Abstract
Purpose of review:
Frailty is an emerging concept in liver transplantation. We reviewed the literature for the tests of frailty which have been validated to predict outcomes in patients with cirrhosis evaluated for liver transplantation.
Recent findings:
Several tools have been developed to further stratify risk of adverse events independent of liver disease severity. These include tests of disability such as activities of daily living and the Karnofsky Performance Score as well as tests of physical frailty such as the Liver Frailty Index (hand-grip, chair stands, and balance)
Summary:
Increasingly, the frailty assessment is seen as a critical component of the liver transplant evaluation. Further research is needed to clarify the optimal timing of testing and the implications of longitudinal change.
Keywords: Liver Disease, Cirrhosis, Portal Hypertension, Hepatic Encephalopathy
Introduction
Liver transplantation is the only cure for end-stage liver disease. Yet, liver allografts are a limited, public good. To maximize outcomes for both patients and society, transplant clinicians must work to match donor grafts with the recipients who benefit the most. We must therefore determine which patients are most likely to thrive post-transplantation. Whereas the model for endstage liver disease (MELD) helps stratify benefit from transplantation, it does not fully capture the risk of harm or lack of benefit from transplant. Chief among these non-MELD considerations is the impact of frailty.
Frailty is an emergent concept in liver transplantation, however it has deep roots. A syndrome of decreased physiologic reserve, frailty increases the risk of adverse health outcomes.3 Adjusting for MELD, frailty is associated with mortality before and after liver transplant. Although there has been a recent flurry of interest in frailty in transplant medicine, it has been an integral, albeit unlabeled component of clinical care for decades. Indeed, before Pugh replaced it with prothrombin time, the 1964 Child classification used ‘nutritional status’, an eye-ball test of the patients’ status, itself a rudimentary frailty assessment.(1) In fact, the eye-ball test still performs well in outcome prediction when compared to more extensive modern classifications of frailty. (2) However, if at a local-level we ration transplants on the basis of frailty, if patients can be said to be “too-frail for transplant,” then the process of frailty determination must be fair and just. To maintain equity in transplant patient-selection we should strive to use reproducible, generalizable metrics. Herein, we will review the definitions and impact of frailty assessments in the pre-liver transplant patient.
A conceptual model of frailty
A useful model of frailty in patients with liver failure should accomplish several things. First, it should instruct an accurate (i.e. associated with important outcomes), reliable (limited interobserver variation) and validates classification of ‘frail vs not-frail’. Second, it should clarify the sources and potential reversibility of frailty. Third, it should be straightforward to operationalize the concept of frailty as a measure that can be obtained in the course of clinical practice.
Frailty exists as a spectrum. Yet, to be useful clinically, we must convert it into categories. A patient who is bedbound, unable to perform activities of daily living (ADL), is clearly frail; they are disabled. A patient who can perform their ADLs but falls routinely and therefore walks very slowly is also frail. However, the latter patient may escape the ‘eye-ball’ test. Frailty can be rendered into tiers: disability and physical frailty. To expose physical frailty may require testing of function (e.g. hand-grip) while testing the function of a disabled person is unlikely to add actionable information.
As with non-cirrhotic patients sarcopenia, progressive immobility, decreased energy expenditure, and malnutrition may lead to frailty. Cirrhosis poses unique problems.(Figure 1) Alcohol use, when present, confounds all factors. Hepatic encephalopathy (HE) is associated with poor co-ordination, disorientation, falls, and sarcopenia and actually confounds the interpretation of frailty(3); ascites limits physical function and accelerates sarcopenia through hyperammonemia. The management of frailty is therefore directed in part towards the optimization of cirrhotic decompensations. Relatedly, the frailty assessment is fundamentally a snapshot of the patient’s dynamic health status. It varies with the fluctuations of encephalopathy and ascites and changing drastically in response to treatment (e.g. alcohol abstinence, treatment of ascites/HE) and illness (e.g. infection, falls, hospitalization). Accordingly, within the context of a liver failure, the reliability of a frailty tool is defined in part by its robustness given the cirrhosis patients’ variable health status.
Figure 1: A Conceptual Model of Frailty in Patients with End-stage Liver Disease.
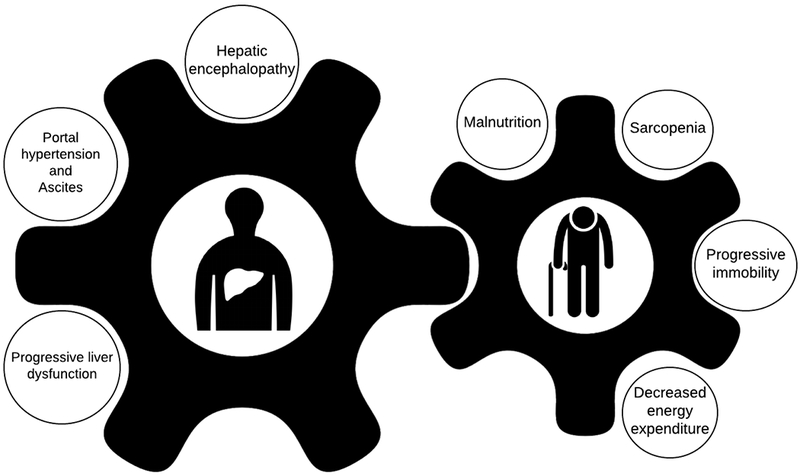
Patients with cirrhosis have unique risk factors for frailty. These include complications with physical limitations such as ascites and overt hepatic encephalopathy. However, beyond the manifestations of advanced liver disease, the metabolic perturbations associated with liver dysfunction and portal hypertension contribute to catabolism, muscle wasting and weakness as well as maladaptive symptoms such as anorexia. These factors cause and compound the factors which define the frailty phenotype: malnutrition and weight loss, sarcopenia, immobility and decreased activity.
Tools for the diagnosis of frailty
There is no one ‘perfect’ test of frailty. Each of the tools available for the diagnosis of frailty each have strengths and weaknesses. It is possible, however, to exploit each test’s strengths for a complementary, comprehensive approach to the identification of frailty. Some – such as those designed to classify disability – are highly specific but insensitive. Similarly, many tests for disability are subjective, relying on patient report or clinician assessment. Objective tests – such as those performed to identify physical frailty, e.g. Liver Frailty Index (LFI)(4) – have less inter-observer variability(5) and tend to be sensitive for clinical outcomes such as delisting but require some dedicated effort by trained staff.
In Table 1, we review tests of disability and frailty. Note is made of those which are conventionally performed in outpatients as well as those tests which have been linked with outcomes before and after transplantation. Whereas the association with post-transplant outcomes has not been explored for some tests, this does not explicitly suggest that they are not applicable for that purpose.
Table 1:
Tools for the Assessment of Disability and Frailty in Patients Evaluated for Liver Transplantation
| Tool | Refs | Where has it been studied? | Special devices/training | Outcome prediction before or after transplant | How to perform |
|---|---|---|---|---|---|
| Subjective | |||||
| Eye-ball test | (2) | (Inpatient) or outpatient | No | Before | Experienced clinicians’ general assessment during examination |
| Activities of Daily Living (ADL) | (6–8) | Inpatient, outpatient | No | Before / After | Grade ability to independently (1 point) or require assistance with (0 points) bathing, dressing, toileting, transferring, maintain continence, and feeding self |
| Clinical Frailty Scale (CFS) | (9) | (Inpatient) or Outpatient | No | Before | Grade patients on scale of 1 (Very Fit) to Terminally ill (9) based on impact of illness and ability to manage symptoms and daily activities. |
| Karnofsky Performance Scale (range A-C or 0-100) | (10, 11, 13, 25) | Inpatient, outpatient | No | Before / After | Grade function as a percentage from 0% (dead) to 100% (working, thriving); Grade A (80-100) normal activity with variable symptoms; B (50-70) requiring assistance and variable medical care; C (10-40), disabled, seriously ill. |
| Mixed subjective and objective | |||||
| Braden Scale Range 6-23 | (8, 15) | Inpatient, (outpatient) | No | Before / After | Grade pressure ulcer risk from 6-23. Higher scores, lower risk. Sensory perception (1, unresponsive, - 4,no impairment); skin moisture (1,constantly moist – 4, rarely); activity (1, bedfast – 4, walks frequently); mobility (1, immobilized – 4, no limits); nutrition (1, poor intake – 4 excellent); transferring (1, difficult – 4, no problem) |
| Fried Frailty Index (FFI) Range 0-5 | (6, 9, 16) | Inpatient, outpatient | Yes | Before | Frailty defined by poor performance on ≥3 of: weight loss (>101bs in year), exhaustion (>3 days/week of feeling ‘everything was an effort’ and ‘could not get going’, hand-grip strength in gender-BMI stratified 20th percentile, 15 ft walk-speed in gender-height stratified 20th percentile, activity on Minnesota Leisure Time Activity scale in gender-stratfied 20th percentile. |
| 6 Minute Walk-Distance (6MWD) Meters walked | (22, 23, 26) | Outpatient | No | Before | Meters walked in 6 minute timed trial |
| Gait speed (meters / second) | (24) | Outpatient | No | Before | Meters per second to walk set distance (e.g. 10 meters). |
| Short Physical Performance Battery (SPPB) Range 0-12 | (6, 9) | Outpatient | Yes | Before | Three tests of balance (yes/no 10 seconds) with variable foot placement (side-by-side, semi-tandem, tandem), 4-meter walk-speed, chair-stands (time to performed 5 rises from a chair) |
| Liver Frailty Index Range 1-7 | (5) | Outpatient | Yes | Before | Three tests of balance (yes/no 10 seconds) with variable foot placement (side-by-side, semi-tandem, tandem), hand-grip, chair-stands (time to performed 5 rises from a chair). |
| Cardio pulmonary Exercise Testing mL/kg/min | (21, 27) | Outpatient | Yes | Before / After | CPET is performed on a treadmill or in a stationary cycle ergometer by increasing the patient’s workload with continuous heart-rate, blood pressure, and electrocardiographic monitoring. Through a respirator, gas exchange is measured to yield indices of oxygen uptake, ventilation, and carbon dioxide output (VC02) |
Subjective Tests
The eye-ball test was best studied by Lai et al as a one-question likert scale ‘How would you rate your patient’s overall health today (0 = excellent, 5 = very poor)?’(2) A score of ≥3 was associated with a MELD, and age-adjusted odds ratio of 2.25 (95% CI 1.22–4.15) death or delisting. Patient reported ADL performance obtained by clinical nurses (or research assistants) has also been associated with a host of pre-transplant outcomes including death, delisting, discharge to a nursing facility and 30-day readmission after discharge.(6–8) Two hybrids combining patient-reported outcomes and clinical assessments have been validated in patients with cirrhosis. The Clinical Frailty Scale, pioneered in this this context by Tandon et al grades patients from very fit (1) to terminally ill (9) based on the severity of disease and performance of ADLs.(9) A CFS score >4 is associated with unplanned hospitalization or death in a cohort of pre-transplant patients with decompensated cirrhosis.(9)
Finally, the Karnofsky Performance Scale (KPS) has been extensively validated in patients with cirrhosis. It is universally collected United Network for Organ Sharing (UNOS). The KPS is both a scale from 0 (death) to 100 (perfect health) and a trichotomized scale A (able to work), B (unable to work but completes ADLs), C (disabled). Based on work from the United Network for Organ Sharing (UNOS) database of waitlisted patients and the North American Consortium for the Study of Endstage Liver Disease, poor KPS is clearly associated with pre-transplant mortality.(10, 11) Serper and colleagues in Pennsylvania recently showed that, adjusting for MELD, age and donor factors, a pre-transplant KPS of 10-40 was significantly associated with increased healthcare costs in the first year after transplant.(12) Dolgin showed that pre-transplant KPS was linked with risk of 30-day mortality after transplant.(13) These data were recently extended in a study by Thuluvath et al.(14) Re-examining the UNOS data, the authors found that a decline in KPS from registration to transplant as well as poor KPS after transplant, was strongly, independently associated with graft and patient survival.
Mixed subjective and objective tests
Two frailty indices combine subjective assessments with objective, quantitative measurements. First, the Braden Scale is a standard index of pressure ulcer risk that is widely used by inpatient nurses. Its dimensions include an assessment of sensory perception, skin moisture, activity, mobility, and nutritional intake. The Braden was found to be associated with mortality in pre-transplant patients.(8) Following transplant, Braden scores were linked with prolonged hospital length of stay, nonambulatory status at discharge, and discharge to a rehabilitation facility.(15)
Second, the Fried physical frailty phenotype (PFP) is the most widely validated tool for frailty assessment. Consisting of patient reported weight loss, activity, and exhaustion as well as measured weakness (hand-grip) and walking speed, the PFP is scored from 0 to 5 based on frail performance for each categories with scores ≥3 generally indicating frailty. Though lacking for post-transplant outcome prediction, there is substantial data with respect to associations with important pre-transplant outcome. Lai and colleagues demonstrated that each incremental point in the FFI was associated with a 45% increase in the MELD-adjusted risk of waitlist mortality.(6) In an expanded pre-transplant cohort, Sinclair and Lai later showed that that frailty was associated with the MELD, albumin, and gender-adjusted risk of hospitalization and hospital utilization.(16) Tandon confirmed these findings in a Canadian cohort.(9) Beyond these outcomes, in a cohort of patients evaluated for liver transplant in Michigan, Derck(17) and Cron(18) demonstrated that, more so than severity of liver disease, frailty was a better indicator of depression(18) and diminished health-related quality of life.(17)However, the PFP can be confounded by disease factors. Our group recently showed that the PFP was not predictive of transplant-free survival in patients with treated hepatic encephalopathy.(19)
Objective Tests
Cardiopulmonary exercise testing (CPET) is a direct assessment of physical function in a controlled setting that has been explored in transplant candidates.(20, 21) CPET is performed on a treadmill or in a stationary cycle ergometer by increasing the patient’s workload with continuous heart-rate, blood pressure, and electrocardiographic monitoring. Through a respirator, gas exchange is measured to yield indices of oxygen uptake, ventilation, and carbon dioxide output (VCO2). Peak oxygen uptake is an example of the outputs used to classify physical function. In the largest study of CPET, Bernal and colleagues demonstrated that aerobic capacity by the CPET was associated with both pre- and post-transplant survival.(21)
One of the first tests of frailty studied in transplant waitlisted patients was the 6-minute walking distance (6MWD, meters). Carey and colleagues performed the 6MWD in 121 transplant candidates when their MELD reached ≥15.(22) Each 100 meters walked was associated with incrementally improved MELD-adjusted survival. However, no specific distance-walked has been statistically or externally validated as a cut-off(23) Although it is a component of the FFI, Dunn et al evaluated walk-speed as a stand-alone assessment. In 373 wait-listed patients, walk-speed was associated with hospital bed-days, adjusting for MELD and Child classification.(24) The Short Physical Performance Battery (SPPB) is a frailty assessment derived from the geriatric literature includes 3 tests of balance, walking-speed, and chair-stands; it has been evaluated in two large cohorts of patients with cirrhosis in Alberta and San Francisco.(6, 9) Scored on a scale of 12 to 0, each point decrement was associated with an increased risk of waitlist mortality in a cohort of 294 listed patients with cirrhosis awaiting transplantation (MELD adjusted hazard ratio 1.19, 95%CI 1.07-1.32).(6)
Batteries of tests are felt to provide adequate range to discriminate risk across a population and allow for longitudinal tracking of changes within patients. A promising battery developed by Dr. Jennifer Lai extends the applications of the SPPB by deriving and validating the components in patients awaiting liver transplantation. In her landmark study of the Liver Frailty Index (LFI), she derived a final battery including chair-stands, hand-grip and balance from a broader set that also included the PFP’s subjective domains, ADL performance, and walk-speed.(5) Compared to the eye-ball test, the LFI outperforms the correct classification of predicted waitlist mortality.(4) Hand-grip and chair-stands without balance have equal performance for the prediction of mortality compared to the LFI,(4) however the three assessments together are felt to provide a greater range for the discrimination of risk within a cohort.
Limits
There are 3 key limitations of frailty assessments. First, cutoffs that are valid in one population may not translate to another. Second, a central component of frailty that is rarely examined is the trajectory of illness. The implications of disability in a patient who was active and working immediately prior to acute-on-chronic liver failure are likely different than disability in a patient which chronic liver failure who has been unable to perform ADLs for months. Unfortunately, most studies do not account for trajectory of illness or report changes in (and the import of) frailty assessments over time. Third, the clinical interpretation of longitudinal changes in most frailty assessments has not been established.
Conclusion
Frailty is an important concept and captures meaningful information to inform pre-transplant decision making. The frail patient requires closer observation, intensified nutrition and management of cirrhotic complications. Further, the frail patient is a high risk of adverse pre-and post-transplant outcomes which must be taken into account in the process of matching allograft types and stratifying risk for transplant waitlisting.
Frailty: How to do it
Frailty assessments should be applied in a fair and uniform fashion. Regardless of age, all patients should be assessed for disability and/or frailty. In practice we use the KPS. The performance of physical frailty tests often requires a dedicated staff. As most, save for the CPET, are straightforward, this could be completed by the clinicians or a medical assistant during clinical sessions. Purchasing a dynamometer for hand-grip and putting stop-watches in clinic rooms are simple tools to remind clinicians to perform the test. For many centers, frailty assessments have been relegated to research protocols. Frailty assessments should be recorded in clinical notes and preferable in a searchable component of the electronic health record, not unlike a vital sign.
Acknowledgments
Funding: Elliot Tapper receives funding from the National Institutes of Health through the Michigan Institute for Clinical and Health Research (KL2TR002241).
Conflict of Interest
Elliot Tapper reports grants from Valeant and Gilead and personal fees from Novartis for a fatty liver disease study.
Footnotes
Disclosure:
Elliot Tapper is the guarantor of this article
Conflict of Interest: None.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Child CG. Surgery and portal hypertension. The liver and portal hypertension. 1964. [Google Scholar]
- 2.Lai JC, Covinsky KE, Hayssen H, Lizaola B, Dodge JL, Roberts JP, et al. Clinician assessments of health status predict mortality in patients with end-stage liver disease awaiting liver transplantation. Liver International. 2015;35(9):2167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic Encephalopathy Impacts the Predictive Value of the Fried Frailty Index. American Journal of Transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** In this prospective cohort study, the prediction of mortality using the frailty phenotype described by Fried in patients with cirrhosis was confounded by a history of hepatic encephalopathy
- 4.Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. The American journal of gastroenterology. 2018;113(2):235. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The liver frailty index, a composite of hand-grip, chair-stands, and balance was shown to improve the prediction of mortality over clinician assessments of frailty (eye-ball test) in outpatients listed for liver transplant.
- 5.Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai JC, Feng S, Terrault N, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. American Journal of Transplantation. 2014;14(8):1870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samoylova ML, Covinsky KE, Haftek M, Kuo S, Roberts JP, Lai JC. Disability in patients with end-stage liver disease: Results from the functional assessment in liver transplantation study. Liver Transplantation. 2017;23(3):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]; * An assessment of activities of daily living provides improved risk-assessment for mortality in a cohort of inpatients with cirrhosis
- 9.Tandon P, Tangri N, Thomas L, Zenith L, Shaikh T, Carbonneau M, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the Clinical Frailty Scale. The American journal of gastroenterology. 2016;111(12):1759. [DOI] [PubMed] [Google Scholar]
- 10.Orman ES, Ghabril M, Chalasani N. Poor performance status is associated with increased mortality in patients with cirrhosis. Clinical Gastroenterology and Hepatology. 2016;14(8):1189–95. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tandon P, Reddy KR, O’leary JG, Garcia-Tsao G, Abraldes JG, Wong F, et al. A Karnofsky performance status–based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65(1):217–24. [DOI] [PubMed] [Google Scholar]; * The karnofsky performance status at the time of hospital discharge was associated with improved risk prediction of mortality in a cohort consisting primarily of patients with acute-on-chronic liver failure
- 12.Serper M, Bittermann T, Rossi M, Goldberg DS, Thomasson AM, Olthoff KM, et al. Functional status, healthcare utilization, and the costs of liver transplantation. American Journal of Transplantation. 2018;18(5):1187–96. [DOI] [PubMed] [Google Scholar]
- 13.Dolgin NH, Martins PN, Movahedi B, Lapane KL, Anderson FA, Bozorgzadeh A. Functional status predicts postoperative mortality after liver transplantation. Clinical transplantation. 2016;30(11):1403–10. [DOI] [PubMed] [Google Scholar]
- 14.Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky Performance Status Before and After Liver Transplantation Predicts Graft and Patient Survival. Journal of hepatology. 2018. [DOI] [PubMed] [Google Scholar]
- 15.Sundaram V, Lim J, Tholey DM, Iriana S, Kim I, Manne V, et al. The Braden scale, a standard tool for assessing pressure ulcer risk, predicts early outcomes after liver transplantation. Liver Transplantation. 2017. [DOI] [PubMed] [Google Scholar]
- 16.Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World journal of gastroenterology. 2017;23(5):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derck JE, Thelen AE, Cron DC, Friedman JF, Gerebics AD, Englesbe MJ, et al. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation. 2015;99(2):340–4. [DOI] [PubMed] [Google Scholar]
- 18.Cron D, Friedman J, Winder G, Thelen A, Derck J, Fakhoury J, et al. Depression and Frailty in Patients With End-Stage Liver Disease Referred for Transplant Evaluation. American Journal of Transplantation. 2016;16(6):1805–11. [DOI] [PubMed] [Google Scholar]
- 19.Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. American Journal of Transplantation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharancy S, Lemyze M, Boleslawski E, Neviere R, Declerck N, Canva V, et al. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation. 2008;86(8):1077–83. [DOI] [PubMed] [Google Scholar]
- 21.Bernal W, Martin-Mateos R, Lipcsey M, Tallis C, Woodsford K, Mcphail MJ, et al. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transplantation. 2014;20(1):54–62. [DOI] [PubMed] [Google Scholar]
- 22.Carey EJ, Steidley DE, Aqel BA, Byrne TJ, Mekeel KL, Rakela J, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transplantation. 2010;16(12):1373–8. [DOI] [PubMed] [Google Scholar]
- 23.Yadav A, Chang YH, Carpenter S, Silva AC, Rakela J, Aqel BA, et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clinical transplantation. 2015;29(2):134–41. [DOI] [PubMed] [Google Scholar]
- 24.Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. The American journal of gastroenterology. 2016;111(12):1768. [DOI] [PubMed] [Google Scholar]
- 25.Malinis MF, Chen S, Allore HG, Quagliarello VJ. Outcomes among older adult liver transplantation recipients in the model of end stage liver disease (MELD) era. Annals of transplantation: quarterly of the Polish Transplantation Society. 2014;19:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faustini Pereira JL, Galant LH, Rossi D, da Rosa T, Henrique L, Garcia E, et al. Functional capacity, respiratory muscle strength, and oxygen consumption predict mortality in patients with cirrhosis. Canadian Journal of Gastroenterology and Hepatology. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ney M, Haykowsky M, Vandermeer B, Shah A, Ow M, Tandon P. Systematic review: pre-and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Alimentary pharmacology & therapeutics. 2016;44(8):796–806. [DOI] [PubMed] [Google Scholar]


