Abstract
Background
Schistosomiasis is a neglected tropical disease caused by Schistosoma parasites. Intervention relies on identifying high-risk regions, yet rapid Schistosoma diagnostics (Kato-Katz stool assays (KK) and circulating cathodic antigen urine assays (CCA)) yield different prevalence estimates. We mapped S. mansoni prevalence and delineated at-risk regions using a survey of schoolchildren in Rwanda, where S. mansoni is an endemic parasite. We asked if different diagnostics resulted in disparities in projected infection risk.
Methods
Infection data was obtained from a 2014 Rwandan school-based survey that used KK and CCA diagnostics. Across 386 schools screened by CCA (N = 19,217). To allow for uncertainty when interpreting ambiguous CCA trace readings, which accounted for 28.8% of total test results, we generated two presence-absence datasets: CCA trace as positive and CCA trace as negative. Samples (N = 9,175) from 185 schools were also screened by KK. We included land surface temperature (LST) and the Normalized Difference Vegetation and Normalized Difference Water Indices (NDVI, NDWI) as predictors in geostatistical regressions.
Findings
Across 8,647 children tested by both methods, prevalence was 35.93% for CCA trace as positive, 7.21% for CCA trace as negative and 1.95% for KK. LST was identified as a risk factor using KK, whereas NDVI was a risk factor for CCA models. Models predicted high endemicity in Northern and Western regions of Rwanda, though the CCA trace as positive model identified additional high-risk areas that were overlooked by the other methods. Estimates of current burden for children at highest risk (boys aged 5–9 years) varied by an order of magnitude, with 671,856 boys projected to be infected by CCA trace as positive and only 60,453 projected by CCA trace as negative results.
Conclusions
Our findings show that people in Rwanda’s Northern, Western and capital regions are at high risk of S. mansoni infection. However, variation in identification of environmental risk factors and delineation of at-risk regions using different diagnostics likely provides confusing messages to disease intervention managers. Further research and statistical analyses, such as latent class analysis, can be used to improve CCA result classification and assess its use in guiding treatment regimes.
Author summary
Schistosomiasis control efforts rely on rapid diagnostic tests to generate maps of disease prevalence. This is so that high-risk areas can be identified and provided with treatments. A range of tests are available, yet it is unclear how their differing results can inform disease mitigation. This study compared the results of diagnostic methods for uncovering areas at risk of Schistosoma mansoni infection in Rwanda. A national survey of Rwandan schoolchildren used two detection methods: a test detecting a schistosome antigen in urine (CCA) and a test to detect parasite eggs in stool (KK). We divided the CCA test into two datasets, as there is large uncertainty when interpreting its reading. We found that these three datasets all tell different stories about the distribution of S. mansoni in Rwanda. While all tests found similar high-risk areas where prevalence reaches 50%, such as Northern and Western regions, their overall messages are mixed. Some urine tests find areas that are high-risk, while stool tests in the same area may consider it low-risk. This makes it difficult to design budgets for treatment programs, as it is unclear where resources should go. More research and analyses are needed to determine how these tests can be used as tools for schistosomiasis risk mapping. Furthermore, the interpretations of mapping outcomes will also likely rest on national decisions regarding programmatic goals.
Introduction
Schistosomiasis, caused by parasitic trematode species of the genus Schistosoma, is an infectious disease affecting people throughout the world’s tropical and sub-tropical regions [1, 2]. Chronic infections can lead to impaired growth and development in children, making schistosomiasis one of the world’s most important neglected tropical diseases [3]. Schistosomiasis is often linked to absence of access to latrines and / or clean water, which can lead to the use of freshwater bodies that can become contaminated with parasite eggs when infected people urinate or defecate while bathing, washing and swimming. Freshwater snails, often present in these water bodies, act as intermediate hosts for the parasites. Following infection of appropriate snail hosts, asexual replication occurs followed by the development of the infectious stages of the parasite which can be released into the water and infect people. Consequently, infection-related morbidity is especially common in poor agricultural areas that rely on unsanitized freshwater [4, 5]. Globally, it is estimated that more than 779 million people live in areas with high risk of human Schistosoma transmission [6]. Infection risk is particularly high in sub-Saharan Africa, where up to 90% of the world’s infections occur [6, 7]. Burdens in this region are enormous, matching or exceeding those related to malaria and HIV/AIDS [3]. An estimated 300,000 people die from schistosomiasis in Africa each year [8].
Mass delivery of anthelminthic treatment can reduce Schistosoma prevalence and facilitate major improvements to public health outcomes [9]. Mass administration of praziquantel can be cost effective, especially in areas with relatively high prevalence of infection and high transmission intensity [7, 10]. Yet burdens are so great that mass drug administration remains unaffordable for most low-income endemic countries [9, 11]. Steps have been taken by Merck KGaA, the United States Agency for International Development, the Bill and Melinda Gates Foundation, the British Department of International Development and the Global Network for Neglected Tropical Diseases to increase delivery of doses to sub-Saharan African countries [12].
Designing effective drug delivery programmes to reduce Schistosoma transmission is complicated by inadequate understanding of regional burdens and risk factors [13, 14]. The geographical distributions and transmission rates of Schistosoma parasites are poorly studied in many endemic regions but are broadly known to be driven by environmental heterogeneity [15–17]. Climate or environmental variables that influence soil moisture and composition can reflect variability in molluscan host distributions and parasite larval survival rates [18–20]. Data-driven approaches to identify environmental correlates of infection risk in understudied endemic regions are imperative to design mitigation procedures.
In addition to climatic and environmental heterogeneity in infection risk, uncertainty surrounding regional infection prevalence is a barrier to treatment design. The World Health Organization (WHO) recommends that treatment for schistosomiasis should be provided to areas of high endemism in efforts to reduce morbidity. Such treatment is important not only for morbidity control but also for current pushes to eliminate schistosomiasis where feasible [21]. Developing treatment regimens to achieve these goals relies on gathering accurate estimates of infection prevalence. Moreover, with targets for 2020–2030 now under public consultation [21], critical assessments of inferences resulting from different diagnostic tests are needed. This is particularly true considering that different diagnostics may be chosen depending on whether a country’s specified goal is elimination as a public health problem (morbidity control) or interruption of transmission. However, gathering estimates of prevalence is challenging, particularly since symptoms of intestinal schistosomiasis are incredibly variable and can include headache, fever, rash, anaemia, bloody diarrhoea and abdominal pain, hepatosplenomegaly, blood in urine, burning sensation during urination, fibrosis of the bladder, and specifically in females, genital lesions which may lead to irreversible consequences, including infertility [2, 22]. Use of rapid diagnostic tests is therefore recommended for generating estimates of prevalence. For intestinal schistosomiasis, detection of eggs in the faeces (primarily using the Kato-Katz (KK) method, which involves microscopic examination of faecal smears [23]) or of circulating cathodic antigen (CCA) in urine are the primary methods of choice for diagnosis of infections [24, 25]. However, the two methods can return different prevalence estimates, with CCA generally yielding higher sensitivity [26, 27]. The KK method may show particularly low sensitivity in low to moderate endemic areas, especially when there are many low-intensity infections [28].
Without an accurate understanding of current burdens and environmental correlates, identifying areas in need of treatment remains difficult. Geostatistical models that use up-to-date infection data and account for environmental risk factors are useful tools for producing evidence-based projections of populations at risk of Schistosoma infection [29, 30]. Modelling assessments should ideally be formulated following adequate scrutiny of assessment of the performances and inferences that are provided by different available diagnostic methods.
Rwanda is a landlocked central African country bordered by Tanzania to the East, Uganda to the North, the Democratic Republic of the Congo (DRC) to the West, and Burundi to the South. With a land area of 26,338 square kilometres and a population of over 10.7 million, Rwanda is one of the most densely populated countries in Africa [31]. The economy is mostly agriculture-based and the average life expectancy is 63 years [32]. Rwanda sustained widespread public health disruption as a result of political and social unrest throughout the 1990’s [33]. Health workforce training and public health outcomes have since improved, however, infectious disease monitoring and control still present major challenges [34–36]. Previous surveys suggest that Schistosoma mansoni is highly endemic and hence a parasite of high public health importance in Rwanda [34, 37]. While some evidence suggests intestinal schistosomiasis reaches 60% prevalence or higher in certain areas, research on infection rates is limited to small-scale regional studies [1, 38–40]. Consequently, we have a poor understanding of risk factors for schistosomiasis in Rwanda.
This study aims to understand geographical variation in S. mansoni infection risk in Rwanda and to provide new insights into uncertainties in treatment pathways that can arise from variation in chosen on-the-ground diagnostic procedures. To accomplish these aims, we outline two key objectives. First, we apply geostatistical models to data from a national school-based survey in Rwanda to identify S. mansoni risk factors, map infection prevalence and delineate endemic clusters of high risk. Second, we compare geographical patterns in projected infection risk arising from the use of the CCA and KK methods. We hypothesize that infection risk will be spatially clustered within the country but that geographical disparities in the size and intensity of these clusters will arise when relying on different diagnostics, providing confusing messages to policymakers designing treatment guidelines.
Methods
Ethics statement
Ethical clearance for this analytical study was provided by The National Ethics Committee in Rwanda (Sep 2014, Ref No: 261/RNEC/2014).
Detecting Schistosoma mansoni infections in Rwandan schoolchildren
Schistosoma mansoni presence-absence data was obtained from a nationwide school-based survey undertaken in Rwanda between June and mid-July 2014. Methods for school selection were as follows: a national sampling effort was carried out following the first nationwide surveys for prevalence of intestinal schistosomiasis and soil-transmitted helminthiasis (STH) in Rwandan schoolchildren, conducted between 2007 and 2008 [1]. This sampling scheme was designed to (1) provide insights into endemicity of S. mansoni across the country and (2) guide the decision of new treatment and surveillance strategies for Rwanda, in alignment with WHO guidelines [41]. The scheme took into consideration groups of sectors as administrative mapping units. Schools were chosen by selecting units likely to have similar S. mansoni transmission characteristics (based on epidemiological characteristics such as nearby perennial water bodies) and to ensure sample sizes in each mapping unit were statistically representative.
To assess possible geographical disparities in risk mapping arising from choice of diagnostic method, infection data was obtained using both the circulating cathodic antigen (CCA; detected in urine) and Kato-Katz (KK; detected in faeces) diagnostic methods. Specifically, CCA was used to survey S. mansoni infection in schoolchildren (aged 5 to 18 years) across 386 schools. A single urine sample was collected from each randomly-selected participant and tested with a point of care CCA rapid test (Rapid Medical Diagnostics, South Africa). A single-use pipette was used to add the drop of urine to the test cassette well, followed by a drop of the provided buffer. It should be noted that CCA tests returns negative, trace, 1+, 2++ or 3+++ readings, which are designed to give an indication of the strength of the reading. Results were read after 20 minutes and graded into one of four intensities: 0 = negative; trace; 1+; 2++; 3+++ using a reference image showing representative incremental readings (Supporting Information, S1 Fig). All CCA kits were from Rapid Medical Diagnostics Batch number 33955, ensuring we did not need to account for possible batch-to-batch variation in trace readings. We did not use a band density reader to avoid observational bias, as it would have been extremely expensive to provide such a tool to every team in the field. Moreover, we note that even though there is an mReader that Mobile Assay and SCORE, developed in 2016 for providing quantitative CCA test results, this mReader cannot distinguish between a trace ‘true positive’ and a trace ‘false positive’. Therefore, during this study and indeed to this day, the visual read of the intensity of the test band on the strip compared to the supplied control image is still considered standard practice in Rwanda.
The KK method was also used in 174 of these schools (concurrent urine and faecal samples were collected from the same participants), while a further 11 schools were surveyed using only KK (bringing the total number of schools surveyed using KK to 185). For this test, a single stool specimen was collected from each participant and duplicate thick smears were prepared for microscopic examination. Smears were assessed for presence of S. mansoni eggs by trained technicians, with each smear assessed by at least two different technicians. Quality control was performed by independent external consultants to ensure accurate diagnosis from KK microscopy. This involved random allocation of 10% of the smears for re-examination by two independent experts.
For each of the visited schools, up to 50 students per school were sampled for parasite infection. However, while the original CCA datasets included 19,371 children, only children with complete information were included in the analysis (children without age, sex, or geocoordinates recorded were excluded). As a result, the total number of children included in CCA analyses was 19,217. Following removal of children with missing data, the total number of pupils ranged from 25–50 per school for CCA sampling (mean = 49.78, sd = 1.89) and from 25–50 for KK sampling (mean = 49.59, sd = 2.05). Across the 174 schools sampled by all three methods, the number of pupils ranged from 44–50 (mean = 49.70, sd = 1.01).
Extraction of environmental variables
Environmental measurements were extracted at the school level to be included as covariates in analyses. We extracted information for three variables likely to influence Schistosoma spp. survival and transmission. These included: average land surface temperature (LST), extracted from the WorldClim database (www.worldclim.org), and the Normalized Difference Vegetation and Normalized Difference Water Indices (NDVI and NDWI), both extracted from the National Oceanographic and Atmospheric Administrations’ Satellite and Information Services database (NOAASIS) (http://noaasis.noaa.gov/). LST was included because this can influence both the density of intermediate molluscan hosts and the rate of schistosomal development within the molluscan host [42]. NDVI and NDWI variables capture local variation in vegetation, moisture and the presence of water bodies, which can impact the distributions of intermediate molluscan hosts [16, 42, 43]. Environmental variables were obtained using the Google Earth Engine in ArcGIS version 10.4.0.5524 [44] at 1km x 1km grid cell resolution and were standardized to unit variance prior to analysis.
Statistical analysis to identify infection risk factors
Our analysis assessed the presence of S. mansoni infection in 19,217 students from 386 schools who provided a urine sample for CCA assay analysis. While trace readings are recommended by the manufacturer and by the WHO [45] to be considered as positive infections, some programs have reported weaker trace readings as negative because they may not reliably confirm the presence or absence of infection [46]. For our analysis, we created two datasets for statistical analyses to account for this uncertainty when categorizing trace readings [47]. To do this, CCA tests were stratified into two separate presence-absence datasets, namely CCA with trace as positive (i.e. only those readings of 0 were considered negative, while readings of trace, 1+, 2+ or 3++ were considered positive) and CCA with trace as negative (i.e. readings of either 0 or trace were considered negative, while all other readings were considered positive). In addition, 9,175 students had stool specimens collected for Kato-Katz analyses, collected from 185 of the 386 schools. Data regarding school geolocation and participant demography (i.e. age and sex) was included for all observations. We stratified ages of participants into a three-level categorical variable (5 to 9 years old; 10 to 14; and 15 to 18 years old). The average age of participants included in analyses was 13.36 years. School polygon centroids were estimated from a shapefile representing Rwanda’s current administrative units (obtained from the geographic data warehouse DIVA GIS (www.diva-gis.org/Data)). Parasite infection and environmental covariate data were linked to their corresponding school centroids.
To assess evidence for spatial autocorrelation in infection probability, we fit logistic regression models (binomial errors with logit link function) using participant sex and age (categorical variables) and the scaled environmental predictors LST, NDVI and NDWI. Correlations between environmental covariates were investigated using Pearson’s correlations. Residuals were extracted and examined with semivariograms (calculated using functions in the ‘geoR’ package [48]) in R version 3.1.1 (The R foundation for statistical computing, Vienna, Austria, http://www.R-project.org). A semivariogram is a graphical representation of the spatial variation in an outcome variable; residual semivariograms represent spatial variation that is left unexplained after accounting for predictors. A semivariogram is characterised by three parameters: the partial sill, representing the spatially structured semivariance component and indicating the tendency for geographical clustering; the nugget, representing the spatially unstructured semivariance component (representing either small-scale spatial variability, measurement error or random variation); and the range, representing the pairwise distance above which two locations can be considered independent (indicative of the average size of geographical clusters). We estimated the tendency for geographical clustering within a region (i.e. proportion of variation due to spatial proximity) by dividing the partial sill by the sum of the nugget plus the partial sill [49]. Separate models were developed using KK, CCA with trace as positive and CCA with trace as negative presence-absence datasets.
Geostatistical models
Examination of spatial autocorrelation revealed some level of residual spatial clustering for each model (see Results below). We implemented geostatistical models to account for our covariates and to simultaneously explore this autocorrelation. Bayesian logistic models with geostatistical random effects were built for CCA tests using the open software OpenBUGS [50]. We assumed the observed presence-absence vectors for each diagnostic group were random draws from an underlying infection probability according to a Bernoulli distribution. Using a logit link function, we modelled this probability as a linear regression that included an intercept, our fixed predictors and a multivariate normal geostatistical random effect capturing distances (km) between pairs of locations (using the spatial.exp function BUGS language, which is essentially a Bayesian kriging model). Note that due to a low overall prevalence in the KK dataset, we had inadequate infection data to generate precise estimates of spatial effects. We instead investigated possible spatial clustering using aggregated data by classifying locations as a binomial variable based on whether that location’s mean observed prevalence was higher or lower than the mean prevalence (1.95%) observed in the entire dataset (i.e. each survey location was categorised using 1 or 0 based on whether its prevalence was ≥ 1.95% or < 1.95%).
Geostatistical models were estimated in a Bayesian framework using Markov Chain Monte Carlo (MCMC) sampling based on the Gibbs sampler in OpenBUGS [50]. Normal priors with mean = 0 and variance = 100 (i.e. precision = 0.01) were specified for intercepts and regression coefficients. Geostatistical random effects were assumed to follow a normal distribution with mean = 0 and variance = 1/tau, where tau was drawn from a gamma distribution (shape = 0.001, scale = 0.001). For CCA models, a burn-in of 5,000 MCMC iterations was used followed by 5,000 iterations. For the KK model, a burn-in of 2,000 iterations was used followed by another 3,300 iterations. Convergence for all models was assessed visually based on inspection of posterior density and trace plots. Significance of predictor effects was inferred based on whether 95% confidence intervals of posterior estimates did not include zero.
Model predictions were used to generate representative maps of the prevalence of S. mansoni infections across Rwanda for boys aged between 5–9 years, the subgroup with the highest overall prevalence of S. mansoni infection in our dataset (though it should be noted that age was not a significant predictor of infection probability, see Results below). Predictions were made at the nodes of a 0.03 × 0.03 decimal degree grid (approximately 3km2). The mean and standard deviation were extracted from the posterior distributions of predicted risk. Marginal predictions of risk were calculated using the spatial.unipred command in OpenBUGS, which carries out single site predictions to yield marginal prediction intervals for each sample site.
Estimation of the number of infections in population at risk of Schistosoma mansoni infections in Rwanda in 2018
To assess geographical discrepancies in S. mansoni endemicity and the estimated numbers of at-risk individuals across the three diagnostic datasets, we selected the highest risk group in our dataset, represented as boys aged between 5 to 9 years old. A raster map of the estimated total population size of this select group (estimated for the year 2018) was multiplied by the predicted prevalence of S. mansoni in the at-risk group to produce a map of the total number of infected children in each grid cell. To create the 2018 population raster, we retrieved a 2015 population density raster from the Center for International Earth Science Information Network (CIESIN) [51], which was originally estimated using National Institute of Statistics Rwanda’s Fourth Population and Housing Census 2012 data. This raster was multiplied by the reported United Nations Development Programme (UNDP) average annual rate of population change (i.e. 2.53%), which was then multiplied by the proportion of 5 to 9 year-olds (i.e. 13.5%) to produce a raster map of the estimated densities of children in this focal group in 2018. All spatial calculations and plots were conducted in ArcGIS version 10.4.0.5524 [44]. Data required to replicate analyses is included in Supporting Information, S1 Data.
Results
Summary statistics of Schistosoma mansoni prevalence in Rwandan schoolchildren
A total of 174 schools were tested for the prevalence of S. mansoni infection using all three methods, with 8,647 pupils providing both stool and urine samples. Across these samples, observed prevalence using CCA with trace as positive was 37.5%, 8.6% when using CCA with trace as negative and only 2% when using KK (Supporting Information, S1 Table). Results were broadly similar when taken across the entire dataset: only 1.95% of the 9,175 children tested with the KK method were diagnosed as infected (Table 1), while observed prevalence was 35.93% for CCA with trace as positive and 7.21% for CCA with trace as negative (N = 19,218; Table 1).
Table 1. Summary statistics of observed Schistosoma mansoni prevalence across Rwandan schools recorded using different diagnostic methods.
A total of 5,547 CCA results were categorised as ‘trace’. The CCA with trace as positive dataset considered these as positive, while the CCA with trace as negative dataset considered these as negative. CCA; Circulating Cathodic Antigen.
| Kato-Katz | CCA with trace as positive | CCA with trace as negative | |
|---|---|---|---|
| Number of schools sampled | 185 | 386 | 386 |
| Number of pupils positive | 179 | 6,906 | 1,385 |
| Number of pupils negative | 8,996 | 12,311 | 17,832 |
| Mean prevalence (%) | 1.95% | 35.93% | 7.21% |
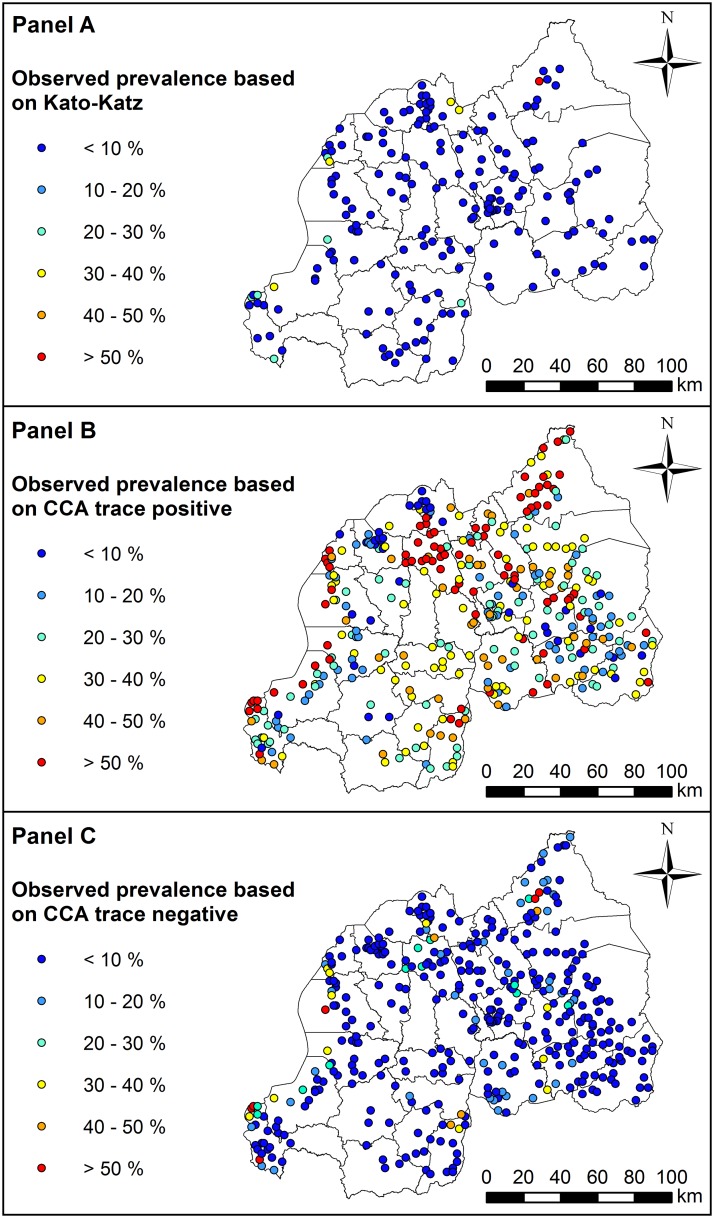
Despite these large discrepancies in diagnosed outcomes, some spatial agreement was evident across tests. Prevalence was generally highest in Northern and Western regions of the country for all three diagnostic groups (Fig 1: Panel A to C). However, this pattern of spatial variation was more evident for the CCA with trace as positive group, with many high-burden locations (i.e. with prevalence >50%) occurring within close proximity of one another in the Northern and Western regions of Rwanda (Fig 1: Panel B). The KK and CCA with trace as negative groups tended to show very low prevalence in most regions, with only a few high-prevalence locations occurring in the Northern and Western Regions (Fig 1: Panel A and C, respectively).
Fig 1. Observed prevalence of Schistosoma mansoni infection in Rwandan schoolchildren estimated using three different diagnostic methods: Kato-Katz (Panel A), CCA with trace as positive (Panel B), and CCA with trace as negative (Panel C).
Refer to S3 Fig in Supporting Information for names of geographical districts. This figure was produced in ArcMap 10.4 (ESRI, Redlands, CA) using a shapefile representing Rwanda’s current administrative units (obtained from the geographic data warehouse DIVA GIS (www.diva-gis.org/Data)). CCA; Circulating Cathodic Antigen.
Spatial clustering in Schistosoma mansoni prevalence and risk prediction
Semivariograms based on residuals from non-spatial models revealed a tendency for spatial clustering for all diagnostic groups, warranting the need for geostatistical methods (Supporting Information, S2 Fig). Sex of participants was an important risk factor for S. mansoni infection across all models, with males exhibiting higher risk compared to females (odds ratios of increased risk for males = 1.71, 1.08 and 1.35 for KK, CCA with trace as positive and CCA with trace as negative, respectively; Table 2). However, environmental predictors differed among methods. We found a positive association between LST and infection risk when using the KK test, whereas NDVI was positively associated with risk for both CCA groups (Table 2).
Table 2. Predictor associations with an individual child’s probability of being infected with Schistosoma mansoni, estimated using three different diagnostic methods to account for possible discrepancies in diagnostic performance and estimates of geographical endemicity.
For each diagnostic method, a geostatistical model was built to estimate predictor effects while accounting for spatial autocorrelation. Presented are effect means and 95% credible intervals (in brackets). KK; Kato-Katz. CCA; Circulating Cathodic Antigen.
| Variable | KK | CCA with trace as positive | CCA with trace as negative |
|---|---|---|---|
| Age 10–14 y (versus 5–9 y) | 1.47 (-4.75, 10.15) | 0.01 (-0.55, 0.57) | 1.11 (-0.20, 2.73) |
| Age 15–18 y (versus 5–9 y) | 0.91 (-5.35, 9.56) | -0.16 (-0.76, 0.46) | 1.08 (-0.29, 2.72) |
| Male (versus female) | 0.54 (0.21, 0.88) | 0.08 (0.02, 0.15) | 0.30 (0.18, 0.43) |
| Land surface temperaturea | 0.91 (0.14, 1.73) | 0.08 (-0.03, 0.20) | 0.12 (-0.06, 0.30) |
| Normalized difference vegetation indexa | 0.93 (-0.08, 2.00) | 0.23 (0.08, 0.38) | 0.28 (0.03, 0.54) |
| Normalized difference water indexa | -0.36 (-1.59, 0.80) | -0.10 (-0.29, 0.08) | -0.25 (-0.56, 0.06) |
| ϕ (phi: rate of decay of spatial correlation)b | 11.90 (4.24, 19.34) | 17.36 (13.10, 19.88) | 14.81 (9.06, 19.54) |
| σ2 (sigma: variance of spatial random effect)b | 11.74 (5.93, 23.15) | 1.17 (0.95, 1.46) | 2.65 (2.00, 3.62) |
| Tau (precision) | 0.10 (0.04, 0.17) | 0.86 (0.69, 1.05) | 0.39 (0.28, 0.50) |
| Intercept | -9.57 (-18.32, -2.99) | -0.80 (-1.42, -0.19) | -4.87 (-6.54, -3.48) |
a Variables were standardized to have mean = 0 and standard deviation = 1
b Measured in decimal degrees and 3/phi determines the cluster size; one decimal degree is approximately 111km at the Equator (the size of the radii of the clusters).
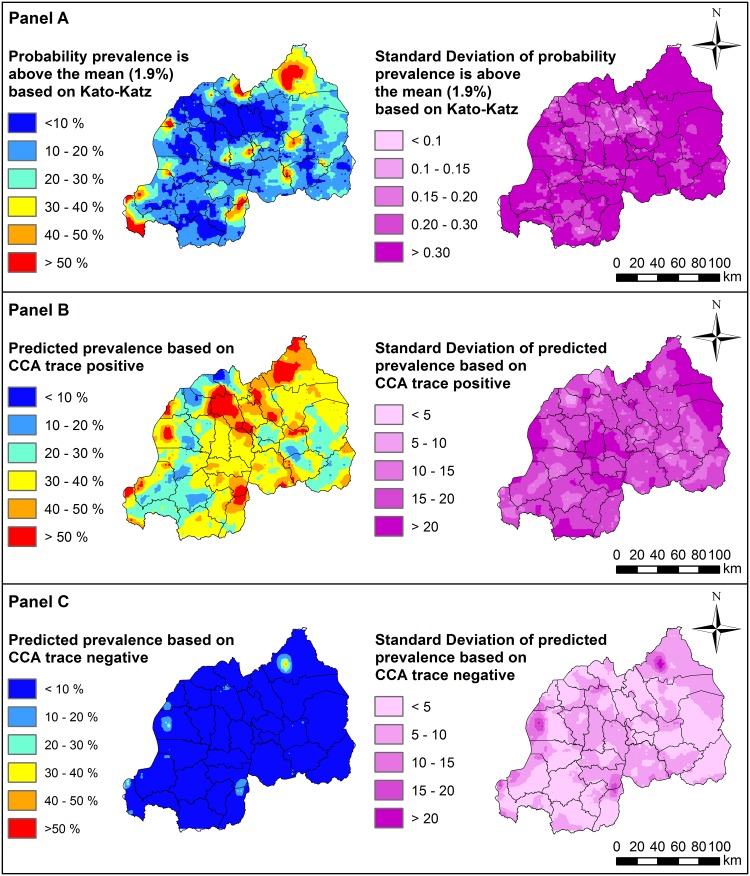
For the KK method, regions with highest probability of harbouring above-average prevalence generally occurred in the Northern region, Nyagatare and Burera districts, the Western region such as Rusizi districts and also in inland-areas of Rwanda such as Kamonyi and Rwamagana (Fig 2: Panel A; see S3 Fig in Supporting Information for locations of districts). Predicted prevalence of S. mansoni using CCA with trace as positive was mostly between 30 to 40% across Rwanda, with highest prevalence (i.e. with >50% predicted prevalence) occurring in Northern districts (including Nyagatare, Gicumbi and Gakenke) as well as Southern districts such as Gisagara and Nyanza (Fig 2: Panel B; S3 Fig). In contrast, predicted prevalence using CCA with trace as negative was mostly <10% across Rwanda, with the highest predicted prevalence observed only in a small localized area in the Northern Nyagatare district (Fig 2: Panel C; S3 Fig). Importantly, vast areas of the country identified as high-risk when considering trace results as positive were estimated to have prevalence <10% when considering trace results as negative.
Fig 2. Predicted prevalence of Schistosoma mansoni infection in Rwandan boys aged 5–9 years, estimated using three different diagnostic methods: Kato-Katz (Panel A), CCA with trace as positive (Panel B), and CCA with trace as negative (Panel C).
Predictions were generated using separate geostatistical models to account for possible discrepancies in diagnostic performance and estimates of geographical endemicity. Refer to S3 Fig in Supporting Information for names of geographical districts. This figure was produced in ArcMap 10.4 (ESRI, Redlands, CA) using a shapefile representing Rwanda’s current administrative units (obtained from the geographic data warehouse DIVA GIS (www.diva-gis.org/Data)). CCA; Circulating Cathodic Antigen.
Comparison of population densities in at-risk regions using different diagnostic tests
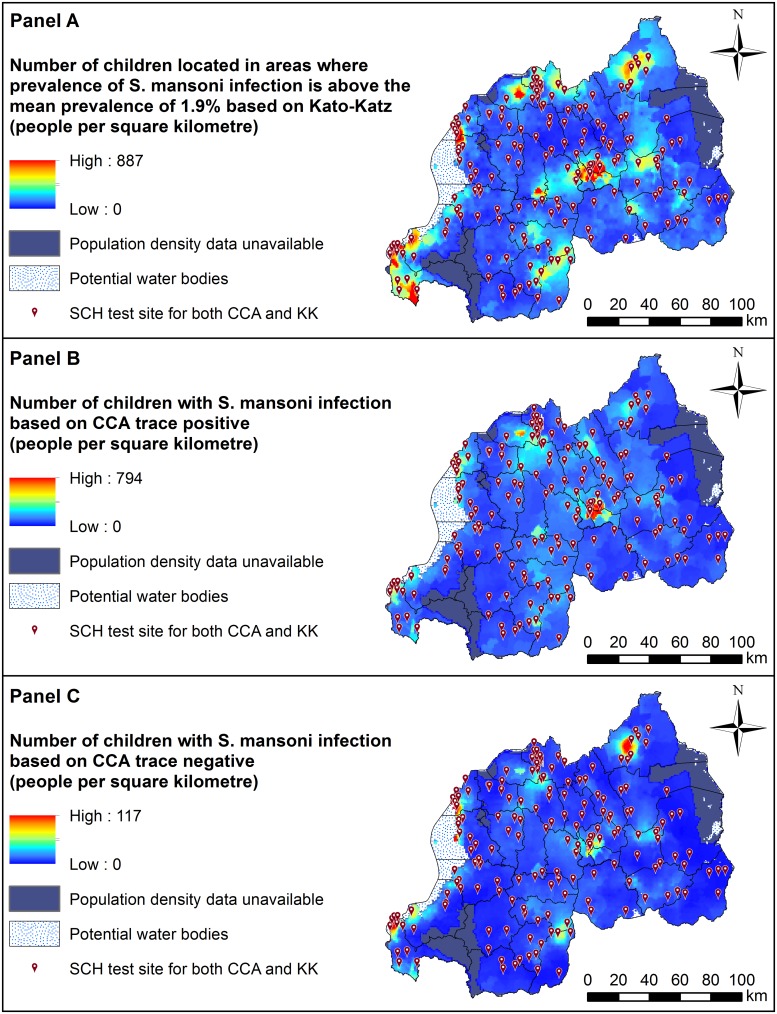
Our spatially-adjusted projection maps identified areas where high densities of individuals in the focal group (boys aged 5 to 9 years) live in risk-prone environments. Projections were broadly in agreement across diagnostic tests, though it should be noted that our KK prediction map is restricted to estimating total at-risk individuals in spatial clusters with above-average predicted prevalence. Areas within and around the capital Kigali in the central area of Rwanda (i.e. the capital city Kigali, and the southwest part of Kigali including Rwezamenyo, Gitega, Kimisagara, Nyakabanda sectors) had high estimated densities of at-risk individuals across all three tests (Fig 3: Panel A to C; S3 Fig). In addition, target areas were also identified in localised areas around Mururu sector in Rusizi district in South-Western province, and Nkombo sector which is located around the southern end of Lake Kivu in Rusizi district.
Fig 3. Distributions of the number of Rwandan boys aged 5–9 years (per square kilometre) estimated to be infected with Schistosoma mansoni based on results from three different diagnostic methods: Kato-Katz (Panel A), CCA with trace as positive (Panel B) and CCA with trace as negative (Panel C).
Estimates were generated using geostatistical predictions applied to a map of Rwanda’s 2018 population. This raster was generated by multiplying National Institute of Statistics Rwanda, Fourth Population and Housing Census 2012 data [51, 52] by the reported United Nations Development Programme (UNDP) average annual rate of population change (i.e. 2.53%), which was then multiplied by the proportion of 5–9 year olds. Refer to S3 Fig in Supporting Information for names of geographical districts. This figure was produced in ArcMap 10.4 (ESRI, Redlands, CA) using a shapefile representing Rwanda’s current administrative units (obtained from the geographic data warehouse DIVA GIS (www.diva-gis.org/Data)). CCA; Circulating Cathodic Antigen. SCH; Schistosoma prevalence mapping unit.
Despite some general agreement, we identified considerable discrepancies in spatial patterns and estimated densities of at-risk children across diagnostic tests. For the CCA with trace as positive test, we estimated that approximately 671,856 boys aged 5 to 9 years are currently infected with S. mansoni in Rwanda (95% CI: 655,349–678,692), while only 60,453 individuals in this target group are predicted to be infected based on the CCA with trace as negative test (95% CI: 52,452–61,116; Table 3). While both CCA datasets identified the Gisenyi sector of the Rubavu district in the Western province as a potential target, estimated densities were much higher when using the CCA with trace as positive data (with up to 887 at-risk individuals per square km; compared to 117 people per square km for CCA with trace as negative; Fig 3: Panel B and C). Moreover, the KK map identified a target area around Rukomo sector in Gicumbi district (North-Eastern region) that was not identified by the CCA tests (Fig 3: Panel A).
Table 3. Predicted number of individual boys aged 5 to 9 years estimated to be harbouring Schistosoma mansoni infection in Rwanda in 2018 based on results from two different diagnostic methods: CCA with trace as positive and CCA with trace as negative.
CCA; Circulating Cathodic Antigen.
| Total population for 2015 (in Thousands)a | Annual population growth rate for 2015–2020 (Percentage)a | Percentage of individuals aged 5-9ya | Predicted number of individuals with S. mansoni infection in 2018 | |
|---|---|---|---|---|
| CCA with as trace positive | CCA with as trace negative | |||
| 11629.6 | 2.53 | 13.85 | 671,856 | 60,453 |
a Source: World Population Prospects 2017 Revision Population Database: Rwanda
CCA; Circulating Cathodic Antigen
Discussion
Our study outlines important risk factors and provides national geostatistical projections of Schistosoma mansoni infection burden in Rwanda. Concordant spatial predictions for diagnostic test groups highlighted areas in most need of interventions. Population-dense areas in the West and around Rwanda’s capital region are projected to exhibit environmental conditions that are conducive to transmission and harbour high densities of at-risk children. Our findings therefore provide useful insights that can be leveraged to devise tailored monitoring and intervention programs. However, our assessment of multiple diagnostic procedures highlights a major issue in efforts to design intervention strategies that can reduce schistosomiasis-related morbidity. Variation in parasite detection rates, identification of different environmental risk factors and geographical discrepancies in at-risk projections suggest that considerably different inferences will be gleaned from surveys using either KK or CCA datasets [27]. This is particularly evident in Rwanda, where the low overall prevalence leads to even greater insensitivity by the KK method and is similar to a comparable country-wide survey done in Burundi [53]. We stress that data collection using multiple diagnostic sources may be necessary to generate accurate infection estimates while dealing with diagnostic test uncertainty.
Identifying areas at greatest need of targeted Schistosoma mansoni mitigation: Discrepancies across Kato-Katz and circulating cathodic antigen surveys
Helminth infection is among the most common causes of anaemia and subsequent hospitalization in Rwanda [31, 54]. As one of Africa’s most densely populated countries and with an economy that relies on agriculture [32], it is unsurprising that up to 36% of schoolchildren are estimated to be infected with S. mansoni. However, infection rates are not homogenous throughout the country. Determining accurate estimates of Schistosoma prevalence is an important step to design mitigation programs, particularly considering that even low infection intensities can cause morbidity [3]. We provide multiple lines of evidence that different diagnostic procedures yield different inferences about S. mansoni infection rates and spatial patterns in Rwandan schoolchildren. Our prevalence estimates varied largely across procedures, with the KK method yielding far lower estimates compared to the CCA datasets. Explaining this discrepancy requires an understanding of what these tests are designed to detect. Positive detection by the KK method is limited to individuals that are infected with egg-producing female worms in sufficient numbers to consistently yield eggs in their faeces. This immediately rules out detection of some infections that are composed of male worms, of worms that have not reached sexual maturity or low numbers of worms that produce low numbers of eggs. Moreover, parasite egg counts in our study (as in most KK studies) were based on a single stool sample, which can limit the ability of technicians to identify infections [28]. Finally, other work suggests that KK detections are strongly associated with infection intensity or egg-laying rates [55]. In contrast, CCA tests are thought to provide a more unbiased estimate across heterogeneous environments [26, 56, 57]. Children who harbour pre-patent adult worms or low densities of egg-producing females are highly likely to be diagnosed as uninfected using the KK method [58], while detection of schistosome-released antigenic proteins using CCA may still be accurate [26].
Running separate analyses using multiple diagnostic procedures may not be cost-effective and clearly can lead to conflicts in resource management when attempting to reduce schistosomiasis in endemic areas. Given that CCA tests do not require a stool sample and have greater capabilities to detect low-intensity infections than KK, implementation of CCA diagnostics could be the best approach for rapid screening during ongoing monitoring programmes. However, the problem of interpretation for CCA tests still remains [56], although a recent systematic modelling paper comparing KK with CCA results from many different countries and endemic levels of infection indicates the relative comparative results of these two assays [27]. Despite the minimal training required for CCA testing, approaches to classify different trace results can be inconsistent [47, 56]. Nevertheless, as previously reported for Burundi [53], comparisons of the KK, CCA and CAA assays by latent class analysis indicate that at least half of trace results are estimated to be true positives.
Analysis of the CCA datasets in our study delivered quite different inferences. The estimated number of 5 to 9 year-old boys currently harbouring infection varied by an order of magnitude, a large difference that could be confusing to decision-makers. It should also be noted that even CCA with trace as positive tests are known to miss some confirmed infections [56], suggesting that our projections of burden in Rwanda could still be conservative. Because many nations where Schistosoma parasites are endemic do not have adequate funds for blanket treatment, this variation in prevalence estimates has important ramifications for the decision-making process. WHO guidelines are used around the world for designing mass drug administration strategies to reduce intestinal helminth infection rates in endemic nations [11, 33, 59]. Current guidelines for reducing schistosomiasis suggest that the prevalence in the at-risk school population should determine the number of interventions to use over the course of a child’s primary school years [41]. For example, areas with estimated prevalence >50% should receive treatment on an annual basis, while areas with prevalence <10% should be treat each child twice during their primary school years [41]. In light of our study, decisions about whether areas are high-risk (including many endemic clusters identified using the CCA with trace positive analysis) or low-risk (covering most of the country when considering the other two analyses) can lead to dramatic differences in the overall cost of treatment across diagnostic methods.
Based on our findings and on previous work suggesting a high sensitivity of CCA tests [25, 47, 57], we suggest treatment should be focused on areas that were identified by the CCA with trace as positive procedure as high-risk. This seems a useful approach to ensure adequate coverage of areas that likely exhibit high prevalence, high average intensity of infection and a relatively large number of infected schoolchildren. Here, our modelling identifies districts around the capital region and along the Western and Northern borders of the country (consisting of mountain highlands, the Virunga volcano range, and Lake Kivu [31]) as harbouring high S. mansoni burdens. These regions are some of the most heavily populated in the country [32, 51], and our projections indicate they contain high densities of at-risk populations. Indeed, using the less conservative CCA with trace as positive test, we estimate that prevalence of S. mansoni reaches >50% among schoolchildren in some of these districts. Targeting these areas will likely be the most cost-effective intervention approach for reducing prevalence and associated morbidity, as programs targeting areas with high transmission risk are expected to be more efficient reduction measures for battling schistosomiasis [10, 59].
In addition to mass drug administration, additional measures should ensure improved access to clean water and environmental measures to control snail abundances and reduce transmission [59, 60]. With malnutrition and diarrhea presenting as two of the most common causes of hospital-based child mortality in Rwanda [34], population densities and lack of adequate sanitation likely play strong roles in driving the observed spatial variation in S. mansoni infection rates. Schistosoma parasites maintain high transmission rates in regions where overcrowding and poor sanitation coincide [61]. For example in Rwanda’s capital city Kigali, where we identified an endemic cluster of high S. mansoni infection risk, construction of adequate sanitation facilities has not kept pace with rapid population expansion in recent years [62]. Instead, many people reside in high-density temporary slums where access to freshwater is limited. However, the issue of poor sanitation is not restricted to urbanised areas. Pit latrines, which facilitate the spread of infectious diseases through environmental contamination, are the most common toilet facilities, while defecation in fields or rivers is also commonplace [63]. Poverty contributes to these poor sanitation practices, as low-income communities are often located in marsh or swamp lands skirting urban centres in Rwanda [62]. In these areas, densities of intermediate snail hosts may be high, leading to high transmission forces.
Risk factors for Schistosoma mansoni infection in Rwandan schoolchildren
Female S. mansoni worms can produce hundreds of eggs per day [64]. When sanitation rates are poor and burdens are high, ecological risk factors that influence vegetation or water properties, both of which impact parasite survival and/or infectivity, become especially important. The widespread availability of satellite imagery has played a key role in identifying ecological correlates of geographical distributions and infection rates for an incredible diversity of pathogens [29, 65–69]. For human helminth parasites, numerous geostatistical analyses have delineated spatial clusters of high infection risk, further indicating a strong role of environmental forces [29, 65, 70, 71]. In our case, we identified LST and NDVI as important predictors of S. mansoni infection probability and spatial clustering. Temperature of the land surface is a key predictor of population dynamics for intermediate snail hosts, while low temperatures can impede the development of Schistosoma parasites within snails [43]. Vegetation indices could reflect distributions of habitats that are suitable for snails, while both variables in tandem may influence vegetation or soil properties that determine the survival of parasite stages in human excreta (Schistosoma eggs that release miracidia) or the infectivity of water-borne stages that penetrate human skin (cercariae) [4, 61]. Identifying key drivers of infection risk using remotely sensed variables presents a major advantage in efforts to provide continuously updated high-resolution projection maps [66, 72–74].
Study limitations
We provide new insights into risk factors and the spatial distribution of S. mansoni infections in Rwanda. However, our study has some weaknesses that should not be ignored. Our dataset did not consider whether infection with other intestinal parasites might have influenced risk of S. mansoni infection. Parasite co-infections are ubiquitous, and there is mounting evidence indicating that biotic parasite associations can have marked influences infection risk and/or disease progression [70, 71, 75–79]. In addition, remote-sensed variables such as those used in our study come with their own levels of uncertainty, though these are commonly ignored when producing raster maps [80, 81]. Moreover, our projection maps of at-risk population densities used population estimates from the UN population database. These estimates may not be entirely accurate, as data on the proportion of persons within our target age group overlaps a number of UN categories. Further uncertainty in our estimates could result from our approach to calculate population sizes using the UNDP average annual rate of population change. This average rate may not reflect spatio-temporal variation in population changes in Rwanda. Finally, while school-based surveys are the primary method of choice for mapping intestinal parasite infections, estimates of infections in adults would provide useful additional information to gain better insights into population-level risk factors [82].
Conclusions
We provide high-resolution predictions of spatial heterogeneity in S. mansoni infection prevalence in Rwandan schoolchildren. Together with our identification of risk factors and data-driven projections of current burdens in at-risk populations, these results can be leveraged to make informed decisions about mass drug treatment regimes. Treatment decisions based on mapping and modelling approaches such as ours could be useful for managers deciding between sustained morbidity control or moving toward elimination [21]. Ongoing monitoring and use of continuously updated geostatistical models will be essential for designing intestinal parasite mitigation programmes and evaluating their efficacy [66, 72–74]. Nevertheless, we highlight important discrepancies in spatial disease projections that rely on different diagnostic procedures. Greater emphasis is needed to develop standardized guidelines for classifying CCA trace results, as our risk maps yielded very different conclusions about areas in need of treatment when considering traces as positive or negative. We hope that our research provides a platform to help mitigate Schistosoma infection burdens and associated morbidity in the tropical and subtropical regions.
Supporting information
(CSV)
CCA; circulating cathodic antigen. The CCA with trace as positive dataset considered readings of ‘trace’ as positive infections, while the CCA with trace as negative dataset considered these as negative.
(DOCX)
From the left to the right: negative (0), trace, positive (1+), double positive (2++), and strong positive (3+++), according to the intensity of the test line.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the many children, parents, schoolteachers and district administrative and health officials who participated in this parasite re-mapping survey in Rwanda. We also thank the district and central level staff, the Rwanda Biomedical Center and the Schistosomiasis Control Initiative (SCI) for providing organizational and administrative support during the field survey.
Data Availability
All data required to replicate analyses is included in the Supporting Information.
Funding Statement
This study received financial support from the END Fund, the Schistosomiasis Control Initiative and the University of Georgia Research Foundation, Inc., which was funded by the Bill & Melinda Gates Foundation for the SCORE Project. SCORE receives its funding from the University of Georgia Research Foundation, Inc., which received funds for SCORE from the Bill & Melinda Gates Foundation. The END Fund and SCORE supported remapping of schistosomiasis through sub-awards to SCI at Imperial College, London. The END Fund also provided a research grant to the University of Queensland for additional data analyses and reporting. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rujeni N, Morona D, Ruberanziza E, Mazigo HD. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: an update on their epidemiology and control. Infect Dis Poverty. 2017;6(1):8 10.1186/s40249-016-0212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke M, Jones M, Gobert G, Li Y, Ellis M, McManus D. Immunopathogenesis of human schistosomiasis. Paras Immunol. 2009;31(4):163–76. [DOI] [PubMed] [Google Scholar]
- 3.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illness. 2008;4(1):65–79. 10.1177/1742395307084407 . [DOI] [PubMed] [Google Scholar]
- 4.Lardans V, Dissous C. Snail control strategies for reduction of schistosomiasis transmission. Parasitology Today. 1998;14(10):413–7. 10.1016/s0169-4758(98)01320-9 [DOI] [PubMed] [Google Scholar]
- 5.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(12):e3296 10.1371/journal.pntd.0003296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. The Lancet Infectious Diseases. 2006;6(7):411–25. 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- 7.Hotez PJ, Fenwick A. Schistosomiasis in Africa: An Emerging Tragedy in Our New Global Health Decade. PLoS Negl Trop Dis. 2009;3(9):e485 10.1371/journal.pntd.0000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JDF, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Tropica. 2003;86(2–3):125–39. 10.1016/s0001-706x(03)00029-9 [DOI] [PubMed] [Google Scholar]
- 9.Hotez P. Mass Drug Administration and Integrated Control for the World’s High‐Prevalence Neglected Tropical Diseases. Clinical Pharmacology & Therapeutics. 2009;85(6):659–64. [DOI] [PubMed] [Google Scholar]
- 10.Turner HC, Truscott JE, Fleming FM, Hollingsworth TD, Brooker SJ, Anderson RM. Cost-effectiveness of scaling up mass drug administration for the control of soil-transmitted helminths: a comparison of cost function and constant costs analyses. The Lancet Infectious Diseases. 2016;16(7):838–46. 10.1016/S1473-3099(15)00268-6 [DOI] [PubMed] [Google Scholar]
- 11.Fenwick A, Rollinson D, Southgate V. Implementation of human schistosomiasis control: challenges and prospects. Adv Parasitol. 2006;61:567–622. 10.1016/S0065-308X(05)61013-5 [DOI] [PubMed] [Google Scholar]
- 12.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. New Engl J Med. 2007;357(10):1018–27. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- 13.Jordan P, Webbe G. Schistosomiasis: epidemiology, treatment and control: London, UK; William Heinemann Medical Books Ltd; 1982. [Google Scholar]
- 14.Rudge JW, Stothard JR, Basáñez M-G, Mgeni AF, Khamis IS, Khamis AN, et al. Micro-epidemiology of urinary schistosomiasis in Zanzibar: Local risk factors associated with distribution of infections among schoolchildren and relevance for control. Acta Tropica. 2008;105(1):45–54. 10.1016/j.actatropica.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 15.Woolhouse ME, Dye C, Etard J-F, Smith T, Charlwood J, Garnett G, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Nat Acad Sci. 1997;94(1):338–42. 10.1073/pnas.94.1.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bethony J, Williams JT, Kloos H, Blangero J, Alves‐Fraga L, Buck G, et al. Exposure to Schistosoma mansoni infection in a rural area in Brazil. II: household risk factors. Trop Med Int Health. 2001;6(2):136–45. 10.1046/j.1365-3156.2001.00685.x [DOI] [PubMed] [Google Scholar]
- 17.King CH. Epidemiology of schistosomiasis: determinants of transmission of infection. Schistosomiasis: World Scientific; 2001. p. 115–32.
- 18.Schur N, Hürlimann E, Stensgaard A-S, Chimfwembe K, Mushinge G, Simoonga C, et al. Spatially explicit Schistosoma infection risk in eastern Africa using Bayesian geostatistical modelling. Acta Tropica. 2013;128(2):365–77. 10.1016/j.actatropica.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 19.Michelson EH. Studies on the biological control of schistosome-bearing snails. Predators and parasites of fresh-water mollusca: A review of the literature. Parasitol. 1957;47(3–4):413–26. [DOI] [PubMed] [Google Scholar]
- 20.Dillon RT. The ecology of freshwater molluscs: Cambridge University Press; 2000. [Google Scholar]
- 21.Fenwick A, Jourdan P. Schistosomiasis elimination by 2020 or 2030? Int J Parasitol. 2016;46(7):385–8. 10.1016/j.ijpara.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Danso‐Appiah A, De Vlas S J, Bosompem KM, Habbema JDF. Determinants of health‐seeking behaviour for schistosomiasis‐related symptoms in the context of integrating schistosomiasis control within the regular health services in Ghana. Trop Med Int Health. 2004;9(7):784–94. 10.1111/j.1365-3156.2004.01267.x [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Basic laboratory methods in medical parasitology. Geneva: World Health Organization; 1991. [Google Scholar]
- 24.Shane HL, Verani JR, Abudho B, Montgomery SP, Blackstock AJ, Mwinzi PNM, et al. Evaluation of Urine CCA Assays for Detection of Schistosoma mansoni Infection in Western Kenya. PLoS Negl Trop Dis. 2011;5(1):e951 10.1371/journal.pntd.0000951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, et al. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Tropica. 2006;97(2):219–28. 10.1016/j.actatropica.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 26.Kittur N, Castleman JD, Campbell CH Jr, King CH, Colley DG. Comparison of Schistosoma mansoni prevalence and intensity of infection, as determined by the circulating cathodic antigen urine assay or by the Kato-Katz fecal assay: a systematic review. Am J Trop Med Hyg. 2016;94(3):605–10. 10.4269/ajtmh.15-0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bärenbold O, Garba A, Colley DG, Fleming FM, Haggag AA, Ramzy RMR, et al. Translating preventive chemotherapy prevalence thresholds for Schistosoma mansoni from the Kato-Katz technique into the point-of-care circulating cathodic antigen diagnostic test. PLoS Negl Trop Dis. 2018;12(12):e0006941 10.1371/journal.pntd.0006941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krauth SJ, Coulibaly JT, Knopp S, Traoré M, N’Goran EK, Utzinger J. An in-depth analysis of a piece of shit: distribution of Schistosoma mansoni and hookworm eggs in human stool. PLoS Negl Trop Dis. 2012;6(12):e1969 10.1371/journal.pntd.0001969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooker S. Spatial epidemiology of human schistosomiasis in Africa: risk models, transmission dynamics and control. Trans Roy Soc Trop Med Hyg. 2007;101(1):1–8. 10.1016/j.trstmh.2006.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chammartin F, Hürlimann E, Raso G, N’Goran EK, Utzinger J, Vounatsou P. Statistical methodological issues in mapping historical schistosomiasis survey data. Acta Tropica. 2013;128(2):345–52. 10.1016/j.actatropica.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 31.ICF International. Rwanda Demographic and Health Survey 2014–15, National Institute of Statistics of Rwanda; Rockville, Maryland, USA: 2016. [Google Scholar]
- 32.World Bank. The World Bank in Rwanda 2014 [May 2018]. http://www.worldbank.org/en/country/rwanda/overview.
- 33.World health report. Working together for health. Geneva http://www.who.int/whr/2006/en: World Health Organization; 2006.
- 34.Rwanda Human Resources for Health Program. Strategic plan 2011–2016. Kigali http://www.brown.edu/academics/medical/bright/sites/brown.edu.academics.medical.bright/files/uploads/MOH%20Rwanda%20HRH%20Strategic%20Plan%202011%20-%202016.pdf: Ministry of Health of the Republic of Rwanda; 2011.
- 35.Binagwaho A, Kyamanywa P, Farmer PE, Nuthulaganti T, Umubyeyi B, Nyemazi JP, et al. The human resources for health program in Rwanda—a new partnership. New Engl J Med. 2013;369(21):2054–9. 10.1056/NEJMsr1302176 [DOI] [PubMed] [Google Scholar]
- 36.Ruberanziza E, Owada K, Clark NJ, Umulisa I, Ortu G, Lancaster W, et al. Mapping Soil-Transmitted Helminth Parasite Infection in Rwanda: Estimating Endemicity and Identifying At-Risk Populations. Trop Med Infect Dis. 2019;4(2):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruberanziza E, Kabera M, Ortu G, Kanobana K, Mupfasoni D, Ruxin J, et al. Nkombo Island: the most important Schistosomiasis mansoni focus in Rwanda. American Journal of Life Sciences. 2015;3(1):27–31. [Google Scholar]
- 38.Mupfasoni D, Karibushi B, Koukounari A, Ruberanziza E, Kaberuka T, Kramer MH, et al. Polyparasite Helminth Infections and Their Association to Anaemia and Undernutrition in Northern Rwanda. PLoS Negl Trop Dis. 2009;3(9):e517 10.1371/journal.pntd.0000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clerinx J, Bottieau E, Wichmann D, Tannich E, Van Esbroeck M. Acute schistosomiasis in a cluster of travelers from Rwanda: diagnostic contribution of schistosome DNA detection in serum compared to parasitology and serology. Journal of Travel Medicine. 2011;18(6):367–72. 10.1111/j.1708-8305.2011.00552.x [DOI] [PubMed] [Google Scholar]
- 40.Lai Y-S, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. The Lancet Infectious Diseases. 2015;15(8):927–40. 10.1016/S1473-3099(15)00066-3 [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Helminth control in school-age children: a guide for managers of control programmes, 2nd edition Geneva: World Health Organization; 2002. [Google Scholar]
- 42.Appleton C. Review of literature on abiotic factors influencing the distribution and life cycles of bilharziasis intermediate host snails. 1978;11:1–25. [Google Scholar]
- 43.Sturrock RF. The intermediate hosts and host–parasite relationships. Jordan P, Webbe G, Sturrock RF, editors. Wallingford: CAB International; 1993. [Google Scholar]
- 44.Environmental Systems Research Institute. ArcGIS Desktop: Release 10.4. Redlands, California, USA: Environmental Systems Research Institute; 2015. [Google Scholar]
- 45.World Health Organization. Scientific working group on Schistosomiasis. Geneva: World Health Organization: 2005. [Google Scholar]
- 46.Casacuberta M, Kinunghi S, Vennervald BJ, Olsen A. Evaluation and optimization of the Circulating Cathodic Antigen (POC-CCA) cassette test for detecting Schistosoma mansoni infection by using image analysis in school children in Mwanza Region, Tanzania. Parasite Epidemiology and Control. 2016;1(2):105–15. 10.1016/j.parepi.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortu G, Ndayishimiye O, Clements M, Kayugi D, Campbell CH, Lamine MS, et al. Countrywide reassessment of Schistosoma mansoni infection in Burundi using a urine-Circulating Cathodic Antigen rapid test: informing the National Control Program. Am J Trop Med Hyg. 2017;96(3):664–73. 10.4269/ajtmh.16-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro PJ, Diggle PJ. geoR: Analysis of Geostatistical Data R package version 1.7–5.2. 2016.
- 49.Soares Magalhães RJ, Clements ACA, Patil AP, Gething PW, Brooker S. The applications of model-based geostatistics in helminth epidemiology and control. Adv Parasitol. 2011;74:267–96. 10.1016/B978-0-12-385897-9.00005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D. The BUGS Book: A practical introduction to Bayesian analysis: CRC Press; 2012. [Google Scholar]
- 51.Center for International Earth Science Information Network (CIESIN). Documentation for the Gridded Population of the World, Version 4 (GPWv4) Columbia University, Palisades, New York: NASA Socioeconomic Data and Applications Center (SEDAC)2016 [21st January 2018]. 10.7927/H4D50JX4. [DOI]
- 52.Population Division of the Department of Economic SAotUNS. World Population Prospects 2017 Revision Population Database: Rwanda. https://esa.un.org/unpd/wpp/DataQuery/2017.
- 53.Clements MN, Corstjens PLAM, Binder S, Campbell CH Jr., de Dood CJ, Fenwick A, et al. Latent class analysis to evaluate performance of point-of-care CCA for low-intensity Schistosoma mansoni infections in Burundi. Paras Vect. 2018;11(1):111-. 10.1186/s13071-018-2700-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colón-González FJ, Tompkins AM, Biondi R, Bizimana JP, Namanya DB. Assessing the effects of air temperature and rainfall on malaria incidence: an epidemiological study across Rwanda and Uganda. Geospatial Health. 2016;11(1s). [DOI] [PubMed] [Google Scholar]
- 55.Foo KT, Blackstock AJ, Ochola EA, Matete DO, Mwinzi PN, Montgomery SP, et al. Evaluation of point-of-contact circulating cathodic antigen assays for the detection of Schistosoma mansoni infection in low-, moderate-, and high-prevalence schools in western Kenya. Am J Trop Med Hyg. 2015;92(6):1227–32. 10.4269/ajtmh.14-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clements MN, Donnelly CA, Fenwick A, Kabatereine NB, Knowles SCL, Meité A, et al. Interpreting ambiguous ‘trace’ results in Schistosoma mansoni CCA Tests: Estimating sensitivity and specificity of ambiguous results with no gold standard. PLoS Negl Trop Dis. 2017;11(12):e0006102 10.1371/journal.pntd.0006102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colley DG, Binder S, Campbell C, King CH, Tchuenté L-AT, N’Goran EK, et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am J Trop Med Hyg. 2013;88(3):426–32. 10.4269/ajtmh.12-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berhe N, Medhin G, Erko B, Smith T, Gedamu S, Bereded D, et al. Variations in helminth faecal egg counts in Kato–Katz thick smears and their implications in assessing infection status with Schistosoma mansoni. Acta Tropica. 2004;92(3):205–12. 10.1016/j.actatropica.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 59.WHO Expert Committee on the Control of Schistosomiasis. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva: World Health Organization; 2002. [PubMed] [Google Scholar]
- 60.Campbell SJ, Savage GB, Gray DJ, Atkinson J-AM, Soares Magalhães RJ, Nery SV, et al. Water, Sanitation, and Hygiene (WASH): A Critical Component for Sustainable Soil-Transmitted Helminth and Schistosomiasis Control. PLoS Negl Trop Dis. 2014;8(4):e2651 10.1371/journal.pntd.0002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Paras Vect. 2015;8(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsinda A, Abbott P, Pedley S, Charles K, Adogo J, Okurut K, et al. Challenges to Achieving Sustainable Sanitation in Informal Settlements of Kigali, Rwanda. Int J Env Res Public Health. 2013;10(12):6939 10.3390/ijerph10126939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasana J, Morin J, Ndikuyeze A, Kamoso P. Impact of water supply and sanitation on diarrheal morbidity among young children in the socioeconomic and cultural context of Rwanda (Africa). Environ Res. 2002;90(2):76–88. 10.1006/enrs.2002.4394 [DOI] [PubMed] [Google Scholar]
- 64.Moore DV, Sandground JH. The Relative Egg Producing Capacity of Schistosoma Mansoni and Schistosoma Japonicum. Am J Trop Med Hyg. 1956;5(5):831–40. 10.4269/ajtmh.1956.5.831 [DOI] [PubMed] [Google Scholar]
- 65.Assoum M, Ortu G, Basáñez M-G, Lau C, Clements ACA, Halton K, et al. Spatiotemporal distribution and population at risk of soil-transmitted helminth infections following an eight-year school-based deworming programme in Burundi, 2007–2014. Paras Vect. 2017;10(1):583 10.1186/s13071-017-2505-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooker S, Michael E. The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Adv Parasitol. 2000;47:245–88. [DOI] [PubMed] [Google Scholar]
- 67.Clark NJ, Seddon JM, Kyaw-Tanner M, Al-Alawneh J, Harper G, McDonagh P, et al. Emergence of canine parvovirus subtype 2b (CPV-2b) infections in Australian dogs. Infect Gen Evol. 2018;58:50–5. 10.1016/j.meegid.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 68.Clark N, Soares Magalhães RJ. Airborne geographical dispersal of Q Fever from livestock holdings to human communities: a systematic review and critical appraisal of evidence. BMC Infect Dis. 2018: 10.1186/s12879-018-3135-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wells K, Gibson DI, Clark NJ, Ribas A, Morand S, McCallum HI. Global spread of helminth parasites at the human–domestic animal–wildlife interface. Glob Change Biol. 2018: 10.1111/gcb.14064 [DOI] [PubMed] [Google Scholar]
- 70.Soares Magalhães RJ, Biritwum N-K, Gyapong JO, Brooker S, Zhang Y, Blair L, et al. Mapping helminth co-Infection and co-intensity: geostatistical prediction in Ghana. PLoS Negl Trop Dis. 2011;5(6):e1200 10.1371/journal.pntd.0001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raso G, Vounatsou P, Singer BH, Eliézer K, Tanner M, Utzinger J. An integrated approach for risk profiling and spatial prediction of Schistosoma mansoni–hookworm coinfection. Proc Nat Acad Sci. 2006;103(18):6934–9. 10.1073/pnas.0601559103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pullan RL, Gething PW, Smith JL, Mwandawiro CS, Sturrock HJ, Gitonga CW, et al. Spatial modelling of soil-transmitted helminth infections in Kenya: a disease control planning tool. PLoS Negl Trop Dis. 2011;5(2):e958 10.1371/journal.pntd.0000958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Truscott J, Turner H, Farrell S, Anderson R. Soil-transmitted helminths: Mathematical models of transmission, the impact of mass drug administration and transmission elimination criteria Adv Parasitol. 94: Elsevier; 2016. p. 133–98. 10.1016/bs.apar.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 74.Basáñez M-G, McCarthy JS, French MD, Yang G-J, Walker M, Gambhir M, et al. A research agenda for helminth diseases of humans: modelling for control and elimination. PLoS Negl Trop Dis. 2012;6(4):e1548 10.1371/journal.pntd.0001548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clark NJ, Wells K, Dimitrov D, Clegg SM. Co-infections and environmental conditions drive the distributions of blood parasites in wild birds. J Anim Ecol. 2016;85:1461–70. 10.1111/1365-2656.12578 [DOI] [PubMed] [Google Scholar]
- 76.Clark NJ, Wells K, Lindberg O. Unravelling changing interspecific interactions across environmental gradients using Markov random fields. Ecol. 2018: 10.1002/ecy.2221 [DOI] [PubMed] [Google Scholar]
- 77.Muturi EJ, Jacob BG, Kim C-H, Mbogo CM, Novak RJ. Are coinfections of malaria and filariasis of any epidemiological significance? Parasitol Res. 2008;102(2):175–81. 10.1007/s00436-007-0779-1 [DOI] [PubMed] [Google Scholar]
- 78.Tollenaere C, Susi H, Laine A-L. Evolutionary and Epidemiological Implications of Multiple Infection in Plants. Trends Plant Sci. 2016;21(1):80–90. 10.1016/j.tplants.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 79.Furuya-Kanamori L, Liang S, Milinovich G, Soares Magalhaes RJ, Clements ACA, Hu W, et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16(1):84 10.1186/s12879-016-1417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hay S, Tatem A, Graham A, Goetz S, Rogers D. Global environmental data for mapping infectious disease distribution. Adv Parasitol. 2006;62:37–77. 10.1016/S0065-308X(05)62002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–61. 10.1016/S0065-308X(05)62007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campbell S, Osei-Atweneboana M, Stothard R, Koukounari A, Cunningham L, Armoo S, et al. The COUNTDOWN Study Protocol for Expansion of Mass Drug Administration Strategies against Schistosomiasis and Soil-Transmitted Helminthiasis in Ghana. Trop Med Infect Dis. 2018;3(1):10 10.3390/tropicalmed3010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
CCA; circulating cathodic antigen. The CCA with trace as positive dataset considered readings of ‘trace’ as positive infections, while the CCA with trace as negative dataset considered these as negative.
(DOCX)
From the left to the right: negative (0), trace, positive (1+), double positive (2++), and strong positive (3+++), according to the intensity of the test line.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data required to replicate analyses is included in the Supporting Information.