision. 2014. [Google Scholar]
- 18. Holm J. Tuberculosis control in Denmark. Public Health Rep 1946; 61:1426–43. [Google Scholar]
- 19. Schmidt M, Schmidt SA, Sandegaard JL, et al. . The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 21. Holden IK, Lillebaek T, Seersholm N, et al. . Predictors for pulmonary tuberculosis treatment outcome in Denmark 2009–2014. Sci Rep 2019; 9:12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danish Health Authority. Alcohol Available at: https://www.sst.dk/en/health-and-lifestyle/alcohol. Accessed August 1, 2019.
- 23. Statistics Denmark Available at: https://www.dst.dk/en. Accessed January 15, 2019.
- 24. epi-news Available at: https://en.ssi.dk/news/epi-news. Accessed February 1, 2019.
- 25. Statens Serum Institut. Tuberkulose 2012. EPI-NYT. Copenhagen, Denmark: Statens Serum Institut; 2014. [Google Scholar]
- 26. Culqui-Levano DR, Rodriguez-Valin E, Donado-Campos JM. Analysis of extrapulmonary tuberculosis in Spain: 2007–2012 National Study. Enferm Infecc Microbiol Clin 2017; 35:82–7. [DOI] [PubMed] [Google Scholar]
- 27. Houston A, CliveMacallan D. Extrapulmonary tuberculosis. Medicine 2014; 42:18–22. [Google Scholar]
- 28. Sama JN, Chida N, Polan RM, et al. . High proportion of extrapulmonary tuberculosis in a low prevalence setting: a retrospective cohort study. Public Health 2016; 138:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qian X, Nguyen DT, Lyu J, et al. . Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect 2018; 7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J 2008; 31:99–105. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Andersen AB, Lillebaek T, et al. . Effect of sex, age, and race on the clinical presentation of tuberculosis: a 15-year population-based study. Am J Trop Med Hyg 2011; 85:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Musellim B, Erturan S, Sonmez Duman E, Ongen G. Comparison of extra-pulmonary and pulmonary tuberculosis cases: factors influencing the site of reactivation. Int J Tuberc Lung Dis 2005; 9:1220–3. [PubMed] [Google Scholar]
- 33. Ohene SA, Bakker MI, Ojo J, et al. . Extra-pulmonary tuberculosis: a retrospective study of patients in Accra, Ghana. PLoS One 2019; 14:e0209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. te Beek LA, van der Werf MJ, Richter C, Borgdorff MW. Extrapulmonary tuberculosis by nationality, the Netherlands, 1993–2001. Emerg Infect Dis 2006; 12:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Svensson E, Millet J, Lindqvist A, et al. ; Western Sweden Tuberculosis Epidemiology Study Group Impact of immigration on tuberculosis epidemiology in a low-incidence country. Clin Microbiol Infect 2011; 17:881–7. [DOI] [PubMed] [Google Scholar]
- 36. Dedicoat MJ, Günther G, Crudu V, et al. . Tuberculosis treatment outcomes in Europe: based on treatment completion, not cure. Am J Respir Crit Care Med 2017; 196:1222–4. [DOI] [PubMed] [Google Scholar]
- 37. Ditah IC, Reacher M, Palmer C, et al. . Monitoring tuberculosis treatment outcome: analysis of national surveillance data from a clinical perspective. Thorax 2008; 63:440–6. [DOI] [PubMed] [Google Scholar]
- 38. Rubel AJ, Garro LC. Social and cultural factors in the successful control of tuberculosis. Public Health Rep 1992; 107:626–36. [PMC free article] [PubMed] [Google Scholar]
- 39. Suarez I, Maria Funger S, Jung N, et al. . Severe disseminated tuberculosis in HIV-negative refugees. Lancet Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 40. Thrane FD, Andersen PH, Johansen IS, Holden IK.. Underreporting of patients diagnosed with tuberculosis in the region of Southern Denmark. Scand J Public Health. Forthcomming 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.