Abstract
Superoxide dismutases (SODs), as a family of metalloenzymes related to the removal of reactive oxygen species (ROS), have not previously been investigated at genome-wide level in tea plant. In this study, 10 CsSOD genes were identified in tea plant genome, including 7 Cu/Zn-SODs (CSDs), 2 Fe-SODs (FSDs) and one Mn-SOD (MSD), and phylogenetically classified in three subgroups, respectively. Physico-chemical characteristic, conserved motifs and potential protein interaction analyses about CsSOD proteins were carried out. Exon-intron structures and codon usage bias about CsSOD genes were also examined. Exon-intron structures analysis revealed that different CsSOD genes contained various number of introns. On the basis of the prediction of regulatory miRNAs of CsSODs, a modification 5’ RNA ligase-mediated (RLM)-RACE was performed and validated that csn-miR398a-3p-1 directly cleaves CsCSD4. By prediction of cis-acting elements, the expression patterns of 10 CsSOD genes and their regulatory miRNAs were detected under cold, drought, exogenous methyl jasmonate (MeJA) and gibberellin (GA3) treatments. The results showed that most of CsSODs except for CsFSD2 were induced under cold stress and CsCSDs may play primary roles under drought stress; exogenous GA3 and MeJA could also stimulated/inhibited distinct CsSODs at different stages. In addition, we found that csn-miR398a-3p-1 negatively regulated the expression of CsCSD4 may be a crucial regulatory mechanism under cold stress. This study provides a certain basis for the studies about stress resistance in tea plants, even provide insight into comprehending the classification, evolution, diverse functions and influencing factors of expression patterns for CsSOD genes.
Introduction
Tea plant (Camellia sinensis) is an important commercial crop and has been cultivated for more than 2000 years in China [1]. In recent years, nevertheless, whose quality and spatial distribution have been constantly affected by several abiotic stresses with the tea-producing regions expanding to the north and the global extreme climate occurring frequently, especially cold and drought [2–3]. According to previous research, cold stress is a serious environmental stress that damage cells and limits plant growth [4], while drought stress reduced tea production and increased tea plant mortality [5]. The most common effects of such stress is the generation of toxic reactive oxygen species (ROS), including superoxide radicals (O·2-), hydroxyl radical (OH·) and hydrogen peroxide (H2O2) [6]. Excessive ROS could result in cell membrane damage, protein oxidation and DNA damage, and eventually lead to metabolic disorder and apoptosis in plants, which has irreversible adverse effects on plant yield. To alleviate the oxidative damage caused by excessive ROS, an efficient and complex antioxidant ROS scavenging system was formed in the long-term process of plant development, including many non-enzymatic and enzymatic defense systems. Superoxide dismutases (SODs), act as the first line of defense in antioxidant system, play a significant role in catalyzing the dismutation of superoxide free radicals and protecting plant cells from oxidative damage [7]. SOD is a family of metalloenzymes related to the removal of ROS in aerobic organisms. Based on the type of metal cofactor, the SODs have been classified into three categories, Cu/Zn-SOD (CSD), Fe-SOD (FSD) and Mn-SOD (MSD), respectively [8]. Moreover, another type of SOD, Ni-SOD (NSD), was also discovered in streptomyces, but have not ever been found in plant [9]. SOD plays an important role in scavenging ROS and maintaining the balance of active oxygen via catalyzes the conversion or dismutation of toxic superoxide anion radicals to synthesize O2 and H2O2 in plants [10]. Under normal circumstance, the synthesis and homeostasis of ROS maintaining a dynamic balance and will not cause damage to plants. However, the dynamic balance will be destroyed under a series of stress. Once the free radical accumulation exceeds the threshold, it will poison the cells [11]. Hitherto, SOD genes family have been widely studied in many plant species, including arabidopsis (Arabidopsis thaliana) [12], tobacoo (Nicotiana tabacum) [13], populus (Populus trichocarpa) [14], longon (Dimocarpus longan) [15], soybean (Glycine max) [16], sorghum (Sorghum bicolor) [17], banana (Musa acuminate) [18], diploid cotton (Gossypium raimondii and G. arboretum) [19], upland cotton (G. hirsutum) [20], cucumber (Cucumis sativus) [21], foxtail millet (Setaria italica) [22], grapevine (Vitis vinifera) [9], japanese larch (Larix kaempferi) [23], and rapeseed-mustard crops (Brassica juncea and Brassica rapa) [24], etc. In previous studies, in A. thaliana, the transcription and translation level of SOD genes increased significantly after subjected to oxidative stress [12]; in G. hirsutum, under drought stress, the expression of three types of SOD genes was significantly up-regulated; but under cold stress, only the expression of CSD genes were increased, and the photosynthetic rate and soluble sugar were also increased [25]; after transfer MSD gene from pea into rice, the drought tolerance was significant enhanced and also exhibited less injury [26]; in tea plant, under the cold stress, the content of ROS was higher together with lower SOD activity, and the leaf damage was more serious in the cold-sensitive cultivar compared to the cold-resistant cultivar [27]. Thus, increasing the activity of SOD is one of the effective ways for plants to resist a series of abiotic stress.
MicroRNAs (miRNAs), a group of 20–24 nt in length, single-stranded non-coding RNAs that negatively regulate gene expression at the post-transcriptional levels [28]. Evidence is accumulating that miRNAs play key roles in the regulation of abiotic responses in plant. For example, miR398 negatively regulate the expression level of CSDs by cold and oxidative stress in A. thaliana [29]; miR165/166 involved in drought resistance each in A. thaliana [30] and O. sativa [31]. In 2010, 13 miRNAs were computational identified. Among them, 11 miRNAs targeted to 37 potential targets in tea plant [32–33]. This was the first finding for the prediction and analysis of tea plant miRNAs through bioinformatics tools, and it provide insight into understanding the function and processing of tea miRNAs. Subsequently, there were also many miRNAs have been found that related to stress resistance in tea plant, such as miR156, miR166a, and miR398 [2–3]. However, the regulatory miRNAs of CsSOD genes are still unknown.
To date, there was no research about the identification and analysis of SOD genes at genome-wide level in tea plant. It is possible that identify all of the SOD family members at genome-wide level with the genome data of tea plant (cultivar ‘Yunkang 10’ and cultivar ‘Shuchazao’) were made available to the public [34–35]. In this study, we performed a research to identify and analyze all the members of SOD in tea plant, including physico-chemical characteristics, phylogenetic relationships, exon-intron structure, motif composition, potential protein interaction, and codon usage bias (CUB). By using miRNA database from previous published article [3], we predicted the regulatory miRNAs of CsSOD genes, then, the modification 5’ RNA ligase-mediated (RLM)-RACE was carried out to verify the putative cleavage sites of CsSODs. Moreover, the cis-acting elements of CsSODs promoters involved in stress responses and hormone stimuli were also predicted to further clarify the regulatory mechanisms of CsSODs expression. Refer to the predicted results, we investigated the expression patterns of the CsSOD genes and their regulatory miRNAs under cold, drought, exogenous gibberellin (GA3), and methyl jasmonate (MeJA) treatments via quantitative real-time polymerase chain reaction (qRT-PCR). This systematic study about CsSOD gene family will provide a certain basis for the studies about exploration of CsSODs diverse functions.
Materials and methods
Retrieval of SOD genes in tea plant
Known AtSOD amino acid sequences downloaded from NCBI as baits, the local BLASTP was executed to search the SOD proteins in two tea plant genomes (cultivar ‘Yunkang 10’ and cultivar ‘Shuchazao’). Functional annotations were filtered for Pfam identifiers of the SOD domains (PF00080, PF00081, and PF02777). Then, the filtered sequences were uploaded to Simple Modular Architecture Research Tool (SMART) (http://smart.embl-heidelberg.de/) [36] and Conserved Domain Database (CCD) (https://www.ncbi.nlm.nih.gov/cdd/) [37], respectively, to further verify the accuracy of these sequences. Information about the length of CDS and amino acid sequences of CsSOD was obtained from the tea plant genome database [34–35]. The WoLF PSORT web server (https://wolfpsort.hgc.jp/) [38] and ProtParam tool in ExPASy web (http://www.expasy.org/) [39] were used to predict the subcellular localizations and the physico-chemical characteristics of CsSOD proteins, respectively.
Phylogenetic tree construction
The full-length of SOD protein sequences from monocotyledonous plant (O. sativa and M. acuminata), and dicotyledonous plant (A. thaliana, C. sinensis, D. longan, and P. trichocarpa) were aligned using the Muscle program with default parameters [40]. For step further classification, we constructed a phylogenetic tree using MEGA5.1 software with the neighbor-joining (NJ) algorithm and setting 1000 bootstrap replicates, other parameters were all default [41]. Then, using iTOL web tool (https://itol.embl.de/) [42] to visualize the phylogenetic tree.
Analysis of exon-intron structure and conserved motifs
A separate clustering diagram for CsSOD and AtSOD proteins was built by MEGA 5.1 software [41]. The exon-intron structures of SOD genes from tea plant and A. thaliana were identified via upload the Genetic Feature Format (GFF3) file from tea plant and A. thaliana genome data to TBtools program for analysis [43]. The sequences of CsSOD and AtSOD proteins were analyzed by MEME web server (http://meme-suite.org/tools/meme) [44] to predicted conserved motifs. Selecting “5 motifs should MEME find” mode and other parameters were left as default. Then, using TBtools program to combine the results of phylogenetic tree, exon-intron structures and conserved motifs from tea plant and A. thaliana into one map.
Analysis of potential protein interaction
The SOD protein interaction network was constructed by the STRING 11.0 (https://string-db.org/cgi/input.pl) [45] on the basis of the orthologs in known AtSOD. Setting the network edges as confidence, the parameters as medium confidence parameter (0.400) and no more than 10 interactors to show.
Analysis of codon usage bias about CsSOD genes
The measured parameters of CUB, including ENC, RSCU, CAI, GC and GC3s were calculated by CodonW program [46], and the data processed in Excel 2013.
Prediction of regulatory elements of CsSOD genes
For cis-acting elements prediction, we extracted the upstream genome sequence within 2000 bp of the start codon from each CsSOD gene as putative promoter region, then using the PlantCARE serve (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [47] to predicted cis-acting elements. CsSOD genes targeted by miRNAs were predicted via searching the CDS sequences of all CsSOD genes for complementary sequences of the tea plant miRNAs using the psRNATarget (https://plantgrn.noble.org/psRNATarget/analysis?function=3) [48] with default parameters, and any targets with an overall score below 5.0 was considered to be a credible miRNA target. The miRNA database was from previous published article [3].
Tea plant materials and experimental treatments
This experiment employed 2-year-old seedlings of tea plant cultivar ‘Tieguanyin’, which were cultivated in a conservatory belong to college of horticulture of Fujian Agriculture and Forestry University, Fuzhou, China (26°05′N, 119°18′E). The conservatory is a place for teaching and scientific research, so, there were no specific permissions were required, and this experiment did not involve any endangered or protected plant materials. The conservatory ambient conditions maintain 25°C ± 2°C temperature, 14/10 h light/dark photoperiod, and 70% relative humidity. Then, those seedlings were subject to different treatments, including cold and drought, and hormone treatments (MeJA and GA) refer to previous reported procedures with minor revise [49–50]. Before these processing, the second leaves from the seedlings were collected at “0 h” as CK. For cold treatments, the temperature in the conservatory was modulated to 4°C and other conditions remain unchanged. For drought treatments, the seedling was irrigated with 15% (w/v) PEG 4000 to simulate drought stress. For exogenous MeJA and GA3 treatments, freshly prepared working solutions of 1 mmol/L MeJA and GA3 were sprayed on 2 different seedlings, respectively. Then, second tender leaves from each seedling were sampled after 12 hours, 24 hours, 36 hours, and 48 hours, respectively. Then, frozen all the samples in liquid nitrogen and stored at −80°C immediately. Three independent biological replicates were employed.
RNA isolation, primers designing, and qRT-PCR analysis
Total RNA from all the samples were isolated using Transzol up (TransGen, Beijing, China). The integrity of the isolated RNA samples was detected by 0.8% agarose gel electrophoresis, and purity and concentration were tested by NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, USA). Those samples whose OD260/OD280 ratios between 1.90 and 2.10 were selected for reverse transcribe to cDNA for CsSOD genes and first-strand cDNA of miRNA using the Prime-Script™ Reagent Kit (Takara, Otsu, Japan) and TransScript miRNA First-strand cDNA Synthesis Super Mix (TransGen, Beijing, China), respectively.
The GAPDH (accession no. GE651107) and β-Actin (accession no. LOC114296039) genes in tea plant were both used as reference genes [51] for CsSODs, while U6 and 5.8s were both used as internal references for regulatory miRNAs of CsSODs. Specific primers of CsSOD genes and reference genes (S5 Table) for qRT-PCR system were designed and verified using tea plant information archive (TPIA) platform [52] and DNAMAN 9 software, respectively, while the miRNA universal primer was from TransScript miRNA First-strand cDNA Synthesis Super Mix (TransGen, Beijing, China), and the specific primers of miRNA and their internal references were shown in S5 Table. The qRT-PCR detection about CsSOD genes and their regulatory miRNAs under different treatments were conducted on LightCycler 480 (Roche Applied Sciences, Basel, Switzerland) platform using Hieff qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) and TransStart Tip Green qPCR SuperMix (TransGen, Beijing, China), respectively. Relative expression level was calculated by 2−ΔCt method [53]. Statistical analyses were conducted using SPSS 25 software, and the data were analyzed by one-way analysis of variance followed by Tukey’s post-hoc test [51].
Cleavage site identification with modified 5’ RLM-RACE
To verified the miRNA cleavage sites of CsCSD4, CsCSD7 and CsFSD2 respectively, a modified 5’ RLM-RACE experiment was performed using First-Choice RLM-RACE Kit (Thermo Fisher Scientific, carlsbad, USA) with the manufacturer’ s instructions. In brief, total RNA (5 μg) from equal mixtures of all the RNA samples were ligated to the adapter for 5’ RACE without treatment with dephosphorylation and decapped. The first round PCR amplification of cDNA fragments was performed using the 5’ RACE outer primer from the manufacturer and gene-specific outer primer (after putative complementary regions of miRNAs about 200 bp) (S5 Table). Then, the first round PCR products were used as the template of nested PCR with 5' RACE inner primer from the manufacturer and gene-specific inner reverse primers (S5 Table). Finally, the resulting PCR fragments were gel-purified by EasyPure Quick Gel Extraction Kit (TransGen, Beijing, China), then cloned into pMD 18-T Vector (Takara, Otsu, Japan) for sequencing (BioSune, Fuzhou, China).
Results
A total of 10 SOD genes were identified in tea plant
In this study, we searching the tea plant genome database (cultivar ‘Yunkang 10’ and cultivar ‘Shuchazao’) using Pfam identifiers of CSD domain (PF00080) and FSD / MSD domain. Both FSD and MSD had the FSD/MSD N-terminal domain (PF00081) and the FSD/MSD C-terminal domain (PF02777). Finally, we both ascertained a total of 10 SOD genes in two tea plant genomes, of which, 7 members belong to CSDs, 2 members belong to FSDs, and only one member was MSD. Based on their scaffold ID order and Pfam types, we named these 10 CsSOD genes as CsCSD1 to CsCSD7, CsFSD1 to CsFSD2 and CsMSD1, respectively. The gene names, transcript IDs, scaffold IDs, coding sequences (CDS), and amino acid sequences were listed in S1 Table. After performed BLASTP on NCBI, we found that the matching rate of part CsSODs in cultivar ‘Shuchazao’ genome (TEA012288, TEA005110, TEA023332, TEA012017) were low compared to known CsSOD genes (only 68%, 25%, 33%, and 54%, respectively). Thus, the subsequent analyses all use CsSOD sequences in cultivar ‘Yunkang 10’ genome.
The physico-chemical characteristics of identified CsSOD proteins analyses, including the lengths, molecular weights (MWs), isoelectric points (pIs), instability index, grand average of hydropathicity (GRAVY), aliphatic index, and subcellular prediction of CsSOD proteins were showed in Table 1. All the 10 CsSOD proteins ranged from 139 (CsCSD5) to 333 (CsFSD1) aa in length, and have MWs from 14.20 (CsCSD5) to 37.42 (CsFSD1) kDa, pIs from 4.90 (CsCSD6) to 7.14 (CsFSD2), instability index from 14.34 (CsCSD5) to 42.49 (CsFSD2), GRAVY from -0.546 (CsFSD1) to 0.326 (CsCSD6), and aliphatic index from 69.79 (CsFSD1) to 92.36 (CsCSD7), respectively. The pI values showed that all members of CsSOD except CsFSD2 were acidic. The values of instability index < 40 or > 40 are determine the protein will be stable in a test tube or not [54]. Other than CsFSD2, most CsSOD proteins were predicted to be stable. According to the result of putative subcellular localization, CsCSD1, CsCSD2, CsCSD3, CsCSD5, CsCSD6 and CsFSD2 proteins were localized in cytoplasm while CsCSD4, CsCSD7 and CsFSD1 proteins were in chloroplasts; and CsMSD1 protein was in mitochondrion (Table 1). The information of previous studies about SOD subcellular localization [55] corroborated our findings.
Table 1. Physico-chemical characteristics of identified CsSOD proteins.
| Name | Protein(aa) | MW(kDa) | pI | Instability index | GRAVY | Aliphatic index | Subcellular location |
|---|---|---|---|---|---|---|---|
| CsCSD1 | 143 | 24.03 | 6.39 | 22.49 | -0.196 | 84.56 | Cytoplasm |
| CsCSD2 | 204 | 21.16 | 5.72 | 20.64 | 0.116 | 90.29 | Cytoplasm |
| CsCSD3 | 152 | 15.47 | 5.62 | 18.22 | -0.135 | 81.97 | Cytoplasm |
| CsCSD4 | 219 | 22.62 | 6.82 | 21.17 | -0.045 | 88.72 | Chloroplast |
| CsCSD5 | 139 | 14.20 | 5.91 | 14.34 | -0.188 | 76.40 | Cytoplasm |
| CsCSD6 | 249 | 25.71 | 4.90 | 20.19 | 0.326 | 86.10 | Cytoplasm |
| CsCSD7 | 208 | 21.49 | 6.44 | 17.71 | -0.080 | 92.36 | Chloroplast |
| CsFSD1 | 333 | 37.42 | 5.42 | 35.36 | -0.546 | 69.79 | Chloroplast |
| CsFSD2 | 243 | 28.26 | 7.14 | 42.49 | -0.516 | 79.05 | Cytoplasm |
| CsMSD1 | 205 | 22.41 | 6.22 | 30.25 | -0.201 | 90.93 | Mitochondrion |
Phylogenetic classification of SOD proteins in different plants
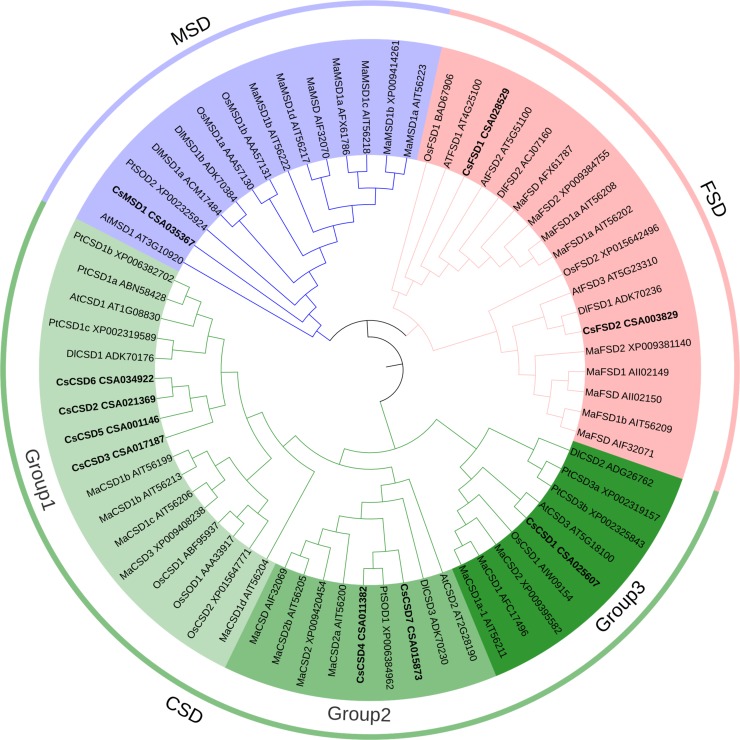
To investigate the evolutionary relationship of SOD in different plant species, we aligned and constructed a phylogenetic tree based on full-length of SOD protein sequences from monocotyledonous plants, O. sativa and M. acuminata, and dicotyledonous plants, C. sinensis, A. thaliana, D. longan and P. trichocarpa (Fig 1). The result showed that all the SOD proteins from different plant species were clustered into three major categories, which in line with their metal cofactor types, Cu/Zn- (CSD), Fe- (FSD) and Mn- (MSD), respectively. Further analysis, MSDs and FSDs were highly similar, and belong to the same subgroup, while the CSDs were quite different from them. The CSD proteins of each species gathered together and showed cluster distribution, indicating that CSDs were highly conserved in different plants. Notably, CsCSD1 and AtCSD3, CsCSD4 and PtSOD1 were in a smallest clade respectively, further indicate that CSD proteins were highly conserved in dicotyledon.
Fig 1. Phylogenetic tree of SOD proteins from O. sativa, M. acuminata, A. thaliana, D. longan, P. trichocarpa, and C. sinensis.
Os: Oryza sativa; Ma: Musa acuminata; At: Arabidopsis thaliana; Dl: Dimocarpus longan; Pt: Populus trichocarpa; Cs: Camellia sinensis.
To step further analysis of clustering result, CSD proteins were subdivided into 3 subgroups (Group1, Group2 and Group3). CsCSD2, CsCSD3, CsCSD5 and CsCSD6 proteins were in Group 1; CsCSD4 and CsCSD7 proteins were in Group 2; and CsCSD1 protein was in Group 3. It was also found in Group 1 that CSD members from O. sativa and M. acuminata were clustered into a small branch, while the CSD members from tea plant, A. thaliana, D. longan and P. trichocarpa were in another small branch. It is speculated that there may be some different evolution strategies in CSD protein sequences between monocotyledonous and dicotyledonous plants.
Exon-intron structures and conserved Motifs of CsSOD and AtSOD
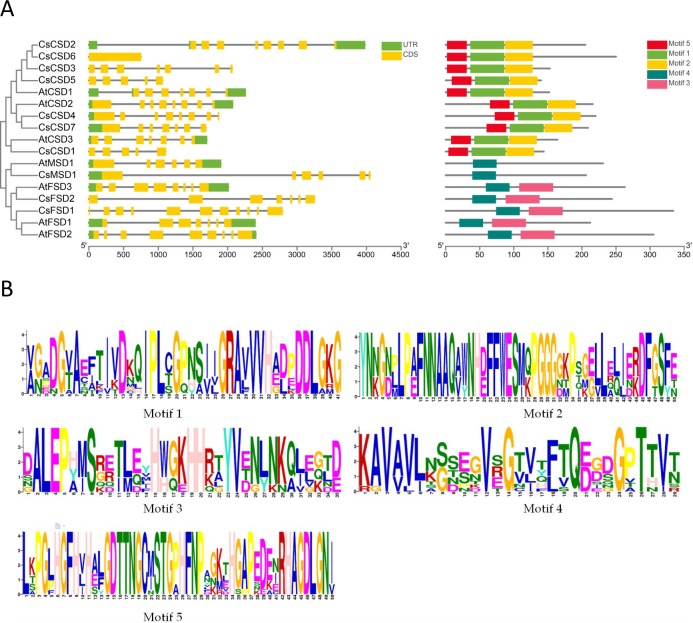
A separate cluster of SOD proteins from C. sinensis and A. thaliana (CsSOD and AtSOD) was shown in Fig 2A. To step further clarify the structure features of CsSODs, a comparative analysis of exon-intron structure was executed between CsSOD and AtSOD genes (Fig 2A). The CsSOD genes exhibited different exon-intron organizational patterns and not in accord with phylogenetic clustering. CsSOD genes contained introns from 0 (CsCSD2) to 10 (CsFSD1) and AtSOD genes contained introns from 5 (AtMSD1) to 8 (AtFSD2). Compare to AtSOD, the number of intron varies greatly among different CsSOD genes. Further analysis, all exon-intron junction sites of CsSOD genes were spliced consistent with the eukaryotic GT-AG splice rules [56].
Fig 2. Phylogenetic tree, conserved motifs, and motif logos of CsSOD and AtSOD proteins and exon-intron structures of CsSOD and AtSOD genes.
(A) Phylogenetic tree, exon-intron structures, conserved motifs of CsSOD and AtSOD proteins; (B) Motif logos of CsSOD and AtSOD proteins.
Furthermore, we also searched for the presence of conserved motifs in all members of CsSOD proteins compare to A. thaliana (Fig 2B). Motif 1, 2 and 5 were found in all CSD proteins while all FSD proteins possess motif 3 and 4. Both CsMSD1 and AtMSD1 proteins contain only motif 4 (Fig 2A). According to the results of CDD annotation, Motif 1, 2 and 5 were all belong to “Cu_Zn Superoxide Dismutase superfamily”. Motif 3 and 4 were both belong to “Sod_Fe_C superfamily”. Different motifs were specifically present in CSD, FSD and MSD, respectively, in accord with their phylogenetic clustering.
Potential CsSOD protein–protein interaction
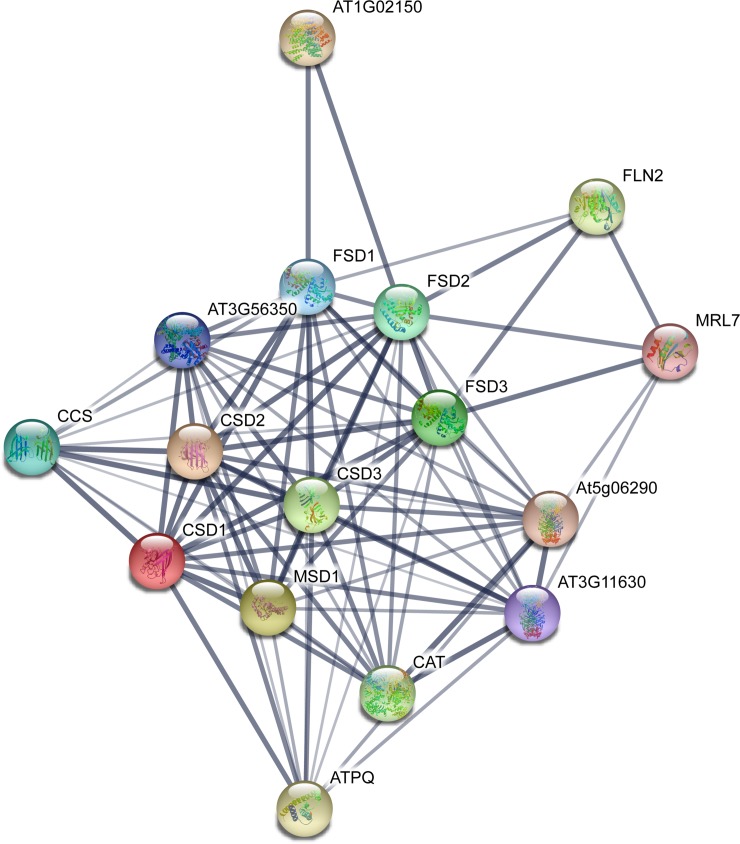
On the basis of the orthologs in A. thaliana, the potential CsSOD protein–protein interaction was analyzed. The homologous AtSOD proteins with the highest identity were regarded as “STRING proteins”. As shown in Fig 3, all the 10 CsSOD proteins associated with 6 known AtSOD proteins in the interaction network. For CSDs, CsCSD2, CsCSD3, CsCSD5, and CsCSD6 in connection with AtCSD1; CsCSD4 and CsCSD7 associated with AtCSD2; and AtCSD3 was the highest homologous protein of CsCSD1. For FSDs, CsFSD1 and CsFSD2 corresponding to AtFSD2 and AtFSD3, respectively. And CsMSD1 associated with AtMSD1. As expected, the corresponding result was exactly same as the phylogenetic classification, which CsSOD proteins corresponding to three types of AtSOD proteins were also divided into same subgroup in the phylogenetic tree (Fig 1). Three types of SOD proteins involved in a strong interaction networks, they may play a regulatory role through the formation of protein complexes. Moreover, CSDs were involved in a strong interaction with copper chaperone for superoxide dismutase (CCS). CCS is responsible for the transfer of Cu2+ into cytoplasm, which increases the concentration of Cu2+ in the cytoplasm and thereby promoting the expression of CSD1 [57]. In addition, the high level of interaction between CSDs and catalase (CAT) implied various enzymes in plant antioxidant enzyme system might respond to various biological and abiotic stresses by synergetic response.
Fig 3. Potential protein–protein interaction network of CsSODs.
CsCSD2, CsCSD3, CsCSD5, and CsCSD6 were displayed as homologous CSD1 protein in A. thaliana; CsCSD4 and CsCSD7 were displayed as homologous CSD2 in A. thaliana; and CsCSD1 was displayed as homologous CSD3 in A. thaliana; CsFSD1 and CsFSD2 were displayed as homologous FSD2 and FSD3 proteins in A. thaliana, respectively. And CsMSD1 was displayed as homologous MSD1 protein in A. thaliana. The higher the interaction coefficient, the thicker the line between proteins, vice versa.
Codon usage bias of CsSOD genes
The phenomenon of CUB can reflect the origin, evolution and mutation patterns of genes and given an important reference value for analysis of gene function and expression [58–59]. Thus, analysis of CUB patterns could provide necessary theoretical guidance for the further studies of species evolution law, gene function and gene expression. In this study, the values of ENC, CAI, GC and GC3s of CsSOD genes were shown in Table 2. We can observe that the ENC values of CsSOD genes were between 42.12 and 58.09 and the average value was 50.12, indicating that the codons of CsSOD genes have no obvious bias and relatively low expression level in tea plant, same as CAI value validation. The CAI values of CsSOD genes varied from 0.19 (CsFSD1) to 0.26 (CsMSD1), which were far less than 0.5. GC and GC3s analyses showed that the GC values of CsCSD4 and CsCSD7 were greater than 0.5, accounting for only 20% of all the subjects studied, and only the GC3s value of CsMSD1> 0.5. We calculated that the average value of GC3s was 0.41 at whole-genome scale in tea plant cultivar ‘Yunkang 10’ and cultivar ‘Shuchazao’, meaning that CsSOD genes except CsMSD1 were consistent with tea plant genome on CUB pattern. Moreover, RSCU values of 59 codons in all CsSOD genes showed that there were 34 optimal codons, including CUU, GUU, ACC, and AGA in CsCSD1; CCU, GCU, and AGG in CsCSD2; CUU, GUU, AGC, GCU, and AGG in CsCSD3; CUC, UCC, and CCA in CsCSD4; CUU, AUU, GUU, ACA, and GCU in CsCSD5; CCU in CsCSD6; CUC, UCC, and CGU in CsCSD7; AGU, CGU, ACA, and AGA in CsFSD1; CUU, ACA, and AGA in CsFSD2; AGC and CGG in CsMSD1, meaning CsSOD genes has stronger bias on these codons, and these codons may be the optimal codons of the CsSOD genes (S2 Table).
Table 2. The values of CAI, ENC, GC, and GC3s of CsSOD genes, ‘Yunkang 10’ and ‘Shuchazao’ genome.
| Name | CAI | ENC | GC | GC3s |
|---|---|---|---|---|
| CsCSD1 | 0.23 | 50.98 | 0.49 | 0.44 |
| CsCSD2 | 0.24 | 45.71 | 0.48 | 0.39 |
| CsCSD3 | 0.25 | 46.48 | 0.49 | 0.33 |
| CsCSD4 | 0.22 | 49.39 | 0.52 | 0.44 |
| CsCSD5 | 0.23 | 42.12 | 0.46 | 0.27 |
| CsCSD6 | 0.22 | 55.95 | 0.48 | 0.35 |
| CsCSD7 | 0.20 | 49.29 | 0.51 | 0.43 |
| CsFSD1 | 0.19 | 51.05 | 0.44 | 0.32 |
| CsFSD2 | 0.20 | 52.16 | 0.42 | 0.37 |
| CsMSD1 | 0.26 | 58.09 | 0.50 | 0.51 |
| ‘Yunkang 10’ genome | 0.20 | 51.91 | 0.45 | 0.41 |
| ‘Shuchazao’ genome | 0.20 | 52.02 | 0.41 | 0.44 |
Various functions of cis-acting elements in CsSOD promoters
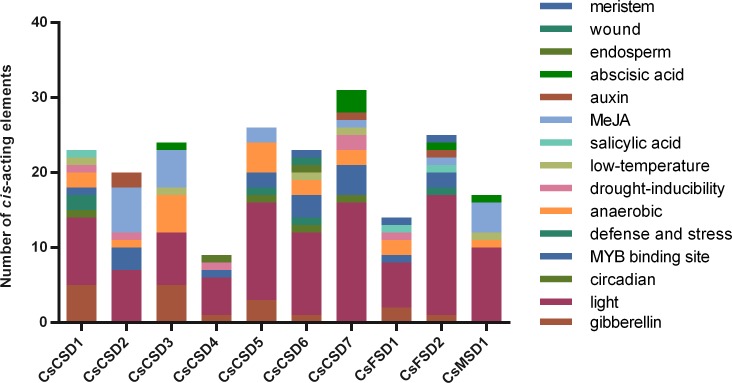
For seeking cis-acting elements of CsSOD genes, we extracted the upstream 2000 base pairs (bp) genome sequences of the start codon from each CsSOD gene as putative promoter region. We filtered the unknown elements because of the information about promoter sequences of CsCSD2, CsCSD4, CsFSD2 and CsMSD1 were insufficient (existing 710 bp, 1117 bp, 130 bp and 533 bp N-containing sequences, respectively). Thus, we found a total of 34 types cis-acting elements about abiotic stress and hormone responses in putative promoters of all CsSOD genes (S3 Table). On analysis, CAAT box and TATA box which related to common promoter and enhancer regions and core promoter element of transcription start, respectively, were found in all promoter regions. Moreover, a large number of light-responsive elements were also been found in all promoters and the number varied from 5 (CsCSD4) to 16 (CsCSD7 and CsFSD2). Other functions of predicted cis-acting elements were showed in Fig 4.
Fig 4. Different cis-acting elements in putative CsSOD promoters which associated with abiotic stresses, hormone responses, growth and development.
These hormone-responsive elements, including P-box, TATC-box and GARE-motif; TCA-element; CGTCA-motif and TGACG-motif; TGA-element; and ABRE, which associated with GA3, salicylic acid (SA), MeJA, Auxin and abscisic acid (ABA) response, respectively. Of which, more than half of members contain GA- and MeJA- responsive elements. For stress-responsive elements, TC-rich repeats; ARE and GC-motif; MYB binding site (MBS); low temperature responsiveness (LTR); and WUN-motif, related to defense and stress, anaerobic induction, drought inducibility, low-temperature inducibility, and wound stress, respectively. Therein, drought inducibility and low-temperature inducibility elements were found in over half of all CsSOD members. Some regulatory elements for plant growth and development, including GCN4-motif and CAT-box were allied to endosperm expression and meristem expression have also been found in CsCSD4, CsCSD6, CsFSD1 and CsFSD2.
Such number of cis-acting elements in typical CsSOD promoter regions manifesting that CsSOD genes should be involved in distinct regulatory mechanisms in stress resistance and the growth development in tea plant.
Validation of csn-miR398a-3p-1 cleavage of CsCSD4
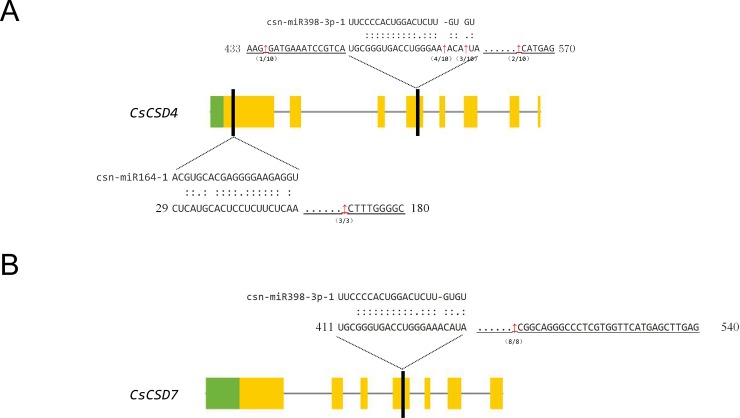
As a class of single-stranded, non-coding small RNAs, miRNAs can suppress the expression of specific target genes by guiding the cleavage of target mRNA or inhibiting them translate into homologous proteins [60]. Hence, predicting the regulatory miRNA of CsSOD genes is crucial way for understanding the regulatory factor about CsSOD expression pattern at posttranscriptional level. By prediction, five miRNAs (miR398a-3p-1, miR164-1, novel-miR54, miR166-5d-1, and miR159a-1) regulate three CsSODs (CsCSD4, CsCSD7 and CsFSD2) by triggering the cleavage (S4 Table). To be specific, CsCSD4 was targeted by csn-miR164-1, and csn-miR398a-3p-1; CsCSD7 was targeted by csn-miR159a-1, csn-miR398a-3p-1, and novel-miR54; and CsFSD2 was targeted by csn-miR166d-5p-1. To further identify cleavage sites, fragments of the CsCSD4, CsCSD7, and CsFSD2 mRNAs were detected using the modification 5’ RLM-RACE. The electrophoretogram (S2 Fig) showed that the nested PCR productions of CsCSD4 was between 100 bp and 250 bp, others were all shorter than 100 bp. Sequencing result showed that no cleavage products cloned from the novel-miR54 and miR159a-1 complementary region of CsCSD7, and miR166d-5p-1 complementary region of CsFSD2 were detected. Moreover, the cleavage sites all mapped outside the complementary regions of csn-miR398a-3p-1 and miR164-1 complementary regions in CsCSD7 and CsCSD4, respectively (Fig 5). Most fraction of cleavage products (7/10) cloned from CsCSD4 were detected between csn-miR398a-3p-1 complementary region (Fig 5A). This result firmly proved that CsCSD4 is directly cleaved by csn-miR398a-3p-1. The expression of each miRNAs and their target genes need to detected in further experiments to determine how they exert specific functions in tea plant.
Fig 5. Cleavage sites mapping of CsCSD4 and CsCSD7 genes.
(A) The cleavage sites mapping of CsCSD4 was aligned with miR164-1 and csn-miR398a-3p-1. (B) The cleavage site mapping of CsCSD7 was aligned with csn-miR398a-3p-1. Numbers in bracket indicate the number of cleavage site, and the cleavage sites were displayed by red arrow. Spots between miRNA and target genes represent complementary sequence.
Expression patterns of CsSOD genes and their regulatory miRNAs under different treatments
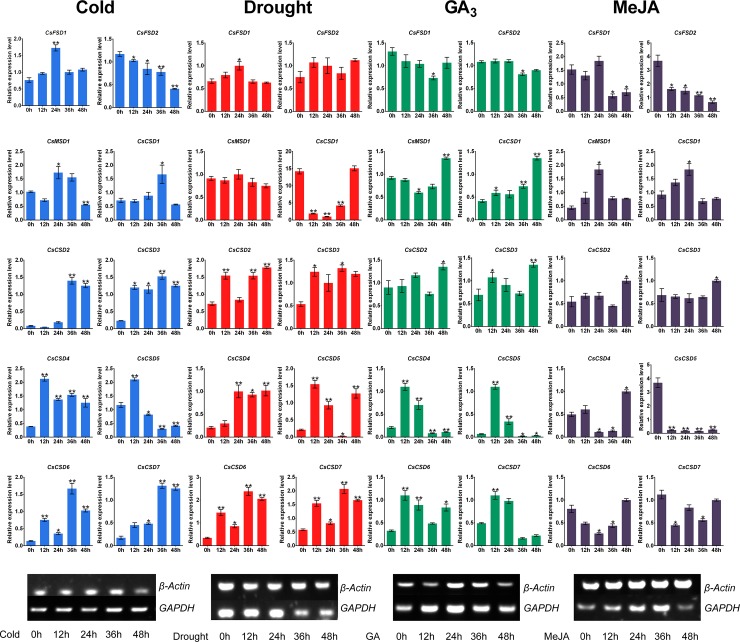
To clarify the potential roles of CsSOD genes involved in abiotic stresses and hormone stimuli, we used qRT-PCR to determine the expression levels of CsSOD genes under cold, drought, exogenous GA3 and MeJA treatments from 0 h to 48 h (Fig 6 and S6 Table). The expression level at 0 h as the control check (CK).
Fig 6. Expression patterns of CsSOD genes under cold, drought, exogenous GA3 and MeJA treatments in tea plant cultivar ‘Tieguanyin’.
The electrophoretograms of GAPDH and β-Actin under different treatments were showed on the bottom of figure. Data shown were the mean of three independent repeated experiments ± standard deviation (SD), and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. * indicates significant difference (p<0.05) and ** indicates extremely significant difference (p<0.01).
Treated by cold stress (4°C), all members of CsSOD genes were significantly induced or suppressed. Apart from CsFSD2 was inhibited and its expression bottoming at 48 h, the other 9 CsSOD genes expression was up-regulated at different stages, such as the expression of CsCSD4 and CsCSD5 peaked at 12 h; CsFSD1 and CsMSD1 reached the peak at 24 h; other members but CsFSD2 were at the highest point at 36 h.
To simulated drought stress, the ‘Tieguanyin’ seedling was irrigated with 15% (w/v) polyethylene glycol 4000 (PEG 4000). Then, we found that the expression trends of CsFSD1 and CsCSD1 were just reverse, which revealed “up-down-up” and “down-up-down” trends from 0 h to 48 h, respectively. Furthermore, CsCSD2, CsCSD3, CsCSD6, and CsCSD7 showed a similar trend of expression, which the expression levels at 24 h were lower than 12 h, 36 h, and 48 h but still higher than 0 h. While the expression of CsFSD2 and CsMSD1 were relatively stable.
Treated by exogenous GA3 (1 mmol/L), both the expressions of CsFSD1 and CsFSD2 were down-regulated at 36 h; CsMSD1, CsCSD1, CsCSD2, and CsCSD3 were up-regulated and peaked at 48 h; and from 0 h to 12 h, 12 h to 36 h, 36 h to 48 h, both CsCSD6 and CsCSD7 showed a “up-down-up” trend. Moreover, CsCSD4 and CsCSD5 were highly induced genes, the highest point (12 h) and lowest point (36 h) were over 12-fold and 55-fold, respectively.
Treated by exogenous MeJA (1 mmol/L), we found that both the expressions of CsMSD1 and CsCSD1 were significantly increased from 0 h to 24 h then decreased after 24 h, but the expression of CsCSD6 just opposite; CsFSD1 and CsCSD7 also have a similar expression profile but CsFSD1 still maintained relatively low expression at 48 h; CsFSD2 and CsCSD5 were markedly suppressed and maintained relatively low expression levels; CsCSD2 and CsCSD3 showed relatively stable; and CsCSD4 was highly induced gene, its expression was down-regulated from 24 h to 36 h but significantly up-regulated at 48 h.
Taken together, most CsSOD genes were significantly induced/repressed by diversiform treatments. It speculated that distinct CsSOD members had specific functions and adopt different coping strategies to response to stress or phytohormone signal at different stages in tea plant.
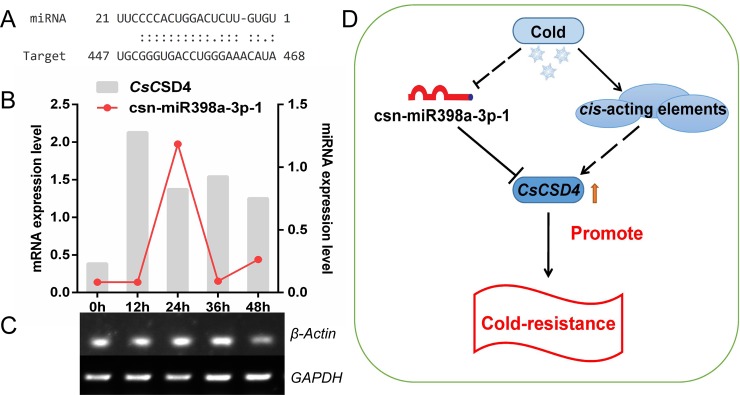
To step further understanding post-transcriptional regulation of CsSOD genes, we also detected the expression levels of predicted target miRNAs by triggering CsSOD genes cleavage under mentioned above treatments (S1 Fig). Finally, we found that the expression trend of csn-miR398-3p-1 just the opposite to CsCSD4 under cold treatment (Fig 7B).
Fig 7.
Csn-miR398a-3p-1 and CsCSD4 aligned fragments (A), expression trends of CsCSD4 and csn-miR398-3p-1 under cold stress (B), the electrophoretogram of β-Actin and GAPDH under cold treatment (C), a model of the expression of CsCSD4 regulated by csn-miR398a-3p-1 and cis-acting elements (D). Arrows: positive regulation; blunt-ended arrows: inhibition; dotted lines: untested.
Discussion
Acting as the first line of defense in antioxidant system, SODs play significant roles in catalyzing the dismutation of superoxide free radicals and protecting plant cells from oxidative damage [7]. Thus, analysis of all the members of CsSOD was important for provide a certain basis to understand and improve stress resistance in tea plants. The publication of tea plant genome data makes it possible to identify all CsSOD genes at the whole-genome level. In this research, we identified a total of 10 CsSOD genes based on tea plant genome, including 7 CsCSDs, 2 CsFSDs, and 1 CsMSD. Previous research found that there were 7 CSDs, 2 FSDs and 2 MSDs in P. trichocarpa [14]; 6 CSDs, 2 FSDs and 4 MSDs in M. acuminata [18]; 5 CSDs, 2 FSDs and 2 MSDs each in G. raimondii and G. arboretum [19]; 10 CSDs, 4 FSDs and 4 MSDs in G. hirsutum [20]; 4 CSDs, 1 FSD and 1 MSD in L. kaempferi [23]; 6 CSDs, 2 FSDs and 2 MSDs in V. vinifera [9]. The proportion of three types of SOD members in different plants could reflecting CSD genes are usually the most abundant SOD genes in different plants. In the analysis of potential protein interaction, we found that CsSODs with their “STRING proteins” in A. thaliana were just subdivided in same subgroups (Fig 1), indicating that different SOD members in same subgroup were highly homologous.
Diversity of intron number in CsSOD gene family
In eukaryotes, gene structure generally contain exons and introns, and are divided into intron-containing genes and intronless genes according to the presence or absence of introns [61]. It is generally believed that the number of introns is closely related to the complexity of the eukaryote’s genome, and most eukaryotes have two or more introns [62]. In our study, we found that the intron numbers of 10 CsSOD genes were quite different, which varied between 0 (CsCSD6) and 10 (CsFSD1), notably, CsCSD6 has no intron and its gene length was significantly shorter than that of the intron-containing CsSOD genes, indicating that the length of the exons was limited to a shorter range than that of the intron [63]. However, the intron numbers of 7 AtSOD genes varied from 5 to 8, with highest intron number in AtMSD1 and lowest intron number in AtFSD2. These findings in accord with previous view that there were no similar SOD genes structures showed in different species [7]. Several lines of evidence suggest that structural divergences perhaps attribute to three main mechanisms: exon/intron gain/loss, exonization/pseudoexonization and insertion/deletion, which bring about orthologous genes evolving different number of intron, each of which contributed underlying structural divergences were different. And, structure divergences occurred proportionally to evolutionary time [64]. We hypothesize that various intron numbers in CsSOD genes may be caused by interaction of the three mechanisms in the course of long-term evolution. Intron is beneficial to species evolution, increasing the length of gene, the recombination frequency between genes, and has the function of regulation; while intronless gene has no advantage for species evolution and recombination [65]. By lateral comparison, it was showed that the expression of CsCSD6 in tea plant presented at a relative low level among CsCSD genes (Fig 5). We inferred that this may be due to the simpler structure of CsCSD6 genes and it is an older gene. This is consistent with the results of studies on intronless genes in O. sativa and A. thaliana [66].
CsSOD genes play key roles in response to stress-resistance in tea plant
Excess ROS caused by abiotic stresses like cold and drought could pose threats to tea yield. SODs involved in scavenging ROS caused by several abiotic stresses in plant [10]. However, the specific responsiveness for cold, drought stress and GA3, MeJA stimuli in each CsSOD genes still unknown. Thus, the qRT-PCR detection could provide pivotal chew for understanding the potential functions of CsSOD genes under these stresses. In this study, the expression levels of most CsSOD genes revealed dynamic trends at certain timing from 0 h to 48 h under abiotic stresses (cold and drought), except for CsFSD2 and CsMSD1 when treated by drought. Evidence is accumulating that SODs hammer at overcome cold and drought stresses in S. lycopersicum [67], G. hirusutm [20], Brassica juncea and B. rapa [24]. It was also reported that increased expression of squash SOD genes along with increased activity of the SOD involved in detoxifying ROS in response to these stresses [68]. In our study, functions of cis-acting elements about cold- and drought-responsiveness exist in over half member promoters. However, drought-related elements have not been found in CsFSD2 and CsMSD1 and their expression levels were relatively stable under drought treatment. It speculated that CsFSD2 and CsMSD1 may not respond to drought stress. Furthermore, one study by over expressing CSD genes of transgenic N. tabacum showed that plants damage caused by drought stress has been mitigated to some extent [13]. In the present study, we found that CsCSD genes revealed remarkably expression change compared to CsFSD1, CsFSD2, and CsMSD1 under drought stress, this observation was similar to the expression of SOD gens in other plants [18, 20, 69], and indicated that CsCSD genes may play primary roles in drought-resistance in tea plant. In addition, under cold stress, the expression trends of most CsSOD genes expect for CsFSD2 were up-regulated comparing with 0 h at different stages. These results indicated that CsSOD genes could respond to cold and drought signal and adopt different strategies at distinct stages.
Studies have shown that phytohormone could activate signal transduction pathway bringing about plant stress responses [70]. For instance, it has been found that the suppression of GA3 signalling is a general response to abiotic stress in A. thaliana [71]; in tobacco, GA3 signaling pathway could regulate ROS scavenge enzyme, such as SOD, peroxidase (POD) and catalase (CAT) to enhance the susceptibility of pathogen infection by overexpressing GhMPK11 [72]. In addition, MeJA responses to biotic and abiotic stresses, which has been widely used to investigate jasmonate signaling pathways and activate defense system [73]. In our research, we have found GA-related elements exist in CsCSD1, CsCSD3, CsCSD4, CsCSD5, CsCSD6, CsFSD1, and CsFSD2 promoters, MeJA-related elements exist in CsFSD2, CsMSD1, CsCSD2, CsCSD3, CsCSD5, and CsCSD7. These genes were significant response to exogenous GA3 and MeJA stimuli. We conjecture that exogenous GA3 and MeJA could act as signal factors in stress-resistance, and then stimulate the transcript level of CsSOD genes at different stages in tea plant.
Csn-miR398a-3p-1 negatively regulated the expression of CsCSD4 may be a crucial regulatory mechanism in tea plant under cold stress
Previous studies have explored the relationships between miRNA and plant stress-responsive, among them, miR398 was known as related to stress regulation and regulates plant responses to a series of stresses [74]. Till now, target genes of miR398, including CSD1, CSD2, CCS1 have been found in many plants [75]. In A. thaliana, chilling resistance could occurs via negatively regulates miR398 expression and the expressions of AtCSD1 and AtCSD2 was induced accordingly [76]; in Triticum aestivum, the expression of miR398 was inhibited by cold stress and CSD gene was up-regulated synchronously [77]; in O. sativa, the expressions of miR398 and its targets (OsCSD1 and OsCSD2) were inhibited and increased under cold stress, respectively [78]. In the present study, we perform the modification 5’ RLM-RACE experiment to verified the putative regulatory miRNAs of CsSODs. And we found that the cleavage site outside the complementary regions between csn-miR164-1 and CsCSD4, csn-miR398a-3p-1 and CsCSD7, and only found csn-miR398a-3p-1 directly cleaves CsCSD4. This result accord with previous views that many prediction tools of plant miRNA target exist a large number of false positive in non-Arabidopsis species [79]. The qRT-PCR experiment showed that the expression level of CsCSD4 gene under cold stress was increased after 0 h as a whole. But from 12 h to 48 h, the expression revealed a “inverted W shape” trend. It speculated that the expression of CsSOD gene may regulated by multiple regulatory factors. To understand the regulation of CsSOD genes in post-transcriptional level, we detected the expression level of regulatory miRNAs of CsSOD genes. Finally, we only found that the expression trends of CsCSD4 and csn-miR398a-3p-1 from 0 h to 48 h under cold stress were just reverse. It suggests that csn-miR398a-3p-1 may be a key regulatory factor of CsCSD4 expression. Although csn-miR164-1 was also predicted regulatory miRNA of CsCSD4, but we not found cleavage sites in their complementary region. Similar case occurs between CsCSD7 and csn-miR398a-3p-1. These maybe due to low psRNATarget screening criteria, which can reduce false positive by increasing screening standards. On the basis of these results, we proposed a hypothetical model to explain potential regulatory factor affecting the expression of CsCSD4 under cold stress (Fig 7D). Cis-acting elements harbored cold-related function could respond to cold signal and promote CsCSD4 expression at transcriptional level; then csn-miR398-3p-1 inhibited CsCSD4 expression at post-transcriptional level. However, with these two regulatory factors, the expression of CsCSD4 revealed a up-regulated trend under cold stress and devote in enhanced cold-resistance in tea plant eventually.
Conclusions
A systematic analyses and research about SOD gene family in tea plant have conducted. We identified 10 CsSOD genes in total, including 7 CsCSDs, 2 CsFSDs and 1 CsMSD. Physico-chemical characteristic, phylogenetic classification, conserved motifs and potential protein interaction analyses about CsSOD proteins were carried out. Exon-intron structures and CUB pattern about CsSOD genes were also examined. In addition, on the basis of cis-acting elements, the expression profiles of CsSOD genes were detected by qRT-PCR under cold, drought, exogenous GA3 and MeJA treatments. The results showed that CsSOD genes play vital roles in regulating responses to these treatments. Moreover, we also detected the expression levels of predicted regulatory miRNAs of CsSOD genes, and we found that the expression trends of CsCSD4 and csn-miR398a-3p-1 were just reverse under cold stress. The modification 5’ RLM-RACE experiment was performed and validated that CsCSD4 was cleaved by csn-miR398a-3p-1. We conjecture csn-miR398a-3p-1 negatively regulated the expression of CsCSD4 may be a crucial regulatory mechanism in tea plant under cold stress. This work provides a certain basis for the studies about stress resistance in tea plants, even provide insight into comprehending the classification, evolution, diverse functions and influencing factors of expression pattern for CsSOD genes.
Supporting information
(TIF)
1 to 5 represent fragments of CsCSD4 cleaved by miR164-1 and csn-miR398a-3p-1, CsCSD7 cleaved by novel-miR54 and csn-miR398a-3p-1, and CsFSD2 cleaved by miR166d-5p-1, respectively. M represents Trans 2K DNA Marker (TransGen, Beijing, China).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank to Yukun Chen, PhD, from college of horticulture, Fujian Agriculture and Forestry University, for editing the English text of a draft of this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by the Natural Science Foundation of Fujian Province (2018J01701), the Earmarked Fund for China Agriculture Research System (CARS-19), the Special Fund Project for Scientific and Technological Innovation of Fujian Agriculture and Forestry University (CXZX2017164, CXZX2018069), the Rural Revitalization Tea Industry Technical Service Project of Fujian Agriculture and Forestry University (11899170102), and the Construction of Plateau Discipline of Fujian Province (102/71201801101). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhao L, Jiang XL, Qian YM, Xie DY, Gao LP, Xia T. Metabolic Characterization of the Anthocyanidin Reductase Pathway Involved in the Biosynthesis of Flavan-3-ols in Elite Shuchazao Tea (Camellia sinensis) Cultivar in the Field. Molecules. 2017;22(12):2241 10.3390/molecules22122241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhu X, Chen X, Song C, Zou Z, Wang Y, et al. Identification and characterization of cold-responsive microRNAs in tea plant (Camellia sinensis) and their targets using high-throughput sequencing and degradome analysis. BMC Plant Biol. 2014;14:271 10.1186/s12870-014-0271-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo YQ, Zhao S, Zhu C, Chang XJ, Yue C, Wang Z, et al. Identification of drought-responsive miRNAs and physiological characterization of tea plant (Camellia sinensis L.) under drought stress. BMC Plant Biol. 2017;17(1):211 10.1186/s12870-017-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng C, Zhao L, Wang Y, Shen JZ, Zhang YF, Jia SS, et al. Integrated RNA-Seq and sRNA-Seq Analysis Identifies Chilling and Freezing Responsive Key Molecular Players and Pathways in Tea Plant (Camellia sinensis). PLOS ONE 2015;10(4):e0125031 10.1371/journal.pone.0125031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheruiyot EK, Mumera LM, Ng'Etich WK, Hassanali A, Wachira FN. HIGH FERTILIZER RATES INCREASE SUSCEPTIBILITY OF TEA TO WATER STRESS. J Plant Nutr. 2009;33(1):115–129. 10.1080/01904160903392659 [DOI] [Google Scholar]
- 6.Apel K, Hirt H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu Rev Plant Biol. 2004;55(1):373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- 7.Fink RC, Scandalios JG. Molecular Evolution and Structure-Function Relationships of the Superoxide Dismutase Gene Families in Angiosperms and Their Relationship to Other Eukaryotic and Prokaryotic Superoxide Dismutases. Arch Biochem Biophys. 2002;399(1):19–36. 10.1006/abbi.2001.2739 [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Xia MX, Chen J, Yuan R, Deng FN, Shen FF. Gene Expression Characteristics and Regulation Mechanisms of Superoxide Dismutase and Its Physiological Roles in Plants under Stress. Biochemistry (Mosc). 2016;81(5):465–80. 10.1134/S0006297916050047 [DOI] [PubMed] [Google Scholar]
- 9.Hu XX, Hao CY, Cheng ZM, Zhong Y. Genome-Wide Identification, Characterization, and Expression Analysis of the Grapevine Superoxide Dismutase (SOD) Family. Int J Genomics. 2019;2019:1–13. 10.1155/2019/7350414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill SS, Anjum NA, Gill R, Yadav S, Hasanuzzaman M, Fujita M, et al. Superoxide dismutase-mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut R. 2015;22(14):10375–10394. 10.1007/s11356-015-4532-5 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plantarum. 2006;126(1):45–51. 10.1111/j.0031-9317.2005.00582.x [DOI] [Google Scholar]
- 12.Kliebenstein DJ, Monde RA, Last RL. Superoxide Dismutase in Arabidopsis: An Eclectic Enzyme Family with Disparate Regulation and Protein Localization1. Plant Physiol. 1998;118(2):637–650. 10.1104/pp.118.2.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, et al. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62(8):2599–2613. 10.1093/jxb/erq432 [DOI] [PubMed] [Google Scholar]
- 14.Molina-Rueda JJ, Tsai CJ, Kirby EG. The Populus Superoxide Dismutase Gene Family and Its Responses to Drought Stress in Transgenic Poplar Overexpressing a Pine Cytosolic Glutamine Synthetase (GS1a). PLOS ONE. 2013;8(2):e56421 10.1371/journal.pone.0056421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YL, Lai ZX. Superoxide dismutase multigene family in longan somatic embryos: a comparison of CuZn-SOD, Fe-SOD, and Mn-SOD gene structure, splicing, phylogeny, and expression. Mol Breeding. 2013;32(3):595–615. 10.1007/s11032-013-9892-2 [DOI] [Google Scholar]
- 16.Gopavajhula VR, Chaitanya KV, Akbar AKP, Shaik JP, Reddy PN, Alanazi M. Modeling and analysis of soybean (Glycine max. L) Cu/Zn, Mn and Fe superoxide dismutases. Genet Mol Biol. 2013;36(2):225–36. 10.1590/S1415-47572013005000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FİLİZ E, TOMBULOĞLU H. Genome-wide distribution of superoxide dismutase (SOD) gene families in Sorghum bicolor. Turk J Biol. 2015;39:49–59. 10.3906/biy-1403-9 [DOI] [Google Scholar]
- 18.Feng X, Lai ZX, Lin YL, Lai GT, Lian CL. Genome-wide identification and characterization of the superoxide dismutase gene family in Musa acuminata cv. Tianbaojiao (AAA group). BMC genomics 2015;16(1):823 10.1186/s12864-015-2046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Xia MX, Chen J, Deng FN, Yuan R, Zhang XP, et al. Genome-wide analysis of superoxide dismutase gene family in Gossypium raimondii and G. arboreum. Plant Gene. 2016;6:18–29. 10.1016/j.plgene.2016.02.002 [DOI] [Google Scholar]
- 20.Wang W, Zhang XP, Deng FN, Yuan R, Shen FF. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genomics. 2017;18(1):376 10.1186/s12864-017-3768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Hu LF, Wu H, Jiang LW, Liu SQ. Genome-Wide Identification and Transcriptional Expression Analysis of Cucumber Superoxide Dismutase (SOD) Family in Response to Various Abiotic Stresses. Int J Genomics. 2017;2017:1–14. 10.1155/2017/7243973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Song H, Zhang BH, Lu QW, Liu Z, Zhang SL, et al. Genome-wide identification, characterization, and expression analysis of superoxide dismutase (SOD) genes in foxtail millet (Setaria italica L.). 3 Biotech. 2018;8(12). 10.1007/s13205-018-1502-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han XM, Chen QX, Yang Q, Zeng QY, Lan T, Liu YJ. Genome-wide analysis of superoxide dismutase genes in Larix kaempferi. Gene. 2019;686:29–36. 10.1016/j.gene.2018.10.089 [DOI] [PubMed] [Google Scholar]
- 24.Verma D, Lakhanpal N, Singh K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genomics. 2019;20(1):227 10.1186/s12864-019-5593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang DY, Yang HL, Li XS, Li HY, Wang YC. Overexpression of Tamarix albiflonum TaMnSOD increases drought tolerance in transgenic cotton. Mol Breeding. 2014;34(1):1–11. 10.1007/s11032-014-0015-5 [DOI] [Google Scholar]
- 26.Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol. 2005;162(4):465–472. 10.1016/j.jplph.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Yao LN, Hao XY, Li NN, Wang YC, Ding CQ, et al. Transcriptional and physiological analyses reveal the association of ROS metabolism with cold tolerance in tea plant. Environ Exp Bot. 2019;160:45–58. 10.1016/j.envexpbot.2018.11.011 [DOI] [Google Scholar]
- 28.Sunkar R. Novel and Stress-Regulated MicroRNAs and Other Small RNAs from Arabidopsis. THE PLANT CELL ONLINE. 2004;16(8):2001–2019. 10.1105/tpc.104.022830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura K, Furumoto T. Cold Signaling and Cold Response in Plants. Int J Mol Sci. 2013;14(3):5312–5337. 10.3390/ijms14035312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan J, Zhao CZ, Zhou JP, Yang Y, Wang PC, Zhu XH, et al. The miR165/166 Mediated Regulatory Module Plays Critical Roles in ABA Homeostasis and Response in Arabidopsis thaliana. PLOS Genet. 2016;12(11):e1006416 10.1371/journal.pgen.1006416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JS, Zhang H, Srivastava AK, Pan YJ, Bai JJ, Fang JJ, et al. Knockdown of Rice MicroRNA166 Confers Drought Resistance by Causing Leaf Rolling and Altering Stem Xylem Development. Plant Physiol. 2018;176(3):2082–2094. 10.1104/pp.17.01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhyay M, Mondal TK, Chand PK. Biotechnological advances in tea (Camellia sinensis [L.] O. Kuntze): a review. Plant Cell Rep. 2016, 35(2):255–287. 10.1007/s00299-015-1884-8 [DOI] [PubMed] [Google Scholar]
- 33.Das A, Mondal TK. Computational identification of conserved microRNAs and their targets in tea (Camellia sinensis). American Journal of Plant Sciences. 2010, 01(02):77–86. 10.4236/ajps.2010.12010 [DOI] [Google Scholar]
- 34.Xia EH, Zhang HB, Sheng J, Li K, Zhang QJ, Kim CH, et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Mol Plant. 2017;10(6):866–877. 10.1016/j.molp.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 35.Wei C, Yang H, Wang S, Zhao J, Liu C, Gao L, et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc Natl Acad Sci USA. 2018;115(18):E4151–E4158. 10.1073/pnas.1719622115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letunic I, Bork P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46(D1):D493–D496. 10.1093/nar/gkx922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45(D1):D200–D203. 10.1093/nar/gkw1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(Web Server):W585–W587. 10.1093/nar/gkm259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, et al. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–52. 10.1385/1-59259-584-7:531 [DOI] [PubMed] [Google Scholar]
- 40.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011;28(10):2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44(W1):W242–W245. 10.1093/nar/gkw290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen C, Xia R, Chen H, He Y. TBtools, a Toolkit for Biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv. 2018:289660 10.1101/289660 [DOI] [Google Scholar]
- 44.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server):W202–W208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan LL, Wang Y, Hu JH, Ding ZT, Li C. Analysis of codon use features of stearoyl-acyl carrier protein desaturase gene in Camellia sinensis. Journal of Theoretical Biology. 2013;334:80–86. 10.1016/j.jtbi.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 47.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic acids res. 2002;30(1):325–327. 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai X, Zhuang Z, Zhao PX. psRNATarget: a plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018;46(W1):W49–W54. 10.1093/nar/gky316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang PJ, Chen D, Zheng YC, Jin S, Yang JF, Ye NX. Identification and Expression Analyses of SBP-Box Genes Reveal Their Involvement in Abiotic Stress and Hormone Response in Tea Plant (Camellia sinensis). Int J Mol Sci. 2018;19:3404 10.3390/ijms19113404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W, Xu Y, Mao J, Hao W, Liu Y, Ni D, et al. Cloning and expression patterns of VQ-motif-containing proteins under abiotic stress in tea plant. Plant Growth Regul 2019;87(2):277–286. 10.1007/s10725-018-0469-2 [DOI] [Google Scholar]
- 51.Guo YQ, Chang XJ, Zhu C, Zhang ST, Li XZ, Fu HF, et al. De novo transcriptome combined with spectrophotometry and gas chromatography-mass spectrometer (GC-MS) reveals differentially expressed genes during accumulation of secondary metabolites in purple-leaf tea (Camellia sinensis cv Hongyafoshou). Journal of Horticultural Science&Biotechnology. 2019;94(3):349–367. 10.1080/14620316.2018.1521708 [DOI] [Google Scholar]
- 52.Xia EH, Li FD, Tong W, Li PH, Wu Q, Zhao HJ, et al. Tea Plant Information Archive: a comprehensive genomics and bioinformatics platform for tea plant. Plant Biotechnol J. 2019. 10.1111/pbi.13111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin YL, Lai ZX. Reference gene selection for qPCR analysis during somatic embryogenesis in longan tree. Plant Science. 2010;178(4):359–365. 10.1016/j.plantsci.2010.02.005 [DOI] [Google Scholar]
- 54.Guruprasad K, Reddy BV, Pandit MW. Correlation between stability of a protein and its dipeptide composition: a novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990;4(2):155–61. 10.1093/protein/4.2.155 [DOI] [PubMed] [Google Scholar]
- 55.Moran JF. Functional Characterization and Expression of a Cytosolic Iron-Superoxide Dismutase from Cowpea Root Nodules. Plant Physiol. 2003;133(2):773–782. 10.1104/pp.103.023010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002;30(1):13 10.1038/ng0102-13 [DOI] [PubMed] [Google Scholar]
- 57.Casareno RLB, Waggoner D, Gitlin JD. The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J Biol Chem. 1998;273(37):23625–23628. 10.1074/jbc.273.37.23625 [DOI] [PubMed] [Google Scholar]
- 58.Hiraoka Y, Kawamata K, Haraguchi T, Chikashige Y. Codon usage bias is correlated with gene expression levels in the fission yeast Schizosaccharomyces pombe. Genes Cells. 2009;14(4):499–509. 10.1111/j.1365-2443.2009.01284.x [DOI] [PubMed] [Google Scholar]
- 59.Wright F. The 'effective number of codons' used in a gene. Gene 1990;87(1):23–29. 10.1016/0378-1119(90)90491-9 [DOI] [PubMed] [Google Scholar]
- 60.Lauressergues D, Couzigou J, San Clemente H, Martinez Y, Dunand C, Bécard G, et al. Primary transcripts of microRNAs encode regulatory peptides. Nature. 2015;520(7545):90 10.1038/nature14346 [DOI] [PubMed] [Google Scholar]
- 61.Sakharkar MK, Chow VTK, Kangueane P. Distributions of exons and introns in the human genome. In silico biology. 2004;4(4):387–393. [PubMed] [Google Scholar]
- 62.Rogozin IB, Sverdlov AV, Babenko VN, Koonin EV. Analysis of evolution of exon-intron structure of eukaryotic genes. Brief Bioinform. 2005;6(2):118–134. 10.1093/bib/6.2.118 [DOI] [PubMed] [Google Scholar]
- 63.Deutsch M, Long M. Intron-exon structures of eukaryotic model organisms. Nucleic Acids Res. 1999;27(15):3219–28. 10.1093/nar/27.15.3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu G, Guo C, Shan H, Kong H. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci USA. 2012;109(4):1187–1192. 10.1073/pnas.1109047109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shabalina SA, Ogurtsov AY, Spiridonov AN, Novichkov PS, Spiridonov NA, Koonin EV. Distinct patterns of expression and evolution of intronless and intron-containing mammalian genes. Mol Biol Evol. 2010;27(8):1745–9. 10.1093/molbev/msq086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jain M, Khurana P, Tyagi AK, Khurana JP. Genome-wide analysis of intronless genes in rice and Arabidopsis. Funct Integr Genomic. 2008;8(1):69–78. 10.1007/s10142-007-0052-9 [DOI] [PubMed] [Google Scholar]
- 67.Feng K, Yu JH, Cheng Y, Ruan MY, Wang RQ, Ye QJ, et al. The SOD Gene Family in Tomato: Identification, Phylogenetic Relationships, and Expression Patterns. Front Plant Sci. 2016;7 10.3389/fpls.2016.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chiang CM, Kuo WS, Lin KH. Cloning and gene expression analysis of sponge gourd ascorbate peroxidase gene and winter squash superoxide dismutase gene under respective flooding and chilling stresses. Horticulture, Environment, and Biotechnology. 2014;55(2):129–137. 10.1007/s13580-014-0116-4 [DOI] [Google Scholar]
- 69.Liu ZB, Zhang WJ, Gong XD, Zhang Q, Zhou LR. A Cu/Zn superoxide dismutase from Jatropha curcas enhances salt tolerance of Arabidopsis thaliana. Genet Mol Res. 2015;14(1):2086–98. 10.4238/2015.March.20.19 [DOI] [PubMed] [Google Scholar]
- 70.Raja V, Majeed U, Kang H, Andrabi KI, John R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ Exp Bot. 2017;137:142–157. 10.1016/j.envexpbot.2017.02.010 [DOI] [Google Scholar]
- 71.Colebrook E.H.; Thomas S.G.; Phillips A.L.; Hedden P. The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol. 2013, 217, 67–75. 10.1242/jeb.089938 [DOI] [PubMed] [Google Scholar]
- 72.Wang F, Wang C, Yan Y, Jia H, Guo X. Overexpression of Cotton GhMPK11 Decreases Disease Resistance through the Gibberellin Signaling Pathway in Transgenic Nicotiana benthamiana. Front Plant Sci. 2016;7:689 10.3389/fpls.2016.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang D, Yan SC. MeJA is more effective than JA in inducing defense responses in Larix olgensis. Arthropod-Plant Inte. 2018;12(1):49–56. 10.1007/s11829-017-9551-3 [DOI] [Google Scholar]
- 74.Zhu C, Ding Y, Liu H. MiR398 and plant stress responses. Physiol Plant. 2011;143(1):1–9. 10.1111/j.1399-3054.2011.01477.x [DOI] [PubMed] [Google Scholar]
- 75.Song X, Li Y, Cao X, Qi Y. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu Rev Plant Biol. 2019. 10.1146/annurev-arplant-050718-100334 [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Jiang J, Song A, Chen S, Shan H, Luo H, et al. Ambient temperature enhanced freezing tolerance of Chrysanthemum dichrum CdICE1 Arabidopsis via miR398. BMC Biol 2013;11:121 10.1186/1741-7007-11-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang B, Sun YF, Song N, Wei JP, Wang XJ, Feng H, et al. MicroRNAs involving in cold, wounding and salt stresses in Triticum aestivum L. Plant Physiol Bioch. 2014;80:90–96. 10.1016/j.plaphy.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 78.Xu S, Jiang YL, Cui WT, Jin QJ, Zhang YH, Bu D, et al. Hydrogen enhances adaptation of rice seedlings to cold stress via the reestablishment of redox homeostasis mediated by miRNA expression. Plant and Soil. 2017;414(1–2):53–67. 10.1007/s11104-016-3106-8 [DOI] [Google Scholar]
- 79.Srivastava P.K., Moturu T.R., Pandey P., Baldwin I.T., Pandey S.P. A comparison of performance of plant miRNA target prediction tools and the characterization of features for genome-wide target prediction. Bmc Genomics 2014; 348(15):15348 10.1186/1471-2164-15-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
1 to 5 represent fragments of CsCSD4 cleaved by miR164-1 and csn-miR398a-3p-1, CsCSD7 cleaved by novel-miR54 and csn-miR398a-3p-1, and CsFSD2 cleaved by miR166d-5p-1, respectively. M represents Trans 2K DNA Marker (TransGen, Beijing, China).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.