Abstract
Data on integrase resistance patterns in low- and middle-income countries (LMICs) is scarce. We assessed genotypic drug resistance in 43 patients with virological failure on integrase strand transfer inhibitors (INSTIs) containing regimens as part of the third-line treatment program in South Africa. Of the raltegravir (RAL)-exposed patients 20 of 34 (59%) had ≥1 major INSTI mutation, including 2 (6%) with dolutegravir (DTG) cross-resistance. Dolutegravir resistance was detected in 1 of 4 DTG-exposed patients. Replacing RAL with DTG may reduce the risk of INSTI mutations. We recommend DTG drug resistance monitoring when DTG is introduced at a larger scale in LMICs.
Keywords: dolutegravir, HIV-1 antiretroviral drug resistance, INSTI-based treatment failure, raltegravir, South Africa
Integrase strand transfer inhibitors (INSTI) are a key component of third-line antiretroviral drug regimens in people living with HIV in low and middle income countries. This study describes drug resistance profiles in patients failing INSTI-based third-line treatment in South Africa which has the largest third-line treatment program globally.
South Africa carries the largest human immunodeficiency virus (HIV) burden globally with an estimated 7.2 million people with HIV (PWH) at the end of 2017 and approximately 4 million people on antiretroviral treatment (ART) [1]. Despite South Africa hosting the largest HIV treatment program in the world, with universal ART eligibility, overall treatment coverage is only 56%. Most PWH on treatment receive a nonnucleoside reverse-transcriptase (NNRTI)-based first-line regimen, whereas those who fail NNRTI-based regimes are switched to a protease inhibitor (PI)-containing regimen. In the public sector, third-line drugs such as ritonavir-boosted darunavir (DRV/r) and raltegravir (RAL) have been available since May 2013, and dolutegravir (DTG) has been available since July 2016. Third-line ART (TLART) in the public sector is accessed through a centralized national committee. Individuals are eligible if they have been exposed to a PI-based regimen for at least 1 year, present with virologic failure despite adherence optimization, and have genotypic evidence of PI resistance. A TLART application is then submitted to the national TLART committee that assesses eligibility and makes a regimen recommendation on a case-by-case basis. All patients eligible for TLART receive DRV/r in combination with a dual nucleoside reverse-transcriptase backbone, with or without an integrase strand transfer inhibitor (INSTI), based on the genotypic profile. The number of patients in need of TLART in South Africa is estimated to be 2800, based on the assumption that 170 000 patients receive a PI-based regimen, 10% of whom are assumed to have virological failure with a 16% prevalence of major PI mutations as seen in a national survey [2]. However, as of October 2018, only 1606 patients were switched to a TLART regimen, including 1084 patients on an INSTI-based regimen (650 on DTG and 434 on RAL) (TLART committee, written personal communication, 10 May 2019.
Evidence of TLART outcomes in low- and middle-income countries are limited. In Botswana, 80% of 93 highly treatment-experienced patients suppressed on a DTG-based salvage regimen [3]. In South Africa 78% to 83% of patients obtain virological suppression on TLART [4–6]. A study from Uganda reported prevalence of INSTI resistance in RAL-based third-line failures at 47% (24 of 51) with RAL and/or elvitegravir (EVG) resistance and 4% (2 of 51) with DTG resistance [7]. Whereas an Argentinian survey reported 72% of patients (41 of 57) on RAL-based salvage ART presented with RAL and/or EVG resistance and 32% presented with with intermediate (29%) to high-level (2%) DTG cross-resistance [8], data from Botswana show that only 4 of 20 (20%) patients failing DTG-based salvage regimens had INSTI resistance [3].
This study aims to assess INSTI resistance among patients failing INSTI-based TLART in the South African public sector.
MATERIALS AND METHODS
We conducted a retrospective analysis of all HIV drug resistance (HIVDR) data obtained from patients who had an INSTI genotyping test request between of January 1, 2015 and of October 31, 2018 at any of the National Health Laboratory Service facilities. For patients with more than 1 INSTI genotype request, only the most recent sequence was included. Demographic and clinical information such as age, ART regimen, and time on INSTI regimen were collected from paper-based laboratory request forms. The latest viral load measurements were extracted from the electronic laboratory information system. A patient file review to obtain detailed treatment regimen information was not permitted due to ethical restrictions.
Integrase strand transfer inhibitor resistance testing was conducted at Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg or Tygerberg Hospital, Stellenbosch. Each laboratory generated integrase sequences using their respective validated, population-based, in-house genotyping method [9]. Both laboratories successfully participated in ≥1 international external quality assurance panel per year. The Stanford HIVdb v8.7 tool (http://sierra2.stanford.edu/sierra/servlet/JSierra) was used to identify HIVDR mutations and generate resistance profiles and categorized as susceptible, intermediate-level, or high-level resistance. Sequences were aligned using MEGA and a phylogenetic tree constructed to detect cross-contamination and omit duplicates from analyses. Subtyping was performed using the Rega HIV subtyping tool v3.0 (http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/).
The pol nucleotide sequences were submitted to GenBank using Bankit (https://www.ncbi.nlm.nih.gov/WebSub/; accession numbers MK899435 to MK899477).
Statistical analyses were performed using GraphPad Prism 8. Two-sided Fisher’s exact tests were used to compare mutation prevalence between groups, considering P < .05 as statistically significant. Confidence intervals (CIs) at 95% were calculated using the modified Wald method.
This study was conducted with Ethical clearance by the Research on Human Subjects (Medical) Committee at the University of the Witwatersrand (Clearance Number M1802227). Due to the retrospective nature of this study, no informed consent was obtained from the patients. This is in line with the Research on Human Subjects (Medical) Committee policy, which states that informed consent is not required for this type of study and hence a waiver was granted.
RESULTS
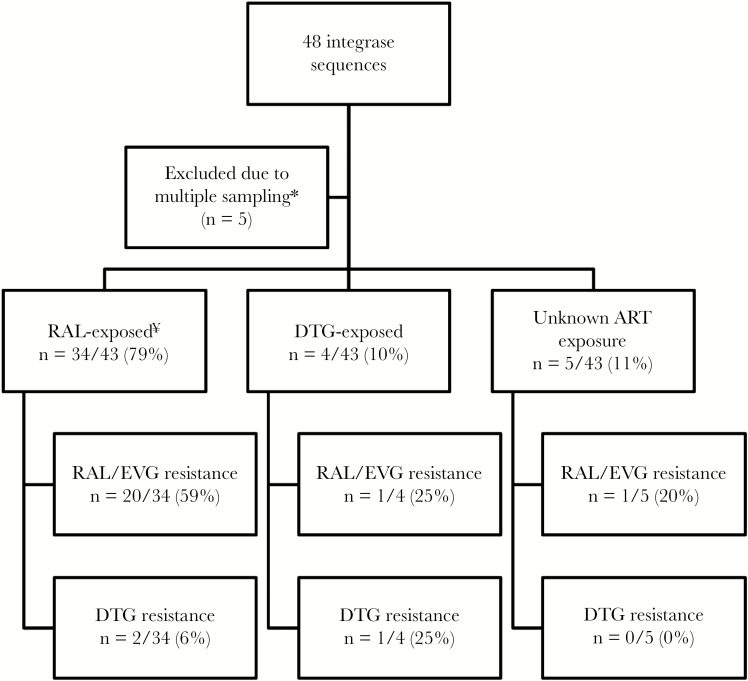
A total of 48 integrase sequences were submitted for review, 5 of which were excluded due to multiple sampling, leaving a cohort of 43 unique patients for final analysis. Integrase resistance was therefore assessed in 43 of 1084 patients on INSTI regimens in South Africa (4%) INSTI-exposed patients. Most integrase sequences, 98% (42 of 43), were classified as subtype C, 1 sequence belonged to subtype G. The cohort comprised 51% males, had a median age of 41 years (interquartile range [IQR], 26–48), and included 2 children (3 and 5 years old) and 6 adolescents. The median HIV viral load at time of HIVDR testing was 4.6 log copies/mL (IQR, 3.8–5.2). Most patients had been exposed to RAL-based regimens (79%, n = 34), including 3 patients who had been exposed to RAL in the past but were not receiving any INSTI at time of HIVDR testing. Four (9%) patients were exposed to DTG. Five cases (12%) had no treatment regimen available at time of testing (Figure 1). Most patients (58%, n = 25) received a combination of an INSTI with DRV/r, whereas 10 patients (23%) were exposed to ritonavir-boosted lopinavir (LPV/r); 1 patient received ritonavir-boosted atazanavir, and 5 patients (12%) received an INSTI regimen without a boosted PI. Duration of INSTI exposure was known for 27 cases (63%) with a median of 25 months (IQR, 14–34).
Figure 1.
Flow-chart of patients failing integrase strand transfer inhibitor (INSTI)-based treatment in South Africa. *, For patients with ≥1 genotype request, only the most recent result was included. ¥, Three of 34 patients had been exposed to raltegravir (RAL) in the past but have no record on INSTI exposure at time of genotyping. ART, antiretroviral treatment; DTG, dolutegravir; EVG, elvitegravir.
Resistance to first-generation INSTIs (RAL and EVG) was detected in 51% (22 of 43; 95% CI, 37%–65%) of all cases. All 22 cases presented with high-level resistance to RAL, whereas 9 and 12 cases showed intermediate and high-level resistance to EVG, respectively. Resistance to first-generation INSTIs was detected in 20 of 34 (59%; 95% CI, 42%–74%) in the RAL-exposed group (P = .6473).
Three patients (7%) presented with intermediate to high-level resistance to DTG (Table 1). All 3 patients with DTG resistance also showed reduced activity to their boosted PI, but none of these patients had lost complete activity to the respective PIs (Table 1). All 3 patients had been on ART for a prolonged period. Patient 25 was initiated on d4T-3TC-NVP and remained on this regimen for 8 years before being switched to a dual regimen of RAL and LPV/r (5 years). It is unknown why this patient only received a dual regimen. Patient 9 had been exposed to d4T-3TC-EFV and TDF-3TC-EFV for an unknown period before being switched to ABC-3TC-DRV/r-RAL (2 years). Patient 8 started on TDF-3TC-NVP (6 years), after which NVP was replaced by LPV/r (4 years); eventually the patient was changed to TDF-3TC-DRV/r-DTG for 22 months before genotyping. Overall, complete loss of boosted PIs was only seen in 8% (2 of 25) of DRV/r-exposed patients and 50% (n = 5 of 10) of the LPV/r-exposed patients.
Table 1.
Demographic, Clinical, and Resistance Profiles for Patients With Integrase Resistance Testing
| Patient | Age | RAL/DTG Exposure | Time on INSTIs (Months) | ART Regimen at Time of HIVDR Testing | HIVVL (Copies/mL) | RAL | EVG | DTG | ATV/r | DRV/r | LPV/r |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 52 | RAL | 24 | ABC-3TC-DRV/r-RAL | 2430 | HLR | HLR | HLR | HLR | IR | HLR |
| 25 | 35 | RAL | 60 | LPV/r-RAL | 23 233 | HLR | HLR | IR | IR | S | IR |
| 1 | 17 | RAL | 25 | TDF-FTC-DRV/r-ETR-RAL | 3459 | HLR | HLR | S | IR | IR | HLR |
| 14 | 43 | RAL | 48 | TDF-3TC-RAL | 3070 | HLR | HLR | S | IR | IR | HLR |
| 11 | 52 | RAL | 69 | DRV/r-RAL | 270 252 | HLR | HLR | S | NA | NA | NA |
| 6 | 51 | RAL | 36 | TDF-3TC-DRV/r-RAL | 27 500 | HLR | HLR | S | HLR | IR | HLR |
| 26 | 46 | RAL | Unknown | LPV/r-RAL | 2316 | HLR | HLR | S | S | S | S |
| 4 | 31 | RAL | 5 | d4T-3TC-DRV/r-RAL | 58 200 | HLR | HLR | S | HLR | IR | HLR |
| 12 | 43 | RAL | 12 | TDF-3TC-DRV/r-RAL | 159 000 | HLR | HLR | S | IR | IR | HLR |
| 10 | 61 | RAL | 20 | 3TC-LPV/r-RAL | 92 800 | HLR | HLR | S | HLR | S | HLR |
| 16 | 45 | RAL | 32 | TDF-FTC-DRV/r-RAL | 13 000 | HLR | HLR | S | S | S | S |
| 42 | 12 | RAL | Unknown | ABC-3TC-DRV/r-RAL | 1843 | HLR | IR | S | HLR | IR | HLR |
| 18 | 44 | RAL | 23 | TDF-3TC-RAL | 778 000 | HLR | IR | S | S | S | S |
| 2 | 47 | RAL | 18 | TDF-3TC-DRV/r-RAL | 27 727 | HLR | IR | S | HLR | S | HLR |
| 7 | 20 | RAL | 22 | LPV/r-RAL | 211 000 | HLR | IR | S | HLR | IR | HLR |
| 27 | 51 | RAL | 25 | TDF-LPV/r-RAL | 1 030 000 | HLR | IR | S | IR | IR | HLR |
| 13 | 35 | RAL | 34 | ETR- RAL-DRV/r | 190 000 | HLR | IR | S | HLR | IR | HLR |
| 32 | 52 | RAL | Unknown | AZT-3TC-DRV/r-RAL | 7190 | HLR | IR | S | HLR | HLR | HLR |
| 43 | 45 | RAL | Unknown | AZT-3TC-RAL | 159 000 | HLR | IR | S | HLR | S | S |
| 40 | 34 | RAL | Unknown | AZT-3TC-RAL | 67 816 | HLR | S | S | S | S | S |
| 24a | 45 | RAL | 3 | AZT-3TC-LPV/r(AZT-LPV/r-RAL) | 2520 | S | S | S | S | S | S |
| 37 | 35 | RAL | Unknown | ETR-DRV/r-RAL | 9337 | S | S | S | S | S | S |
| 29 | 14 | RAL | 13 | AZT-3TC-DRV/r-RAL | 42 900 | S | S | S | S | S | S |
| 5a | 58 | RAL | 25 | ABC-3TC-ATZ/r(TDF-3TC-RAL-DRV/r) | 386 000 | S | S | S | S | S | S |
| 28 | 18 | RAL | 27 | AZT-3TC-DRV/r-RAL | 1140 | S | S | S | S | S | S |
| 17 | 39 | RAL | 33 | ABC-TDF-3TC-DRV/r-RAL | 64 200 | S | S | S | IR | IR | HLR |
| 19 | 54 | RAL | 42 | TDF-FTC-DRV/r-RAL | 140 714 | S | S | S | HLR | IR | HLR |
| 22 | 37 | RAL | 53 | AZT-3TC-TDF-DRV/r-RAL | 6990 | S | S | S | HLR | HLR | HLR |
| 31a | 50 | RAL | 70 | ABC-3TC-LPV/r(TDF-FTC-LPV/r-RAL) | 1 370 000 | S | S | S | HLR | IR | HLR |
| 20 | 21 | RAL | Unknown | TDF-3TC-ETR-DRV/r-RAL | 14 900 | S | S | S | S | S | S |
| 41 | 16 | RAL | Unknown | 3TC-DRV/r-RAL | 30 663 | S | S | S | NA | NA | NA |
| 33 | 12 | RAL | Unknown | ETR-DRV/r-RAL | 2530 | S | S | S | HLR | IR | HLR |
| 34 | 10 | RAL | Unknown | AZT-3TC-DRV/r-RAL | 868 | S | S | S | IR | S | IR |
| 38 | 3 | RAL | Unknown | ABC-3TC-LPV/r-RAL | 88 821 | S | S | S | S | S | S |
| 8 | 38 | DTG | 22 | TDF-3TC-DRV/r-DTG | 311 000 | HLR | HLR | IR | IR | IR | HLR |
| 15 | 45 | DTG | 4 | TDF-3TC-DRV/r-DTG | 39 400 | S | S | S | IR | IR | HLR |
| 30 | 43 | DTG | 10 | AZT-3TC-DTG | 6500 | S | S | S | S | S | S |
| 39 | 31 | DTG | 1 | ABC-3TC-ATV/r-DTG | 117 274 | S | S | S | S | S | S |
| 35 | 55 | Unknown | Unknown | Unknown | 429 241 | HLR | IR | S | HLR | HLR | HLR |
| 3 | 57 | Unknown | Unknown | AZT-EFV-LPV/r | 222 604 | S | S | S | S | S | S |
| 21 | 5 | Unknown | Unknown | Unknown | 6990 | S | S | S | IR | S | IR |
| 23 | 41 | Unknown | Unknown | AZT-3TC-LPV/r | 19 400 | S | S | S | IR | S | HLR |
| 36 | 39 | Unknown | Unknown | TDF-FTC-DRV/r | 139 609 | S | S | S | HLR | IR | HLR |
Abbreviations: ABC, abacavir; ART, antiretroviral treatment; ATV/r, ritonavir-boosted atazanavir; AZT, zidovudine; d4T, stavudine; DTG, dolutegravir; DRV/r, ritonavir-boosted darunavir; ETR, etravirine; EVG, elvitegravir; FTC, emtricitabine; HIV, human immunodeficiency virus; HIVDR, HIV drug resistance; HIVVL, HIV viral load; HLR, high-level resistance; INSTIs, integrase strand transfer inhibitors; IR, intermediate resistance; LPV/r, ritonavir-boosted lopinavir; NA, not applicable, no protease and reverse-transcriptase genotype available; RAL, raltegravir; S, susceptible; TDF, tenofovir; 3TC, lamivudine.
aThree patients were not receiving an INSTI at time of genotyping, but their previous regimen (in italics) indicate exposure to INSTI.
The most common major INSTI mutations were Y143C/R/H (n = 12) and N155H (n = 9); the accessory T97A mutation was detected in 13 cases. The complete integrase, protease, and reverse-transcriptase mutation profile is presented in Supplementary Table 1.
Only 4 patients in the cohort had recorded exposure to DTG, and 3 of them presented without any INSTI mutations after 1, 4, and 10 months of DTG-exposure, respectively. The fourth patient presented with intermediate resistance to DTG after 22 months of DTG exposure (Table 1).
Discussion
This retrospective study provides important insights into the INSTI resistance profiles in patients failing INSTI-based TLART in the South African public sector. The presence of INSTI resistance is common in patients failing RAL-based regimens (59%) after a median of only 25 months of RAL-exposure, confirming the low genetic barrier of first-generation INSTIs. These results are lower than those observed in Argentina where 72% of heavily treated patients presented with RAL and/or EVG resistance after a median of 22.5 months of RAL exposure [8]. Our findings are more similar to those from a Ugandan cohort of 51 patients failing RAL-based TLART where 47% of patients presented with RAL and/or EVG resistance [7]. The prevalence of HIV-1 subtypes differs globally and among studies reporting INSTI resistance: HIV-1 subtype B/F and B predominated in the Argentinian cohort versus A, D, and A/D in Uganda and subtype C in our study. Human immunodeficiency virus-1 subtype may influence INSTI development. However, our study was not able to establish subtype associations due to the small sample and many possible confounders such as regional differences in treatment regimens, virological failure monitoring, and concomitant medication. Therefore, it is not known whether the higher frequency of multiple INSTI mutations in the Argentinian cohort (49%), compared with 40% in our study, might indicate a subtype association. In our study, all patients with only RAL/EVG resistance presented with a single major INSTI mutation with (n = 16) or without (n = 5) accessory INSTI mutations. In contrast, the 3 patients with DTG resistance presented with at least 2 major INSTI mutations, indicating that accumulation of INSTI mutations should be avoided. The most common mutations in RAL-exposed patients were Y143C/R/H and N155H (29% each), which are known to be common in RAL-exposed patients [10]. The third signature RAL mutation, Q148R, which is commonly observed in subtype B, was only observed once in this study. The R263K mutation was not observed in our cohort, which was also reported by Argentinian and Ugandan studies [7, 8]. Cross-resistance to DTG remains limited to 6% in RAL-exposed individuals, which is also consistent with the Ugandan findings (4%) [7], but this is substantially lower compared with the findings from the Argentinian cohort (32%), where poor adherence was reported by most patients [8].
Our results suggest that most patients failing RAL regimens can still benefit from a DTG-based regimen, provided limited accumulation of INSTI mutations. The Viking 3 study showed that 69% of patients, who previously failed RAL-based ART with resistance mutations, obtained virological suppression at week 24 on DTG-based regimens, administering DTG 50 mg bid. In addition, an Italian, real-life cohort analyzed virological outcomes in RAL/EVG-exposed patients who switched to DTG-salvage regimens [11]. None of the 79 patients who remained on DTG after a median of 18 months in this study experienced virological failure at the end of the follow-up period. Despite a favorable prognosis of viral suppression for RAL-experienced patients on DTG-regimens, there is concern that those patients who remain on a failing RAL-based regimen are at risk of accumulating multiple INSTI mutations and thereby reducing DTG activity. Appropriate interruption of RAL could prevent accumulation of mutations, hence optimizing the effect of DTG in RAL-exposed patients. The current recommendation from the TLART committee is to administer DTG twice daily in patients with confirmed or suspected INSTI resistance [12].
Although the group of DTG-based failures is very small (n = 4), one of these patients presented with DTG resistance after only 22 months of DTG exposure with multiple mutations: T66A, E138K, Y143R, S147G, Q95K, and T97A. A recent case report from Botswana also described DTG resistance in a highly treatment-experienced, DTG-exposed patient with 5 INSTI mutations (E138K, G140A, S147G, Q148R, T97A) but without R263K [13]. The variability of resistance pathways selected under DTG pressure was also confirmed in various DTG mono or “less-drugs trials”, which were reviewed by Blanco et al [14]. The signature DTG mutation R263K was not detected in any of the patients exposed to DTG. This might be due to the small sample size, but it has also been shown that the prevalence of R263K in subtype C might be lower compared with subtype B because this mutation is shown to be more deleterious in subtype C [15].
The study presented is limited by the lack of an accurate measure of the frequency of patients currently failing INSTI-based ART, and subsequently those that are referred for genotyping, in the South African HIV programme, which would permit an estimation of representativeness. However, integrase resistance testing for the public sector is only offered in 2 laboratories; all data from these laboratories were collated to make the study as representative as possible. Treatment history and duration of INSTI exposure were only extracted from drug resistance test request forms, which were limited. Information on treatment adherence was not available; however, these patients are likely to be considered as adherent because intensified adherence counseling is recommended before PI resistance can be confirmed by genotyping. Therefore, these findings are not necessarily generalizable to other populations that might receive INSTI-based regimens in the future. Finally, mutations in the 3’-polypurine tract, which have also been reported to contribute to DTG resistance, were not assessed [16]. Despite these drawbacks, the study provides current analysis of integrase resistance in South Africa.
Conclusions
Overall, our findings indicate that patients failing RAL-containing regimens are prone to the development of resistance, albeit limited observed cross-resistance to DTG. Although patients with pre-existing RAL mutations are likely to respond well to DTG-based salvage regimens, it is of concern that further accumulation of mutations may lead to DTG resistance. Since July 2016, DTG has been the preferred INSTI for TLART in South Africa. Now that DTG is available in the public sector, we recommend that, unless clinically contraindicated, RAL should be replaced by DTG in all patients on salvage regimens, to prevent potential accumulation of INSTI resistance mutations. The risk of development of resistance under DTG pressure cannot be estimated from this retrospective study; however, it is clear that in the absence of adequately powered prospective controlled studies, surveillance programs should be implemented once DTG is introduced on a larger scale in these settings, particularly because it is being considered for first-line treatment.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 27th International Workshop on HIV Drug Resistance and Treatment Strategies, Johannesburg, South Africa; Southern African HIV Clinicians Society (SAHCS) Conference 2018, Johannesburg, South Africa.
References
- 1. PEPFAR. South Africa Country Operational Plan 2018, Strategic Direction Summary. PEPFAR; 2018. Available at: https://www.pepfar.gov/documents/organization/285854.pdf. Accessed 10 May 2019. [Google Scholar]
- 2. Steegen K, Bronze M, Papathanasopoulos MA, et al. Prevalence of antiretroviral drug resistance in patients who are not responding to protease inhibitor-based treatment: results from the first national survey in South Africa. J Infect Dis 2016; 214:1826–30. [DOI] [PubMed] [Google Scholar]
- 3. Seatla KK, Avalos A, Mphoyakgosi T, et al. Preliminary virologic outcomes and prevalence of integrase strand transfer inhibitor resistance mutations among highly treatment experienced patients receiving dolutegravir in Botswana. In: 22nd International AIDS Conference; 23–27 July 2018; Amsterdam, The Netherlands. [Google Scholar]
- 4. Evans D, Hirasen K, Berhanu R, et al. Predictors of switch to and early outcomes on third-line antiretroviral therapy at a large public-sector clinic in Johannesburg, South Africa. AIDS Res Ther 2018; 15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meintjes G, Dunn L, Coetsee M, et al. Third-line antiretroviral therapy in Africa: effectiveness in a Southern African retrospective cohort study. AIDS Res Ther 2015; 12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moorhouse M, Maartens G, Venter WDF, et al. Third-line antiretroviral therapy program in the South African public sector: cohort description and virological outcomes. J Acquir Immune Defic Syndr 2019; 80:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ndashimye E, Avino M, Kyeyune F, et al. Absence of HIV-1 drug resistance mutations supports the use of dolutegravir in Uganda. AIDS Res Hum Retroviruses 2018; 34:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cecchini DM, Castillo S, Copertari G, et al. Resistance to HIV integrase strand transfer inhibitors in Argentina: first interim survey. Rev Esp Quimioter 2019; 32:263–7. [PMC free article] [PubMed] [Google Scholar]
- 9. Van Laethem K, Schrooten Y, Covens K, et al. A genotypic assay for the amplification and sequencing of integrase from diverse HIV-1 group M subtypes. J Virol Methods 2008; 153:176–81. [DOI] [PubMed] [Google Scholar]
- 10. Malet I, Delelis O, Valantin MA, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother 2008; 52:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rusconi S, Adorni F, Tau P, et al. Dolutegravir (DTG)-containing regimens after receiving raltegravir (RAL) or elvitegravir (EVG): durability and virological response in a large Italian HIV drug resistance network (ARCA). J Clin Virol 2018; 105:112–7. [DOI] [PubMed] [Google Scholar]
- 12. Castagna A, Ferrara M, Galli L, et al. Long-term efficacy of dolutegravir in treatment-experienced subjects failing therapy with HIV-1 integrase strand inhibitor-resistant virus. J Antimicrob Chemother 2018; 73:177–82. [DOI] [PubMed] [Google Scholar]
- 13. Seatla KK, Avalos A, Moyo S, et al. Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana. AIDS 2018; 32:1899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blanco JL, Marcelin AG, Katlama C, Martinez E. Dolutegravir resistance mutations: lessons from monotherapy studies. Curr Opin Infect Dis 2018; 31:237–45. [DOI] [PubMed] [Google Scholar]
- 15. Mesplède T, Quashie PK, Hassounah S, et al. The R263K substitution in HIV-1 subtype C is more deleterious for integrase enzymatic function and viral replication than in subtype B. AIDS 2015; 29:1459–66. [DOI] [PubMed] [Google Scholar]
- 16. Wijting IEA, Lungu C, Rijnders BJA, et al. HIV-1 resistance dynamics in patients with virologic failure to dolutegravir maintenance monotherapy. J Infect Dis 2018; 218:688–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.