Abstract
HIV nucleotide sequences generated through routine drug resistance testing (DRT) and reported to Maryland’s Molecular HIV Surveillance system are most effective for elucidating transmission patterns and identifying outbreaks if DRT is ordered promptly and sequences are reported completely. Among reported cases of HIV infection newly diagnosed during 2011–2013 in Maryland residents aged >13 years, we assessed sequence ascertainment completeness. To better understand which populations were most likely to have a sequence, we examined associations between sequence ascertainment and clinical and demographic characteristics. During 2011–2013, 4423 new HIV infection diagnoses were reported; sequences were ascertained for 1282 (29.0%). Among 3267 cases with complete data, odds for having a sequence ascertained were highest for cases in persons living inside Maryland’s Central Region with initial CD4 counts <500 cells/mm3 (adjusted odds ratio [aOR] 2.4, 95% confidence interval [CI] 1.9–3.1). Sequence ascertainment did not vary significantly by patient age, sex, race/ethnicity or HIV transmission category. Educational interventions, policy changes and improved processes to increase timely DRT and subsequent sequence reporting with a focus on testing at entry to care, particularly for those with higher CD4 counts and those living outside the Central Region, might improve ascertainment completeness.
Keywords: HIV, drug resistance testing, surveillance, molecular epidemiology
Background
As part of caring for persons living with HIV in the United States, clinical providers order drug resistance testing (DRT), which determines nucleotide sequences of parts of the HIV-1 pol gene that are important anti-HIV drug targets. Pre-2007 U.S. Department of Health and Human Services (HHS) guidelines (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2006) recommended that providers order DRT immediately before starting antiretroviral therapy (ART) as determined by a patient’s CD4 count. Recommendations regarding when to initiate ART and order DRT have changed over time. During December 2009–March 2012, guidelines strongly recommended ART initiation for anyone with a CD4 count <350 cells/mm3. For persons with a CD4 count 350–500 cells/mm3, the panel was split between a moderate and strong recommendation to initiate ART, and 50% of the panel considered ART initiation optional for a CD4 count >500 cells/mm3 (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2009). Beginning March 2012, ART initiation was strongly recommended for everyone with a CD4 count ≤500 cells/mm3 (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2012). Furthermore, since December 2007, guidelines have recommended ordering DRT on all patients with viral load (VL) >1000 copies/mL when they enter care, regardless of starting ART, effectively delinking ordering DRT and initiating ART. DRT was discouraged and many labs would not perform DRT for persons with VL <1000 copies/mL due to unreliable viral amplification (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2008).
In 2013, Maryland received funding to participate in the Centers for Disease Control and Prevention’s (CDC’s) Molecular HIV Surveillance (MHS) system, an optional component of the National HIV Surveillance System (NHSS) at the time; Maryland had participated in MHS during 2011–2012 without federal funds. Maryland regulations require that laboratories report positive HIV tests, nucleotide sequences from DRT, CD4 counts and VL counts to the Maryland Department of Health (MDH) (Code of Maryland Regulations 10.18.02). MDH requires all laboratories performing DRT (6–8 labs during 2011–2013) to report sequences electronically to maximize data integrity and completeness. Although MHS was designed to evaluate HIV genetic diversity and monitor drug resistance (CDC and CSTE, 2015), MHS also aims to elucidate transmission networks (Oster et al., 2015; Smith et al., 2009) and identify disease clusters and outbreaks (France et al., 2016).
To identify clusters or outbreaks, nucleotide sequences from all possible two-way pairings of cases are compared. Cases with highly similar sequences are considered possibly linked and might have occurred in transmission partners, or if indirectly linked, in persons part of the same transmission cluster. Once clusters are identified, investigations can help elucidate transmission factors so as to guide interventions.
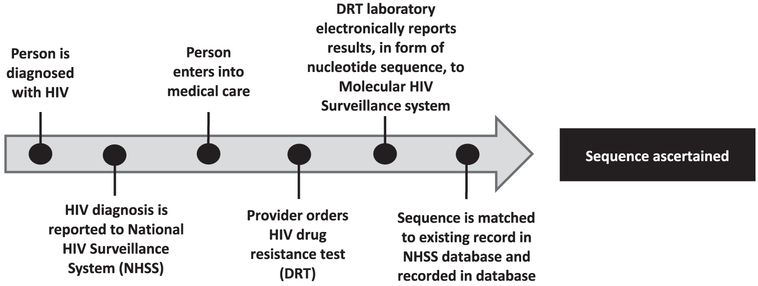
In 2015, CDC analyzed national HIV sequence data (Oster et al., 2016, February) and identified a Maryland cluster of five cases. Despite extensive interviews, no epidemiologic links were identified, likely because of missing sequence information. Reasons for missing data could include problems anywhere along the continuum from HIV diagnosis and reporting to NHSS, to entry to care, to ordering of DRT, to reporting of a sequence to the MHS system and matching it with a case in the NHSS (Figure 1).
Figure 1.
HIV sequence ascertainment timeline of events in the MHS system. Sequences might not be ascertained by the system due to patient- or provider-level factors that prevent patients from having DRT ordered or collected, such as loss to follow-up after diagnosis or tests not being ordered at entry to medical care. Surveillance system factors, such as ordered sequences not being reported to the system, might also lead to missing sequence data.
We examined the completeness of Maryland’s HIV sequence data ascertained by the MHS system and aimed to characterize cases with missing sequence data. A better understanding of populations at highest risk for lacking sequence data might allow targeting of interventions to increase sequence ascertainment so as to fully describe transmission networks, ultimately aiding our ability to stop ongoing transmission. We sought to (1) identify the percentage of HIV cases newly diagnosed in Maryland that had a nucleotide sequence ascertained by MHS and (2) identify disparities in nucleotide sequence ascertainment by examining demographic and clinical characteristics of Maryland patients with newly diagnosed and reported HIV infection cases that did and did not have sequences in the MHS system.
Methods
Population for analysis
We identified cases of HIV infection reported to MDH as part of routine HIV surveillance that were newly diagnosed during 1 January 2011–31 December 2013 in Maryland residents aged ≥13 years. The CDC/Council of State and Territorial Epidemiologists (CSTE) HIV surveillance case definitions in effect during our analysis period were used (Schneider et al., 2008). Cases in persons previously diagnosed with HIV infection outside of Maryland were excluded.
Measures
We determined the percentage of cases of newly reported HIV infection for which at least one nucleotide sequence was ascertained as of 1 February 2015. For cases with a nucleotide sequence and a complete HIV diagnosis date, including day, month and year, we calculated time from diagnosis to specimen collection for HIV DRT. If cases had more than one sequence in the MHS system, the date for the earliest sequence was used. We described cases with and without nucleotide sequences by patient demographic and clinical characteristics, as defined by NHSS protocols. Demographic characteristics included age at diagnosis, sex at birth, race/ethnicity, region of residence in the state, and US-born or foreign-born. Race/ethnicity categories included American Indian/Alaska Native, Asian, black or African American (henceforth referred to as black), Hispanic/Latino, Native Hawaiian/other Pacific Islander, white and multiracial; these categories were mutually exclusive. Clinical characteristics included initial CD4 count, typically collected at entry to care, first VL and HIV transmission category. Transmission categories included male-to-male sexual contact (men who have sex with men or MSM), heterosexual contact, persons who inject drugs (PWID), MSM who inject drugs, other (including perinatal and transfusion-related), and none identified or reported (CDC, 2016).
Statistical analyses
After preliminary analysis, demographic and clinical variables other than year of diagnosis and HIV transmission category were dichotomized for univariate and multivariate analyses. Because most cases were in persons of black race, we grouped cases in persons from all other racial/ethnic categories and conducted subsequent analyses comparing cases in persons of black versus non-black race. Final analyses compared cases in persons aged 13–49 years with those aged ≥50 years. Transmission was categorized as MSM, heterosexual contact, PWID (including MSM who inject drugs, because heterosexuals who inject drugs would be also assigned to this group by surveillance definitions) and none/other (combined due to small numbers of other). For univariate analysis, the none/other category was further stratified by sex. Initial CD4 count was dichotomized to ≤500 cells/mm3 and >500 cells/mm3 and VL to <1000 copies/mL and ≥1000 copies/mL.
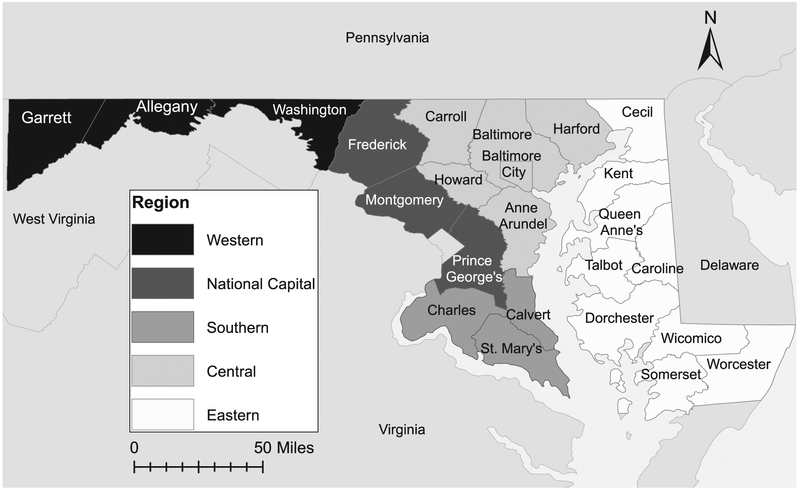
Most cases included in this study were in residents of the Central Region (comprising the city of Baltimore and Carroll, Howard, Anne Arundel, Baltimore, and Harford counties) or the National Capital Region (comprising Frederick, Montgomery, and Prince George’s counties) (Figure 2). A limited number of patients resided in other regions. Final analyses compared Central Region cases to cases from all other regions.
Figure 2.
Maryland counties by region.
Analyses examining associations between patient demographic or clinical characteristics and sequence ascertainment were performed using chi-square tests; prevalence ratios (PRs) and 95% confidence intervals (CIs) were calculated. Multivariate logistic regression models were constructed in STATA 12.0 (StataCorp, 2011; Stata Statistical Software: Release 12. College Station, TX: Stata-Corp LP) to further examine these associations. The demographic and clinical characteristics considered were those that were determined a priori to be potentially related to DRT ordering or nucleotide sequence reporting, based on available literature and an understanding of HIV care. To identify characteristics useful in guiding interventions to improve sequence ascertainment, all variables identified a priori were included in the models to control for potential confounding and all possible two-way interactions were examined. The analysis was not limited to cases with VL ≥1000 copies/mL, as our primary objective was to examine whether a sequence was ascertained rather than the quality of sequences ascertained. The final model did not include VL given its inherent close association with the outcome but included all other a priori variables and significant interactions.
Results
We identified 4423 reported new diagnoses of HIV infection in persons aged ≥13 years in Maryland during 2011–2013 (Table 1). Diagnosis dates were equally distributed across 2011 (34.0%), 2012 (33.6%) and 2013 (32.5%). Approximately 70% of patients were male and approximately 75% black. There was a peak in those aged 20–29 years, fewer cases in persons <20 years, and the remainder evenly distributed across the other age groups. Approximately 38% had no identified or reported transmission category, and most others were MSM only (33.6%) or heterosexual (23.1%). Approximately 84% of patients had an initial CD4 count; of those, 31.6% had an initial count >500 cells/mm3. Most patients resided in Maryland’s Central (49.5%) and National Capital (43.4%) regions.
Table 1.
Demographic and clinical characteristics of Maryland HIV cases newly diagnosed during 2011–2013.
|
n (%) Total N = 4423 |
|
|---|---|
| Year of HIV diagnosis | |
| 2011 | 1502 (34.0) |
| 2012 | 1485 (33.6) |
| 2013 | 1436 (32.5) |
| Sex at birth | |
| Male | 3144 (71.1) |
| Female | 1279 (28.9) |
| Race/ethnicity | |
| White | 628 (14.2) |
| American Indian/Alaska Native | 7 (0.2) |
| Asian | 50 (1.1) |
| Black/African American | 3309 (74.8) |
| Hispanic ethnicity | 275 (6.2) |
| Native Hawaiian/Pacific Islander | 0 (0) |
| Multiple races | 154 (3.5) |
| Age (years) at HIV diagnosis | |
| 13–19 | 200 (4.5) |
| 20–29 | 1332 (30.1) |
| 30–39 | 960 (21.7) |
| 40–49 | 1005 (22.7) |
| ≥50 | 926 (20.9) |
| Transmission category | |
| Persons who inject drugs | 189 (4.3) |
| Male-to-male sex (MSM) | 1488 (33.6) |
| MSM who inject drugs | 34 (0.8) |
| Heterosexual | 1020 (23.1) |
| None identified/reported | 1684 (38.1) |
| Other | 8 (0.2) |
| CD4 at diagnosis (cells/mm3) | |
| <350 | 1818 (41.1) |
| 350–500 | 727 (16.4) |
| >500 | 1174 (26.5) |
| Unknown | 704 (15.9) |
| First viral load (copies/mL) | |
| <1000 | 748 (16.9) |
| ≥1000 | 2727 (61.7) |
| Unknown | 948 (21.4) |
| Region of residence | |
| Central | 2189 (49.5) |
| Eastern Shore | 124 (2.8) |
| National Capital | 1918 (43.4) |
| Southern | 135 (3.1) |
| Western | 57 (1.3) |
| Place of birth | |
| Foreign-born | 355 (8.0) |
| US-born | 3,535 (79.9) |
| Unknown | 533 (12.1) |
Among cases of newly diagnosed HIV infection, 1282 (29.0%) had HIV DRT performed with ≥1 nucleotide sequence ascertained in the MHS system. Among 1275 cases with complete data available for dates of HIV diagnosis and DRT specimen collection, the median time from diagnosis to collection was 29 days (range 0–1441 days, interquartile range 8–176 days), and 872 (68.4%) had a specimen collected ≤90 days from diagnosis.
No significant differences were found by year of HIV diagnosis, race/ethnicity or sex at birth for cases with and without an HIV nucleotide sequence (Table 2). Early analyses showed that the probability of having a sequence ascertained changed near age 50 years; thus final analyses compared persons aged 13–49 years to those ≥50 years. Sensitivity regression analyses using cutoffs of 45 and 55 years had similar findings (data not shown). Among cases in persons aged 13–49 years, 30.0% had a sequence ascertained, compared with 25.1% of cases in persons aged ≥50 years (PR 1.2; 95% CI 1.1–1.4; p = 0.003). Compared with cases in the MSM category (31.4% with a sequence), 32.5% in the heterosexual transmission category had a sequence (PR 1.0; 95% CI 0.9–1.2; p = 0.5), 30.9% in the PWID category had a sequence (PR 1.0; 95% CI 0.8–1.2; p = 0.9), and 24.5% in the none/other transmission category had a sequence (PR 0.8; 95% CI 0.7–0.9; p < 0.001). The none/other transmission category was further examined by sex; similar percentages of males (25.0%) and females (24.1%) had sequences ascertained. Among 3719 cases with initial CD4 count, 35.5% of those with a count ≤500 cells/mm3 had a sequence, compared with 27.6% of those with a count >500 cells/mm3 (PR 1.3; 95% CI 1.2–1.4; p < 0.001). Cases with first VL ≥1000 copies/mL were more likely to have a sequence ascertained (38.0% vs. 21.0%; PR 1.8; 95% CI 1.6–2.1; p < 0.001). Sequence ascertainment differed significantly by residence region, with 33.1% of cases in persons living inside the Central Region having a sequence compared to 24.9% of cases in persons living outside the region (PR 1.3; 95% CI 1.2–1.5; p < 0.001). Finally, location of birth was available for 3890 patients; for cases in persons born in the United States, 29.4% had a sequence ascertained, compared with 23.1% of those born outside the United States (PR 1.3; 95% CI 1.0–1.6; p = 0.01).
Table 2.
Characteristics of Maryland HIV cases newly diagnosed during 2011–2013 by nucleotide sequence ascertainment in the MHS system, N = 4423.
| Sequence ascertained n (%) N = 1282 |
Sequence not ascertained n (%) N = 3141 |
Prevalence ratio (PR) | 95% CIa | p-Value | |
|---|---|---|---|---|---|
| Year of HIV diagnosis | |||||
| 2011 | 452 (30.1) | 1050 (69.9) | Ref | N/A | N/A |
| 2012 | 429 (28.9) | 1056 (71.1) | 1.0 | (0.9–1.1) | 0.5 |
| 2013 | 401 (27.9) | 1035 (72.1) | 0.9 | (0.8–1.0) | 0.2 |
| Age at diagnosis, years | |||||
| ≥50 | 232 (25.1) | 694 (74.9) | Ref | N/A | N/A |
| 13–49 | 1050 (30.0) | 2447 (70.0) | 1.2 | (1.1–1.4) | 0.003 |
| Sex at birth | |||||
| Male | 909 (28.9) | 2235 (71.1) | Ref | N/A | N/A |
| Female | 373 (29.2) | 906 (70.8) | 1.0 | (0.9–1.1) | 0.9 |
| Race/ethnicity | |||||
| Non-black/African American | 341 (30.6) | 773 (69.4) | Ref | N/A | N/A |
| Black/African American | 941 (28.4) | 2368 (71.6) | 0.9 | (0.8–1.0) | 0.2 |
| Transmission category | |||||
| Male-to-male sex (MSM) | 467 (31.4) | 1021 (68.6) | Ref | N/A | N/A |
| Heterosexual | 332 (32.5) | 688 (67.5) | 1.0 | (0.9–1.2) | 0.5 |
| Persons who inject drugs (PWID)b | 69 (30.9) | 154 (69.1) | 1.0 | (0.8–1.2) | 0.9 |
| Other/none | 414 (24.5) | 1278 (75.5) | 0.8 | (0.7–0.9) | <0.001 |
| CD4 at diagnosis (cells/mm3)c | |||||
| >500 | 324 (27.6) | 850 (72.4) | Ref | N/A | N/A |
| ≤500 | 904 (35.5) | 1641 (64.5) | 1.3 | (1.2–1.4) | <0.001 |
| First viral load (copies/mL) d | |||||
| <1000 | 157 (21.0) | 591 (79.0) | Ref | N/A | N/A |
| ≥1000 | 1036 (38.0) | 1691 (62.0) | 1.8 | (1.6–2.1) | <0.001 |
| Region of residence | |||||
| Outside Central Region | 557 (24.9) | 1677 (75.1) | Ref | N/A | N/A |
| Inside Central Region | 725 (33.1) | 1464 (66.9) | 1.3 | (1.2–1.5) | <0.001 |
| Region of residence stratified by CD4 at diagnosis (cells/mm3)c | |||||
| Outside Central Region | |||||
| CD4 > 500 | 117 (20.4) | 456 (79.6) | Ref | N/A | N/A |
| CD4 ≤ 500 | 413 (31.6) | 895 (68.4) | 1.5 | (1.3–1.9) | <0.001 |
| Inside Central Region | |||||
| CD4 > 500 | 207 (34.4) | 394 (65.6) | Ref | N/A | N/A |
| CD4 ≤ 500 | 491 (39.7) | 746 (60.3) | 1.2 | (1.0–1.3) | 0.03 |
| Place of birthe | |||||
| Foreign-born | 82 (23.1) | 273 (76.9) | Ref | N/A | N/A |
| US-born | 1040 (29.4) | 2495 (70.6) | 1.3 | (1.0–1.6) | 0.01 |
CI, confidence interval.
Includes MSM who inject drugs.
Excludes persons with unknown CD4 count (n = 704).
Excludes persons with unknown first viral load (n = 948).
Excludes persons with unknown place of birth (n = 533).
We conducted multivariate analysis for 3267 cases with complete data for demographic and clinical characteristics (Table 3). Because of significant interaction between residence region and initial CD4 count, the effects of these two variables were considered together in our final model. Cases in persons residing inside the Central Region of Maryland with initial CD4 counts ≤500 cells/mm3 had the highest adjusted odds of having a sequence ascertained compared to cases in persons residing outside the Central Region with initial CD4 counts >500 cells/mm3 (adjusted odds ratio (aOR) 2.4; 95% CI 1.9–3.1; p < 0.001). Cases in persons residing inside Maryland’s Central Region with initial CD4 count >500 cells/mm3 also had increased adjusted odds for having a sequence ascertained (aOR 2.0; 95% CI 1.5–2.6; p < 0.001). Cases in persons born in the United States had higher adjusted odds of having a sequence ascertained than cases in foreign-born persons (aOR 1.5; 95% CI 1.1–2.0; p = 0.004).
Table 3.
Final multivariable logistic regression model with adjusteda odds for sequence ascertainment by demographic and clinical characteristics among Maryland HIV cases newly diagnosed during 2011–2013, N = 3267.
| Independent variable | Adjusted OR |
95% CIb | p-Value |
|---|---|---|---|
| Year of HIV diagnosis | |||
| 2011 | Ref | N/A | N/A |
| 2012 | 0.9 | (0.8–1.1) | 0.5 |
| 2013 | 0.9 | (0.7–1.0) | 0.1 |
| Age <50 years at diagnosis | 1.2 | (1.0–1.4) | 0.1 |
| Female sex | 1.0 | (0.9–1.2) | 0.8 |
| Black/African American race | 1.0 | (0.8–1.1) | 0.6 |
| Transmission category | |||
| Male-to-male sex (MSM) | Ref | N/A | N/A |
| Heterosexual | 1.2 | (1.0–1.5) | 0.1 |
| Persons who inject drugs (PWID)c | 1.1 | (0.8–1.6) | 0.5 |
| None reported/other | 0.9 | (0.7–1.0) | 0.1 |
| Interaction between region of residence and initial CD4 count | |||
| Outside Central Region and CD4 > 500 mm3 | Ref | N/A | N/A |
| Outside Central Region and CD4 ≤ 500 mm3 | 1.8 | (1.4–2.3) | <0.001 |
| Inside Central Region and CD4 > 500 mm3 | 2.0 | (1.5–2.6) | <0.001 |
| Inside Central Region and CD4 ≤ 500 mm3 | 2.4 | (1.9–3.1) | <0.001 |
| US-born | 1.5 | (1.1–2.0) | 0.004 |
Final model included all variables identified a priori and significant two-way interactions.
CI, confidence interval.
Includes MSM who inject drugs.
Discussion
Fewer than one-third of Maryland residents with newly diagnosed HIV infection during 2011–2013 had nucleotide sequences in the MHS system by 1 February 2015, which is low given the recommendation that all persons with HIV infection should have DRT ordered at entry to care (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2015). One-third also falls short of CDC’s standard that ≥50% of cases with newly diagnosed HIV have a specimen collected for sequencing within 3 months and reported within 12 months following diagnosis (CDC and CSTE, 2015). Specimen collection occurred within 3 months of diagnosis for approximately two-thirds of Maryland cases with a specimen (CDC and CSTE, 2015). Only 13.8% of the cases lacking sequence data also lacked both CD4 and VL, suggesting that failure to engage in care does not explain the majority of missing sequence data.
Despite differences in sequence ascertainment by age and transmission category on univariate analysis, these differences did not persist in our multivariable model. On multivariate analysis, residents of Maryland’s Central Region (Baltimore City and Baltimore County) were more likely to have a sequence ascertained than residents of other regions, especially when initial CD4 count was ≤500 cells/mm3. This finding cannot be explained by regional differences in age distribution, racial/ethnic composition, or transmission categories. These differences might reflect variations in provider practices in DRT or reporting in different regions of the state and for patients with different initial CD4 counts. Another recent study found that approximately one-third of persons living with HIV in 2013 were not receiving DRT at entry to care as recommended since 2007 (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2007) and in some jurisdictions were less likely to receive it at entry to care if initial CD4 counts were ≥500 cells/mm3 (Dasgupta et al., 2017). Most persons with HIV infection in Maryland’s MHS system who live outside the Central Region live close to Washington, DC and northern Virginia. Sequencing performed in those jurisdictions might not be reported to Maryland, resulting in lower ascertainment outside the Central Region. However, recent data exchanges with Virginia and Washington, DC health departments revealed that <3% of cases with sequences were identified via this mechanism.
We also found higher sequence ascertainment for cases born in the United States, compared with foreign-born persons. Evidence shows that foreign- born persons are less likely to have insurance (Grieco, 2004; Ku, 2009; Thamer, Richard, Casebeer, & Ray, 1997) and have lower rates of health care utilization (Ku, 2009), which might result in lower DRT. Additionally, foreign-born cases might have earlier, unreported out-of-country HIV diagnoses that are not reflected in this surveillance system, and, therefore might be misclassified as a new diagnosis.
Prior to 2007, guidelines recommended DRT immediately before initiation of ART, resulting in a close temporal association between the two. Because guidance changes might take time to penetrate clinical practice, one might expect disparities in DRT (and thus in sequence ascertainment) that are similar to those observed for rates of filled ART prescriptions or retention in care. Previous studies covering similar periods as this evaluation have shown higher rates of retention in care among women, whites, persons ≥50 years and MSM (Yehia et al., 2012); lower rates of retention in care among blacks (Dasgupta, Oster, Li, & Hall, 2016) and higher rates of filled ART prescriptions among whites, men and persons ≥55 years (Horberg, Hurley, Klein et al., 2015). In contrast to these studies, however, our evaluation did not identify differences in sequence ascertainment by age, sex or transmission category after adjusting for other covariates. One previous study did observe lower rates of retention in care for patients with initial CD4 counts >500 cells/mm3 (Yehia et al., 2012), similar to our finding that cases with higher initial CD4 counts residing outside Maryland’s Central Region had lower odds of having a sequence ascertained.
Some components of the HIV care continuum, such as linkage to and retention in care, measurement of CD4 counts and VL and rates of filled ART prescriptions, are used as indicators of the quality of care for persons living with HIV (Althoff et al., 2014; Horberg, Hurley, Towner et al., 2011; Valdiserri, Forsyth, Yakovchenko, & Koh, 2013). Given incentives for providers to demonstrate high-quality care, data for these variables are well documented and available for study. In contrast, DRT has not often been used as an indicator, which might, in part, explain why less is known about persons receiving DRT or timing of DRT. More recently, however, researchers have started to examine DRT patterns. While we did not demonstrate differences by transmission categories, Dasgupta et al. found that male PWID were less likely to receive DRT compared to MSM (2017).
This evaluation was subject to several limitations. During 2011–2013, one large academic center (in Maryland’s Central Region) that performs HIV DRT had not established electronic laboratory reporting. A review of reports to MHS for June-December 2017 showed that 8.2% of sequences came from this lab,. suggesting that their lack of reporting during 2011–2013 resulted in missing data. Additionally, in March 2012, HHS recommended starting ART for all persons living with HIV, regardless of CD4 count (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2012). Thus provider practices regarding the timing of DRT during the analysis period might not reflect current practices. Finally, insufficient funding during 2011–2012 might have reduced MDH’s capacity to collect and analyze data, even if providers were routinely ordering DRT.
In summary, during 2011–2013, HIV nucleotide sequence ascertainment was low in Maryland, particularly among foreign-born persons and those with higher CD4 counts who live outside the Central Region. Interventions to increase DRT in these populations (particularly at entry to care) and sequence reporting might improve data completeness. Interventions might include social media or educational campaigns targeting patients and providers at Federally Qualified Health Centers where foreign-born persons are more likely to receive care or provision of resources to improve electronic laboratory reporting. Increased data linkage and sharing with neighboring jurisdictions might improve reporting for out-of-state care. Additionally, DRT use as a quality indicator for reimbursement might incentivize provider ordering and provider and laboratory reporting. Resulting improvements in sequence ascertainment can increase understanding of drug resistance and HIV transmission patterns and might ultimately lead to reduced HIV transmission.
Acknowledgments
The authors gratefully acknowledge the valuable contributions of the following persons to this manuscript: Andrea Winquist, Harold Boykin, Jami Stockdale, Molly Gribbin, Lucy Wilson, and Alexa Oster.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Althoff KN, Rebeiro P, Brooks JT, Buchacz K, Gebo K, Martin J, … North American Aids Cohort Collaboration on Research Design. (2014). Disparities in the quality of HIV care when using U.S. Department of Health and Human Services Indicators. Clinical Infectious Diseases, 58, 1185–1189. doi: 10.1093/cid/ciu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). Terms, definitions, and calculations used in CDC HIV surveillance publications. Retrieved from https://www.cdc.gov/hiv/statistics/surveillance/terms.html
- Centers for Disease Control and Prevention and Council of State and Territorial Epidemiologists. (2015). Technical guidance for HIV surveillance programs: molecular HIV surveillance. [Google Scholar]
- Dasgupta S, Hall HI, Hernandez AL, Ocfemia MCB, Saduvala N, & Oster AM (2017). Receipt and timing of HIV drug resistance testing in six U.S. Jurisdictions. AIDS Care, 1–8. doi: 10.1080/09540121.2017.1316356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Oster AM, Li J, & Hall HI (2016). Disparities in consistent retention in HIV care - 11 states and the District of Columbia, 2011–2013. MMWR Morbidity and Mortality Weekly Report, 65, 77–82. doi: 10.15585/mmwr.mm6504a2 [DOI] [PubMed] [Google Scholar]
- France AM, Panneer N, Wertheim JO, Ocfemia MCB, Hernandez AL, & Oster AM (2016). Identifying growing clusters of recent, rapid HIV transmission to target prevention. Oral presentation presented at the International HIV Transmission Workshop, Chicago, IL. [Google Scholar]
- Grieco E (2004). Health insurance coverage of the foreign born in the United States: Numbers and trends. Retrieved from http://www.migrationpolicy.org/research/health-insurance-coverage-foreign-born-united-states-numbers-and-trends
- Horberg MA, Hurley LB, Klein DB, Towner WJ, Kadlecik P, Antoniskis D, … Silverberg MJ (2015). The HIV care cascade measured over time and by age, sex, and race in a large National Integrated Care System. AIDS Patient Care and STDs, 29, 582–590. doi: 10.1089/apc.2015.0139 [DOI] [PubMed] [Google Scholar]
- Horberg M, Hurley L, Towner W, Gambatese R, Klein D, Antoniskis D, … Johnson M (2011). HIV quality performance measures in a large integrated health care system. AIDS Patient Care and STDs, 25, 21–28. doi: 10.1089/apc.2010.0315 [DOI] [PubMed] [Google Scholar]
- Ku L (2009). Health insurance coverage and medical expenditures of immigrants and native-born citizens in the United States. American Journal of Public Health, 99, 1322–1328. doi: 10.2105/AJPH.2008.144733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster AM, Wertheim JO, Hernandez AL, Ocfemia MC, Saduvala N, & Hall HI (2015). Using molecular HIV surveillance data to understand transmission between subpopulations in the United States. Journal of Acquired Immune Deficiency Syndromes, 70, 444–451. doi: 10.1097/QAI.0000000000000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster AM, Xu S, Peters PJ, Galang R, Patel M, Switzer W, … Hall HI (2016, February). Identifying growing HIV clusters among persons who inject drugs - United States. Oral and poster presentations presented at the International HIV Drug Resistance Workshop, Boston, MA. [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2006). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL000629.pdf [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2007). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL000721.pdf [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2008). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL000988.pdf [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2009). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001561.pdf [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2012). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003093.pdf. [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. (2015). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Retrieved from https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003400.pdf [Google Scholar]
- Schneider E, Whitmore S, Glynn MK, Dominguez K, Mitsch A, & McKenna MT (2008). Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years – United States, 2008. MMWR Morbidity and Mortality Weekly Report, 57(RR10), 1–8. [PubMed] [Google Scholar]
- Smith DM, May SJ, Tweeten S, Drumright L, Pacold ME, Kosakovsky Pond SL, … Little SJ (2009). A public health model for the molecular surveillance of HIV transmission in San Diego, California. AIDS, 23, 225–232. doi: 10.1097/QAD.0b013e32831d2a81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamer M, Richard C, Casebeer AW, & Ray NF (1997). Health insurance coverage among foreign-born US residents: The impact of race, ethnicity, and length of residence. American Journal of Public Health, 87, 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiserri RO, Forsyth AD, Yakovchenko V, & Koh HK (2013). Measuring what matters: Development of stan-dard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Reports, 128, 354–359. doi: 10.1177/003335491312800504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, & Berry SA (2012). Comparing (HIV Research Network) different measures of retention in outpatient HIV care. AIDS, 26, 1131–1139. doi: 10.1097/QAD.0b013e3283528afa [DOI] [PMC free article] [PubMed] [Google Scholar]