Abstract
BACKGROUND:
Oral chronic graft-versus-host disease (cGvHD) impairs oral function and patients’ quality of life. Some lesions are refractory to local and systemic immunosuppressive therapy, and new therapeutic modalities are required. The aim of the study was to assess the efficacy and safety of topical application of autologous platelet gel (PG) in patients with oral cGvHD.
STUDY DESIGN AND METHODS:
PG was prepared from autologous blood and applied on ulcerous lesions using an automated system. The oral cGvHD was assessed using the 273-point Oral Mucositis Rating Scale (OMRS) prior and after completion of the PG treatment. The overall response to treatment of particular topography expressed as the total score on OMRS was compared to total score on National Institutes of Health cGvHD Oral Mucosal Score (NIH OMS). The pain intensity was measured by the Numeric Pain Rating Scale (NRS).
RESULTS:
In five patients, 12 autologous blood collections were performed; median 3 (range 1–3) per patient, and 26 PG applications were performed; median 6 (range 2–8) per patient. PG applications reduced lesions in oral cGvHD: median OMRS total score was reduced for 43.2% (range 9.6%−47.3%), and median NIH OMS total score for 27.3% (range 20.0%−50.0%) from baseline values. Median of pain intensity reduction on NRS scale was 57.1% (range 50%−100%). No side effects were observed.
CONCLUSION:
Application of autologous PG in oral cGvHD showed as an efficient and safe treatment option for patients who do not respond to standard local treatment.
Chronic graft-versus-host disease (cGvHD) is a serious late immunologic complication of allogeneic hematopoietic stem cell transplantation (alloHSCT) that affects many organs and tissues. The oral cavity is the second most commonly affected organ, with a wide variety of lesions that contribute to significant morbidity and late complications that heavily impair oral function and quality of life.1 In 2005, the National Institutes of Health Consensus Working Group for Diagnosis and Staging of cGvHD standardized the criteria for the diagnosis of oral cGvHD,2 which were revised in 2014.3 For the quantitative measurement of oral cGvHD, several scoring scales have been proposed by NIH Consensus criteria based on clinical presentation and functional impact, designed for clinical use at clinical visits and for research purposes.4 For oral cGvHD activity scoring by dental or oral medicine specialists in research settings, a 273-point Oral Mucositis Rating Scale (OMRS) is recommended, which rates seven oral mucosa changes on 13 intraoral locations on a scale of 0 to 3, with final score ranging from 0 to 273.4,5 For scoring of treatment response by transplant clinicians in clinical trials the NIH cGvHD Oral Mucosal Score (NIH OMS) was developed and validated with scores ranging from 0 to 15.1,6,7
In addition to the systemic therapy, topical corticosteroid application is a mainstay of oral lesion treatment, although it is often accompanied by numerous side effects.4 Since some oral lesions are refractory even to the combination of systemic and local immunosuppressive therapy, new therapeutic modalities are required. Proven biological properties of platelets in tissue healing and regeneration through the release of potent growth factors and cytokines have made platelet derivatives a very popular biological therapy in many fields of medicine and dental medicine.8,9 Picardi and colleagues have shown that platelet gel (PG) prepared from allogeneic blood may be used as a safe and effective tool in the management of skin and oral lesions related to acute as well as chronic GvHD.10 The aim of this study was to assess the feasibility, efficacy, and safety of the topical application of autologous PG in the management of cGvHD oral ulcers. The current study reports the use of autologous blood as a source of PG in the treatment of oral cGvHD assessed by NIH criteria.
MATERIALS AND METHODS
A multidisciplinary team for cGvHD was established at the University Hospital Center in Zagreb, Croatia, in 2013 to study patients according to the NIH Consensus Criteria.11 Until January 2017, five patients with oral cGvHD ulcerous lesions resistant to standard oral treatment were treated with autologous PG. The protocol was approved by the Ethics Committee of University Hospital Center Zagreb and School of Medicine, University of Zagreb, and all participants signed informed consent. The clinical diagnosis of oral cGvHD was established according to 2005 NIH Consensus criteria, as well as the use of the OMRS and OMS NIH scoring.2–4,6 A detailed medical history was taken, and clinical examination and laboratory testing were performed to evaluate whether patients fulfilled the criteria for autologous blood donation.12 The platelet count should be ≥150 × 109/L. Serologic tests for bloodborne pathogens hepatitis B, hepatitis C, and human immunodeficiency virus were carried out. Microbiology assessment of oral mucosa was performed to exclude infection with Pseudomonas aeruginosa, which was considered a contraindication for platelet derivative application.13 In case Pseudomonas, Klebsiella, or Enterococcus was detected, the application was postponed until the infection was eradicated.13,14 As some growth factors are influenced by the lower pH,15 oral mucosa pH was tested with pH Test Papers (Macherey-Nagel GmbH & Co.) before treatment.
Activity of oral cGvHD was assessed on the 273-point OMRS before each application and upon completion of the treatment.4,5,16 Oral lesions were coded according to World Health Organization (WHO) topography17 and assessed with the same 273-point OMRS to enable measurement of clinical changes on a particular oral location.
Platelet gel was produced and applied using Vivostat system (Vivostat A/S) devices, as described by Picardi and colleagues10 In a closed-system platelet-rich fibrin (PRF) preparation kit, 120 mL of autologous blood was drawn and processed automatically in the Vivostat system. A total dose of 6 mL of platelet concentrate was prepared. Depending on the extension and localization of the lesions, the whole dose of PG was applied at once, or using the Vivostat Split Kit divided into two or three equal parts. One aliquot was applied fresh, and others were frozen and stored with the assistance of the transfusion service at −80°C for the following applications.
Before application, the frozen aliquots were thawed at room temperature for 40 minutes. A Vivostat applicator unit and Spraypen application device were used for PG application. If necessary, the debridement of the lesion by standard oral care modalities was performed to remove the mucous saliva layer prior to PG application.18 PG was delivered to lesions in liquid form and solidified on contact with tissue, covering the surface of the lesion. Treatment response was evaluated qualitatively on the basis of lesion size reduction, the appearance of granulation tissue, and pain assessment. For the quantitative evaluation of overall therapeutic response (complete or partial), OMRS and NIH OMS were used. In cases of total reepithelization of the lesion, the response was defined as complete and partial when islets of granulation tissue appeared on the surface of ulcerous lesion covered with pseudomembrane. The primary endpoint was to compare the total score response for a particular topographic site on the 273-point OMRS to the total NIH OMS score. The pain intensity was measured according to Numeric Pain Rating Scale (NRS) before and after PG application.19
RESULTS
Five patients with severe global NIH cGvHD and multiple oral ulcerous lesions were treated with topical application of autologous PG. Patients’ characteristics are presented in Table 1. All patients had diagnostic signs of oral cGvHD: two had severe and three moderate oral cGvHD as measured on NIH OMS. At the time of enrollment, patients used no oral local immunosuppressive or other local therapy. Systemic immunosuppressive therapy is presented in Table 1. Patient 5 was without systemic immunosuppressive therapy in order to maintain the graft-versus-leukemia effect and to avoid the next relapse of the disease.
TABLE 1.
Patient characteristics and treatment results
| Patient no. (sex/age, yrs) | Diagnosis | AlloHSCT (yr) HSC source, donor type | Systemic immune- suppressive therapy | Oral cGvHD manifestations | Topography of oral mucosa*18 | Number of blood collections/ number of applications | OMRS score prior/after PG for each oral topographic site | Lesions assessment scales prior/after PG (% reduction) | Pain assessment NRS scale20 prior/ after PG (% reduction) | |
|---|---|---|---|---|---|---|---|---|---|---|
|
OMRS4,5 |
NIH OMS6 |
|||||||||
| 1. (F/59) | OMF | First 2012. PBSC, MUD, graft rejection; Second 2012. PBSC, same MUD | CSP, methylprednisolone | Atrophy, pseudo- membrane, erythema, lichen planus–like changes, ulcers | 19; 20; 17 | 3/8 | 11; 9; 6/9; 6; 7 | 73/66 (9.6%) | 13/9 (30.8%) | 7/3 (57.1%) |
| 2. (M/61) | AML | 2013. PBSC, MUD | CSP | Atrophy, erythema, lichen planus changes, mucocele | 20; 53; 54 | 1/3 | 8; 5; 8/9; 2;3 | 36/19 (47.2%) | 10/8 (20.0%) | 7/0 (100%) |
| 3. (F/58) | AA | 2014. PBSC, MRD | CSP, methylprednisolone | Atrophy, pseudo- membrane, erythema, lichen planus–like changes, ulcers | 13; 14 | 3/6 | 11; 10/9; 3 | 86/50 (41.9%) | 13/10 (23.1%) | 4/2 (50%) |
| 4. (M/27) | CML Ph+ | 2013. PBSC, MUD | Methylprednisolone, CSP, MMF | Atrophy, pseudo- membrane, ery- thema, lichen planus–like changes, ulcers | 19; 20; 17 | 3/7 | 8; 7; 5/9; 3; 4 | 37/21 (43.2%) | 11/8 (27.3%) | 8/4 (50%) |
| 5. (F/49) | AML | First 2015. PBSC MRD 2015. DLI 3×, relapse; Second 2016. PBSC, MUD, 2016 DLI | None | Atrophy, erythema, lichen planus–like changes, ulcers | 15; 19; 40 | 2/2 | 6; 8; 9/2; 5; 7 | 38/20 (47.4%) | 6/3 (50.0%) | 7/3 (57.1%) |
WHO oral topography codes by Roed-Petersen and Roenstrup: vermilion border–upper (13), lower (14), labial commissures–right (15), labial mucosa–upper (17), buccal mucosa–right (19), left (20), dorsum of the tongue–right (39), left (40), soft palate–right (53), left (54).
AA= aplastic anemia; AML =acute myeloid leukemia; CML =chronic myeloid leukemia; CSP =cyclosporine; DLI =donor lymphocyte infusion; F =female; M =male; MMF =mycophenolate mofetil; MRD =matched related donor; MUD = matched unrelated donor; OMF = osteomyelofibrosis; PBSC = peripheral blood stem cells.
The median time from alloHSCT to oral ulcer onset was 15 months (range 5–26 months), and the median duration of lesions before PG application was 40 days (range 11–180 days). All patients fulfilled the criteria for autologous blood donation.12 No clinical or laboratory signs of infection were present.
A total of 12 autologous blood collections were performed, with a median of 3 (range 1–3) per patient, without any side effects. Venous access was obtained through peripheral veins, and all patients tolerated repeated phlebotomies well. The interval between two blood collections was 2 to 4 weeks.
The treatment results are presented in Table 1. A total of 26 PG applications were performed, with a median of 6 (range 2–8) per patient. The median interval between applications was 7 days (range 1–21 days). The oral mucosal pH was measured before the first application, and pH was found in the range of acidic (pH < 6). During PG treatment, reduction of lesions was observed. The pseudomembranes became thinner and interspersed with islets of granulation tissue. The erythema around the edges intensified as a sign of the tissue response, and the ulcer area was reduced. Expansion of treated lesions was not found. The oral lesions covered with mucous saliva healed slower due to mechanical barrier and lower pH. The partial response with decrease in NIH OMS score of 2 to 4 points was observed in all five patients. A complete response with resolution of lesions was observed in Patient 3 (Fig. 1) and Patient 5. Median OMRS scores before and after treatment were 38 (range 36–86) and 21 (range 19–66), respectively, with 43.2% (range 9.6%−47.4%) reduction. Median NIH OMS scores before and after treatment were 7 (range 4–8) and 3 (range 0–4), respectively, with 27.3% (range 20%−50%) reduction. The pain had decreased since the first application in all patients. The median pain intensity according to NRS scale before and after treatment was 7 (range 4–8) and 3 (range 0–4), respectively, with 57.1% (range 50%−100%) reduction.
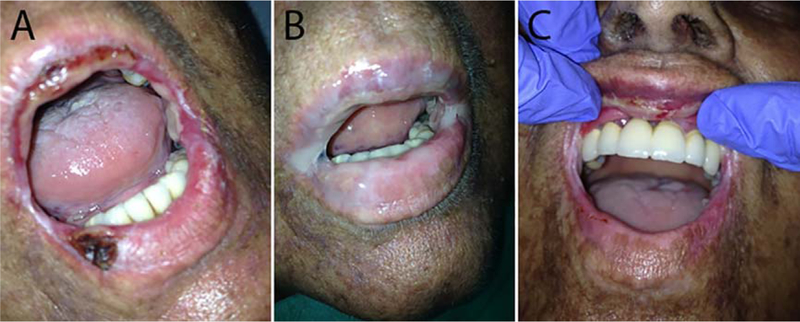
Fig. 1.
Hemorrhagic ulcer on lower labial mucosa (Patient 3). (A) Before first platelet gel application (oral signs of cGvHD: OMRS = 86; NIH OMS = 13). (B) Platelet gel application on both lips. (C) Complete regression of lower lip lesions 23 days after third platelet gel application (oral signs of cGvHD: OMRS = 50; NIH OMS = 10).
No side effects were observed during application. The main inconvenience was the restriction on eating and drinking for at least 2 hours after application to prevent removal of the PG from the lesions. The median follow-up was 14 months (range 6–31 months). In four patients, global cGvHD disease was stable during the follow-up, except the one in whom lung cGvHD developed. In two patients with complete response, no recurrence of oral ulcers was observed.
DISCUSSION
This study reports the successful use of autologous blood as a source of PG in the treatment of five patients with oral cGvHD defined by NIH criteria. Oral cGvHD activity was assessed according to the 273-point OMRS and severity by the NIH OMS, which provided the opportunity to quantitatively evaluate treatment results with PG using contemporary standard criteria.
Platelet derivatives that contain abundant growth factors have been widely used in different areas of medicine due to their regenerative, anti-inflammatory, and analgesic properties.13,20,21 The antimicrobial effect was proved against most bacteria except Klebsiella, Enterococcus, and Pseudomonas.13,14 Since some growth factors are influenced by the decreased pH of the tissue, the acidic surface of oral mucosa and the saliva may decrease the effect of platelet derivatives.15
Platelet derivatives can be produced by various techniques, either in a blood bank environment with use of good manufacturing practice, or in a clinical point of care, with use of automatic devices.20 They vary in their structure and composition, and observed variation in therapeutic efficacy may result from the lack of standardized preparation and application protocol and in proper use of terminology. In order to provide an objective assessment of treatment results, they are classified depending on fibrin density and WBC content into four categories, and Vivostat PRF is classified as pure platelet-rich plasma gel.22
The literature search on the platelet derivatives found no evidence of systemic or local effects that might limit their use if the risk of infections related to contamination during collection and preparation was excluded.8 In our study, all patients responded well to the treatment, without systemic or local adverse reactions. The pain reduction was observed even after the first application and the healing of the lesions began. The number of required applications was related to the extent of the lesions, and usually more than one application was necessary. Depending on the number of applications, the results were in the range of partial to complete healing response, with improved oral intake and quality of life. This study used NIH criteria for the assessment of therapeutic response, as well as the NRS for pain rating, which enables the reproducibility and comparison of study results.4,16,19
Nowadays, autologous platelet derivatives are preferred to allogeneic, mainly due to immunocompatibility, no risk of cross infections, and easy availability; only when autologous cells have been limited or unavailable have allogeneic cells been used.20 However, Picardi and colleagues used PG produced from allogeneic blood to treat mucosal and skin lesions caused by acute or chronic GvHD, and excluded the patients as donors for autologous gel preparation because of their underlying hematologic disease, which could impair the efficacy of the products.10 The current study showed, however, that even patients with hematologic diseases can be candidates for autologous PG treatment when they fulfill the criteria for autologous blood donation, considering blood counts and absence of acute infections, and ability to tolerate repeated phlebotomy, blood loss, and the resultant hematological and cardiovascular challenges. Results presented here demonstrate that autologous blood donation is safe even in patients with active cGvHD, and no blood transfusion was needed after donation.
Other studies also report on successful use of autologous blood derivatives in cGvHD patients. Del Fante and colleagues used autologous platelet lysate mucoadhesive formulation in the treatment of oral cGvHD, and they used allogeneic blood only in patients with thrombocytopenia.23 Pezzotta and coworkers used autologous platelet lysate drops for treatment of ocular cGvHD.24 Tahmaz and colleagues demonstrated that the application of autologous serum eye drops in patients with ocular cGvHD was safe and efficient with no side effects in treatments lasting 6 months, although they collected 300 to 450 mL of autologous blood, which was twice the volume collected in our patients.25
Although the sample size is small, this study supports autologous PG being a promising treatment option for oral cGvHD in patients who do not respond to standard treatments. Other authors have considered autologous blood as an inadequate source for platelet derivatives; however, this study demonstrated that autologous PG is efficient, feasible, and safe even in patients with cGvHD. The measurement of treatment response per standardized NIH criteria is essential for quantitative and reproducible assessment of therapeutic outcome. In the future, randomized prospective studies at larger patient cohorts are necessary to further examine the effectiveness of autologous PG in oral cGvHD.
ACKNOWLEDGMENTS
We would like to thank many members of the multidisciplinary team for cGvHD at the University Hospital Center Zagreb, Croatia.
This work was supported by the Unity Through Knowledge Fund project entitled “Clinical and Biological Factors Determining Severity and Activity of Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation.” This work was also supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
SZP is an employee of the US government, and as such this work was done in that capacity. The views expressed do not necessarily represent the views of the National Institutes of Health or the US government.
ABBREVIATIONS:
- alloHSCT
allogeneic hematopoietic stem cell transplantation
- cGvHD
chronic graft versus host disease
- NIH OMS
National Institutes of Health cGvHD Oral Mucosal Score
- NRS
Numeric Pain Rating Scale
- OMRS
Oral Mucositis Rating Scale
- PG
platelet gel
- PRF
platelet-rich fibrin.
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Bassim CW, Fassil H, Mays JW, et al. Validation of the National Institutes of Health chronic GvHD oral mucosal score using component-specific measures. Bone Marrow Transplant 2014;49:116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945–56. [DOI] [PubMed] [Google Scholar]
- 3.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mays JW, Fassil H, Edwards DA, et al. Oral chronic graft-versus- host disease: current pathogenesis, therapy, and research. Oral Dis 2013;19:327–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert MM, Williams BE, Lloid ME, et al. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplant: development of an oral mucositis index. Cancer 1992;69:2469–77. [DOI] [PubMed] [Google Scholar]
- 6.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant 2006;12:252–66. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant 2015;21:984–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Pascale MR, Sommese L, Casamassimi A, et al. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev 2015;29:52–61. [DOI] [PubMed] [Google Scholar]
- 9.Cohn CS, Lockhart E. Autologous platelet-rich plasma: evidence for clinical use. Curr Opin Hematol 2015;22: 527–32. [DOI] [PubMed] [Google Scholar]
- 10.Picardi A, Lanti A, Cudillo L, et al. Platelet gel for treatment of mucocutaneous lesions related to graft-versus-host disease after allogeneic hematopoietic stem cell transplant. Transfusion 2010;50:501–6. [DOI] [PubMed] [Google Scholar]
- 11.Pulanic D, Desnica L, Vrhovac R, et al. Chronic graft-vs-host disease in 2016: a major challenge and an opportunity. Croat Med J 2016;57:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Directorate for the Quality of Medicines & Health- Care, Council of Europe. Guide to the preparation, use and quality assurance of blood components. Principles of autologous transfusion 19th ed. Strasbourg: European Directorate for the Quality of Medicines & HealthCare, Council of Europe; 2017. p. 193–8. [Google Scholar]
- 13.Drago L, Bortolin M, Vassena C, et al. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielecki TM, Gazdzik TS, Arendt J, et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br 2007;89:417–20. [DOI] [PubMed] [Google Scholar]
- 15.Wahlstrom O, Linder C, Kalen A, et al. Acidic preparations of platelet concentrates release bone morphogenetic protein-2. Acta Orthop 2008;79:433–7. [DOI] [PubMed] [Google Scholar]
- 16.Baird K, Steinberg SM, Grkovic L, et al. National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant 2013;19:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer IR, Pindborg JJ, Bezroukov V, et al. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Community Dent Oral Epidemiol 1980;8:1–26. [DOI] [PubMed] [Google Scholar]
- 18.Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med 2010;83:1–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Farrar JT, Young JP Jr., LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- 20.Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus 2016; 25:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterman C, McCarthy MB, Cote MP, et al. Platelet-rich plasma increases anti-inflammatory markers in a human coculture model for osteoarthritis. Am J Sports Med 2015;43: 1474–84. [DOI] [PubMed] [Google Scholar]
- 22.Dohan Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 2014;4:3–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Del Fante C, Perotti C, Bonferoni MC, et al. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS PharmSciTech 2011;12:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pezzotta S, Del Fante C, Scudeller L, et al. Long-term safety and efficacy of autologous platelet lysate drops for treatment of ocular GVHD. Bone Marrow Transplant 2017;52:101–6. [DOI] [PubMed] [Google Scholar]
- 25.Tahmaz V, Gehlsen U, Sauerbier L, et al. Treatment of severe chronic ocular graft-versus-host disease using 100% autologous serum eye drops from a sealed manufacturing system: a retrospective cohort study. Br J Ophthalmol 2017;101: 322–6. [DOI] [PubMed] [Google Scholar]