Abstract
Parenting is a critical factor in adolescent social-emotional development, with maladaptive parenting leading to risk for the development of psychopathology. However, the emotion-related brain mechanisms underlying the influence of parenting on psychopathology symptoms are unknown. The present study utilized functional magnetic resonance imaging and laboratory measures to examine sex-differentiated associations among parenting, adolescent emotion-related brain function, and substance use and psychopathology symptoms in 66 12–14 year olds. Maternal parenting behaviors (warmth, negative parenting) were observed in a laboratory task. Adolescent brain responses to negative emotional stimuli were assessed in emotion processing regions of interest (left [L] and right [R] amygdala, anterior insula, anterior cingulate cortex [ACC]). Adolescents reported on substance use and depressive, anxiety, and externalizing symptoms. Maternal negative parenting predicted adolescent brain activation differently by sex. For girls, negative parenting predicted heightened R ACC activation to negative emotional stimuli. For boys, negative parenting predicted blunted L and R anterior insula and L ACC activation. Furthermore, for girls, but not boys, heightened L anterior insula and heightened L and R ACC activation were associated with substance use and depressive symptoms, respectively. Findings suggest neural response to negative emotion as a possible sex-specific pathway from negative parenting to psychopathology.
Keywords: Parenting, Emotion, Adolescence, fMRI, Developmental Psychopathology
One of the most important influences on child and adolescent development is parenting behavior. Parenting is particularly critical in early adolescence, when youth begin significant emotional, cognitive, and social changes (Steinberg, 2001) and experience increases in substance use and some forms of psychopathology (Hankin et al., 1998; Johnston et al., 2018). Adaptive parenting during early adolescence may buffer youth from psychopathology. In contrast, maladaptive parenting (e.g., low warmth, high negative parenting) during early adolescence predicts longitudinal increases in substance use and internalizing (e.g., depression, anxiety) and externalizing symptoms into middle adolescence (e.g., Barnes, Riefman, Farrell, & Dintcheff, 2000; Pettit, Laird, Dodge, Bates, & Criss, 2001).
Despite the association between parenting and psychopathology, the neural mechanisms by which parenting impacts the development of psychopathology are unknown. Recently, researchers have hypothesized that maladaptive parenting affects the developing brain during childhood and adolescence, leading to alterations in social, emotional, and cognitive development and to psychopathology risk (Belsky & de Haan, 2010). However, little research has examined associations between parenting and adolescent brain function as these relate to psychopathology. The present study examined: 1) Associations between maternal parenting behaviors and early adolescents’ emotion-related neural responses, and 2) Associations between early adolescents’ emotion-related neural responses and substance use and psychopathology symptoms. Given sex differences in emotional reactivity (e.g., Chaplin & Aldao, 2013), we examined whether adolescent sex moderated these associations.
Parenting and Adolescent Emotion-Related Brain Function
Starting in early adolescence, there is significant development of structure, function, and connectivity of brain networks involved in emotional arousal (Casey, Jones, & Hare, 2008; Pfeifer & Allen, 2012). This period also may be a time of vulnerability of brain networks to external influence, including parenting (Teicher & Sansom, 2016). Indeed, animal studies find that experimental alterations in maternal care lead to alterations in offspring emotion-related brain function (Caldji, Diorio, & Meaney, 2003), and human behavioral studies find that maladaptive parenting predicts emotional arousal and regulation problems in children and adolescents (Morris, Silk, Steinberg, Myers, & Robinson, 2007).
Studies have found that a history of child abuse is associated with adolescent brain structure, including amygdala and hippocampus volume (e.g., Whittle et al., 2013). However, few studies have examined links between a range of non-abusive parenting behaviors and adolescent brain function. One study found that greater observed maternal negative emotion in a parent-adolescent interaction with 11–17 year olds was correlated with adolescents’ decreased amygdala, anterior insula, subgenual anterior cingulate cortex (ACC), and nucleus accumbens activation to a peer rejection task (Tan et al., 2014). A second study of older adolescents found that higher maternal self-reported authoritarian (harsh, punitive) parenting was associated with decreased caudate response to a peer rejection task for all adolescents and with decreased ventrolateral (vl)PFC response for adolescents with behavioral inhibition (Guyer et al., 2015). Third, a study of 9–16 year olds found that higher child-reported parental psychological over-control, but not lower parental warmth, was associated with lower insula response to an emotional conflict task (Marusak, Thomason, Sala-Hamrick, Crespo, & Rabinak, 2017). Fourth, Romund and colleagues (2016) found that lower adolescent-reported maternal warmth/support was correlated with higher amygdala response to negative emotional faces in 13–16 year olds. Studies also have found associations between maternal warmth and adolescent brain structure (e.g., Whittle et al., 2014) and reward processing (e.g., Morgan, Shaw, & Forbes, 2014). In sum, initial research suggests that parenting behavior is associated with altered adolescent emotion-related brain function. However, more research is needed, particularly examining associations with adolescent outcomes.
Emotion-Related Brain Function and Adolescent Substance Use and Symptoms
Emotional reactivity and regulation have been proposed as trans-diagnostic processes underlying substance abuse and psychopathology (Aldao, Nolen-Hoeksema, & Schweizer, 2010), with different emotion-related profiles associated with internalizing symptoms (e.g., depression, anxiety) versus externalizing symptoms (e.g., acting out behaviors) (Chaplin & Cole, 2005), and with substance use considered as either internalizing or externalizing. In response to negative emotional stimuli, humans engage an integrated network, including amygdala, anterior insula, ACC, and orbitofrontal cortex (OFC), as well as lateral PFC and striatal regions. The present study focuses on three key emotion processing regions that specifically support negative emotional arousal: amygdala, anterior insula, and ACC. The amygdala is involved in rapid processing of and reactivity to motivationally-relevant information including negative emotional stimuli, anterior insula in perception and interoceptive awareness of emotional (and other somatic) experiences, and ACC in regulating attention in the service of emotional and other responses (e.g., Lindquist, Wager, Kober, Bliss-Moreau, & Barrett, 2012).
For substance use, theorists have proposed that youth may show a pattern of either excessive emotional arousal or blunted emotional arousal, which may lead them to use substances to either relieve excessive negative emotion or to up-regulate blunted arousal (Donohew et al., 1999; Sinha, 2008). These two different pathways to substance use are consistent with substance use representing either an internalizing or externalizing problem. There are few studies of neural emotion processing and substance use in adolescents. One found that decreased amygdala, ACC, hippocampus, and OFC responses to stress imagery were associated with illicit drug use in middle adolescents who were prenatally cocaine exposed (Yip, Lacadie, Sinha, Mayes, & Potenza, 2016). A second found that increased amygdala response to negative emotion faces was associated with greater problem drinking in late adolescent college students (Nikolova, Knodt, Radtke, & Hariri, 2016). Thus, either blunted or heightened emotion-related brain activation may be associated with substance use.
Heightened neural activation in emotional arousal-related brain regions to negative emotional stimuli is theorized to contribute to internalizing disorders. Specifically, theories propose that depressive symptoms are linked to higher amygdala, ACC (especially ventral ACC), anterior insula, and OFC and blunted dorsolateral [dl] PFC activation (Mayberg, 1997). Empirically, depressive symptoms have been associated with heightened amygdala activation and altered (sometimes higher, sometimes lower) insula, ACC (both ventral ACC and full ACC), and OFC activation to negative emotional stimuli in early through late adolescence (e.g., Hall et al., 2014; Tao et al., 2012; Yang et al., 2010). Adolescent anxiety symptoms have been correlated with heightened amygdala reactivity to fear faces and heightened amygdala and vlPFC activation to threatening faces (e.g., Killgore & Yurgelon-Todd, 2005; Monk et al., 2006).
Altered emotion processing has also been implicated in externalizing symptoms (Jones, Laurens, Herba, Barker, & Viding, 2009). Empirical studies have found that some adolescents with externalizing symptoms (especially those with callous-unemotional traits) show decreased amygdala and ACC activation to negative emotional stimuli (e.g., Jones et al., 2009; Stadler et al., 2007).
Sex Differences in Brain Pathways from Parenting to Adolescent Adjustment
Adolescent sex is important to consider in understanding emotion-related pathways from parenting to substance use and psychopathology. First, girls and boys show different courses and correlates of substance use (Amaro, Blake, Schwartz, & Flinchbaugh, 2001). Second, there are sex differences in depressive symptoms (girls > boys) in adolescence and in anxiety symptoms (girls > boys) and externalizing symptoms (boys > girls) in childhood (Hankin et al., 1998; Kimonis, Frick, & McMahon, 2014; Lewinsohn, Gotlib, Lewinsohn, Seeley, & Allen, 1998). Third, there are sex differences in emotional arousal, with girls expressing negative emotions (especially sadness and fear) more intensely than boys (Chaplin & Aldao, 2013), and women showing greater neural responses to negative emotional stimuli than men (Stevens & Hamann, 2012). This sex difference may be due to biologically-based differences, different socialization of boys versus girls, or both (Chaplin & Aldao, 2013).
We propose that there may be sex differences in emotion-related neural pathways from parenting to psychopathology (see Figure 1). Maladaptive parenting may lead to altered neural responding to negative emotional stimuli, differently for girls versus boys. For girls, given their tendency to allow negative emotions, maladaptive parenting may lead to heightened activation in emotion-related brain networks to negative emotional stimuli, leading girls to risk for internalizing symptoms and for using substances to down-regulate negative arousal. For boys, given their tendency to limit negative emotions, maladaptive parenting may lead to blunted neural activation to negative emotional stimuli, leading boys to engage in substance use and acting-out/risky behaviors to up-regulate arousal. Supporting this, Potenza and colleagues (2012) found that heightened emotion-related brain activation was associated with cocaine dependence in women, but not men. Another study found that prenatal cocaine exposure (and associated maladaptive parenting) predicted heightened emotional responses to stress for adolescent girls, but blunted salivary alpha amylase (sAA) responses for adolescent boys, and that heightened emotional responses predicted substance use for girls and blunted sAA responses predicted substance use for boys (Chaplin et al., 2015).
Figure 1.
Conceptual model. Hyp is an abbreviation for hypothesis.
The Present Study
The present study used laboratory and fMRI methodology to examine associations among observed maternal parenting behaviors, adolescent neural responses to negative emotional stimuli, and substance use and psychopathology symptoms in early adolescents. Because youth are just beginning to use substances and develop psychopathology in early adolescence, our study examined neural markers before the brain is affected by entrenched substance use/psychopathology. We used an observational measure of parenting behaviors to tap in-the-moment behavior that may be outside of parents’ awareness. We limited the study to mothers, consistent with prior studies, to limit variability due to parent sex.
We hypothesized that maladaptive parenting (lower warmth, higher negative parenting) would interact with adolescent sex to be associated with adolescents’ responses to negative emotional stimuli in three regions of interest (ROIs) (amygdala, anterior insula, ACC), with maladaptive parenting associated with heightened ROI activation to negative emotional stimuli for girls and blunted activation for boys. Second, we hypothesized a brain activation by sex interaction such that heightened activation in the ROIs to negative emotional stimuli for girls and blunted activation for boys would be linked to substance use, internalizing (depressive, anxiety), and externalizing symptoms.
Method
Participants
Participants were sixty six 12–14 year olds (34 boys [sex was determined by parent report]; mean age = 12.59, SD = 0.70) and their mothers, recruited from a larger behavioral study to participate in this MRI study. Families were recruited for the larger study through mailings to households in a suburban area in the mid-Atlantic United States to participate in a study of sex, emotion, and adolescent development. Inclusion criteria for this MRI study was age 12–13 years for adolescent at time of recruitment (8 youth turned 14 before the MRI session), IQ >= 80 for adolescent (on Wechsler Abbreviated Scale of Intelligence), adequate English proficiency to complete questionnaires for adolescent and mother, and MRI safe for adolescent (e.g., no metal in body). Exclusion criteria were history of prenatal substance exposure, autism spectrum disorder, psychotic disorder, congenital brain defect, or traumatic brain injury for adolescent and current psychiatric medication use for adolescent (due to effects of psychiatric medication on brain function), which were assessed by parent report. The first eighty one adolescents from the larger study (N = 249) who met criteria and were interested and MRI safe participated in this MRI study. Of these, 15 were excluded due to inability to complete the MRI scan (n = 9) or excessive head motion during the fMRI emotion task (n = 6), leaving a final N of 66. The 66 adolescents were not significantly different from the 15 who were excluded on demographics, parenting behavior, or substance use/psychopathology symptoms.
Race/ethnicity was representative of the community, with 71.2% non-Hispanic White, 9.1% Hispanic, 10.6% more than one race, and 9.0% Asian, African-American or Other. Consistent with the local community, family income level was mostly (77%) greater than or equal to $100,000 per year. Mothers were 95% biological mothers and 5% non-biological [e.g., adoptive] mothers.
Procedure
Families attended three sessions. In the first, adolescents completed questionnaires, interviews, and breath/urine screens assessing demographics, substance use, and psychopathology symptoms. In the second, the adolescent and mother completed the parent-adolescent interaction task (PAIT). In the third, adolescents completed the MRI scan. The three sessions were scheduled about 1–2 weeks apart. For 4 adolescents, fMRI sessions were delayed 4–6 months due to adolescents using orthodontic braces. For those adolescents, substance use and psychopathology measures were taken again at the same time as the fMRI session and those measures used in analyses. Study procedures were approved by the University Institutional Review Board. Informed parental consent and adolescent assent were obtained.
Laboratory Parent-Adolescent Interaction Task (PAIT)
Upon arriving for the second session, adolescents and mothers went to separate rooms and each completed the Issues Checklist (IC; Prinz, Foster, Kent, & O’Leary, 1979), a checklist of common family conflict topics (e.g., cleaning bedroom). Participants endorsed topics discussed in the past month and the anger level they felt during the discussion. After completing the IC, there was a 30-minute adaptation period during which participants listened to two 5-minute relaxation audio recordings and were told to relax quietly.
Following this, the mother was brought into the adolescent’s room and was seated next to the adolescent. The dyad engaged in two challenging 10 minute discussions (a conflict discussion and a discussion about drug use), with discussion order randomly assigned (there were no discussion order effects on parenting behaviors). The discussions were video-recorded and later coded for parenting behaviors. In the conflict discussion, the mother and adolescent discussed their mutually highest-rated conflict issue from the IC. In the drug use discussion, the adolescent and mother were asked to “discuss the topic of using alcohol, tobacco, marijuana, or any other drug for 10 minutes” (Boone & Lefkowitz, 2007). If the dyad finished either discussion early, they were asked to keep discussing the topic(s) for the full 10 minutes.
Observed parenting behaviors.
Maternal parenting behaviors during the two discussions were coded by trained coders using the PAIT Coding System (Chaplin, 2010). Maternal warmth (e.g., mother nods head, makes eye contact, praises adolescent) and negative parenting (e.g., mother criticizes, mocks, or, interrupts adolescent, uses harsh vocal tone) were coded, based on the parenting literature (e.g., Gottman, Katz, & Hooven, 1997). Coders viewed each discussion and rated each parenting behavior once for each 10 minute discussion on a scale from 1–5 (“none present” to “high level”), based on facial expressions, behaviors, vocal tone, and speech content. Ratings for the two discussions were summed. Twenty five percent of the videotaped interactions were randomly chosen, double-coded, and checked for inter-rater reliability. Intra-class correlation coefficients were moderate for warmth (ICC = 0.68) and were good for negative parenting (ICC = 0.77) (Koo & Li, 2016).
fMRI Session
Upon arrival, adolescents completed nine practice items for the emotion task on a computer outside of the scanner. They then completed the 60-minute MRI session, which included the emotion task, two other tasks, and a T1-weighted structural scan. Scans were acquired on a Siemens 3T Allegra MR scanner with a standard single-channel birdcage head coil.
Emotion task.
Adolescents viewed negative, neutral, and positive International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) pictures. The IAPS is a standardized method to evoke emotions by viewing pictures of emotionally-laden scenes. IAPS pictures have been found to elicit emotion-related brain activation in adolescents (McRae et al., 2012). We used the same negative IAPS pictures used in a prior study of adolescents, which were selected to be developmentally appropriate (McRae et al., 2012). We then selected developmentally-appropriate neutral and positive IAPS pictures matched to the negative pictures on subject type, color, and luminescence. IAPS pictures were presented using a rapid event-related design in a pseudo-randomized order, with trial order and timing determined with Optseq2 (Dale, 1999). A total of 81 trials (27 negative, 27 positive, 27 neutral) were presented across 3 runs of approximately 6.5 minutes each, with 27 trials per run and a balanced number of trial types per run. Each trial consisted of viewing a picture (4s), youth rating their intensity of negative emotion (2s) and positive emotion (2s) on a scale from 1 to 4 using a button box, and an inter-trial interval period (viewing crosshairs) jittered between 2s and 12s. Analyses focused on negative trials minus neutral trials. Both negative and neutral trials had the same rating period, thus the contrast allows for a focus on the difference between viewing negative vs viewing neutral pictures.
MRI Image acquisition.
Functional images of the hemodynamic response during the emotion task were collected using gradient-echo echoplanar images (GE-EPI) (TR/TE: 2250/30ms; flip = 70o; FOV: 192mm; matrix size: 64 × 64; 40 axial 3mm thick/1mm gap slices). For structural imaging, we acquired a whole-head anatomical scan using a T1-weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) pulse sequence (TR/TE = 2300/3ms; FOV = 260mm; matrix size = 256 × 256; 160 1mm thick slices).
MRI preliminary analyses.
MRI data was analyzed with FSL 5.0 (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012) and MATLAB (Mathworks, Natick, MA). Data were motion corrected, slice time corrected, and B0 unwarped. Runs with motion > 3mm at any TR in any direction were excluded (6 adolescents) except for 2 children who had 1–2 TR spikes of between 3 and 6mm. Those spikes were scrubbed with FSL motion outlier function. For analyses, we included motion correction parameters as regressors in first-level GLM analyses run in FEAT. Regressors for the timing of events of interest (viewing negative and neutral pictures) were created. These were convolved with a double gamma hemodynamic response function to create explanatory variables. Data were smoothed with a 6mm full width half maximum (FWHM) Gaussian kernel, and then linear regression was run at each voxel, using generalized least squares with a voxel-wise, temporally and spatially regularized autocorrelation model, drift fit with Gaussian-weighted running line smoother (96s FWHM). Data were co-registered to that participant’s MPRAGE image, and then to the MNI template.
Questionnaire/Interview Measures
Substance use.
Adolescent lifetime substance use was assessed in the first study session with a combination of: (1) self-report on the Youth Risk Behavior Survey 2011 National Version (YRBS; Brener et al., 2004), (2) interview with the Teen Addiction Severity Index (T-ASI; Kaminer, Bukstein, & Tarter, 1991), (3) urine screens with the TESTCUP5 for opiates, cocaine, THC, PCP, barbiturates and cotinine, and (4) breath screens with the Alcosensor III Intoximeter (for alcohol) and a CO monitor (for smoked tobacco). Youth were considered positive for substance use if they endorsed lifetime use of any substance (nicotine, alcohol, marijuana, inhalants, and others) on the YRBS or T-ASI or if they had a positive urine or breath screen. Thus, our substance use variable was a yes (lifetime use) vs no (no use) categorical variable. In the present study, 12 adolescents reported lifetime substance use (18.2%, 6 boys, 6 girls), consistent with national rates for early adolescents (e.g., Johnston et al., 2018) and prior studies of early adolescents (Chaplin et al., 2012; Kaplow, Curran, & Dodge, 2002). Although rates at this age are low, it is still important to examine predictors of substance use in early adolescence given that early substance use onset (<= age 14) has been shown to predict substance use disorders into adulthood (e.g., Chassin, Pitts, & Prost, 2002; Glied & Pine, 2002).
Depressive symptoms.
Adolescents completed the Children’s Depression Inventory (CDI, Kovacs & Staff, 2003), a widely used 27-item report of depressive symptoms in the previous 2-weeks. Items are scored from 0 to 2 and summed. In the present study, CDI scores ranged from 0 to 36 (M = 5.79, SD = 6.92), consistent with other community samples (Masip, Amador-Campos, Gómez-Benito, & del Barrio Gándara, 2010). Four youth (all girls) were in the clinical range (score >=19).
Anxiety symptoms.
Adolescents completed the Revised Children’s Manifest Anxiety Scale (RCMAS; Reynolds & Richmond, 1985), a widely-used 28-item self-report of anxiety symptoms. Items are scored from 0 to 1 and summed to create a total score. In the present study, RCMAS scores ranged from 0 to 23 (M = 6.53, SD = 5.38), somewhat lower than other community samples. Two youth (both girls) were in the clinical range (score >=19).
Externalizing symptoms.
Adolescent externalizing symptoms were assessed by parent-report on the Child Symptom Inventory (CSI, Gadow & Sprafkin, 2002), a widely-used measure with good psychometric properties. Symptoms are rated from 0 “never” to 3 “very often.” Total symptom severity scores ranged from 0 to 17 (M = 5.34, SD = 3.74) for Oppositional Defiant Disorder (ODD; 8 items) and from 0 to 5 (M = 0.75, SD = 1.12) for Conduct Disorder (CD; 15 items), consistent with or slightly higher than means from other community samples (Gadow, Nolan, Sprafkin, & Schwartz, 2002). CD and ODD scores were z-scored (to put them on the same scale) and summed to create a composite score for externalizing symptoms. Three youth (all boys) were in the clinical range (>=2 SDs above mean).
Covariate measures.
As a measure of parent substance use frequency, mothers reported on their use of alcohol, marijuana, and other illicit substances in the past 30 days on the Addiction Severity Index (ASI; McLellan, Luborsky, O’Brien, & Woody, 1980), a semi-structured interview. For our measure of parent substance use frequency, we took the maximum number of days using in past 30 days across alcohol, marijuana, and other substances (for all mothers, their max use was for alcohol or marijuana). Mean parent substance use frequency scores were 6.31 days per month (SD = 8.56), with a range from 0 to 30 days per month. Scores were skewed and so a square root transformation was used in analyses.
As a measure of family stress, adolescents reported on negative life events in the past year on the Negative Life Events Inventory (NLEI; Wills, Sandy, Yaeger, Cleary, & Shinar, 2001). We summed all of the family-related events to create a family stress score. The events in this score included martial conflict, divorce, death in family, serious illness in family, and family move(s) (items 1, 2, 3, 6, 6, 8, 10, 14, 18, and 19). Items were rated as 1 (happened in past year) or 0 (did not happen), thus total family stress scores could range from 0 to 10. Mean family stress scores were 1.29 (SD = 1.64), with a range from 0 to 8. Scores were skewed and so a square root transformation was used in analyses.
Results
Analysis Plan
ROI data.
Analyses focused on responses in ROIs (bilateral amygdala, anterior insula, and ACC). Data on whole brain activation to the emotion task are provided in Table 1. ROIs were created from FSL’s Harvard-Oxford atlas (for amygdala and anterior insula) and the Automated Anatomical Labels (Tzourio-Mazoyer et al., 2002) (for ACC) in MNI standard space. Follow-ups examined ACC sub-regions (dorsal [d]ACC, pregenual [pg]ACC, and subgenual [sg]ACC), created following Rotge et al. (2014). Coefficient of parameter estimate (COPE) values for negative and neutral trials were extracted and averaged across these subject-specific ROIs. We calculated the difference between negative trials (8 sec) minus neutral trials (8 sec) as our contrast of interest (following McRae e al., 2012). This allowed us to isolate BOLD response to negative pictures, controlling for BOLD response to images in general.
Table 1.
Peak Activation in the Emotion Task
| Contrast and Cluster Name | This cluster also includes: | Size (vx.) | Z max. | MNI X | MNI Y | MNI Z |
|---|---|---|---|---|---|---|
| 1. Negative >Neutral | ||||||
| Left Paracingulate Gyrus | Anterior Cingulate Cortex, Right Superior Frontal Gyrus | 2100 | 6.07 | 50 | 88 | 38 |
| Right Orbitofrontal Cortex | Right Temporal Pole, Right Anterior Middle Temporal Gyrus | 677 | 4.57 | 34 | 71 | 26 |
| Left Orbitofrontal Cortex | Left Temporal Pole | 423 | 4.82 | 59 | 69 | 24 |
| Right Cerebellum | 318 | 4.74 | 35 | 25 | 18 | |
| Left Cerebellum | 194 | 4.45 | 54 | 25 | 17 | |
| Left Amygdala | Left Hippocampus | 120 | 4.46 | 58 | 60 | 26 |
| 2. Positive >Neutral | ||||||
| Frontal Pole | Frontal Medial Cortex, Paracingulate Gyrus | 2341 | 4.61 | 47 | 94 | 36 |
Note. vx=voxel; MNI=Montreal Neurological Institute; max=maximum. FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Z statistic images were thresholded using clusters determined by Z >3.3 and a (corrected) cluster significance threshold of P=0.05 (Worsley 2001).
Covariates.
We did not covary race or age given our fairly homogenous sample. We considered including menstrual cycle phase, pubertal level, handedness (4 youth were left-handed), non-psychiatric medication use on fMRI day, parent substance use in past month and family stress in past year as covariates if they were correlated with any of the dependent variables. A description of parent substance use and family stress measures is in the Supporting Information file. Medication use was correlated with higher R anterior insula activation and higher depressive symptoms, parent substance use was correlated with higher L ACC activation, and family stress was correlated with substance use. Thus, these variables were covaried in analyses predicting these outcomes below (e.g., medication use was covaried in regressions predicting depressive symptoms).
Main analyses.
Main analyses were conducted in SPSS with extracted ROI scores. To test hypothesis 1 (that parenting would interact with adolescent sex to be associated with adolescents’ neural responses to negative emotional stimuli), 2 sets of regressions (one for warmth, one for negative parenting) were conducted. In these regressions, observed maternal parenting behavior (warmth or negative parenting), adolescent sex, and parenting behavior X sex interactions predicted negative (-neutral) ROI activation scores. In each set of regressions, 6 regressions were done, one for each ROI (L amygdala, R amygdala, L anterior insula, R anterior insula, L ACC, R ACC). False discovery rate (FDR; Benjamini & Hochberg, 1995) correction was used to correct the regression coefficient p-values from the 6 regressions. To test hypothesis 2 (that neural responses to negative emotional stimuli would interact with adolescent sex to be associated with adolescents’ substance use, internalizing, and externalizing symptoms), 4 sets of regressions were conducted (for substance use, depressive, anxiety, and externalizing symptoms) with negative (-neutral) ROI activation scores, sex, and ROI score X sex interactions predicting lifetime substance use vs. non-use (with logistic regression) and depressive, anxiety, and externalizing symptoms (with linear regression). In each set of regressions, 6 regressions were done (L amygdala, R amygdala, L anterior insula, R anterior insula, L ACC, R ACC). FDR correction was used to correct the regression coefficient p-values from the 6 regressions. Significant ACC findings were followed up with exploratory analyses for ACC sub-regions, with FDR correction. If there was an interaction with sex at an FDR-corrected p < .05, follow-up regressions were conducted for boys and girls separately. Simple slope plots were created for figures with PROCESS macro in SPSS 18.0 (Hayes, 2013).
Exploratory moderated mediation analyses.
When there was a significant parenting X sex effect on ROI activation and a significant ROI activation X sex effect on adolescent substance use/psychopathology symptoms for the same ROI, we conducted exploratory moderated mediation analyses using the PROCESS Macro for SPSS with process model 58 and 5,000 bootstrap samples. Notably, because data were all collected at the same time-point, this would not reflect true mediation, but may be useful information for future longitudinal studies.
Preliminary Analyses
Continuous variables were examined for outliers (values > 3 SDs above the mean). Maternal negative parenting, bilateral amygdala, anterior insula, and ACC ROI data, and depressive symptoms each had 1–2 outlier cases. These cases were winsorized (set to equal 3 SDs above the mean), consistent with prior research (Kertes & Gunnar, 2004). After winsorization, depressive symptoms were still skewed (skewedness > 1) and so square root transformed symptoms were used in analyses. Untransformed means are used in the tables and figure for ease of interpretation. One participant was missing externalizing symptom data and thus was not included in externalizing analyses. Sex differences in mean levels of variables were examined and none were found. Adolescents’ negative emotion expression in PAIT was also coded, and there was no sex difference in this. Adolescents’ mean behavioral negative emotion rating for negative emotional pictures was 3.40 on a 1–4 scale (SD = .46) and for neutral pictures was 1.48 (SD = .44) (difference: t = 26.34, p < .001). Correlations among study variables are shown in Table 2.
Table 2.
Intercorrelations, Means, and Standard Deviations for Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | M | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex | -- | N/A | N/A | |||||||||||
| 2. Warmth | .02 | -- | 3.13 | 0.81 | ||||||||||
| 3. Neg Par | .12 | −.24 | -- | 1.41 | 0.57 | |||||||||
| 4. R Amy | −.00 | .29* | −.10 | -- | 3.49 | 10.40 | ||||||||
| 5. L Amy | .09 | .18 | −.01 | .76*** | -- | 5.02 | 10.04 | |||||||
| 6. R AI | −.05 | .19 | −.16 | .35** | .35** | -- | 0.49 | 11.73 | ||||||
| 7. L AI | .03 | .04 | −.19 | .40** | .53*** | .78*** | -- | 1.10 | 13.07 | |||||
| 8. R ACC | −.15 | −.01 | .04 | .25* | .36** | .54*** | .63*** | -- | 1.55 | 10.85 | ||||
| 9. L ACC | −.05 | .17 | −.17 | .42*** | .47*** | .60*** | .67*** | .84*** | -- | 4.23 | 14.39 | |||
| 10. Subst | −.01 | −.34** | .15 | −.22 | −.14 | −.02 | .10 | .16 | .09 | -- | N/A | N/A | ||
| 11. Depr | −.20 | −.25* | −.03 | −.27* | −.07 | .10 | .13 | .23 | .20 | .52*** | -- | 5.61 | 6.21 | |
| 12. Anx | −.22 | −.13 | −.06 | −.18 | −.09 | −.03 | .02 | .08 | .09 | .02 | .78*** | -- | 6.53 | 5.38 |
| 13. Ext | .03 | −.31* | −.03 | −.22 | −.20 | −.07 | −.08 | .02 | −.05 | .31* | .32* | .23 | −0.14 | 1.27 |
Note. ROI scores indicate activation to negative minus neutral stimuli. For sex, 0 = girl, 1 = boy. Neg Par = negative parenting; R = right; L = left; Amy = amygdala; AI = anterior insula; ACC = anterior cingulate cortex; Subst = substance use; Depr = depressive symptoms; Anx = anxiety symptoms; Ext = externalizing symptoms; N/A = not applicable.
p < .05.
p < .01.
p < .001.
Parenting Predicting Brain Response to Negative Emotional Stimuli, by Sex
Maternal negative parenting.
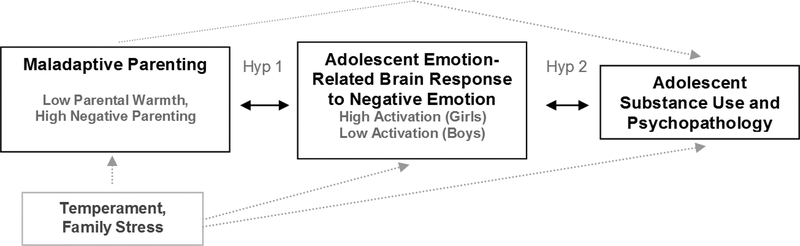
As shown in Figure 2, regressions found significant maternal negative parenting X adolescent sex interactions predicting adolescent activation to negative emotional (minus neutral) stimuli, with FDR correction, for R amygdala (β = −1.67, p = .02), L anterior insula (β = −1.24, p = .02), R anterior insula (β = −1.39, p = .01), L ACC (β = −1.20, p = .02), and R ACC (β = −1.27, p = .02). Main effects of negative parenting and sex on adolescent ROI activation were not significant after FDR correction. Follow-ups indicated that, for girls, higher maternal negative parenting was associated with higher activation to negative emotional (-neutral) stimuli for R ACC (β = 0.51, p = .003), but not R amygdala, L/R anterior insula, or L ACC. For boys, higher negative parenting was associated with lower activation to negative emotional (-neutral) pictures for L anterior insula (β = −0.45, p = .008), R anterior insula (β = −0.39, p = .02), and L ACC (β = −0.47, p = .004), but not R amygdala or R ACC. Although the follow ups for R amygdala were not statistically significant, the pattern of findings was that negative parenting was associated with lower R amygdala activation for boys (see Figure 2). ACC sub-region follow-ups found significant negative parenting X sex interactions for all ACC sub-regions with FDR correction (βs = −1.02 to −1.28, ps = .04 to .02). For girls, negative parenting predicted higher R sACC (β = .40, p = .03) and R pACC (β = .41, p = .02) activation. For boys, negative parenting predicted lower L pACC (B = −.50, p = .002) and L dACC (B = −.42, p = .01) activation.
Figure 2.
plots show simple slopes for boys and girls for significant maternal negative parenting by adolescent sex interactions predicting adolescent brain activation to negative (-neutral) emotional stimuli,for R amygdala, bilateral anterior insula, and bilateral ACC. ROIs were created for bilateral amygdala (centroid: x,y,z=± 23, −5, −18), bilateral anterior insula (x,y,z= ± 37, 11, −3), and bilateral ACC (x,y,z= ± 4, 34, 13). The following were abbreviations: SD (for Standard Deviation), R (for right), L (for left), Ant. (for Anterior).
Maternal warmth.
There were no significant maternal warmth X sex interactions predicting ROI activation. There was a main effect for low maternal warmth to predict lower R amygdala activation to negative emotional (-neutral) stimuli, but this did not survive FDR correction (β = 0.29, uncorrected p = .02, corrected p = .12).
Brain Response to Negative Emotional Stimuli and Adolescent Adjustment, by Sex
Substance use.
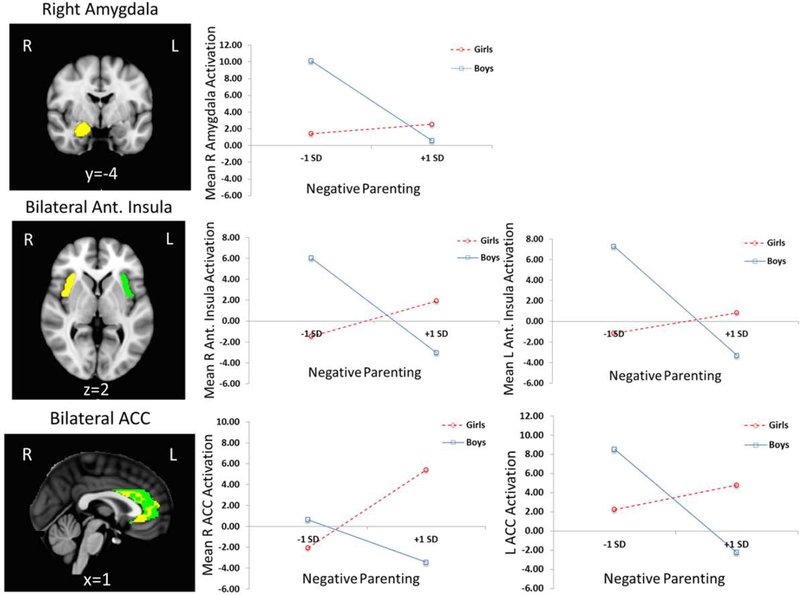
Logistic regressions found brain activation to negative emotional (-neutral) stimuli X adolescent sex interactions predicting adolescent substance use, after FDR correction, for L anterior insula (β = 0.83, p = .04) and R anterior insula (β = 0.83, p = .04), as shown in Figure 3. Main effects of ROI activation and sex on substance use were not significant after FDR correction. Interaction follow-ups found that, for girls, greater activation to negative emotional (-neutral) stimuli was associated with greater likelihood of substance use for L anterior insula (Exp[B] = 1.13 p = .02). For boys, insula activation was not associated with substance use.
Figure 3.
Plots show simple slopes for boys and girls for significant or trend-level adolescent brain activation to negative (-neutral) emotional stimuli by adolescent sex interactions predicting adolescent substance use, for bilateral anterior insula. ROIs were created for bilateral anterior insula (x,y,z= ± 37, 11, −3). The following were abbrevations: SD (for Standard Deviation) and Subst.(for Substance).
Internalizing symptoms.
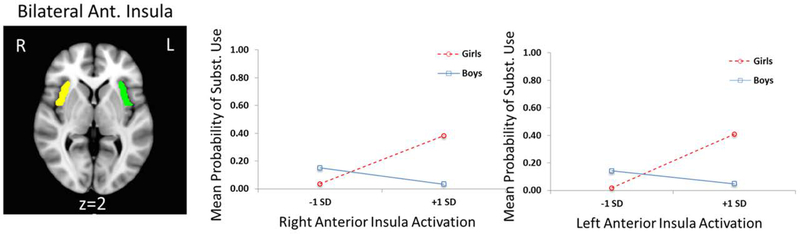
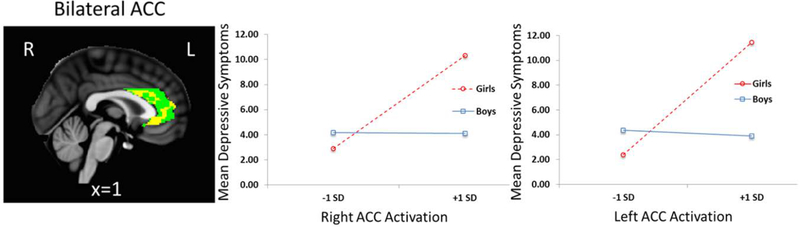
Regressions found significant brain activation to negative emotional (-neutral) stimuli X sex interactions predicting adolescent depressive symptoms, after FDR correction, for L ACC (β = −0.60, p = .02) and R ACC (β = −0.48, p = .02), as shown in Figure 4. There were also significant main effects, with higher L and R ACC activation to negative emotional (-neutral) stimuli associated with higher depressive symptoms after FDR correction (βs = 0.66, 0.58, ps < .01). Interaction follow-ups found that, for girls, greater activation to negative emotional (-neutral) stimuli was associated with greater depressive symptoms for L ACC (β = 0.45, p = .01) and R ACC (β = 0.43 p = .01). For boys, ACC activation to negative (-neutral) emotional stimuli was not significantly associated with depressive symptoms. ACC sub-region follow-ups found significant ACC activation X sex interactions predicting depressive symptoms for bilateral pgACC and dACC (βs = −0.48 to −0.56, ps = .02), but not sgACC (Bs = −0.23, −0.33, ns). For girls, greater bilateral pgACC and dACC activation was associated with greater depressive symptoms (Bs = 0.36 to 0.45, ps = .03 to .01). For boys, pg and dACC activation was not significantly associated with depressive symptoms. There were no significant ROI activation X sex interactions or main effects for anxiety symptoms.
Figure 4.
Plots show simple slopes for boys and girls for significant brain activation to negative (-neutral) emotional stimuli by sex predicting adolescent current depressive symptoms for bilateral ACC. ROIs were created for bilateral ACC (x,y,z= ± 4, 34, 13) SD is an abbreviation for Standard Deviation.
Externalizing symptoms.
There were no significant ROI activation X sex interactions or main effects for externalizing symptoms.
Exploratory Moderated Mediation Analyses
We tested and found evidence for the indirect effect of negative parenting on depressive symptoms through R ACC activation, moderated by sex (Index of Moderated Mediation = −0.55, SE = 0.32, 95% CI = −1.22 to −0.01). For girls, but not boys, there was evidence for the indirect effect through R ACC (Effect = 0.58, SE = 0.31, 95% CI = 0.08 to 1.24).
Discussion
The present study was the first to examine associations among parenting behaviors, adolescent neural responses to negative emotional stimuli, and substance use and psychopathology symptoms in early adolescence. The study found that observed maternal negative parenting behavior during parent-adolescent interactions was associated with adolescent neural activation to negative emotional stimuli, with heightened activation for girls and blunted activation for boys. In addition, heightened neural activation to negative emotional stimuli was associated with substance use and depressive symptoms for girls, but not for boys. Findings suggest a potential sex-differentiated brain-based pathway from maladaptive parenting to adolescent substance use and depressive symptoms.
Parenting and Adolescent Emotion-Related Neural Responses
Maternal negative parenting was associated with heightened R ACC activation (specifically ventral [pg and sg]ACC) to negative emotional stimuli for girls, but with blunted R and L anterior insula and L ACC (specifically pgACC and dACC) activation for boys. Girls’ heightened ventral ACC may reflect that parenting leads to heightened emotional reactivity/emotional awareness in girls. Boys’ blunted anterior insula, pgACC, and dACC may reflect that negative parenting is associated with lowered emotional reactivity/awareness and also less cognitive engagement (through dACC). It is interesting that negative parenting behavior (e.g., harsh tone, criticism) was associated with emotion-related brain activation, but positive parenting (warmth) was not. There was a correlation between low maternal warmth and blunted R amygdala activation, but this did not survive FDR correction. Our stronger findings for negative parenting than warmth are consistent with three of the four prior studies of parenting and adolescent brain function (Guyer et al., 2015; Marusak et al., 2017; Tan et al., 2014), which found that parental negative affect, harsh parenting, and psychological control (but not warmth) predicted altered brain activation to emotional stimuli, but are inconsistent with Romund and colleagues’ (2016) finding that reported maternal warmth (and not negative parenting) was associated with heightened amygdala activation to negative emotional faces. Our measure of observed negative parenting may have reflected negatively emotionally charged parenting, including parents’ negative emotion expressions, which specifically may impact (or be associated genetically with) their children’s neural processing of negative emotions. Also, our inter-rater reliability for warmth was only moderate, which could have contributed to less strong findings for warmth. Future studies should examine whether positive parenting is more linked to other brain functions, such as reward processing.
Negative parenting’s association with altered ACC and insula activation to negative emotion may have several explanations. First, consistent with our conceptual model, harsh parenting may impact children’s developing brain structure and function, leading to alterations in brain networks involved in negative emotion processing. These effects may manifest differently for girls versus boys because of innate and socialized sex differences in children’s emotional reactivity (Chaplin & Aldao, 2013). Second, highly neurally sensitive girls and blunted boys may elicit harsher parenting. Third, negative parenting may reflect a genetically-based tendency toward altered brain reactivity to negative stimuli that is passed on to children (with different expressions for girls versus boys). Our study should be followed up with longitudinal neuro-imaging studies to delineate direction of effects. Prior longitudinal findings that parenting precedes changes in child emotion-related behavior suggest that there is likely to be support for parenting affecting brain development (Belsky & de Haan, 2010).
If future research confirms that negative parenting is associated with emotion-related brain function differently for boys versus girls, there are important implications. For example, parenting-focused interventions should be sensitive to child sex and pay particular attention to girls with heightened emotion-related neural reactivity and boys with blunted reactivity. And these interventions for girls should prioritize reducing negative parenting.
Adolescent Emotion-Related Brain Function and Substance Use
For girls (but not boys), greater L anterior insula activation to negative emotional stimuli was associated with current substance use. Girls’ heightened anterior insula reactivity to negative emotional stimuli may reflect heightened interoceptive awareness of negative emotional experiences, which then may lead them to use substances to manage high emotional arousal. This would be consistent with the hypothesized pattern of girls’ development of internalizing disorders and may suggest that girls take an internalizing pathway toward substance use. Future studies may ask girls and boys to report on reasons for substance use to confirm that girls/women seek out substances to down-regulate arousal. The insula is involved in negative emotion processing and also in craving, and so negative emotional stress may sensitize the insula in girls, leading to greater craving and substance use (Sinha, 2008). Boys with high negative parenting showed blunted insula (and L ACC) activation, but this blunted activation was not associated with boys’ substance use. Boys’ lowered insula activation may become a risk factor later in adolescence as youth transition from early use to heavy use.
Given that our findings are cross-sectional, however, it is also possible that girls’ use of substances may have altered their insula development. This is unlikely given that the youth in this study were in early adolescence and thus had not used large amounts of substances. But, it is possible, and future longitudinal neuro-imaging work should examine this. Either way, the present findings have implications, including that substance use prevention programs should be sensitive to sex and should focus on reducing reactivity to negative emotional stressors for girls. Notably, we examined substance use, and not substance use disorders (SUDs). However, early substance use initiation (<= 14 years) is a risk factor for later SUDs, thus our findings with 12–14 year olds may have implications for preventing SUDs.
Adolescent Emotion-Related Brain Function and Psychopathology Symptoms
Heightened bilateral ACC activation to negative emotional stimuli was associated with depressive symptoms for girls (specifically pgACC and dACC), but not for boys. In addition, moderated mediation was present, with R ACC activation mediating the association between negative parenting and depressive symptoms for girls, but not boys. Notably, there were no significant brain activation-anxiety symptom associations. Past research has found brain activation-anxiety associations, but these all used emotional stimuli that more specifically elicited fear/threat (e.g., viewing threatening faces). Thus, neural processing of negative stimuli may be a trans-diagnostic process for internalizing disorders, but within this umbrella there may be stronger effects for fear-specific stimuli to anxiety symptoms.
Our findings for depressive symptoms are consistent with past research finding that heightened amygdala activation and altered ACC activation to negative emotional stimuli are associated with depressive symptoms (e.g., Hall et al., 2014), although our findings found a stronger role for ACC than amygdala. Amygdala responds to salient stimuli and perhaps was activated commonly to the negative pictures by most of the adolescents in our study. In contrast, neural reactivity related to high affective reactivity (pgACC) and cognitive monitoring/evaluation (dACC) were more specifically linked to depressive symptoms, although interestingly sgACC activation was not (in contrast with other studies suggesting a particular role for sgACC in depression- Hall et al., 2014).
Our findings may reflect that girls take a pathway from high environmental stress to depression characterized by high reactivity to/processing of negative emotion, consistent with rumination and emotional arousal theories of depression. Boys may take a different pathway to depression and future research should examine alternate pathways, perhaps through reward system functioning. It is also possible that depressive symptoms may lead to heightened attention to negative emotional stimuli (for girls), and future longitudinal studies should examine this. Implications of our findings are that interventions should select girls experiencing negative parenting and help them to reduce emotional reactivity to prevent depressive symptoms.
The present study did not find associations between brain activation to negative emotional stimuli and externalizing symptoms. Prior research finds that associations with externalizing symptoms are strongest for youth with callous-unemotional traits, and our study may have had few youth with those traits. Also, our study used parent report of externalizing symptoms, which may have missed some covert externalizing behaviors.
Limitations and Conclusions
There were a few limitations. The sample was small and largely White and middle income, limiting generalizability. The small sample limited power to examine full moderated mediation models. In addition, we focused on maternal parenting behaviors and did not include fathers. Our fMRI task included subjective ratings of negative emotion during trials, which may have attenuated BOLD responses and we did not examine reactivity to the positive emotional stimuli. In addition, although we examined functional activation, future research should examine functional connectivity among regions during emotion processing. Finally, we examined symptoms and not clinical diagnoses, and our sample, consistent with other community samples, had a limited range of symptoms.
In sum, we found that observed maternal negative parenting behaviors during parent-adolescent interactions were associated with adolescent brain activation to negative emotional stimuli differently by sex, with heightened R ACC activation for girls and blunted L ACC and L and R anterior insula activation for boys. Further, heightened anterior insula activation was associated with substance use and heightened ACC activation with depressive symptoms for girls, but not boys. Findings suggest a potential emotion-related brain pathway (sensitization of negative emotion processing brain networks) from negative parenting to substance use and depressive symptoms for girls. If findings are replicated, this suggests early neural markers that can be used to develop gender-sensitive prevention programs.
Acknowledgements
Support for this project was provided by the National Institute on Drug Abuse (NIDA) through grants R01-DA-033431 and R01-DA-033431-S1 (PI: Chaplin) and grant F31-DA-041790 (PI: Turpyn). The authors gratefully acknowledge the study sponsor, the participating families, and the study research assistants, particularly Fran Faundez, Alexandra Martelli, Juliana Jacangelo, Corynne Ross, and Wendy Baccus.
Footnotes
Conflicts of Interest: Author Sinha is on the scientific advisory board for Embera NeuroTherapeutics, Inc. There are no other conflicts of interest to declare.
References
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30, 217–237. doi: 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Amaro H, Blake SM, Schwartz PM, & Flinchbaugh LJ (2001). Developing theory-based substance abuse prevention programs for young adolescent girls. Journal of Early Adolescence, 21, 256–293. doi: 10.1177/0272431601021003002 [DOI] [Google Scholar]
- Barnes GM, Reifman AS, Farrell MP, & Dintcheff BA (2000). The effects of parenting on the development of adolescent alcohol misuse: A six-year wave latent growth model. Journal of Marriage & the Family, 62, 175–186. doi: 10.1111/j.1741-3737.2000.00175.x [DOI] [Google Scholar]
- Belsky J, & de Haan M (2010). Annual research review: Parenting and children’s brain development: The end of the beginning. Journal of Child Psychology and Psychiatry, 52, 409–428. doi: 10.1111/j.1469-7610.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 289–300. doi: 10.2307/2346101 [DOI] [Google Scholar]
- Boone TL, & Lefkowitz ES (2007). Mother-adolescent health communication: Are all conversations created equally? Journal of Youth and Adolescence, 36, 1038–1047. doi: 10.1007/s10964-006-9138-2 [DOI] [Google Scholar]
- Brener ND, Kann L, Kinchen SA, Grunbaum JA, Whalen L, Eaton D, … Ross JG (2004). Methodology of the youth risk behavior surveillance system. Recommendations and Reports/Centers for Disease Control. 53, 1–13. [PubMed] [Google Scholar]
- Caldji C, Diorio J, & Meaney MJ (2003). Variations in maternal care alter GABAA receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology, 28, 1950–1959. doi: 10.1038/sj.npp.1300237 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. doi: 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM (2010). Parent-adolescent interaction task (PAIT) coding system. Unpublished manual, Psychiatry Department, Yale University School of Medicine, New Haven, CT. [Google Scholar]
- Chaplin TM & Aldao A (2013). Gender differences in emotion expression in children: A meta-analytic review. Psychological Bulletin, 139, 735–765. doi: 10.1037/a0030737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM & Cole PM (2005). The role of emotion regulation in the development of psychopathology In Hankin BL, & Abela JRZ (Eds.), Development of psychopathology: A vulnerability-stress perspective (pp. 49–74). Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Chaplin TM, Sinha R, Simmons J, Healy S, Mayes LC, Hommer RE, & Crowley MJ (2012). Parent-adolescent conflict interactions and adolescent alcohol use. Addictive Behaviors, 37, 605–612. doi: 10.1016/j.addbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Visconti KJ, Molfese P, Susman E, Klein LC, Sinha R, & Mayes LC (2015). Prenatal cocaine exposure differentially affects stress responses in girls and boys: Associations with future substance use. Development and Psychopathology, 27,163–180. doi: 10.1017/S0954579414000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, & Prost J (2002). Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology,70, 67. doi: 10.1037/0022-006X.70.1.67 [DOI] [PubMed] [Google Scholar]
- Dale AM (1999). Optimal experimental design for event-related fMRI. Human Brain Mapping, 8, 109–114. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohew RL, Hoyle RH, Clayton RR, Skinner WF, Colon SE, & Rice RE (1999). Sensation seeking and drug use by adolescents and their friends: Models for marijuana and substance. Journal of Studies on Substance and Drugs, 60, 622–631. doi: 10.15288/jsa.1999.60.622 [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sprafkin J, & Schwartz J (2002). Tics and psychiatric comorbidity in children and adolescents. Developmental Medicine and Child Neurology, 44, 330–338. doi: 10.1017/S001216220100216X [DOI] [PubMed] [Google Scholar]
- Gadow KD, & Sprafkin JN (2002). Child symptom inventory 4: Screening and norms manual. Checkmate Plus. [Google Scholar]
- Glied S, & Pine DS (2002). Consequences and correlates of adolescent depression. Archives of Pediatrics & Adolescent Medicine, 156, 1009–1014. doi: 10.1001/archpedi.156.10.1009 [DOI] [PubMed] [Google Scholar]
- Gottman JM, Katz LF, & Hooven C (1997). Meta-emotion: How families communicate emotionally. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Guyer AE, Jarcho JM, Pérez-Edgar K, Degnan KA, Pine DS, Fox NA, & Nelson EE (2015). Temperament and parenting styles in early childhood differentially influence neural response to peer evaluation in adolescence. Journal of Abnormal Child Psychology, 43, 863–874. doi: 10.1007/s10802-015-9973-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LM, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, … Cullen KR (2014). An fMRI study of emotional face processing in adolescent major depression. Journal of Affective Disorders, 168, 44–50. doi: 10.1016/j.jad.2014.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107, 128–140. doi: 10.1037/0021-843X.107.1.128 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, & Smith SM (2012). Fsl. Neuroimage, 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2018). Monitoring the Future national survey results on drug use, 1975–2017: Overview, key findings on adolescent drug use. Retrieved from https://deepblue.lib.umich.edu/bitstream/handle/2027.42/142406/Overview%202017%20FINAL.pdf?sequence=1&isAllowed=y
- Jones AP, Laurens KR, Herba CM, Barker GJ, & Viding E (2009). Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry, 166, 95–102. doi: 10.1176/appi.ajp.2008.07071050 [DOI] [PubMed] [Google Scholar]
- Kaminer Y, Bukstein O, & Tarter RE (1991). The Teen-Addiction Severity Index: Rationale and reliability. Substance Use and Misuse, 26, 219–226. doi: 10.3109/10826089109053184 [DOI] [PubMed] [Google Scholar]
- Kaplow JB, Curran PJ, & Dodge KA (2002). Child, parent, and peer predictors of early-onset substance use: A multisite longitudinal study. Journal of Abnormal Child Psychology, 30, 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, & Gunnar MR (2004). Evening activities as a potential confound in research on the adrenocortical system in children. Child Development, 75, 193–204. doi: 10.1111/j.1467-8624.2004.00663.x [DOI] [PubMed] [Google Scholar]
- Killgore WD, & Yurgelun-Todd DA (2005). Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport, 16, 1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, & McMahon RJ (2014). Conduct and oppositional defiant disorders In Mash EJ & Barkely RA (Eds.), Child Psychopathology (pp. 145–179). New York, NY: Guilford Press. [Google Scholar]
- Koo TK, & Li MY (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15, 155–163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, & Staff MHS (2003). Children’s Depression Inventory (CDI): technical manual update. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical report A-8.
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, & Allen NB (1998). Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of Abnormal Psychology, 107, 109–117. doi: 10.1037/0021-843X.107.1.109 [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, & Barrett LF (2012). The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences, 35, 121–143. doi: 10.1017/S0140525X11000446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Thomason ME, Sala‐Hamrick K, Crespo L, & Rabinak CA (2017). What’s parenting got to do with it: emotional autonomy and brain and behavioral responses to emotional conflict in children and adolescents. Developmental Science. e12605 10.1111/desc.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masip AF, Amador-Campos JA, Gómez-Benito J, & del Barrio Gándara V (2010). Psychometric properties of the Children’s Depression Inventory in community and clinical sample. The Spanish Journal of Psychology, 13, 990–999. doi: 10.1017/S1138741600002638 [DOI] [PubMed] [Google Scholar]
- Mayberg HS (1997). Limbic-cortical dysregulation: A proposed model of depression. The Journal of Neuropsychiatry and Clinical Neurosciences, 9, 471–481. doi: 10.1176/jnp.9.3.471 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, O’Brien CP, & Woody GE (1980). An improved evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous Mental Disorders, 168, 26–33. Retrieved from 10.1097/00005053-198001000-00006 [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, … Ochsner KN (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive Affective Neuroscience, 7, 11–22. doi: 10.1093/scan/nsr093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, … Pine DS (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry, 163, 1091–1097. doi: 10.1176/appi.ajp.163.6.1091 [DOI] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, & Forbes EE (2014). Maternal depression and warmth during childhood predict age 20 neural response to reward. Journal of the American Academy of Child & Adolescent Psychiatry, 53, 108–117. doi: 10.1016/j.jaac.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, & Robinson LR (2007). The role of the family context in the development of emotion regulation. Social Development, 16, 361–388. doi: 10.1111/j.1467-9507.2007.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Knodt AR, Radtke SR, & Hariri AR (2016). Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: Possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Molecular Psychiatry, 21, 348–356. doi: 10.1038/mp.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit GS, Laird RD, Dodge KA, Bates JE, & Criss MM (2001). Antecedents and behavior‐problem outcomes of parental monitoring and psychological control in early adolescence. Child Development, 72, 583–598. doi: 10.1111/1467-8624.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, & Allen NB (2012). Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences, 16, 322–329. doi: 10.1016/j.tics.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KIA, Lacadie CM, Fulbright RK, Tuit KL, & Sinha R (2012). Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. American Journal of Psychiatry, 169, 406–414. doi: 10.1176/appi.ajp.2011.11020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz RJ, Foster S, Kent RN, & O’Leary KD (1979). Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of Applied Behavioral Analysis, 12, 691–700. doi: 10.1901/jaba.1979.12-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, & Richmond BO (1985). Revised children’s manifest anxiety scale. Los Angeles: Western Psychological Services. [Google Scholar]
- Romund L, Raufelder D, Flemming E, Lorenz RC, Pelz P, Gleich T, … Beck A (2016). Maternal parenting behavior and emotion processing in adolescents—An fMRI study. Biological Psychology, 120, 120–125. doi: 10.1016/j.biopsycho.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Rotge JY, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, … & Fossati P (2014). A meta-analysis of the anterior cingulate contribution to social pain. Social Cognitive and Affective Neuroscience, 10, 19–27. doi: 10.1093/scan/nsu110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141, 105–130. doi: 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, & Poustka F (2007). Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: Association with temperament traits. Journal of Psychiatric Research, 41, 410–417. doi: 10.1016/j.jpsychires.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2001). We know some things: Parent-adolescent relationships in retrospect and prospect. Journal of Research on Adolescence, 11, 1–19. doi: 10.1111/1532-7795.00001 [DOI] [Google Scholar]
- Stevens JS, & Hamann S (2012). Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia, 50, 1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Tan PZ, Lee KH, Dahl RE, Nelson EE, Stroud LJ, Siegle GJ, & Silk JS (2014). Associations between maternal negative affect and adolescent’s neural response to peer evaluation. Developmental Cognitive Neuroscience, 8, 28–39. doi: 10.1016/j.dcn.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, … Emslie GJ (2012). Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. American Journal of Psychiatry, 169, 381–388. doi: 10.1176/appi.ajp.2011.11040615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, & Sansom JA (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57, 241–266. doi: 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–289. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Whittle S, Dennison M, Vijayakumar N, Simmons JG, Yücel M, Lubman DI, … Allen NB (2013). Childhood maltreatment and psychopathology affect brain development during adolescence. Journal of the American Academy of Child & Adolescent Psychiatry, 52, 940–952. doi: 10.1016/j.jaac.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MB, … Allen NB (2014). Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Developmental Cognitive Neuroscience, 8, 7–17. doi: 10.1016/j.dcn.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Sandy JM, Yaeger AM, Cleary SD, & Shinar O (2001). Coping dimensions, life stress, and adolescent substance use: a latent growth analysis. Journal of Abnormal Psychology, 110, 309–323. doi: 10.1037//002I-843X.110.2.309 [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, … Wu J (2010). Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 42–51. doi: 10.1016/j.jaac.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Lacadie CM, Sinha R, Mayes LC, & Potenza MN (2016). Prenatal cocaine exposure, illicit-substance use and stress and craving processes during adolescence. Drug and Alcohol Dependence, 158, 76–85. doi: 10.1016/j.drugalcdep.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]