Abstract
Systemic autoimmune diseases (AID) have multiorgan, heterogeneous clinical presentations and are characterized by dysregulation of the immune system, immunodeficiency, irreversible organ damage and increased morbidity and mortality. Preventing or decreasing flares of AID correlate with durable disease control, significant reduction of inflammation and prevention of disability or therapy-related toxicity. There is an urgent need for better treatment of severe, therapy-refractory AID. Extracorporeal photopheresis (ECP) is a cell-based immunomodulatory treatment which has been extensively used in variety of autoimmune disorders for the last two decades. ECP treatment is FDA approved for the treatment of cutaneous T-cell lymphoma (CTCL) with particularly promising results seen in graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HCT). Prolonged therapy is safe, well tolerated and allows reduction of systemic immunosuppression in therapy-refractory patients. Both clinical and experimental evidence suggest that ECP mechanism of action is characterized by apoptosis and phagocytosis of activated cells by antigen-presenting cells (APC), secretion of anti-inflammatory cytokines and stimulation of regulatory T cells (Tregs). The focus of this paper is to review the current evidence of ECP use in the treatment of AID. Here, we summarize the experience of nine major AID from 65 published reports. The key findings demonstrate substantial evidence of ECP feasibility, safety and in some AID also promising efficacy. However, the role of ECP in AID therapy is not established as most published studies are retrospective with limited number of patients and the trials are small or poorly standardized. The available data support future investigations of ECP as a therapeutic modality for the treatment of AID in well-designed prospective clinical studies.
Keywords: photopheresis, extracorporeal, autoimmune disease, cellular therapy, immunomodulation
INTRODUCTION
Current treatments for systemic autoimmune diseases (AID) include prolonged systemic immunosuppression with steroids or other agents or biologicals, which frequently lead to serious side-effects or are ineffective. Novel therapies are necessary for aggressive, therapy-refractory AID to achieve higher rate of sustained responses with a better safety profile. The safety profile and absence of significant late side effects of extracorporeal photopheresis (ECP) therapy is appealing [1]. Substantial evidence suggests that ECP can be used in addition to first line or salvage therapy in AID and is associated with noticeable response rates. However, paucity of well-designed prospective clinical trials is currently a major problem in evaluating the efficacy of ECP in AID. ECP use has been reported in multiple diseases, (Fig. 1) but FDA approved indication is only for the treatment of cutaneous T-cell lymphoma (CTCL). Recently, the European Dermatology Forum with Knobler et al., provided expert recommendations for the general clinical use of ECP [2]. The current review describes in detail ECP trials related to AID only, compared to Knobler et al, which focused primarily on larger trials in CTCL, graft-versus-host disease (GVHD) and transplantation. The lack of double-blinded clinical studies forced centers considering EPC to use mainly data from non-randomized retrospective studies or numerous case reports leading to the conclusion that the efficacy of ECP for major AID is doubtful.
Fig. 1.
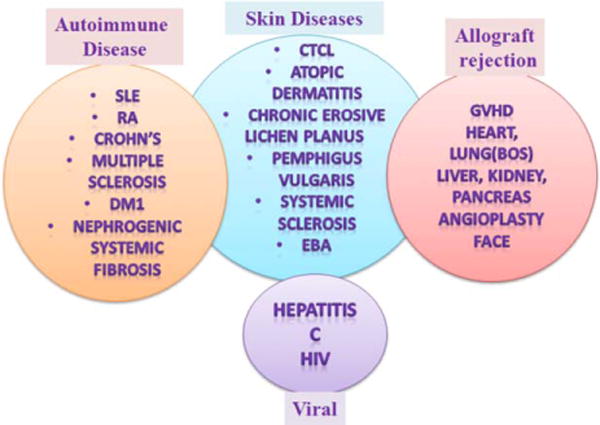
Current applications of ECP. Abbreviations: SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; NSF, nephrogenic systemic fibrosis; MS, multiple sclerosis; DM1, diabetic mellitus type I; CTCL, cutaneous T-cell lymphoma; GVHD, graft-versus-host disease; PV, pemphigus vulgaris; SS, systemic sclerosis; EBA, epidermolysis bullosa acquisita; BOS, bronchiolitis obliterans syndrome; HIV, human immunodeficiency virus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In this review, we summarized the currently published ECP experience and outcomes for the treatment of the following AID: atopic dermatitis, oral lichen planus, systemic sclerosis (SS), systemic lupus erythematous (SLE), nephrogenic systemic fibrosis (NSF), multiple sclerosis (MS), diabetic mellitus type I (DMI), rheumatoid arthritis (RA), and psoriasis (Figs. 2–4). The clinical objective is to select a patient who will benefit from this treatment the most, optimize ECP duration and schedule as well as standardize response criteria to assess the therapeutic benefit.
Fig. 2.
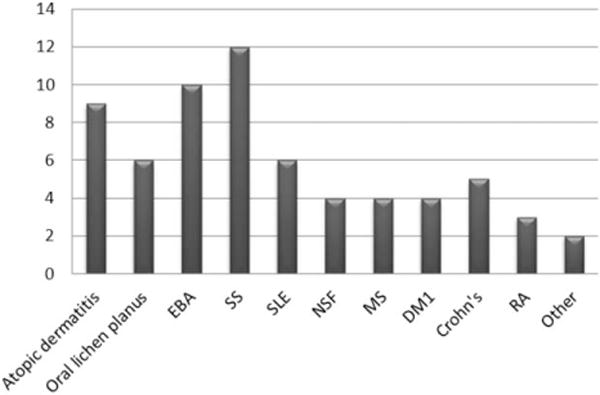
Number of published ECP studies (N = 65) for different AID from 1989–2014. The number of published studies (X-axis) n = 65, is summarized in the diagram. Each bar demonstrates one disease. Other diseases include deep morphea and psoriasis.
Fig. 4.
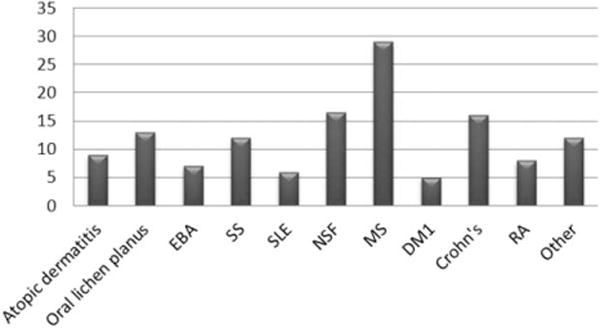
Number of ECP cycles typically performed for specific AID (1 cycle = 2 ECP treatments in 2 sessions). The median number of ECP cycles (X-axis) is summarized in the bar diagram; each bar demonstrates number of cycles for one disease. Other diseases include deep morphea and psoriasis.
ECP PROCEDURE
ECP includes three steps: (1) apheresis—collection of a buffy coat (~200 ml) with plasma (~300 ml), overall, six collections per apheresis procedure, (2) processing the buffy coat cells with eight methoxypsoralen (8-MOP), a photosensitizing agent, and UV-A light ex vivo, and (3) reinfusion of the treated autologous cells. Administration of oral 8-MOP should be avoided due to side effects and insufficient gastrointestinal absorption. Anticoagulants, such as heparin or citrate acid are used during ECP [3]. The duration of one ECP treatment varies between 3 and 6 hours, depending on the device, and is usually performed on two consecutive days, defined as one cycle. The interval between cycles ranges from weekly to every other week or monthly. The currently available in-line, one-step ECP systems are UVAR XTS and Cell-Ex (Therakos™, Exton, PA, Therakos.com). Offline ECP includes three steps which are performed separately; the currently available major offline system is MACOGENIC® G2, MacoPharma (macopharma.com).
MECHANISM OF ACTION
The exact mechanism of action of ECP remains to be elucidated since the key effect may vary between diseases and only 5% of cells in blood circulation undergo direct treatment exposure to UV-A light and 8-MOP. These treated autologous cells express apoptotic markers [4] and the apoptosis occurs gradually over time after ECP [5]. The reinfused cells are phagocytized by antigen-presenting cells (APCs), the latter become activated and express antigens on their surface. Next, anti-inflammatory cytokines such as IL-10 are produced, which promote development of T-regs [6,7]. Modulation of T-regs during ECP likely plays a major role in the suppression of the ongoing immune response.
Further immunological changes were found during ECP therapy: decreased quantities of CD81[8] and Th17[7] cells, decreased soluble IL-2-Ra and TNF-a R1 levels [9], 80% decrease in IFN-γ secreting Th1 cells [10]. On the other hand an increase in IL-4, IL-5, and IL-10 secreting Th2 cells [11], natural killer cells (CD31CD561) [12], and plasmacytoid dendritic cells (pDC) 1 day after ECP [13] was associated with response to ECP treatment. These changes lead to Th1/Th2 balance restoration [14] and normalization of the CD4/CD8 ratio [12].
The ECP treated cells consist of about 30% of monocytes, which are less sensitive to apoptosis and may differentiate into dendritic cells [15]. Bladon et al. showed [16] a significant expansion of CD36 marker on monocytes, an important receptor for the uptake of apoptotic material [17] during ECP, suggesting an enhancement of clearance after ECP. A defect in the clearance of apoptotic cells has been suggested to contribute to the development of pathogenesis in AID [18].
Another mechanism by which ECP may lead to amelioration of AID is via its effect on B cells and B cell survival factor (BAFF), which are recognized mediators of autoimmunity. Recently published data showed normalization of B cells with an atypical CD21low [19] profile and BAFF [20] during ECP, which were associated with response to ECP.
Diminished levels of circulating Tregs have been seen in various AID such as DM1 [21], SLE [22], RA [23], asthma, inflammatory bowel disease [24], primary biliary cirrhosis [25]. Expansion of Tregs [26–28] has been seen in patients receiving ECP treatment and is associated with response to ECP therapy. Therefore, ECP-associated immunomodulation can be defined as an induction of T-regs, alteration of cytokines, modification of DC, direct apoptosis of autoreactive cells and anergy of activated, cytotoxic T cells.
CLINICAL APPLICATION
A substantial number of AID have been treated with ECP. This review summarizes published data of nine major AID including atopic dermatitis, oral lichen planus, SS, SLE, NSF, MS, DM1, RA, and psoriasis. Table I summarizes all 65 studies, however only studies with >3 patients were summarized in the text of the manuscript. Each study description includes authors and year of publication, study design, number of patients, ECP schedule and duration, and reported responses.
TABLE I.
Summary of ECP Studies for AID
| Author, year | Study design | Number of pts | ECP schedule | Duration months | Response |
|---|---|---|---|---|---|
| Atopic Dermatitis | |||||
| Prinz 1994 [29] | Case series | 3 | One cycle every 4 weeks, after 12 ECP every 6 weeks | 12 months | 67% CR, 33% PR, ↓, cutaneous inflammation, ↓, IgE |
| Richter 1998 [30] | Case series | 3 | One cycle every 2 weeks | 5 months | 75% improvement, monotherapy |
| Prinz 1999 [31] | Retrospective open clinical trial | 14 | One cycle every 2 weeks | 3 months | 72% OR, normalization of CD4/CD8 |
| Radenhausen 2003 [32] | Retrospective case series | 10 | One cycle every 2 weeks, oral MOP | 5 months | ↓, in SCORAD 87 to 36, ↓, in eosinophilic cationic protein, sIL-2R, sE-Selectin |
| Radenhausen 2004 [33] | Two-center, open clinical trial | 35 | One cycle every 2 weeks, oral MOP | 5 months | 65% OR, ↓, in SCOPAD 74 to 36 |
| Sand 2007 [34] | Single arm, open-label | One cycle every 2 weeks for 20 weeks | 12 months | ↓, in SCORAD 78 to 56 after 10 cycles, improvement in EACT-G, SE36 | |
| Hjuler 2010 [35] | Retrospective case series | 6 | One cycle every 4 weeks to 8 weeks | 67 months | 100% marked improvement, 1CR |
| Rubegni 2013 [36] | Retrospective case series | 7 | One cycle every 2 weeks | 3 months | 85.7% OR, ↓SCORAD, long lasting stabilization in 57% |
| Wolf 2013 [37] | Prospective | 10 | One cycle every 2 weeks | 5 months | 30% MR, SCOPAD ↓, 65 to 55, no change in SKINDEX, SE-36, FACT scores |
| Summary, Median, range | 9 | 95 3–35 |
One cycle every 2 weeks | 5 3–67 |
84 30–100 |
| Oral lichen planus | |||||
| Gerber 1997 [38] | Case report | 1 | 12 cycles | 6 months | CR |
| Becherel 1998 [39] | Open prospective | 7 | One cycle every 2 weeks, 24 cycles | 12 months | 100% CR after 1.5Mo |
| Kunte 2005 [40] | Case series | 4 | One cycle every 2 weeks | 10 months | 100% nearly CR, Clinical sustained improvement after discontinuation |
| Guyot 2007 [41] | Case series | 12 | Initially one cycle every weeks for 3 weeks, 21 cycles | 11.5 months, (1–36) | 75% CR, 25% PR, Monthly evaluation, recurrence in 11 pt, ↓, lymphocytes in responders |
| Elewa 2011 [42] | Case report | 1 | 6–12 cycles | 6 months | Reduction of ulcers 80% |
| Toberer 2012 [43] | Case report | 1 | 14 cycles, every 2 weeks, after 3mo | 6 months | CR |
| Marchesseau-Merlin 2008 [44] | Case series | 2 | 9, 20 | 75%, Stabilization with subjective improvement, Flare after discontinuation | |
| Summary Median, range | 7 | 28 1–12 |
One cycle every 2 weeks | 6 | 100% |
| Epidermolysis Bullosa Acquisita | |||||
| Rook 1989–90 [45], [46] | Case series | 4 PV | One cycle every 4 weeks, after 7 ECP every 5 weeks | 2 years | 75% CR, Antibody titer ↓, 1280 to 40, Discontinuation of medication |
| Liang 1992 [47] | Case report | 1PV | One cycle every 2 weeks for 2ECP every 3 weeks | 70% OR | |
| Gollnick 1993 [48] | Case report | 1PV | One cycle every 4 weeks | Near CR | |
| Miller 1995 [49] | Case report | 1 | One cycle every 3 weeks | 2 months | Clinical improvement |
| Owsianowski 1996 [50] | Case report | 1 | One cycle every 4 weeks | Clinical improvement | |
| Gordon 1997 [51] | Prospective | 3 | One cycle every 3 weeks | 5 months | 100% objective sustained clinical improvement |
| Azana 1997 [52] | Case report | 1 | One cycle every 4 weeks | 15 months | CR after 5cycle, AB titer ↓, 1000–0, Discontinued IS, sustained response |
| Camara 1999 [53] | Case report | 1 | One cycle every 3 weeks | 24 months | Clinical improvement |
| Wollina 1999 [54] | Case series | One cycle every 4 weeks | 4–42 months | Clinical improvement | |
| Sanli 2010 [55] | Retrospective longitudinal | 8 PV/3EBA | One cycle every 4 weeks, 21–51 cycles | 20 months months | PV in 100% OR after two to six cycles, EBA 2CR, 1PR. Less effective in patients with high autoAB and as monotherapy. Steroid taper |
| Summary Median, range | 10 | 31 1–11 |
One cycle every 4 weeks | 20 1–32 |
100 70–100 |
| Systemic Sclerosis | |||||
| Rook 1989 [56] | Case series | 2 | One cycle every 2 weeks | 12 months | 1CR, 1PR |
| Rook 1992 [57] | Pospective, randomized, single-blind | 31 | One cycle every 4 weeks | 6–10 months | At 6mo: 68% skin vs 32%, 10mo: 69% vs 50%, no difference after 10Mo |
| Cribier 1995 [58] | Open | 9, 2 morphea | One cycle every 2 weeks | 6 months | Unchanged in 3 (38%), aggravated in 38%, progression in 13%, 50% OR morphea |
| Owsianowski 1996 [50] | Retrospective | 10 | One cycle every 4 weeks | 24 months | 50% OR |
| Schwartz 1997 [59] | Retrospective | 5 | One cycle monthly | 59 months (6–21) | 100% Improvement/stabilization in joint mobility |
| Krasagakis, 1998 [60] | Prospective | 16 | One cycle every 4 weeks | 6–5 months | OR 38%, mixed 13%, stable 19%, |
| Enomoto 1999 [61] | Prospective multicenter, randomized crossover. | 19 | One cycle every 4 weeks | 12 months | 5.4% skin improvement. |
| Muellegger 2000 [62] | Single center observational study | 11 | One cycle every 4 weeks | 16–57 months | 45% OR in skin changes and physical performance. Progression in extracutaneous (91%) and QoL (82%) |
| Reich 2003 [63] | Observational | 20 | One cycle every 4 weeks | 12 months | 55% (30%PR, 25% stable). Responders had short PSS-course, moderate ANA titre, normal TNE-alpha, lack of Scl-70 |
| Hashikabe 2005 [64] | Observational | 13/11 ECP | 2 only oral MOP, ointment | Mean 15days | improvement in dermal edema, not fibrosis |
| Knobler 2006, [65] | Multicenter randomized double-blind, placebo-controlled | 27 | One cycle every 4 weeks | 6–12 months | improvement in skin severity, joints; but not between the therapy arms, ↓, in new joints involvement |
| Papp 2012 [66] | Open study with controls | 16 | One cycle in 6 weeks | 9 months | Improve in joints, mobility, ↓, of dermal thickness, ↓, Th17, ↑ Tregs |
| Summary Median, range | 12 | 179 2–31 |
One cycle every 4 weeks | 11 0.5–59 |
60 5.4–100 |
| Systemic Lupus Erythematosus | |||||
| Knobler 1992 [67] | Pilot study | 8 | One cycle monthly | 6 months | 88% OR, ↓, in clinical activity score from 7 to 1 |
| Richter 1998 [68] | Case report | 1 | One cycle monthly | 6 months | CR |
| Wollina 1999 [69] | Case series | 2 | 6–9 cycles | 6 months | CR for 18 and 11 Mo |
| Richard 2002 [70] | Case report | 1 | One cycle monthly | 9 months | OR, but not sustained |
| Morruzzi 2009 [71] | Case series | 4 | Two cycles | 1 months | 50% CR, 50% PR |
| Boeckler 2008 [72] | Case report | 1 | One cycle every 2 weeks | 2 months | CR |
| Summary Median, range | 6 | 17 1–8 |
One cycle monthly | 6 1–9 |
100 88–100 |
| Nephrogenic Systemic Fibrosis | |||||
| Lauchli 2004 [73] | Case report | 1 | Four cycles | Improvement of induration | |
| Gilliet 2005 [74] | Case series | 3 | One cycle every 2–4 weeks | 6 months | 100% improvement skin softening, joint motility (1CR) |
| Richmond 2007 [75] | Case series | 5 | 34 ECP, every 2–3 weeks in 4 pts, 1 pt weekly | Mean 8.5 months | 60% mild benefit in skin tightening, range of motion, functional skin thickening, joint capacity, PET, functional index |
| Mathur 2008 [76] | Case series | 3 | One cycle every 2 weeks | 6 months | 100% clinical improvement |
| Summary Median, range | 4 | 12 1–5 |
One cycle every 2 weeks | 6 6–9 |
100 60–100 |
| Multiple Sclerosis | |||||
| Poehlau 1997 [77] | Case series | 2 | One cycle every 4 weeks, MOP oral | 4y, 1y | ↓, in relapses from 11 to 1 |
| Rostami 1999 [78] | Double blind, placebo-controlled | 16 | One cycle every 4 weeks | 12 months | No difference in EDSS, Ambulation index, Scripp’s |
| Besnier 2002 [79] | Hot study | 4 | 1 ECP Weekly for 6 weeks, monthly for 6Mo | 6 | OR 80% (1PR, 3Stable), 1 Worse. Kurzke, EDSS by independent neurologist |
| Cavaletti 2006 [80] | Pilot study | 5 | One cycle every 2 weeks for 4Mo, 2ECP every 4 weeks for 6mo, 2ECP every 8 weeks for 12Mo | 24 months, 102 ECP | ↓, in relapse rate, EDSS, MRI stabilization |
| Summary Median, range | 4 | 28 | One cycle every 4 weeks | 12 6–24 |
90 0–100 |
| Diabetes Type 1 | |||||
| Ludvigsson 2001 [81] | Randomized double-blind placebo controlled | 19 | Five cycles | 3 months | ↓, insulin need, No difference in HbAlC, weeks eak effect on disease process |
| Ernerudh 2004, [82] | Randomized double-blind placebo controlled | 19 | Five cycles | 3 months | No clinical, cellular difference; increased activated T cells in placebo |
| Faresjo 2005 [83] | Randomized double-blind placebo controlled | 10 | Five cycles | 3 months | Protective role of ECP, ↓, of IFN-g, increase in IL-4 in ECP arm |
| Jonson 2008 [84] | Randomized double-blind placebo controlled | 19 | Five cycles | 3 months | Increase of CD4,CD8, ↓, in CTLA4, TGF-b mRNA in sham arm |
| Summary Median, range | 4 | 29 10–19 |
Five cycles | 3 | n/a |
| Crohn’s disease | |||||
| Reinisch 2001 [85] | Prospective pilot study | 9 | 6 months | OR 44% with discontinued steroids, 44% ↓, steroids (>50%), intestinal homing of ECP-treated cells | |
| Guariso 2003 [86] | Case series | 2 | 22 ECP, Weekly | 3 months | No change |
| Bissaccia 2007 [87] | Prospective | 2 | 30 ECP, every 4 weeks | 6 months | Clinical response for moderate, active refractory disease |
| Abreu 2009 [88] | Multicenter prospective | 28 | Week 1–4: twice weekly, every week; Week 5–12: twice weekly, every other weeks, | 3 months | 50% response CDAI (75% maintain response at 2y), 60% fistula closure |
| Reinisch 2013 [89] | Prospective open-label, multicenter | 31 | 12 ECP | 6 months | 23% discontinued steroids, sign. ↓, of steroids, 10% remain in CR 48 Weeks after ECP |
| Summary Median, range | 5 | 72 2–31 |
One cycle every 4 weeks | 6 3–6 |
33.5 0–50 |
| Rheumatoid Arthritis | |||||
| Malawista 1991 rom | Pilot study | 7 | One cycle monthly | 6 months | 57% OR |
| Vahlquist 1996 [91] | Open study | 8 | One cycle every 2 weeks | 6 months | 50% PR, significant (74%) decrease in the Ritchie articular index, ↑ CD4:CD8 ratio in responders prior ECP |
| Bracaglia 2008 | Case report | 1 | One cycle every week | 4 months | Mo response, no Tregs changes |
| Summary | 3 | 16 | 6 | 36 0–57 |
|
| Deep Morphea, Psoriasis | |||||
| Vonderheid 1990 [92] | Case series | 4 | One cycle every 2 weeks | 6–13 months | 100% clinical improvement PR, flare after discontinuation of Mtx, |
| Neustadter 2009 [93] | Case report | 1 | One cycle every 2 weeks, every 3.4 weeks | 6 months | Clinical improvement within 1–2 months |
| Summary Median, range | 2 | 5 1–4 |
One cycle every 2 weeks | 6 | n/a |
Abbreviations used: MR, minimal response; One cycle = two ECP treatments; OR, overall response; CR, complete response; PR, partial response; sign significant, PV, pemphigus vulgaris; CDAI, Crohn’s disease activity index; W, week; Mo, month; ECP, extracorporeal photopheresis; n/a, not applicable; ↓ decreased, autoAB autoantibodies.
Definition of studies: Case report – is a clinical observation of diagnosis, treatment, follow-up etc without casual conclusions on effectiveness of the intervention. Case series – is a group of cases, without or with controls (case–control observational study), or literature/historical controls involving patients under similar treatment and including clinical descriptive analysis. Open clinical trial is a study where participants know about administered medication. Retrospective study is a longitudinal analysis of patient’s history. Prospective study is a longitudinal observation of newly enrolled patients. Randomized controlled study is a prospective investigation of active experimental intervention with random and equal assignment of patients or controls. Cross-sectional study evaluates the relationship between ECP treated and not treated groups of patients with the defined disease at one specific time point over a short period of time. Longitudinal study is a correlational research including repeated observations of the same variables over prolonged period of time to record the clinical outcome. Observational study includes ECP patients which were passively observed during the treatment to record the clinical outcome but lacking a casual association. Pilot study is a conducted preliminary small scale study to evaluate feasibility, duration, patient size and potential effectiveness in order to prepare a further larger study.
For atopic dermatitis, chronic relapsing inflammatory skin disease, nine studies are reported with overall 95 patients (range 3–35 per study). However the majority of studies included small number of patients. ECP was administered every 2 weeks in the majority of studies, followed by four[29] and eight [35] weeks intervals. The median duration of treatment was 5 months, with the range of 3–67 months. Overall 84% of patients achieved response, (range 30–100%) as measured by SCORAD (SCORing Atopic Dermatitis). A significant reduction in SCORAD, from a median of 76% to 46%, was demonstrated in four studies. However, the results of the single prospective study by Wolf et al. with 10 patients showed [37] a minimal response: less than 30% of patients after 20 weeks of the therapy. This study also measured various laboratory markers during ECP which could be used as secondary endpoint tools, including IgE, eosinophilic cationic protein, soluble E-selectin and IL-2R, where non-responders tend to have higher levels of IgE, eosinophilic cationic protein, soluble E-selectin and IL-2R [32].
Oral lichen planus was investigated in seven studies with 28 patients (range 1–12 per study), however, only three studies (n = 23) had sufficient patient number to evaluate efficacy of ECP. Latter received a median of 21 cycles, usually every 2 weeks for 12 months. Response evaluation was performed monthly. Sustained improvement [40] with significant reduction of ulcers was seen in all treated patients already after 1.5 months of the ECP. Complete resolution of all symptoms was achieved after 12 months. The only large study, with 12 patients, was published by Guyot [41] and ECP treatment resulted in complete resolution in 75% of patients and partial improvement in 25%, however seven had recurrence of symptoms after ECP was stopped.
Thirty-one patients with epidermolysis bullosa acquisita (EBA) including 12 with pemphigus vulgaris from 10 studies received ECP. Four studies [46,51,54,65] (n = 25) were evaluated. The average duration of ECP was 20 cycles and was performed every 4 weeks. Overall response was seen in 88% (75–100%). Immunosuppression was tapered in all patients and continuous responses (>6 months) were achieved after ECP was stopped. ECP was less effective in patients with high autoantibody titer and as a mono-therapy [54].
SS is a multiorgan connective tissue disorder and the AID most commonly treated with ECP. The first patient was treated 25 years ago by Rook et al. [45] and achieved clinical improvement. Eleven of 12 studies were evaluated. One-hundred seventy seven patients (range 5–31 per study) received a median of 12 cycles for a period of 10 months (range 0.5–59 months). The ECP schedule was two treatments per month in the majority of studies. Reported response for these patients was 50% (range 5–100%). Three prospective studies with 31, 16, and 19 patients were evaluated. Overall, a response was seen in 37% of patients (range 5–69%) in these cohorts. Significant clinical improvement as demonstrated by reduction of dermal thickness was achieved in patients with dermal edema, without visceral involvement, compared to those with fibrosis [64]. When compared to D-penicillamine therapy in multicenter trial, at 6 and 10 months, significant clinical improvement in the skin was seen in ECP arm. In another randomized, double-blind, placebo-controlled multicenter study by Knobler et al. [65] 27 patients treated with ECP were compared to 37 receiving sham therapy. ECP arm showed significant improvement in skin severity, assessed in 22 body regions and in joint involvement (60 joints) when compared to baseline. However, there was no difference in skin scores between groups after treatment was stopped. On the other hand, a significant number of joints showed improvement over the 12 months of ECP with a significant decrease in the number of new joints involved, suggesting both stabilization and prevention of further damage by ECP therapy. As a surrogate marker for response, elevation of T-regs and decrease of Th17 cells were seen as early as after two cycles. In addition, increases in IL-10, IL-1Ra, and HGF levels with decreases in CCL2 and TGF-beta levels were seen in the ECP arm.
For SLE from six available studies (n = 17) only 2 (n = 12) can be evaluated [66,69]. ECP was performed for a median of 4 months (range 1–6). All of these patients were females with mild to moderate disease activity at the start of ECP, while 47% had a disease flare. Clinical improvement defined as a reduction of clinical activity score was achieved in the vast majority of patients (94%, range 88–100). ECP-responders were able to withdraw from immunosuppression during ECP with prolonged remissions reported. Side effects such as photosensitivity were reported in 59% of patients without exacerbation of SLE.
NSF was reported in four studies with 12 treated patients. Three studies (n = 11) can be evaluated. The median duration of treatment was 7 months with 18 cycles per patient. Considerable improvement of skin induration and joint mobility was seen in all of the patients. In the largest study of case series with five NSF patients by Richmond et al [75] 60% of patients showed a mild benefit. The response score was classified as worsened, stable, mildly or markedly improved.
ECP was reported in four studies (n = 28, range 2–16 per study) with MS. Three studies (n = 26) were evaluated. These patients received ECP for a median of 12 months (range 6–24) with two treatments per month. Response was defined as reduction in MS relapse rates [77], MRI stabilization [80], and reduction in EDSS [89]. However, Rostami et al. in a double blind, placebo-controlled study [78] demonstrated no difference in EDSS, ambulation, and Scripp’s scores. Moreover, ECP was also ineffective in progressive MS.
ECP was tested in 29 patients with diabetes type I (DM1) in four randomized double-blind studies. Three of them included identical patients [76,82,85]. Patients received a median of five cycles with a median duration of 4 months. It is difficult to evaluate the role of ECP in treatment of DM1 as only mild suppressive effect was associated with lower insulin need and stabilization of disease progression [81] in ECP arm.
ECP for Crohn’s disease, a chronic inflammatory bowel disease, was evaluated in five studies with 72 patients, including 68 patients from three prospective studies. Patients received ECP every 2 weeks with an average of 16 cycles per patient or 6 months of therapy. Overall response was evaluated by Crohn’s disease activity index and in these prospective studies response was seen in 33% of patients. Response was more pronounced in patients with moderately active and refractory disease and were also associated with significant reduction [89] or discontinuation of steroids [85]. Patients remained in remission for 48 weeks after discontinuation of ECP.
Other AID treated with ECP includes RA (n = 16), psoriasis (n = 1) and deep morphea (n = 4). Fifteen patients with RA were evaluated with average of nine ECP cycles for 6 months [90,91]. Overall clinical improvement was seen in 50% of patients. Patients with deep morphea [92] (n = 4) and psoriasis [93] (n = 1) achieved complete resolution after 12 cycles of ECP or 6 months of therapy. Due to small number of patients it is impossible to evaluate the efficacy of ECP in these diseases.
EVALUATION OF EFFICACY
Standardized scoring methods should be used to document severity of the AID at baseline. Furthermore, response to the therapy should be documented by monitoring primary and secondary outcomes at defined intervals, monthly or every 3 months. For example, skin severity scoring including percentages of skin involvement and quantity of lesions should be assessed and documented properly, including photography to allow comparisons within the trial or between different studies. Defined eligibility criteria are critical for study interpretation. Patients with long-lasting atopic dermatitis (>12months), resistant to major immunosuppressants, with a SCORAD >45 are considered for the ECP treatment. ECP administration and response assessment intervals and duration should be precisely defined. ECP response evaluation for atopic dermatitis should be performed by using SCORAD scale [30]. For EBA, global assessment of all lesions assessed by PDAI (pemphigus disease area index), ABSIS (autoimmune bullous skin intensity score) for EBA was recently approved [94] and should be used to monitor response. Based on the available literature, for SS ECP is recommended in early progressive disease, mainly of the skin, typically in combination, as a second line treatment. A standard outcome measure for skin, determined by modified Rodnan skin score and photography, can be used to assess response at baseline and every month during treatment. For psoriasis two scoring parameters were described, the percentage of involved body surface and psoriasis area severity index (PASI) [92]. For NSF the degree of response to ECP can be determined by quantity of skin induration, using modified Rodnan score, range of motion and patient perception of disability [75]. The limited data on Crohn’s disease suggests that patients may benefit from ECP by reduction of steroids and stabilization of active Crohn’s disease can be achieved. Crohn’s disease activity index (CDAI) score can be used to monitor responses to ECP [89]. Dermatological life quality index, SKINEX as well as other commonly used quality of life assessment scores (FACT, SF-36) may be used as additional patient reported outcomes to monitor the response to ECP. The comparison to baseline score is usually performed at 3 months after the start of ECP, as it is not expected that chronic inflammatory diseases will respond within a shorter interval. Furthermore, based on the immunological changes (Tregs, apoptosis etc), it is reasonable to explore the use of these factors as potential biomarkers for monitoring responses to ECP. These biomarkers should be tested at the same time intervals as the evaluation for clinical response.
LIMITATIONS
The decisions that can be made concerning the use of ECP in AID are limited as most studies conducted so far are small, retrospective, included only case reports and used various response criteria. In addition, ECP centers use different devices and methods (inline, offline). Treatment schedules vary from one cycle per week up to one cycle per month or every 6 weeks. Similar variations were seen in the duration of therapy, ranging from 15 days of therapy up to 6 years. This variability might have a significant impact on assessment of clinical response. Patients’ disease courses (long-lasting versus early onset) as well as conventional systemic immunosuppression or monotherapy varied in observed studies. Besides, it is not only important that outcome measures record best clinical improvement but also disease stabilization, prevention from further disability or death from chronic progressive AID are important.
DISCUSSION
There is a need for better and less toxic treatments for severe and therapy-refractory AID. The benefits of ECP are well documented in a substantial number of patients with chronic GVHD, including severe and therapy-refractory cases [95–99] [100,101]. In solid organ transplantation reduction of immunosuppression, resolution of rejection and achievement of sustained response have also been reported [102–106]. The focus of this review was to assess the effects of ECP in patients with AID. Based on reported clinical experience, there is sufficient data available to suggest the potential effectiveness and to support further investigation of ECP in AID. ECP for AID is a feasible, extensively used and promising therapeutic option for certain patients. However, the magnitude of benefit and best patient selection are far from clear and larger studies should offer an opportunity to standardize the ECP process. It is also important to provide better defined guidance with regard to the length and frequency of the procedures, amount of infused cells and number of cycles and concomitant use of immunosuppressive therapy. The beneficial effect of ECP is associated with enhancement and normalization of clearance of excessive apoptotic material which is delayed in AID [107–110]. Down modulation of cell-mediated immunity is associated with the mechanism of ECP action. In addition, modulation of the inflammatory environment, cytokine profile and regulatory cell subsets are observed during ECP and are most pronounced in responders to the therapy. Apoptotic-cell based therapies can be also used for prevention, as it was recently showed in GVHD [111] and treatment of transplant rejection [112]. All these provide a mechanistic rationale for evaluating ECP as a therapeutic approach in AID.
Major limitations of prolonged immunosuppression are the toxicity, infectious complications, and disease flares during attempts for therapy taper. No relation to infectious complications or other significant side effects were reported among summarized studies. Compared to conventional therapies ECP has noticeable advantages as it is not immunosuppressive. Besides, ECP allows tapering and even discontinuing concomitant immunosuppression including steroids. Other important factor supporting ECP therapy is tolerability and safety: no increased incidence of infection or organ damage was seen in these studies. Few side effects such as hypotension, transient anemia, catheter-related complications, and hypersensitivity to 8-MOP were reported, but no long-term complications were seen in these patients. ECP is contraindicated for patients with AID with allergy to 8-MOP, light-sensitive disease, aphakia, and pregnancy. Moreover, monitoring of blood counts with electrolytes is necessary prior and after ECP procedure. Transfusions maybe required when platelets are below 20,000, or hematocrit is less than 33%.
ECP therapy is a time-consuming procedure which requires lengthy treatments, with a median duration up to 6 months to achieve durable response. The need for intravenous access and day-hospital facility in a qualified ECP center with experienced staff are often limiting factors. Moreover, patients with AID may face other important issues such as geographical distance to access the ECP facility and the high costs of this treatment. The latter may be connected with the lack of insurance coverage, as ECP is still an investigational treatment.
In a variety of disease, responders to ECP demonstrate increase in survival rates [113] and quality of life [31,114,115]. Whether such benefit exists in AID has not been documented yet. In the future studies in AID, criteria for efficacy should be better standardized and likely defined by complete response (CR), a resolution of all disease manifestations, partial response (PR), a reduction in more than 50% of manifestations; stable disease (SD), defined as absence of further progression in any organ or site, and progression of disease (PD). Validated, disease-specific tools should be used wherever possible for evaluation of response to the therapy and performed on predefined time intervals. In general for treatment induction it is recommended to administer ECP every 2 weeks on two consecutive days. After evidence of response ECP can be administered once a month till resolution or stabilization as maintenance.
Further requirements to conduct a clinical trial of ECP in AID include defined patient population and standard operating procedures. Patient selection issues include adults or children, their functional status, distance from the ECP center, admission to the day hospital and organ function. Patients may require vascular access which can be peripheral or central line, single or dual needle. The goal of defining patient selection with AID is to improve interpretation of the data and identify the patient group with a likely maximum response rate, which also depends on the duration and stage of the disease. Further eligibility criteria may include patients with increased susceptibility to infections, as ECP doesn’t increase infection rate, patients with AID flares, as ECP demonstrated reduction of flares; and patients with a steroid-refractory disease. The latter allows the tapering of immunosuppression or the switching of patients to another salvage line. ECP could be used to prevent disease relapses such as in MS [80]. The issues related to ECP procedures include the availability of ECP-trained staff, ECP instruments and kits. The number of patients that can be enrolled on a trial also depends on the quantities of instruments, kits and experienced staff available.
There is substantial biological rational and clinical evidence supporting further investigation of ECP in treating AID. AID are heterogeneous and have complex presentations with very few available biological parameters which can be applied for prediction of response and its monitoring. Also multiple confounding factors can influence the outcome of ECP therapy which is all together mandating strict standardization of clinical trials design. It is currently too early to draw any conclusions concerning whether long-term treatment with ECP can effectively lead to clinical benefit. The existing data after 25 years of experience clearly demonstrate safety and feasibility of ECP. However, to better determine its role as a potential standard therapy in AID, randomized and well-planned prospective controlled trials are necessary.
Fig. 3.
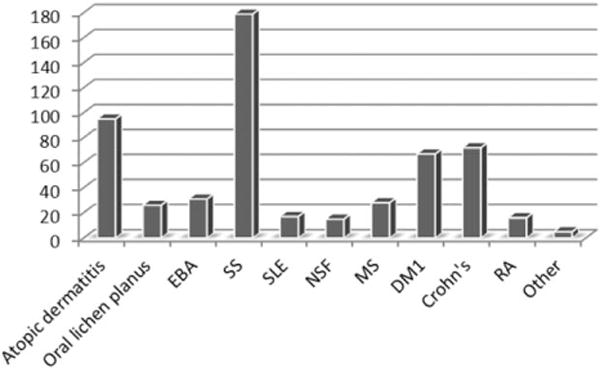
Number of patients (N = 551) with AID treated with ECP. The number of patients studied (X-axis) n = 551 is summarized in the bar diagram, each bar demonstrates the number of patients for each disease. Other diseases include deep morphea and psoriasis.
Acknowledgments
This manuscript was presented in part at the Workshop on Transplant and Cellular Therapy for Autoimmune Diseases held at Froedtert & Medical College of Wisconsin Clinical Cancer Center, Milwaukee, WI, April 20, 2013.
Abbreviations
- 8-MOP
8 methoxypsoralen
- ABSIS
autoimmune bullous skin intensity score
- AID
autoimmune diseases
- APC
antigen-presenting cells
- CDAI
Crohn’s disease activity index
- CR
complete response
- CTCL
cutaneous T-cell lymphoma
- DMI
diabetic mellitus type I
- EBA
epidermolysis bullosa acquisita
- ECP
Extracorporeal photopheresis
- GVHD
graft-versus-host disease
- HCT
hematopoietic stem cell transplantation
- MS
multiple sclerosis
- NSF
nephrogenic systemic fibrosis
- PASI
psoriasis area severity index
- PD
progression of disease
- PDAI
pemphigus disease area index
- pDC
plasmacytoid dendritic cells
- PR
partial response
- RA
rheumatoid arthritis
- SD
stable disease
- SLE
systemic lupus erythematosus
- SS
systemic sclerosis
References
- 1.Klassen J. The role of photopheresis in the treatment of graft-versus-host disease. Curr Oncol. 2010;17:55–58. doi: 10.3747/co.v17i2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knobler R, Berlin G, Calzavara-Pinton P, Greinix H, Jaksch P, Laroche L, Ludvigsson J, Quaglino P, Reinisch W, Scarisbrick J, Schwarz T, Wolf P, Arenberger P, Assaf C, Bagot M, Barr M, Bohbot A, Bruckner-Tuderman L, Dreno B, Enk A, French L, Gniadecki R, Gollnick H, Hertl M, Jantschitsch C, Jung A, Just U, Klemke CD, Lippert U, Luger T, Papadavid E, Pehamberger H, Ranki A, Stadler R, Sterry W, Wolf IH, Worm M, Zic J, Zouboulis CC, Hillen U. Guidelines on the use of extracorporeal photopheresis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 1):1–37. doi: 10.1111/jdv.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber LL, Dunbar NM, Dumont LJ, Szczepiorkowski ZM. Successful use of citrate anticoagulant with heparin bolus for excessive clotting during extracorporeal photopheresis. Transfusion. 2012;52:2494–2495. doi: 10.1111/j.1537-2995.2012.03846.x. [DOI] [PubMed] [Google Scholar]
- 4.Lamioni A, Parisi F, Isacchi G, Giorda E, Di Cesare S, Landolfo A, Cenci F, Bottazzo GF, Carsetti R. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–850. doi: 10.1097/01.tp.0000157278.02848.c7. [DOI] [PubMed] [Google Scholar]
- 5.Daniele N, Del Proposto G, Cerrone P, Sinopoli S, Sansone L, Gadaleta DI, Lanti A, Ferraro AS, Spurio S, Scerpa MC, Zinno F, Adorno G, Isacchi G. Evaluation of cell death after treatment with extracorporeal photopheresis. Transfus Apher Sci. 2012;46:53–57. doi: 10.1016/j.transci.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C, Renoldi G, Parma M, Balduzzi A, Perseghin P, Biondi A. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD41CD251GITR1Foxp31CD62L1 functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 7.Di Biaso I, Di Maio L, Bugarin C, Gaipa G, Dander E, Balduzzi A, Parma M, D’Amico G, Perseghin P, Biondi A, Biagi E. Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation. 2009;87:1422–1425. doi: 10.1097/TP.0b013e3181a27a5d. [DOI] [PubMed] [Google Scholar]
- 8.French LE, Alcindor T, Shapiro M, McGinnis KS, Margolis DJ, Porter D, Leonard DG, Rook AH, Foss F. Identification of amplified clonal T cell populations in the blood of patients with chronic graft-versus-host disease: positive correlation with response to photopheresis. Bone Marrow Transplant. 2002;30:509–515. doi: 10.1038/sj.bmt.1703705. [DOI] [PubMed] [Google Scholar]
- 9.Miracco C, Rubegni P, De Aloe G, D’Ascenzo G, Mazzatenta C, De Santi MM, Fimiani M. Extracorporeal photochemotherapy induces apoptosis of infiltrating lymphoid cells in patients with mycosis fungoides in early stages. A quantitative histological study. Br J Dermatol. 1997;137:549–557. doi: 10.1111/j.1365-2133.1997.tb03785.x. [DOI] [PubMed] [Google Scholar]
- 10.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 11.Klosner G, Trautinger F, Knobler R, Neuner P. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459–462. doi: 10.1046/j.1523-1747.2001.01276.x. [DOI] [PubMed] [Google Scholar]
- 12.Alcindor T, Gorgun G, Miller KB, Roberts TF, Sprague K, Schenkein DP, Foss FM. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001;98:1622–1625. doi: 10.1182/blood.v98.5.1622. [DOI] [PubMed] [Google Scholar]
- 13.Foss FM, Gorgun G, Miller KB. Extracorporeal photopheresis in chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29:719–725. doi: 10.1038/sj.bmt.1703529. [DOI] [PubMed] [Google Scholar]
- 14.Di Renzo M, Rubegni P, De Aloe G, Paulesu L, Pasqui AL, Andreassi L, Auteri A, Fimiani M. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T-cell lymphoma. Immunology. 1997;92:99–103. doi: 10.1046/j.1365-2567.1997.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiue LH, Alousi AM, Wei C, Hosing CM, Duvic M, Ni X. Augmentation of blood dendritic cells by extracorporeal photopheresis in patients with leukemic cutaneous T-cell lymphoma and graft-versus-host disease. J Invest Dermatol. 2013;133:2098–3100. doi: 10.1038/jid.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bladon J, Taylor PC. Lymphocytes treated by extracorporeal photopheresis can down-regulate cytokine production in untreated monocytes. Photodermatol Photoimmunol Photomed. 2005;21:293–302. doi: 10.1111/j.1600-0781.2005.00192.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Renzo M, Rubegni P, Sbano P, Cuccia A, Castagnini C, Pompella G, Pasqui AL, Capecchi PL, Auteri A, Laghi Pasini F, Fimiani M. ECP-treated lymphocytes of chronic graft-versus-host disease patients undergo apoptosis which involves both the Fas/FasL system and the Bcl-2 protein family. Arch Dermatol Res. 2003;295:175–182. doi: 10.1007/s00403-003-0415-6. [DOI] [PubMed] [Google Scholar]
- 18.Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Herrmann M. SLE—a disease of clearance deficiency? Rheumatology. 2005;44:1101–1107. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 19.Kuzmina Z, Greinix HT, Knobler R, Worel N, Kouba M, Weigl R, Kormoczi U, Rottal A, Pohlreich D, Zielinski C, Pickl WF. Proportions of immature CD191CD21- B lymphocytes predict the response to extracorporeal photopheresis in patients with chronic graft-versus-host disease. Blood. 2009;114:744–746. doi: 10.1182/blood-2009-05-221028. [DOI] [PubMed] [Google Scholar]
- 20.Whittle R, Taylor PC. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host disease to extracorporeal photopheresis. Blood. 2011;118:6446–6449. doi: 10.1182/blood-2011-05-354019. [DOI] [PubMed] [Google Scholar]
- 21.Sgouroudis E, Piccirillo CA. Control of type 1 diabetes by CD41Foxp31 regulatory T cells: lessons from mouse models and implications for human disease. Diabetes Metab Res Rev. 2009;25:208–218. doi: 10.1002/dmrr.945. [DOI] [PubMed] [Google Scholar]
- 22.Gerli R, Nocentini G, Alunno A, Bocci EB, Bianchini R, Bistoni O, Riccardi C. Identification of regulatory T cells in systemic lupus erythematosus. Autoimmun Rev. 2009;8:426–430. doi: 10.1016/j.autrev.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Cooles FA, Isaacs JD, Anderson AE. Treg cells in rheumatoid arthritis: an update. Curr Rheumatol Rep. 2013;15:352. doi: 10.1007/s11926-013-0352-0. [DOI] [PubMed] [Google Scholar]
- 24.Kamikozuru K, Fukunaga K, Hirota S, Hida N, Ohda Y, Yoshida K, Yokoyama Y, Tozawa K, Kawa K, Iimuro M, Nagase K, Saniabadi AR, Nakamura S, Miwa H, Matsumoto T. The expression profile of functional regulatory T cells, CD41CD25high1/forkhead box protein P31, in patients with ulcerative colitis during active and quiescent disease. Clin Exp Immunol. 2009;156:320–327. doi: 10.1111/j.1365-2249.2009.03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rong G, Zhong R. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol. 2009;156:217–225. doi: 10.1111/j.1365-2249.2009.03898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt S, Johnson TS, Karakhanova S, Naher H, Mahnke K, Enk AH. Extracorporeal photophoresis augments function of CD41CD251FoxP31 regulatory T cells by triggering adenosine production. Transplantation. 2009;88:411–416. doi: 10.1097/TP.0b013e3181aed927. [DOI] [PubMed] [Google Scholar]
- 27.Quaglino P, Comessatti A, Ponti R, Peroni A, Mola F, Fierro MT, Savoia P, Novelli M, Bernengo MG. Reciprocal modulation of circulating CD41CD251bright T cells induced by extracorporeal photochemotherapy in cutaneous T-cell lymphoma and chronic graft-versus-host-disease patients. Int J Immunopathol Pharmacol. 2009;22:353–362. doi: 10.1177/039463200902200212. [DOI] [PubMed] [Google Scholar]
- 28.Meloni F, Cascina A, Miserere S, Perotti C, Vitulo P, Fietta AM. Peripheral CD4(1)CD25(1) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplant Proc. 2007;39:213–217. doi: 10.1016/j.transproceed.2006.10.227. [DOI] [PubMed] [Google Scholar]
- 29.Prinz B, Nachbar F, Plewig G. Treatment of severe atopic dermatitis with extracorporeal photopheresis. Arch Dermatol Res. 1994;287:48–52. doi: 10.1007/BF00370718. [DOI] [PubMed] [Google Scholar]
- 30.Radenhausen M, Michelsen S, Plewig G, Bechara FG, Altmeyer P, Hoffmann K. Bicentre experience in the treatment of severe generalised atopic dermatitis with extracorporeal photochemotherapy. J Dermatol. 2004;31:961–970. doi: 10.1111/j.1346-8138.2004.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 31.Sand M, Bechara FG, Sand D, Radenhausen M, Tomi NS, Altmeyer P, Hoffmann K. Extracorporeal photopheresis as a treatment for patients with severe, refractory atopic dermatitis. Dermatology. 2007;215:134–138. doi: 10.1159/000104265. [DOI] [PubMed] [Google Scholar]
- 32.Radenhausen M, von Kobyletzki G, Hoxtermann S, Altmeyer P, Hoffmann K. Activation markers in severe atopic dermatitis following extracorporeal photochemotherapy. Acta Derm Venereol. 2003;83:49–50. doi: 10.1080/00015550310002710. [DOI] [PubMed] [Google Scholar]
- 33.Richter HI, Billmann-Eberwein C, Grewe M, Stege H, Berneburg M, Ruzicka T, Krutmann J. Successful monotherapy of severe and intractable atopic dermatitis by photopheresis. J Am Acad Dermatol. 1998;38:585–588. doi: 10.1016/s0190-9622(98)70122-7. [DOI] [PubMed] [Google Scholar]
- 34.Rubegni P, Poggiali S, Cevenini G, D’Ascenzo G, Perrone A, Flori ML, Barbini P, Fimiani M. Long term follow-up results on severe recalcitrant atopic dermatitis treated with extracorporeal photochemotherapy. J Eur Acad Dermatol Venereol. 2013;27:523–526. doi: 10.1111/j.1468-3083.2012.04552.x. [DOI] [PubMed] [Google Scholar]
- 35.Hjuler KP, Vestergaard C, Deleuran M. A retrospective study of six cases of severe recalcitrant atopic dermatitis treated with long-term extracorporeal photopheresis. Acta Derm Venereol. 2010;90:635–636. doi: 10.2340/00015555-0952. [DOI] [PubMed] [Google Scholar]
- 36.Gerber M, Gmeinhart B, Volc-Platzer B, Kalhs P, Greinix H, Knobler R. Complete remission of lichen-planus-like graft-versus-host disease (GVHD) with extracorporeal photochemotherapy (ECP) Bone Marrow Transplant. 1997;19:517–519. doi: 10.1038/sj.bmt.1700661. [DOI] [PubMed] [Google Scholar]
- 37.Wolf PGD, Tomi NS, Schempp CM, Hoffmann K. Extracorporeal photochemotherapy as systemic monotherapy of severe, refractory atopic dermatitis: results from a prospective trial. Photochem Photobiol Sci. 2013;12:174–181. doi: 10.1039/c2pp25203a. [DOI] [PubMed] [Google Scholar]
- 38.Becherel PA, Bussel A, Chosidow O, Rabian C, Piette JC, Frances C. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805. doi: 10.1016/s0140-6736(05)78932-7. [DOI] [PubMed] [Google Scholar]
- 39.Elewa R, Altenburg A, Zouboulis CC. Recalcitrant severe erosive cutaneous lichen planus treated with extracorporeal photopheresis monotherapy. Br J Dermatol. 2011;165:441–443. doi: 10.1111/j.1365-2133.2011.10378.x. [DOI] [PubMed] [Google Scholar]
- 40.Kunte C, Erlenkeuser-Uebelhoer I, Michelsen S, Scheerer-Dhungel K, Plewig G. Treatment of therapy-resistant erosive oral lichen planus with extracorporeal photopheresis (ECP) J Dtsch Dermatol Ges. 2005;3:889–894. doi: 10.1111/j.1610-0387.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 41.Guyot AD, Farhi D, Ingen-Housz-Oro S, Bussel A, Parquet N, Rabian C, Bachelez H, Frances C. Treatment of refractory erosive oral lichen planus with extracorporeal photochemotherapy: 12 cases. Br J Dermatol. 2007;156:553–556. doi: 10.1111/j.1365-2133.2006.07647.x. [DOI] [PubMed] [Google Scholar]
- 42.Toberer F, Naher H. Lichen sclerosus et atrophicans leading to joint contractures: restoration of joint mobility by extracorporeal photopheresis. J Am Acad Dermatol. 2012;67:e269–e271. doi: 10.1016/j.jaad.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Marchesseau-Merlin AS, Perea R, Kanold J, Demeocq F, Souteyrand P, D’Incan M. Photopheresis: an alternative therapeutic approach in corticoresistant erosive oral lichen planus. Ann Dermatol Venereol. 2008;135:209–212. doi: 10.1016/j.annder.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Rook AH, Jegasothy BV, Heald P, Nahass GT, Ditre C, Witmer WK, Lazarus GS, Edelson RL. Extracorporeal photochemotherapy for drug-resistant pemphigus vulgaris. Ann Intern Med. 1990;112:303–305. doi: 10.7326/0003-4819-112-4-303. [DOI] [PubMed] [Google Scholar]
- 45.Rook AHHP, Nahass GT, Jegasothy BV. Treatment of autoimmune disease with extracorporeal photochemotherapy: pemphigus vulgaris—preliminary report. Yale J Biol Med. 1989;62:647–652. [PMC free article] [PubMed] [Google Scholar]
- 46.Liang G, Nahass G, Kerdel FA. Pemphigus vulgaris treated with photopheresis. J Am Acad Dermatol. 1992;26(5 Pt 1):779–780. doi: 10.1016/s0190-9622(08)80560-9. [DOI] [PubMed] [Google Scholar]
- 47.Gollnick HP, Owsianowski M, Taube KM, Orfanos CE. Unresponsive severe generalized pemphigus vulgaris successfully controlled by extracorporeal photopheresis. J Am Acad Dermatol. 1993;28:122–124. doi: 10.1016/s0190-9622(08)80854-7. [DOI] [PubMed] [Google Scholar]
- 48.Miller JL, Stricklin GP, Fine JD, King LE, Arzubiaga MC, Ellis DL. Remission of severe epidermolysis bullosa acquisita induced by extracorporeal photochemotherapy. Br J Dermatol. 1995;133:467–471. doi: 10.1111/j.1365-2133.1995.tb02680.x. [DOI] [PubMed] [Google Scholar]
- 49.Owsianowski M, Garbe C, Ramaker J, Orfanos CE, Gollnick H. Therapeutic experiences with extracorporeal photopheresis. Technical procedure, follow-up and clinical outcome in 31 skin diseases. Hautarzt. 1996;47:114–123. doi: 10.1007/s001050050387. [DOI] [PubMed] [Google Scholar]
- 50.Gordon KB, Chan LS, Woodley DT. Treatment of refractory epidermolysis bullosa acquisita with extracorporeal photochemotherapy. Br J Dermatol. 1997;136:415–420. [PubMed] [Google Scholar]
- 51.Azana JM, de Misa RF, Harto A, Ledo A, Espana A. Severe pemphigus foliaceus treated with extracorporeal photochemotherapy. Arch Dermatol. 1997;133:287–289. doi: 10.1001/archderm.1997.03890390021002. [DOI] [PubMed] [Google Scholar]
- 52.Camara A, Becherel PA, Bussel A, Lagrange S, Chosidow O, Joly P, Piette JC, Frances C. Resistant acquired bullous epidermolysis with severe ocular involvement: the success of extracorporeal photochemotherapy. Ann Dermatol Venereol. 1999;126:612–615. [PubMed] [Google Scholar]
- 53.Wollina U, Lange D, Looks A. Short-time extracorporeal photochemotherapy in the treatment of drug-resistant autoimmune bullous diseases. Dermatology. 1999;198:140–144. doi: 10.1159/000018090. [DOI] [PubMed] [Google Scholar]
- 54.Sanli H, Akay BN, Ayyildiz E, Anadolu R, Ilhan O. Remission of severe autoimmune bullous disorders induced by long-term extracorporeal photochemotherapy. Transfus Apher Sci. 2010;43:353–359. doi: 10.1016/j.transci.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Rook AH. Photopheresis in the treatment of autoimmune disease: experience with pemphigus vulgaris and systemic sclerosis. Ann N Y Acad Sci. 1991;636:209–216. doi: 10.1111/j.1749-6632.1991.tb33452.x. [DOI] [PubMed] [Google Scholar]
- 56.Rook AH, Freundlich B, Jegasothy BV, Perez MI, Barr WG, Jimenez SA, Rietschel RL, Wintroub B, Kahaleh MB, Varga J, et al. Treatment of systemic sclerosis with extracorporeal photochemotherapy. Results of a multicenter trial. Arch Dermatol. 1992;128:337–346. [PubMed] [Google Scholar]
- 57.Cribier B, Faradji T, Le Coz C, Oberling F, Grosshans E. Extracorporeal photochemotherapy in systemic sclerosis and severe morphea. Dermatology. 1995;91:25–31. doi: 10.1159/000246481. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz J, Gonzalez J, Palangio M, Klainer AS, Bisaccia E. Extracorporeal photochemotherapy in progressive systemic sclerosis: a follow-up study. Int J Dermatol. 1997;36:380–385. doi: 10.1046/j.1365-4362.1997.00066.x. [DOI] [PubMed] [Google Scholar]
- 59.Krasagakis K, Dippel E, Ramaker J, Owsianowski M, Orfanos CE. Management of severe scleroderma with long-term extracorporeal photopheresis. Dermatology. 1998;196:309–315. doi: 10.1159/000017927. [DOI] [PubMed] [Google Scholar]
- 60.Enomoto DN, Mekkes JR, Bossuyt PM, Yong SL, Out TA, Hoekzema R, de Rie MA, Schellekens PT, ten Berge IJ, de Borgie CA, Bos JD. Treatment of patients with systemic sclerosis with extracorporeal photochemotherapy (photopheresis) J Am Acad Dermatol. 1999;41:915–922. doi: 10.1016/s0190-9622(99)70246-x. [DOI] [PubMed] [Google Scholar]
- 61.Muellegger RR, Hofer A, Salmhofer W, Soyer HP, Kerl H, Wolf P. Extended extracorporeal photochemotherapy with extracorporeal administration of 8-methoxypsoralen in systemic sclerosis. An Austrian single-center study. Photodermatol Photoimmunol Photomed. 2000;16:216–223. doi: 10.1034/j.1600-0781.2000.160505.x. [DOI] [PubMed] [Google Scholar]
- 62.Reich S, Radenhausen M, Altmeyer P, Hoffmann K. Extracorporeal photopheresis in progressive systemic sclerosis: discrimination of responders and non-responders. J Dtsch Dermatol Ges. 2003;1:945–951. doi: 10.1046/j.1439-0353.2003.03747.x. [DOI] [PubMed] [Google Scholar]
- 63.Papp G, Horvath IF, Barath S, Gyimesi E, Vegh J, Szodoray P, Zeher M. Immunomodulatory effects of extracorporeal photochemotherapy in systemic sclerosis. Clin Immunol. 2012;142:150–159. doi: 10.1016/j.clim.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Hashikabe M, Ohtsuka T, Yamazaki S. Quantitative echographic analysis of photochemotherapy on systemic sclerosis skin. Arch Dermatol Res. 2005;296:522–527. doi: 10.1007/s00403-005-0552-1. [DOI] [PubMed] [Google Scholar]
- 65.Knobler RM, French LE, Kim Y, Bisaccia E, Graninger W, Nahavandi H, Strobl FJ, Keystone E, Mehlmauer M, Rook AH, Braverman I. A randomized, double-blind, placebo-controlled trial of photopheresis in systemic sclerosis. J Am Acad Dermatol. 2006;54:793–799. doi: 10.1016/j.jaad.2005.11.1091. [DOI] [PubMed] [Google Scholar]
- 66.Knobler RM, Graninger W, Lindmaier A, Trautinger F. Photopheresis for the treatment of lupus erythematosus. Preliminary observations. Ann N Y Acad Sci. 1991;636:340–356. doi: 10.1111/j.1749-6632.1991.tb33464.x. [DOI] [PubMed] [Google Scholar]
- 67.Richter HI, Krutmann J, Goerz G. Extracorporeal photopheresis in therapy-refractory disseminated discoid lupus erythematosus. Hautarzt. 1998;49:487–491. doi: 10.1007/s001050050775. [DOI] [PubMed] [Google Scholar]
- 68.Wollina U, Looks A. Extracorporeal photochemotherapy in cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 1999;13:127–130. [PubMed] [Google Scholar]
- 69.Richard MA, Saadallah S, Lefevre P, Poullin P, Buscaylet S, Grob JJ. Extracorporeal photochemotherapy in therapy-refractory subacute lupus. Ann Dermatol Venereol. 2002;129:1023–1026. [PubMed] [Google Scholar]
- 70.Morruzzi C, Liu V, Bohbot A, Cribier B, Lipsker D. Four cases of photopheresis treatment for cutaneous lupus erythematosus refractory to standard therapy. Ann Dermatol Venereol. 2009;136:861–867. doi: 10.1016/j.annder.2009.10.183. [DOI] [PubMed] [Google Scholar]
- 71.Boeckler P, Liu V, Lipsker D. Extracorporeal photopheresis in recalcitrant lupus erythematosus. Clin Exp Dermatol. 2009;34:e295–e296. doi: 10.1111/j.1365-2230.2009.03239.x. [DOI] [PubMed] [Google Scholar]
- 72.Lauchli S, Zortea-Caflisch C, Nestle FO, Burg G, Kempf W. Nephrogenic fibrosing dermopathy treated with extracorporeal photopheresis. Dermatology. 2004;208:278–280. doi: 10.1159/000077321. [DOI] [PubMed] [Google Scholar]
- 73.Gilliet M, Cozzio A, Burg G, Nestle FO. Successful treatment of three cases of nephrogenic fibrosing dermopathy with extracorporeal photopheresis. Br J Dermatol. 2005;152:531–536. doi: 10.1111/j.1365-2133.2005.06434.x. [DOI] [PubMed] [Google Scholar]
- 74.Mathur K, Morris S, Deighan C, Green R, Douglas KW. Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: three case reports and review of literature. J Clin Apher. 2008;23:144–150. doi: 10.1002/jca.20170. [DOI] [PubMed] [Google Scholar]
- 75.Richmond H, Zwerner J, Kim Y, Fiorentino D. Nephrogenic systemic fibrosis: relationship to gadolinium and response to photopheresis. Arch Dermatol. 2007;143:1025–1030. doi: 10.1001/archderm.143.8.1025. [DOI] [PubMed] [Google Scholar]
- 76.Besnier DP, Chabannes D, Mussini JM, Dupas B, Esnault VL. Extracorporeal photochemotherapy for secondary chronic progressive multiple sclerosis: a pilot study. Photodermatol Photoimmunol Photomed. 2002;18:36–41. doi: 10.1034/j.1600-0781.2002.180106.x. [DOI] [PubMed] [Google Scholar]
- 77.Poehlau D, Rieks M, Postert T, Westerhausen R, Busch S, Hoffmann K, Altmeyer P, Przuntek H. Photopheresis—a possible treatment of multiple sclerosis?: report of two cases. J Clin Apher. 1997;12:154–155. doi: 10.1002/(sici)1098-1101(1997)12:3<154::aid-jca9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 78.Rostami AM, Sater RA, Bird SJ, Galetta S, Farber RE, Kamoun M, Silberberg DH, Grossman RI, Pfohl D. A double-blind, placebo-controlled trial of extracorporeal photopheresis in chronic progressive multiple sclerosis. Mult Scler. 1999;5:198–203. doi: 10.1177/135245859900500310. [DOI] [PubMed] [Google Scholar]
- 79.Ernerudh J, Ludvigsson J, Berlin G, Samuelsson U. Effect of photopheresis on lymphocyte population in children with newly diagnosed type 1 diabetes. Clin Diagn Lab Immunol. 2004;11:856–861. doi: 10.1128/CDLI.11.5.856-861.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cavaletti G, Perseghin P, Dassi M, Cavarretta R, Frigo M, Caputo D, Stanzani L, Tagliabue E, Zoia C, Grimaldi M, Isella V, Rota S, Ferrarese C, Frattola L. Extracorporeal photochemotherapy: a safety and tolerability pilot study with preliminary efficacy results in refractory relapsing-remitting multiple sclerosis. Neurol Sci. 2006;27:24–32. doi: 10.1007/s10072-006-0561-7. [DOI] [PubMed] [Google Scholar]
- 81.Ludvigsson J, Samuelsson U, Ernerudh J, Johansson C, Stenhammar L, Berlin G. Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Arch Dis Child. 2001;85:149–154. doi: 10.1136/adc.85.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faresjo MK, Ernerudh J, Berlin G, Garcia J, Ludvigsson J. The immunological effect of photopheresis in children with newly diagnosed type 1 diabetes. Pediatr Res. 2005;58:459–466. doi: 10.1203/01.pdr.0000176906.42001.c3. [DOI] [PubMed] [Google Scholar]
- 83.Jonson CO, Pihl M, Nyholm C, Cilio CM, Ludvigsson J, Faresjo M. Regulatory T cell-associated activity in photopheresis-induced immune tolerance in recent onset type 1 diabetes children. Clin Exp Immunol. 2008;153:174–181. doi: 10.1111/j.1365-2249.2008.03625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guariso G, D’Inca R, Sturniolo GC, Zancan L, Dall’Amico R. Photopheresis treatment in severe Crohn disease. J Pediatr Gastroenterol Nutr. 2003;37:517–520. doi: 10.1097/00005176-200310000-00022. [DOI] [PubMed] [Google Scholar]
- 85.Reinisch W, Nahavandi H, Santella R, Zhang Y, Gasche C, Moser G, Waldhor T, Gangl A, Vogelsang H, Knobler R. Extracorporeal photochemotherapy in patients with steroid-dependent Crohn’s disease: a prospective pilot study. Aliment Pharmacol Ther. 2001;15:1313–1322. doi: 10.1046/j.1365-2036.2001.01054.x. [DOI] [PubMed] [Google Scholar]
- 86.Bisaccia E, Palangio M, Gonzalez J. Extracorporeal photochemotherapy for the treatment of refractory Crohn’s disease. Transfus Apher Sci. 2007;37:171–174. doi: 10.1016/j.transci.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Abreu MT, von Tirpitz C, Hardi R, Kaatz M, Van Assche G, Rutgeerts P, Bisaccia E, Goerdt S, Hanauer S, Knobler R, Mannon P, Mayer L, Ochsenkuhn T, Sandborn WJ, Parenti D, Lee K, Reinisch W. Extracorporeal photopheresis for the treatment of refractory Crohn’s disease: results of an open-label pilot study. Inflamm Bowel Dis. 2009;15:829–836. doi: 10.1002/ibd.20833. [DOI] [PubMed] [Google Scholar]
- 88.EC V. Evaluation of clinical studies of the efficacy of therapeutic apheresis. J Clin Apher. 2000;15:6–17. doi: 10.1002/(sici)1098-1101(2000)15:1/2<6::aid-jca2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 89.Reinisch W, Knobler R, Rutgeerts PJ, Ochsenkuhn T, Anderson F, von Tirpitz C, Kaatz M, Janneke van der Woude C, Parenti D, Mannon PJ. Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn’s disease: an open-label, multicenter, prospective trial. Inflamm Bowel Dis. 2013;19:293–300. doi: 10.1002/ibd.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malawista SE, Trock D, Edelson RL. Photopheresis for rheumatoid arthritis. Ann N Y Acad Sci. 1991;636:217–226. doi: 10.1111/j.1749-6632.1991.tb33453.x. [DOI] [PubMed] [Google Scholar]
- 91.Vahlquist C, Larsson M, Ernerudh J, Berlin G, Skogh T, Vahlquist A. Treatment of psoriatic arthritis with extracorporeal photochemotherapy and conventional psoralen-ultraviolet A irradiation. Arthritis Rheum. 1996;39:1519–1523. doi: 10.1002/art.1780390911. [DOI] [PubMed] [Google Scholar]
- 92.Vonderheid EC, Kang CA, Kadin M, Bigler RD, Griffin TD, Rogers TJ. Extracorporeal photopheresis in psoriasis vulgaris: clinical and immunologic observations. J Am Acad Dermatol. 1990;23(4 Pt 1):703–712. doi: 10.1016/0190-9622(90)70278-p. [DOI] [PubMed] [Google Scholar]
- 93.Neustadter JH, Samarin F, Carlson KR, Girardi M. Extracorporeal photochemotherapy for generalized deep morphea. Arch Dermatol. 2009;145:127–130. doi: 10.1001/archdermatol.2008.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daniel BS, Hertl M, Werth VP, Eming R, Murrell DF. Severity score indexes for blistering diseases. Clin Dermatol. 2012;30:108–113. doi: 10.1016/j.clindermatol.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Apisarnthanarax N, Donato M, Korbling M, Couriel D, Gajewski J, Giralt S, Khouri I, Hosing C, Champlin R, Duvic M, Anderlini P. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: feasibility and results. Bone Marrow Transplant. 2003;31:459–465. doi: 10.1038/sj.bmt.1703871. [DOI] [PubMed] [Google Scholar]
- 96.Bisaccia E, Palangio M, Gonzalez J, Adler KR, Rowley SD, Goldberg SL. Treating refractory chronic graft-versus-host disease with extracorporeal photochemotherapy. Bone Marrow Transplant. 2003;31:291–294. doi: 10.1038/sj.bmt.1703830. [DOI] [PubMed] [Google Scholar]
- 97.Bisaccia E, Palangio M, Gonzalez J, Adler KR, Scarborough R, Goldberg SL, Rowley SD. Treatment of extensive chronic graft-versus-host disease with extracorporeal photochemotherapy. J Clin Apher. 2006;21:181–187. doi: 10.1002/jca.20084. [DOI] [PubMed] [Google Scholar]
- 98.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, Smith V, Khouri I, Giralt S, de Lima M, Hsu Y, Ghosh S, Neumann J, Andersson B, Qazilbash M, Hymes S, Kim S, Champlin R, Donato M. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–3080. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 99.Foss FM, DiVenuti GM, Chin K, Sprague K, Grodman H, Klein A, Chan G, Stiffler K, Miller KB. Prospective study of extracorporeal photopheresis in steroid-refractory or steroid-resistant extensive chronic graft-versus-host disease: analysis of response and survival incorporating prognostic factors. Bone Marrow Transplant. 2005;35:1187–1193. doi: 10.1038/sj.bmt.1704984. [DOI] [PubMed] [Google Scholar]
- 100.Greinix HT, Knobler RM, Worel N, Schneider B, Schneeberger A, Hoecker P, Mitterbauer M, Rabitsch W, Schulenburg A, Kalhs P. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91:405–408. [PubMed] [Google Scholar]
- 101.Greinix HT, Volc-Platzer B, Kalhs P, Fischer G, Rosenmayr A, Keil F, Honigsmann H, Knobler RM. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96:2426–2431. [PubMed] [Google Scholar]
- 102.Jaksch P, Scheed A, Keplinger M, Ernst MB, Dani T, Just U, Nahavandi H, Klepetko W, Knobler R. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2012;31:950–957. doi: 10.1016/j.healun.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 103.Barr ML, Meiser BM, Eisen HJ, Roberts RF, Livi U, Dall’Amico R, Dorent R, Rogers JG, Radovancevic B, Taylor DO, Jeevanandam V, Marboe CC. Photopheresis for the prevention of rejection in cardiac transplantation. Photopheresis Transplantation Study Grou N Engl J Med. 1998;339:1744–1751. doi: 10.1056/NEJM199812103392404. [DOI] [PubMed] [Google Scholar]
- 104.Dall’Amico R, Montini G, Murer L, Andreetta B, Tursi V, Feltrin G, Guzzi G, Angelini A, Zacchello G, Livi U. Benefits of photopheresis in the treatment of heart transplant patients with multiple/refractory rejection. Transplant Proc. 1997;29:609–711. doi: 10.1016/s0041-1345(96)00323-5. [DOI] [PubMed] [Google Scholar]
- 105.Dall’Amico R, Montini G, Murer L, Andreetta B, Zacchello G, Gambino A, Feltrin G, Caforio A, Tursi V, Livi U. Extracorporeal photochemotherapy after cardiac transplantation: a new therapeutic approach to allograft rejection. Int J Artif Organs. 2000;23:49–54. [PubMed] [Google Scholar]
- 106.Dall’Amico R, Murer L. Extracorporeal photochemotherapy: a new therapeutic approach for allograft rejection. Transfus Apher Sci. 2002;26:197–204. doi: 10.1016/s1473-0502(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 107.Hannani D, Merlin E, Gabert F, Laurin D, Dem eocq F, Chaperot L, Kanold J, Plumas J. Photochemotherapy induces a faster apoptosis of alloreactive activated T cells than of nonalloreactive resting T cells in graft versus host disease. Transplantation. 2010;90:1232–1238. doi: 10.1097/tp.0b013e3181fa4eb6. [DOI] [PubMed] [Google Scholar]
- 108.Holtick U, Wang XN, Marshall SR, Scheid C, von Bergwelt-Baildon M, Dickinson AM. In vitro PUVA treatment preferentially induces apoptosis in alloactivated T cells. Transplantation. 2012;94:e31–e34. doi: 10.1097/TP.0b013e31825f4454. [DOI] [PubMed] [Google Scholar]
- 109.Tambur AR, Ortegel JW, Morales A, Klingemann H, Gebel HM, Tharp MD. Extracorporeal photopheresis induces lymphocyte but not monocyte apoptosis. Transplant Proc. 2000;32:747–748. doi: 10.1016/s0041-1345(00)00966-0. [DOI] [PubMed] [Google Scholar]
- 110.Voss CY, Fry TJ, Coppes MJ, Blajchman MA. Extending the horizon for cell-based immunotherapy by understanding the mechanisms of action of photopheresis. Transfus Med Rev. 2010;24:22–32. doi: 10.1016/j.tmrv.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 111.Mevorach D, Zuckerman T, Reiner I, Shimoni A, Samuel S, Nagler A, Rowe JM, Or R. Single infusion of donor mononuclear early apoptotic cells as prophylaxis for graft-versus-host disease in myeloablative HLA-matched allogeneic bone marrow transplantation: a phase I/IIa clinical trial. Biol Blood Marrow Transplant. 2014;20:58–65. doi: 10.1016/j.bbmt.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 112.Morelli AE, Larregina AT. Apoptotic cell-based therapies against transplant rejection: role of recipient’s dendritic cells. Apoptosis. 2010;15:1083–1097. doi: 10.1007/s10495-010-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29:424–431. doi: 10.1016/j.healun.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 114.Mohla G, Horvath N, Stevens S. Quality of life improvement in a patient with severe atopic dermatitis treated with photopheresis. J Am Acad Dermatol. 1999;40(5 Pt 1):780–782. doi: 10.1016/s0190-9622(99)70167-2. [DOI] [PubMed] [Google Scholar]
- 115.Talpur R, Demierre MF, Geskin L, Baron E, Pugliese S, Eubank K, Zic JA, Miller DR, Tharp M, Bohjanen K, Duvic M. Multicenter photopheresis intervention trial in early-stage mycosis fungoides. Clin Lymphoma Myeloma Leuk. 2011;11:219–227. doi: 10.1016/j.clml.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 116.Prinz B, Michelsen S, Pfeiffer C, Plewig G. Long-term application of extracorporeal photochemotherapy in severe atopic dermatitis. J Am Acad Dermatol. 1999;40:577–582. doi: 10.1016/s0190-9622(99)70440-8. [DOI] [PubMed] [Google Scholar]


