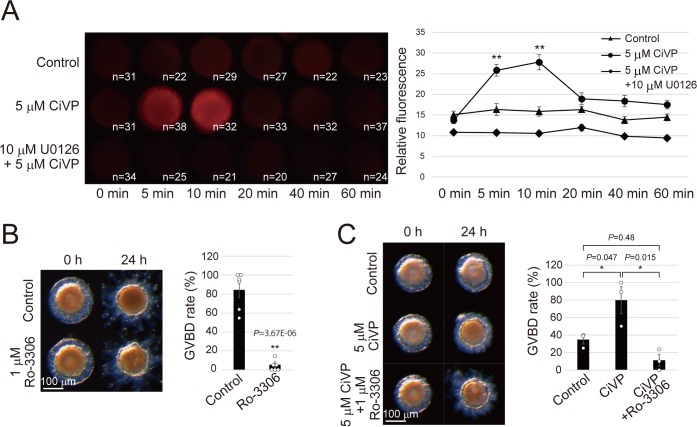
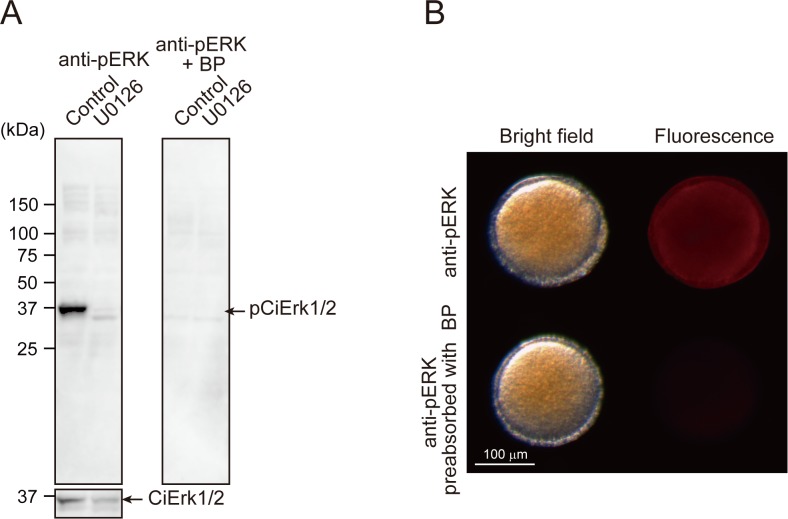
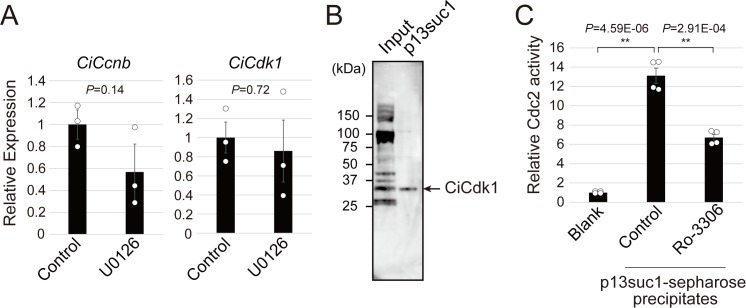
Figure 4. CiVP promotes GVBD via CiErk1/2-CiMPF pathway.
(A) Indicated numbers of numbers of de-folliculated oocytes pretreated with or without 10 μM U0126 were stimulated with 5 μM CiVP for 0, 5, 10, 20, 40, and 60 min. Immunofluorescence using anti-phosphorylated ERK1/2 antibody indicated specific activation of CiErk1/2 at 5 to 10 min after CiVP stimulation (F = 14.91; p=2.12E-12), which was not observed in control (F = 1.05; p=0.39) or U0126-pretreated oocytes (F = 2.13; p=0.065). Representative images are shown (left). Signal intensities were quantified using Fiji software (right), and significant activation was verified by one-way factorial ANOVA followed by Tukey’s post hoc test (**, P<0.01). (B) Treatment with 1 μM Ro-3306, a selective Cdc2 inhibitor, significantly inhibited GVBD in late stage III follicles (t-value: 9.12; **, p=3.67E-06). (C) CiVP-induced GVBD of late stage II follicles (t-value: −2.83; *, p=0.047) was disrupted by Ro-3306 (t-value: 4.12; *, p=0.015). Representative images of follicles and GVBD rates are shown. Data from six (B, n = 6) and three (C, n = 3) independent experiments were analyzed by Student’s t test and are shown as mean ± SEM with data points.