Abstract
Background
Some dental implant failures may be due to bacterial contamination at implant insertion. Infections around biomaterials are difficult to treat, and almost all infected implants have to be removed. In general, antibiotic prophylaxis in surgery is only indicated for patients at risk of infectious endocarditis; with reduced host‐response; when surgery is performed in infected sites; in cases of extensive and prolonged surgical interventions; and when large foreign materials are implanted. A variety of prophylactic systemic antibiotic regimens have been suggested to minimise infections after dental implant placement. More recent protocols recommended short‐term prophylaxis, if antibiotics have to be used. Adverse events may occur with the administration of antibiotics, and can range from diarrhoea to life‐threatening allergic reactions. Another major concern associated with the widespread use of antibiotics is the selection of antibiotic‐resistant bacteria. The use of prophylactic antibiotics in implant dentistry is controversial.
Objectives
To assess the beneficial or harmful effects of systemic prophylactic antibiotics at dental implant placement versus no antibiotic or placebo administration and, if antibiotics are beneficial, to determine which type, dosage and duration is the most effective.
Search methods
We searched the Cochrane Oral Health's Trials Register (to 17 June 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 5), MEDLINE via OVID (1946 to 17 June 2013) and EMBASE via OVID (1980 to 17 June 2013). There were no language or date restrictions placed on the searches of the electronic databases.
Selection criteria
Randomised controlled clinical trials (RCTs) with a follow‐up of at least three months, that compared the administration of various prophylactic antibiotic regimens versus no antibiotics to people undergoing dental implant placement. Outcome measures included prosthesis failures, implant failures, postoperative infections and adverse events (gastrointestinal, hypersensitivity, etc).
Data collection and analysis
Screening of eligible studies, assessment of the risk of bias of the trials and data extraction were conducted in duplicate and independently by two review authors. Results were expressed as risk ratios (RRs) using a random‐effects model for dichotomous outcomes with 95% confidence intervals (CIs). Heterogeneity, including both clinical and methodological factors, was to be investigated.
Main results
Six RCTs with 1162 participants were included: three trials compared 2 g of preoperative amoxicillin versus placebo (927 participants), one compared 3 g of preoperative amoxicillin versus placebo (55 participants), one compared 1 g of preoperative amoxicillin plus 500 mg four times a day for two days versus no antibiotics (80 participants), and one compared four groups: (1) 2 g of preoperative amoxicillin; (2) 2 g of preoperative amoxicillin plus 1 g twice a day for seven days; (3) 1 g of postoperative amoxicillin twice a day for seven days, and (4) no antibiotics (100 participants). The overall body of evidence was considered to be of moderate quality. The meta‐analyses of the six trials showed a statistically significant higher number of participants experiencing implant failures in the group not receiving antibiotics (RR 0.33; 95% CI 0.16 to 0.67, P value 0.002, heterogeneity: Tau2 0.00; Chi2 2.87, df = 5 (P value 0.57); I2 0%). The number needed to treat for one additional beneficial outcome (NNTB) to prevent one person having an implant failure is 25 (95% CI 14 to 100), based on an implant failure rate of 6% in participants not receiving antibiotics. There was borderline statistical significance for prosthesis failures (RR 0.44; 95% CI 0.19 to 1.00), with no statistically significant differences for infections (RR 0.69; 95% CI 0.36 to 1.35), or adverse events (RR 1; 95% CI 0.06 to 15.85) (only two minor adverse events were recorded, one in the placebo group). No conclusive information can be derived from the only trial that compared three different durations of antibiotic prophylaxis since no event (implant/prosthesis failures, infections or adverse events) occurred in any of the 25 participants included in each study group. There were no trials that evaluated different antibiotics or different antibiotic dosages.
Authors' conclusions
Scientific evidence suggests that, in general, antibiotics are beneficial for reducing failure of dental implants placed in ordinary conditions. Specifically 2 g or 3 g of amoxicillin given orally, as a single administration, one hour preoperatively significantly reduces failure of dental implants. No significant adverse events were reported. It might be sensible to suggest the use of a single dose of 2 g prophylactic amoxicillin prior to dental implant placement. It is still unknown whether postoperative antibiotics are beneficial, and which antibiotic is the most effective.
Keywords: Humans; Dental Restoration Failure; Amoxicillin; Amoxicillin/administration & dosage; Anti‐Bacterial Agents; Anti‐Bacterial Agents/administration & dosage; Antibiotic Prophylaxis; Antibiotic Prophylaxis/adverse effects; Bacterial Infections; Bacterial Infections/prevention & control; Dental Implants; Dental Implants/adverse effects; Drug Administration Schedule; Jaw, Edentulous, Partially; Jaw, Edentulous, Partially/surgery; Randomized Controlled Trials as Topic
Plain language summary
Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications
Review question
This review, carried out by authors of the Cochrane Oral Health Group, has been produced to assess the possible benefits of antibiotics taken orally at the time of the placement of a dental implant in order to prevent infection. If antibiotics are shown to be of benefit in preventing infection, this review also seeks to establish which type, dosage and duration of treatment is the most effective. The use of antibiotics to prevent infection in implant dentistry is controversial, and there is a need to answer these questions in order to improve the success rates of dental implants whilst minimising complications, harms or adverse effects.
Background
Missing teeth can sometimes be replaced with dental implants to which a crown, bridge or denture can be attached. Bacteria introduced during the placement of implants can lead to infection, and sometimes implant failure. Infections around biomaterials (such as dental implants) are difficult to treat and almost all infected implants have to be removed, which is why it is so important to prevent infection if possible.
It has been suggested that taking antibiotics orally either before or after placement (or both) can minimise the chances of infection.
Generally the use of antibiotics in surgery in order to prevent infection is only recommended for people at risk, when surgery is extensive, or performed in infected sites, and when large foreign materials are implanted in the body. Recently, a short term course of antibiotics has been recommended when antibiotics have to be used, because sometimes antibiotics can cause side effects that range from diarrhoea to life‐threatening allergic reactions. Another major concern associated with the widespread use of antibiotics is the increase in the appearance of antibiotic‐resistant bacteria.
Study characteristics
The evidence on which this review is based was up to date as of 17 June 2013. Six trials were included with a total of 1162 participants.
All six of these trials compared the use of antibiotics to prevent infection (failures and complications) with no treatment or treatment with a placebo (a fake medicine with no active ingredient). The antibiotic used in all the trials was amoxicillin; doses and timing of doses varied, although most used a single dose taken just before the implant was placed. One of the trials, with 100 participants, also looked at different doses of amoxicillin taken at different times.
There were no trials that looked at alternative antibiotics.
Participants were people over 18 years of age who were able to give consent to taking part in a medical trial. Potential participants were excluded for a variety of reasons that included: if they were at risk of heart disease, had artificial joints, had problems with their immune system, were affected by diabetes, had received radiotherapy in the head and neck area, had need of additional procedures at the time of implant placement, were allergic to penicillin, had chronic/acute infections near the planned implant site, were already receiving antibiotic treatment for any other reasons (or had taken them up to six months previously), had been treated with or were receiving intravenous amino‐bisphosphonates, were pregnant or breast feeding, were receiving long‐term nonsteroidal anti‐inflammatory drug therapy, or had blood clotting problems. The follow‐up period in all the trials was at least three months.
Key results
It appears that the oral administration of two grams of amoxicillin one hour before placement of dental implants is effective in reducing implant failures. More specifically, giving antibiotics to 25 people will avoid one person experiencing early implant losses. It is still unclear whether postoperative antibiotics are beneficial, or which antibiotics work best.
Quality of the evidence
The evidence from the six trials (1162 participants) that compared the use of antibiotics with placebo or no treatment was considered to be of moderate quality. However, the one trial (100 participants) that investigated antibiotics given for different lengths of time was found to be at high risk of bias.
Summary of findings
for the main comparison.
| Antibiotics compared with no antibiotics at placement of dental implants | ||||||
|
Patient or population: people requiring dental implants Settings: dental practice Intervention: prophylactic antibiotics Comparison: no antibiotics or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Without antibiotic | Antibiotic | |||||
| Implant failure at 4 months | Low risk population | RR 0.33 (0.16 to 0.67) | 1162 (6) | ⊕⊕⊕⊝2 moderate quality | ||
| 10 per 10001 | 4 per 1000 (2 to 7) | |||||
| High risk population | ||||||
| 100 per 1000 | 40 per 1000 (16 to 67) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
1The median risk in the control arm was 6% so low risk of 1% and high risk of 10% assumed 2 Downgraded due to three of the six studies being at high risk of bias
CI = confidence interval; RR = risk ratio
GRADE Working Group grades of evidence: High quality (⊕⊕⊕⊕): further research is very unlikely to change our confidence in the estimate of effect Moderate quality (⊕⊕⊕⊝): further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality (⊕⊕⊝⊝): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality (⊕⊝⊝⊝): we are very uncertain about the estimate
Background
Description of the condition
Dental implants are widely used for replacing missing teeth. Despite the high success rates published in the literature, implant failures do occur (Esposito 1998a). It is believed that a certain number of early dental implant losses are due to bacterial contamination at implant insertion (Esposito 1998b). It is known that infections around biomaterials, such as implants, are very difficult to treat and almost all infected implants have to be removed sooner or later (Esposito 1998b). The likelihood of an infection around dental implants is influenced by the surgical skill (traumatic and prolonged surgery is more likely to favour infections) and by the degree of asepsis (sterility). In general, antibiotic prophylaxis in surgery is only recommended in the following situations: for patients at risk of infectious endocarditis, patients with reduced host‐response, when surgery is performed in infected sites, for extensive and prolonged surgical interventions, and when large foreign materials are implanted.
Description of the intervention
In order to minimise infections after dental implant placement various prophylactic systemic (whole body) antibiotic regimens have been suggested. Initially, antibiotics were recommended preoperatively and up to 10 days postoperatively, one of the most commonly followed protocols being the administration of 2 g of phenoxymethylpenicillin (penicillin‐V), orally, about one hour preoperatively and then 2 g twice a day for 10 days (Adell 1985). More recent protocols recommended short‐term prophylaxis (Flemmig 1990): 2 g of penicillin‐V (or amoxicillin or amoxicillin/clavulanate) administered orally, one hour prior to surgery and 500 mg of penicillin‐V four times a day for one day.
How the intervention might work
While it is important to minimise risk of implant failures, it is also sensible to minimise the use of antibiotics, since adverse events may occur. Complications most commonly associated with the use of antibiotics range from diarrhoea to life‐threatening allergic reactions. Another major concern associated with the widespread use of antibiotics is the selection of antibiotic‐resistant bacteria.
Why it is important to do this review
The use of antibiotics in implant dentistry is controversial and some controlled clinical trials yielded contradictory results (Dent 1997; Gynther 1998; Laskin 2000; Binahmed 2005). It would be useful to know whether prophylactic antibiotics are effective in reducing postoperative infections and failures of dental implants; and, if so, which is the most effective antibiotic, at what dose and for what duration.
Objectives
To assess the beneficial or harmful effects of systemic prophylactic antibiotics at dental implant placement versus no antibiotic or placebo administration and, if antibiotics are beneficial, to determine which type, dosage and duration is the most effective.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) with a follow‐up of at least three months.
Types of participants
Any group of people undergoing dental implant placement.
Types of interventions
Administration of prophylactic antibiotics versus no antibiotics/placebo.
Administration of different antibiotics.
Administration of different doses or different durations of the same antibiotic.
Types of outcome measures
Primary outcomes
Implant failure: implant mobility and removal of stable implants dictated by progressive marginal bone loss or infection.
Prosthesis that could not be placed, or prosthesis failure if secondary to implant failures.
Secondary outcomes
Postoperative infections.
Adverse events (gastrointestinal, hypersensitivity, etc).
Search methods for identification of studies
For the identification of studies included or considered for this review, detailed search strategies were developed for each database searched. These were based on the search strategy developed for MEDLINE (OVID), but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2011 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (Higgins 2011). Details of the MEDLINE search are provided in Appendix 1.
Electronic searches
We searched the following electronic databases:
Cochrane Oral Health's Trials Register (17 June 2013) (see Appendix 2);
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 5) (see Appendix 3);
MEDLINE via OVID (1946 to 17 June 2013) (see Appendix 1);
EMBASE via OVID (1980 to 17 June 2013) (see Appendix 4).
We did not place any restrictions on language or date of publication when searching the electronic databases.
Searching other resources
Unpublished studies
We wrote to all the authors of the identified RCTs, we checked the bibliographies of all identified RCTs and relevant review articles, and we used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an Internet discussion group (implantology@yahoogroups.com), however we discontinued this for this update due to poor yield.
Handsearching
Only handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL was included (see the Cochrane Masterlist (http://us.cochrane.org/master‐list) for details of journal issues searched to date).
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether or not the studies met the inclusion criteria. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent validity assessment and data extraction. Studies rejected at this, or subsequent stages, were recorded in the Characteristics of excluded studies table, and reasons for exclusion recorded.
Data extraction and management
Data were extracted by two review authors independently using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreement was discussed and a third review author consulted where necessary. All trial authors were contacted for clarification or missing information.
For each trial the following data were recorded.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics, source of recruitment and criteria for inclusion.
Details of the type of intervention.
Details of the outcomes reported, including method of assessment, and time intervals.
Assessment of risk of bias in included studies
This was conducted using the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011). It is a two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other bias'). Each domain includes one specific entry in a 'Risk of bias' table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, either 'low risk', 'high risk' or, where there is insufficient information on which to base a judgement, 'unclear risk'.
The risk of bias assessment of the included trials was completed independently and in duplicate by two review authors as part of the data extraction process. On occasions when the review authors were also authors of trial reports that needed to be assessed, the reports were independently evaluated only by review authors who had not been involved in the trials.
Summarising risk of bias for a study
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows:
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias. | Plausible bias unlikely to alter the results seriously. | Low risk of bias for all key domains. | Most information is from studies at low risk of bias. |
| Unclear risk of bias. | Plausible bias that raises some doubt about the results. | Unclear risk of bias for one or more key domains. | Most information is from studies at low or unclear risk of bias. |
| High risk of bias. | Plausible bias that seriously weakens confidence in the results. | High risk of bias for one or more key domains. | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results. |
Measures of treatment effect
For each outcome, all of which were binary outcomes, the estimate of effect of an intervention was expressed as risk ratio together with 95% confidence intervals.
Unit of analysis issues
The statistical unit was the participant, and not the prosthesis or implant.
Dealing with missing data
Trial authors were contacted to retrieve missing data where necessary. If agreement could not be reached, data were excluded until further clarification was available. Methods for estimating missing standard deviations in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions would have been used if required (Higgins 2011). An intention‐to‐treat (ITT) analysis was undertaken where data were available and appropriate.
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity. Heterogeneity would have been considered to be significant if the P value was less than 0.1. The I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance, was used to quantify heterogeneity with an I2 value over 50% indicating moderate to high heterogeneity.
Assessment of reporting biases
If there had been a sufficient number of trials (more than 10) in any meta‐analysis we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry (Egger 1997), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry had been identified we would have examined possible causes.
Data synthesis
A meta‐analysis was performed only if there were studies with similar comparisons that reported the same outcome measures. Risk ratios were combined for dichotomous data, using random‐effects models provided there were more than three studies in the meta‐analysis. Numbers needed to treat for an additional harm (NNTH) were calculated for participants affected by implant failures. The recommendations of the Cochrane Handbook for Systematic Reviews of Interventions were followed for studies with zero‐cell counts (Higgins 2011). The fixed value of 0.5 was added to all cells with zero‐cell counts and risk ratios calculated with the Review Manager (RevMan) software (RevMan 2013). If there were no events in both arms, no calculations were undertaken, because in this situation the study does not provide any indication of the direction or magnitude of the relative treatment effect.
Subgroup analysis and investigation of heterogeneity
Clinical heterogeneity was to be assessed by examination of the types of participants and interventions for all outcomes in each study. It was decided to formulate the following hypotheses to be investigated for subgroup analyses. However since the number of trials included in the meta‐analysis was small (less than 10) this was not undertaken. This may be done in future updates of this review for the following subgroups:
single versus multiple implants;
post‐extractive implants versus implants in completely‐ or partially‐healed sites;
long versus short procedures;
complicated versus simple procedures.
Sensitivity analysis
We planned to undertake sensitivity analyses to examine the effect of the study quality assessment on the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was also to be examined, but there were too few trials to undertake these analyses.
Results
Description of studies
Results of the search
Seven randomised controlled trials (RCTs) were identified as potentially eligible for inclusion in this review (Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011; Nolan 2013; Tan 2013). One trial was excluded because the duration of follow‐up was less than three months (eight weeks) (Tan 2013).
Included studies
SeeCharacteristics of included studies table for further details.
Three multicentre trials were conducted in Italy (Esposito 2008a; Esposito 2010a; Caiazzo 2011), one multicentre trial in Spain (Anitua 2009), one single‐centre trial in Belgium (Abu‐Ta'a 2008), and one single‐centre trial in Ireland (Nolan 2013).
Two trials used antibiotics and placebo donated by a drug company manufacturing generic drug (Esposito 2008a; Esposito 2010a); the company was not involved in the design of the study, in the data evaluation, or in commenting on the manuscript. One trial was supported by the implant manufacturer (Anitua 2009). No external funding was received in the other trials (Abu‐Ta'a 2008; Caiazzo 2011; Nolan 2013).
The multicentre trials were conducted in private practices (Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011), and the two single‐centre trials in university hospitals (Abu‐Ta'a 2008; Nolan 2013).
Characterisitics of the participants
All participants included in the trials were adults with different forms of edentulism (missing teeth) with the exception of one trial that included only participants requiring single implant‐supported crowns (Anitua 2009).
Characteristics of the interventions
(1) Antibiotic prophylaxis versus placebo or no treatment: failures and complications (six trials with 1162 participants)
One trial (Abu‐Ta'a 2008), assessed as being at high risk of bias, compared 1 g of amoxicillin given one hour preoperatively plus 500 mg of amoxicillin four times a day for two days versus no antibiotics. An unknown type of dental implant was used.
Two placebo‐controlled trials compared 2 g of amoxicillin given one hour preoperatively with identical placebo tablets (Esposito 2008a; Esposito 2010a). Various implant brands were used (Zimmer Dental, Dentsply Friadent, Nobel Biocare, Intra‐Lock, Camlog, Dyna, Biomet 3i, Endopore, Z‐system, PF Tecom, Ghimas, Silpo, MegaGen and Geass).
One placebo‐controlled trial compared 2 g of amoxicillin given one hour preoperatively with identical placebo tablets (Anitua 2009). BTI dental implants were used.
One trial compared 2 g of amoxicillin given one hour preoperatively versus no antibiotics (Caiazzo 2011). In this trial two additional groups were included that received: 2 g of preoperative amoxicillin plus 1 g twice a day for seven days; and 1 g of postoperative amoxicillin twice a day for seven days. This trial used Biomet 3i Osseotite implants with external connection.
One trial compared 3 g of amoxicillin given one hour preoperatively with placebo tablets (Nolan 2013). A variety of implant brands were used (Biomet 3i, Nobel Biocare, Ankylos, Straumann SLA Implants).
(2) Antibiotic prophylaxis given for different duration (one trial with 100 participants)
One trial compared four interventions (Caiazzo 2011): (1) 2 g of amoxicillin given one hour preoperatively; (2) 2 g of preoperative amoxicillin plus 1 g twice a day for seven days; (3) 1 g of postoperative amoxicillin twice a day for seven days; and (4) no antibiotics. This trial used Biomet 3i Osseotite implants with external connection.
(3) Antibiotic prophylaxis with different antibiotics (no trials)
(4) Antibiotic prophylaxis given at different dosages (no trials)
Characteristics of the outcomes
All trials, except for Nolan 2013, reported all the outcome measures of interest to the present review.
Prosthesis failure: Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011. The authors of one trial were unable to supply this information (Nolan 2013).
Implant failure: Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011; Nolan 2013.
Postoperative infections: Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011; Nolan 2013.
Adverse events: Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011; Nolan 2013.
Duration of follow‐up
Three months after implant placement: Anitua 2009; Caiazzo 2011.
Three to four months after implant placement: Nolan 2013.
Four months after implant placement: Esposito 2008a; Esposito 2010a.
Five months after implant placement: Abu‐Ta'a 2008.
Main inclusion criteria
Any person over 18 years of age, able to sign an informed consent form, undergoing dental implant placement: Esposito 2008a; Esposito 2010a; Nolan 2013; Caiazzo 2011.
People requiring single implants in bone of medium density. Bone density was measured in Hounsfields (HU) on high resolution scans with the BTI Scan® program (BTI, Vitoria, Spain). Medium bone density was defined as from 400 to 1100 HU: Anitua 2009.
Fully‐ or partially‐edentulous participants: Abu‐Ta'a 2008.
Main exclusion criteria
At risk of bacterial endocarditis: Abu‐Ta'a 2008; Esposito 2008a; Esposito 2010a; Nolan 2013.
Having implanted biomaterials in the body (hip or knee prostheses, etc): Esposito 2008a; Esposito 2010a; Nolan 2013.
Immunosuppressed or immunocompromised: Abu‐Ta'a 2008; Esposito 2008a; Esposito 2010a; Nolan 2013.
Affected by diabetes (controlled or uncontrolled): Esposito 2008a; Esposito 2010a; Nolan 2013.
Uncontrolled diabetes mellitus: Abu‐Ta'a 2008.
Received radiotherapy in the head and neck area: Abu‐Ta'a 2008; Esposito 2008a; Esposito 2010a; Nolan 2013; only if more than 5000 rads: Anitua 2009.
Need of augmentation procedure concomitant with implant placement: Esposito 2008a; Esposito 2010a; Nolan 2013.
Allergic to penicillin: Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011; Nolan 2013.
Presence of chronic/acute infections in the vicinity of the planned implant site: Esposito 2008a; Esposito 2010a; Nolan 2013.
Already under antibiotic treatment for any other reasons: Esposito 2008a; Anitua 2009; Esposito 2010a.
Treated or receiving treatment with intravenous amino‐bisphosphonates: Esposito 2008a; Esposito 2010a.
Pregnant or lactating: Esposito 2008a; Esposito 2010a; Caiazzo 2011; Nolan 2013.
Long‐term nonsteroidal anti‐inflammatory drug therapy: Caiazzo 2011.
History of antibiotic therapy six months prior to the study: Caiazzo 2011.
History of systemic steroid medication or recent systemic antibiotic therapy: Nolan 2013.
Blood coagulation impairment: Nolan 2013.
Sample size
A priori calculation for the sample size was undertaken in three trials (Esposito 2008a; Anitua 2009; Esposito 2010a).
Baseline comparability between treatment groups
No major baseline differences were apparent in any of the included trials.
Excluded studies
One trial was excluded because the duration of follow‐up was less than three months (eight weeks) (Tan 2013).
Risk of bias in included studies
Allocation
Sequence generation
All included studies described an adequate method of sequence generation and were assessed as being at low risk of bias for this domain.
Allocation concealment
Allocation concealment was reported as having being done adequately in four (67%) of the included studies (Esposito 2008a; Anitua 2009; Esposito 2010a; Nolan 2013). It was unclear from the trial report and communication with authors whether allocation for one study had been adequately concealed, and this study was assessed as being at unclear risk of bias for this domain (Abu‐Ta'a 2008). No allocation concealment procedures were implemented in one trial (Caiazzo 2011), and this trial was assessed as being at high risk of bias for this domain.
Blinding
Four trials (67%) were placebo controlled (Esposito 2008a; Anitua 2009; Esposito 2010a; Nolan 2013), and operators, participants and outcome assessors were blinded, whereas for the remaining two trials (Abu‐Ta'a 2008; Caiazzo 2011), operators, participants and outcome assessors were not blinded and these studies were considered at high risk of detection bias.
Incomplete outcome data
All outcome data were reported in five trials (83%) (Abu‐Ta'a 2008; Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011). One study excluded all 16 participants who did not attend the two‐ and seven‐day postoperative examinations and did not provide information regarding the fate of the implants for these people (Nolan 2013), therefore, the risk of attrition bias was recorded as high for this trial.
Selective reporting
We assessed all but one of the trials (83%) included in this review as being at low risk of selective reporting bias because they all reported most of the main outcomes of this review. The authors of one trial could not supply information about whether some of the prostheses could not be placed after implant failures without placing additional implants for replacing the failed ones (Nolan 2013), therefore this trial was assessed as being at high risk of bias for this domain.
Other potential sources of bias
No other potential source of bias could be identified.
Overall risk of bias
The final 'Risk of bias' assessment, after incorporating the additional information kindly provided by the authors of three trials (Abu‐Ta'a 2008; Caiazzo 2011; Nolan 2013), is summarised in Figure 1 and Figure 2. For each trial we assessed whether it was at low, unclear or high risk of bias. Three trials were judged to be at low risk of bias (Esposito 2008a, Anitua 2009, Esposito 2010a), and three at high risk of bias (Abu‐Ta'a 2008; Caiazzo 2011; Nolan 2013).
1.
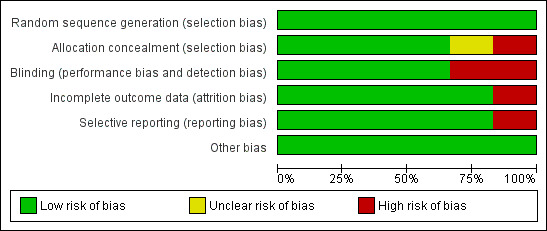
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
2.
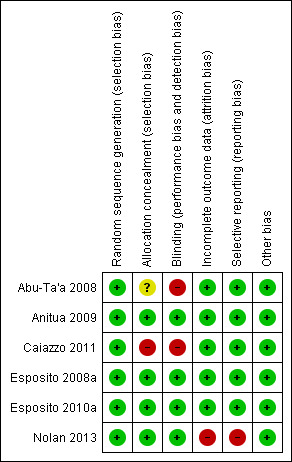
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
See: Table 1
(1) Antibiotic versus placebo or no treatment (six trials with 1162 participants)
One trial assessed as being at high risk of bias, compared 1 g of amoxicillin given one hour preoperatively plus 500 mg of amoxicillin four times a day for two days versus no antibiotics (Abu‐Ta'a 2008). Forty participants were included in each group and none dropped out after five months. No prosthesis failed. Five implants failed in three participants who did not receive antibiotics. One participant in the antibiotic group and four in the control group experienced a postoperative infection. No adverse events were reported. No statistically significant differences were observed for any of the outcome measures.
One placebo‐controlled trial assessed as being at low risk of bias (Esposito 2008a), compared 2 g of amoxicillin given one hour preoperatively with identical placebo tablets. There were 165 participants in each group, but seven from each group had to be excluded from the analyses for various reasons. Two participants in the antibiotic group experienced a prosthesis failure versus four in the placebo group. Two participants (two implants) in the antibiotic group experienced implant losses versus eight participants (nine implants) in the placebo group. Three participants in the antibiotic group presented with signs of infection versus two in the placebo group. One minor adverse event was recorded in each group. No statistically significant differences were observed for any of the outcome measures.
One placebo‐controlled trial assessed as being at low risk of bias (Anitua 2009), compared 2 g of amoxicillin given one hour preoperatively with identical placebo tablets. Fifty‐two participants were included in the antibiotic group and 53 in the placebo group. Two participants in each group experienced an implant/crown failure and six in each group experienced a postoperative infection. No adverse events were reported. No statistically significant differences were observed for any of the outcome measures.
One placebo‐controlled trial, assessed as being at low risk of bias (Esposito 2010a), compared 2 g of amoxicillin given one hour preoperatively with identical placebo tablets. The antibiotic group had 254 participants and the placebo group had 255, but two participants from the antibiotic group and one from the placebo group had to be excluded from the analyses for various reasons. Four participants in the antibiotic group experienced a prosthesis failure versus 10 in the placebo group. Five participants in the antibiotic group experienced seven implant losses versus 12 participants who lost 13 implants in the placebo group. Four participants in the antibiotic group presented with clear signs of infection versus eight in the placebo group. No adverse events were reported. No statistically significant differences were observed for any of the outcome measures.
One trial assessed as being at high risk of bias (Caiazzo 2011), compared 2 g of amoxicillin given one hour preoperatively with no antibiotics. There were two additional intervention groups (2 g amoxicillin given one hour preoperatively plus 1 g twice a day for seven days; and 1 g of amoxicillin given immediately after implantation twice a day for seven days). Twenty‐five participants were included in each group and none dropped‐out after three months. Two single crowns could not be placed in the no antibiotics group because of implant failures. Two single implants failed in two participants in the no antibiotic group versus none in any of the three antibiotic groups. No infections or side‐effects were reported. No statistically significant differences were observed for any of the outcome measures.
One placebo‐controlled trial, assessed as being at high risk of bias (Nolan 2013), compared 3 g of amoxicillin given one hour preoperatively to 27 participants, with identical placebo tablets given to another 28 participants. A study sample of 83 was identified but 28 patients had to be excluded (eight required simultaneous bone grafts, four needed grafting, and 16 patients were unable to attend 2nd and 7th day postoperative visits). It is unknown whether all prostheses could be delivered as planned. No participant in the antibiotic group had implant losses versus five participants who lost five implants in the placebo group. No participant in the antibiotic group presented with clear signs of infection versus two participants in the placebo group. No statistically significant differences were observed for any of the outcome measures.
A total of 1162 participants were analysed in the six included trials, three of which were assessed as being at low risk of bias and three at high risk of bias. The overall body of evidence was considered to be of moderate quality. More participants experienced implant losses in the group that did not receive antibiotics and this was statistically significant (risk ratio (RR) 0.33; 95% CI 0.16 to 0.67, P value 0.002, heterogeneity: Tau2 0.00; Chi2 2.87, df = 5 (P value 0.57); I2 0%) (Figure 3). In order to illustrate the magnitude of the effect of implant failures, the number of people needed to treat for an additional beneficial outcome (NNTB), i.e. given antibiotics, to prevent one person having an implant failure is 25 (95% CI 14 to 100). This is based on an implant failure of 6% in participants not receiving antibiotics, as seen in the meta‐analysis. No heterogeneity was observed in the meta‐analysis. The meta‐analyses for the other outcomes showed borderline statistical significance in favour of antibiotics for prosthesis failures (five trials) (RR 0.44; 95% CI 0.19 to 1.00) (Analysis 1.2), and no statistically significant difference for postoperative infections (six trials) (RR 0.69; 95% CI 0.36 to 1.35) (Analysis 1.3), and adverse events (six trials) (RR 1.0; 95% CI 0.06 to 15.85) (Analysis 1.4).
3.
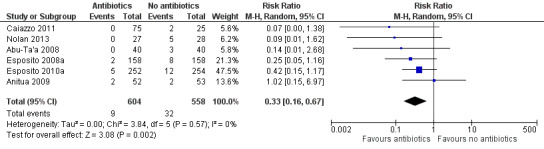
Forest plot of comparison: 1 Antibiotics versus placebo/no antibiotics, outcome: 1.1 Implant failures
1.2. Analysis.
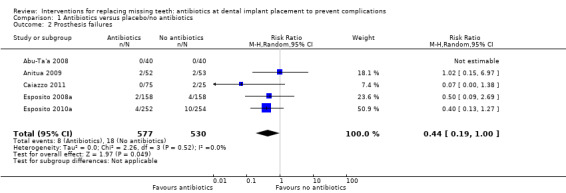
Comparison 1 Antibiotics versus placebo/no antibiotics, Outcome 2 Prosthesis failures.
1.3. Analysis.
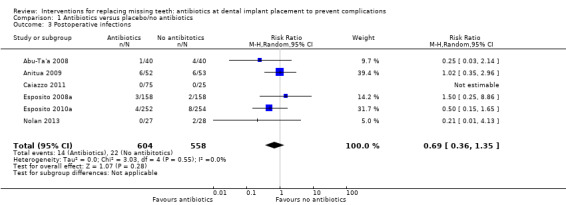
Comparison 1 Antibiotics versus placebo/no antibiotics, Outcome 3 Postoperative infections.
1.4. Analysis.
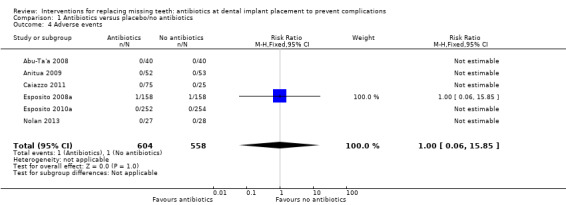
Comparison 1 Antibiotics versus placebo/no antibiotics, Outcome 4 Adverse events.
If data for preoperative antibiotics only are considered, then prophylactic use of these antibiotics confers a benefit for those having dental implant placements, with an RR of 0.37 (95% CI 0.18 to 0.76); this is based on five trials, at unclear or high risk of bias. However, three trials with the great majority of participants were at low risk of bias.
(2) Antibiotic prophylaxis given for different durations (one trial with 100 participants)
One trial (Caiazzo 2011), at high risk of bias, compared four interventions: (1) 2 g of amoxicillin given one hour preoperatively; (2) 2 g of preoperative amoxicillin plus 1 g twice a day for seven days; (3) 1 g of postoperative amoxicillin twice a day for seven days; and (4) no antibiotics (this intervention is not of interest when considering duration of antibiotic use). Twenty‐five participants were included in each group and none dropped‐out after three months. Not a single event occurred in the three antibiotic groups (75 participants).
(3) Antibiotic prophylaxis with different antibiotics (no trials)
(4) Antibiotic prophylaxis given at different dosages (no trials)
Discussion
Summary of main results
The meta‐analysis of six randomised controlled trials (RCTs), three of which were assessed as being at high risk of bias and three at low risk of bias, suggests that short‐term antibiotics, e.g. 2 g (Esposito 2008a; Anitua 2009; Esposito 2010a; Caiazzo 2011), or 3 g (Nolan 2013), of amoxicillin administered one hour prior to implant placement or 1 g of amoxicillin administered one hour prior to implant placement and 500 mg four times a day for two days postoperatively (Abu‐Ta'a 2008), significantly decrease early implant failures (RR 0.33; 95% CI 0.16 to 0.67). This observation has important clinical implications, as the use of antibiotics in this way would prevent one person experiencing an early implant loss for every 25 people receiving antibiotics.
There were borderline statistical significance for prosthesis failures in favour of antibiotics (RR 0.44; 95% CI 0.19 to 1.00), and no statistically significant differences for any of the other outcomes: infections (RR 0.69; 95% CI 0.36 to 1.35) or adverse events (RR 1; 95% CI 0.06 to 15.85). In particular only two minor adverse events were reported, one in the antibiotic group (diarrhoea and somnolence) and one in the placebo group (itching for one day), which suggest that the antibiotic regimens used may not have a significant negative impact on the participants' well‐being. In other words, the benefit of using short‐term antibiotics may outweigh the risks in the short‐term for individual patients. However we need to consider that the sample sizes of all trials together were far too small to be able to catch rare ‐ but life‐threatening ‐ adverse events such as anaphylactic shock.
No conclusive information can be derived from the only trial that compared three different durations of antibiotic prophylaxis, since no event (implant or prosthesis failures, infections or adverse events) occurred in any of the 25 participants included in each study group (Caiazzo 2011).
Overall completeness and applicability of evidence
Results of this review appear to be easily applicable to populations of patients undergoing routine implant placement procedures both in university hospitals and private practices.
Quality of the evidence
Three trials were judged to be at low risk of bias (Esposito 2008a; Anitua 2009; Esposito 2010a), and the remaining three at high risk of bias (Abu‐Ta'a 2008; Caiazzo 2011; Nolan 2013), however the results of all studies were homogenous, and this strengthens confidence in the results. The main issues were the lack of blinding of operators, participants and outcome assessors in two trials (Abu‐Ta'a 2008; Caiazzo 2011), and the withdrawal of randomised participants not attending all follow‐ups in another (Nolan 2013). Lack of blinding may induce surgeons to perform, more 'clean and careful' implantation procedures in people without antibiotic coverage to minimise risks of infection, and patients may report more or fewer adverse events depending on their personal beliefs on the role of antibiotic coverage. Proper blinding remains the only way to minimise these risks, and could have been easily and effectively done by use of a placebo. The withdrawal of participants who did not attend the immediate postoperative visits should not be done, since they attended the most relevant appointments, such as abutment connection, when the main outcome measures could have been recorded (implant/prosthesis success). All data should be recorded and reported, allowing readers to make their own judgements. Theoretically the trial authors could have retrieved this information from the participants' files, however participants were assigned a unique identification number at the beginning of the study and their names were not used, as requested by the ethical committee. Unfortunately, the list identifying participants was deleted recently, so their records could not be retrieved to see the outcome of their implants.
Potential biases in the review process
No particular biases in the review process could be identified.
Agreements and disagreements with other studies or reviews
An earlier systematic review on this topic concluded that there is little evidence for the use of antibiotic prophylaxis in general dentistry, and recommended monitoring of antibiotic use among dental practitioners (Schwartz 2007). One RCT was not included in this review because its follow‐up was too short (eight weeks) (Tan 2013). In this trial 329 healthy adults in need of a single implant‐supported crown were randomly assigned to four groups: 1) preoperatively 2 g of amoxicillin one hour before surgery (81 participants), 2) 2 g of amoxicillin immediately following surgery (82 participants), 3) preoperatively 2 g of amoxicillin one hour before surgery and 500 mg thrice daily on days two and three after surgery (86 participants), 4) preoperatively 2 g of placebo one hour before surgery (80 participants). Subjects were examined clinically by blinded examiners up to crown delivery at eight weeks after implant placement. Implants were not submerged. There was only a significant difference in flap closure at week 4, where 5% of the participants who did not receive antibiotic did not achieve complete wound closure when compared to 0% for the three other groups (P value 0.01). There was only one implant failure, which again occurred in one of the participants who did not received antibiotics. It is worth to observe that this trial reported an extremely low failure rate (0.3% at participant level), which is almost 13 times less than that reported in another trial (3.81% at participant level) that included participants with similar characteristics (Anitua 2009). The latter figures are more representative of what currently happens in 'everyday' conditions. Nevertheless, the findings of the non‐included trial (Tan 2013) agree with the findings of the present review.
There are a few controlled trials that provided contradictory results. The first study on this subject evaluated implant success at abutment connection (four to six months after implant placement) (Dent 1997). This trial compared various dosages and antibiotics given preoperatively and postoperatively, generally compared with no antibiotics or antibiotics given in an insufficient dosage to an unknown number of participants (2641 implants). Significantly fewer failures occurred in the antibiotic group (1.5% versus 4%). The study was updated by a second publication that presented data with a follow‐up of three years after loading (Laskin 2000). This reported 387 participants (1743 implants) in the antibiotic group and 315 participants (1247 implants) in the control group. The results suggested fewer failures when antibiotics were used (4.6% versus 10%). This multicentre trial was initially described as a randomised controlled trial (RCT), but, in reality, dentists were free to choose when to give antibiotics, which antibiotics to give, and which dosage to use. In addition, there was no blinded assessment and participants were not considered as the statistical unit of the analysis, so the possible clustering of failures was not taken into account.
In a retrospective controlled clinical study (Gynther 1998), 147 participants (790 implants) who received 1 g of phenoxymethylpenicillin one hour preoperatively and 1 g every eight hours postoperatively for 10 days were compared with 132 participants (664 implants) who did not receive any antibiotics. Both groups were treated at the same centre, but at different time points (antibiotic group between 1980 and 1985; no antibiotic group between 1991 and 1995). No differences in survival rates of implants were reported. Another prospective multicentre controlled clinical study administered a single preoperative dose of penicillin G or V (1,000,000 units) or 600 mg of clindamycin to all patients, then one group had no postoperative antibiotics, the other receiving 300 mg penicillin V orally four times a day, or in case of penicillin allergy, 150 mg clindamycin orally three times a day for seven days (Binahmed 2005). A single dose was given to 125 participants (445 implants), while long‐term prophylactic antibiotics were given to 90 participants (302 implants). Biological complications only were evaluated at one and two weeks post surgery and just before abutment connection. There were no differences regarding biological complications, which included three wound dehiscences in each group, with one developing an infection in the long‐term antibiotic group. The authors concluded that long‐term prophylactic antibiotic use was of no advantage or benefit over a single dose, however implant success, which should have been the primary outcome measure, was not evaluated. Unfortunately, all these studies were highly biased in their methodology, so the validity of their conclusions should be questioned.
Additional information can be obtained from three double‐blinded RCTs that evaluated the efficacy of prophylactic antibiotics used for bone augmentation procedures prior to implant placement (Lindeboom 2003; Lindeboom 2005; Lindeboom 2006). One pilot placebo‐controlled RCT (Lindeboom 2003) compared a preoperative single dose of 2 g penicillin phenethicillin with a placebo in 20 participants undergoing intraoral buccal onlay grafting with resorbable barriers to allow implant placement. Two participants developed an infection at both the receptor and donor sites; two participants developed a wound infection at the receptor site; and one participant developed an infection at the donor site only. All of these participants (50%) were in the placebo group. No infections were observed in the antibiotic group. It could be concluded that there was a statistically significant increased risk of having an infectious complication after bone augmentation with resorbable barriers without antibiotic prophylaxis. One RCT compared 2 g penicillin phenethicillin versus 600 mg of clindamycin as a single dose in participants treated with block‐shaped bone graft harvested from the mandibular ramus and covered by resorbable barriers (the implants were not placed in the study) (Lindeboom 2006). Each group had 75 participants and the presence of infection was assessed weekly for eight weeks. No statistically significant differences were observed for postoperative infections (four infections at the augmented sites of the penicillin phenethicillin versus two in the clindamycin group, and three infections at the donor site of each group). The findings of this trial suggest that both penicillins and clindamycin are effective in reducing infection at augmented sites. No side effects related to the single‐administration of antibiotics were reported. In another, similar, RCT the same research group evaluated whether it was more effective to use a single dose of 600 mg clindamycin one hour prior to onlay bone grafting procedures followed by either placebo or 300 mg clindamycin every six hours for one day (Lindeboom 2005). Each group had 62 participants. No statistically significant differences were observed for postoperative infections (two infections at the augmented sites of the single dose group versus three infections in the one‐day group, and four infections at the donor sites of the single dose group versus two infections in the one‐day group). Again, no side effects related to the administration of antibiotics were reported.
Authors' conclusions
Implications for practice.
There is evidence from a meta‐analysis including six trials with 1162 participants suggesting that administration of antibiotics significantly reduces early failure of dental implants placed in ordinary conditions. Five of these compared a single administration of preoperative antibiotics (2 g or 3 g of amoxicillin given orally one hour preoperatively) with placebo or no antibiotics. More specifically, giving antibiotics to 25 people will prevent one person experiencing early implant losses. Borderline statistical significance was shown for prosthesis failures. No statistically significant differences in postoperative infections and adverse events were observed. No major adverse events were reported. It might be sensible to suggest routine use of a single dose of 2 g of prophylactic amoxicillin just before placing dental implants. It remains unclear whether an adjunctive use of postoperative antibiotics is beneficial, and which antibiotic is the most effective.
Implications for research.
Priority should be given to large pragmatic double‐blinded RCTs evaluating the efficacy of prolonged antibiotic prophylaxis compared to a single preoperative dose in those subgroups of people where implant failures are more likely to occur, particularly in those receiving immediate post‐extractive implants and augmentation procedures in conjunction with implant placement. It would also be useful to evaluate which antibiotic type is the most effective.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 24 June 2013 | New citation required but conclusions have not changed | Substantive amendment. New authorship. New methods. 2 new included studies. Conclusions not changed. |
| 24 June 2013 | New search has been performed | Search updated to June 2013. |
| 2 May 2008 | New search has been performed | Search updated to January 2008. |
| 2 May 2008 | Amended | Converted to new review format. |
| 2 May 2008 | New citation required and conclusions have changed | Substantive amendment. Title was modified. 2 randomised controlled trials were included. The conclusions changed. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
We wish to thank Anne Littlewood (Cochrane Oral Health) for her assistance with literature searching; Luisa Fernandez Mauleffinch and Phil Riley (Cochrane Oral Health) for their help with the preparation of this review; Paul Coulthard, Vassiliki Loli, Richard Oliver, Minesh Talati and Peter Thomsen for their contributions in previous versions of the present review; Mahmoud Abu‐Ta'a, Gorka Orive and Ioannis Polyzois for providing us with information about their trials. We would also like to thank the following referees: Ian M Brook, Alfonso Caiazzo, Matteo Chiapasco, Sue Furness, Anne‐Marie Glenny, Lee Hooper, Jerome Lindeboom, David R Moles, Ian Needleman, Michele Nieri, Gorka Orive, and Ioannis Polyzois.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Dental Implants/ 2. exp Dental Implantation/ or dental implantation 3. exp Dental Prosthesis, Implant‐Supported/ 4. ((osseointegrated adj implant$) and (dental or oral)) 5. dental implant$ 6. (implant$ adj5 dent$) 7. (((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$) 8. "implant supported dental prosthesis" 9. ("blade implant$" and (dental or oral)) 10. ((endosseous adj5 implant$) and (dental or oral)) 11. ((dental or oral) adj5 implant$) 12. OR/1‐11
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. The Cochrane Oral Health Group's Trials Register search strategy
An updated search was undertaken using the Cochrane Register of Studies and the search strategy below in January 2013:
#1 ("dental implant*" or "oral implant*" or "implant support*" or "endosseous implant*" or "blade implant*") AND (INREGISTER) #2 ((implant* and (oral or dental))) AND (INREGISTER) #3 ("subperiosteal implant*") AND (INREGISTER) #4 ((implant* AND overdenture*)) AND (INREGISTER) #5 (((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*)))) AND (INREGISTER) #6 (#1 or #2 or #3 or #4 or #5) AND (INREGISTER)
A previous search of the Register was undertaken in January 2012 using the Procite software and the search strategy below:
(dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 DENTAL IMPLANTS explode all trees (MeSH) #2 DENTAL IMPLANTATION explode all trees (MeSH) #3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH) #4 ((osseointegrat* near implant*) and (dental* or oral*)) #5 (dental next implant*) #6 (implant* near dent*) #7 dental‐implant* #8 ((overdenture* near dental*) and implant*) #9 ((overdenture* near oral*) and implant*) #10 ((crown* near dental*) and implant*) #11 ((crown* near oral*) and implant*) #12 ((bridge* near dental*) and implant*) #13 ((bridge* near oral*) and implant*) #14 ((prosthesis near dental*) and implant*) #15 ((prosthesis near oral*) and implant*) #16 ((prostheses near dental*) and implant*) #17 ((prostheses near oral*) and implant*) #18 ((restoration* near dental*) and implant*) #19 ((restoration* near oral*) and implant*) #20 (implant next supported next dental next prosthesis) #21 (blade next implant*) #22 ((endosseous near implant*) and dental) #23 ((endosseous near implant*) and oral*) #24 ((dental* near implant*) or (oral* near implant*)) #25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE (OVID) search strategy
1. tooth implantation/ 2. ((implant‐supported or implant$) adj support$).mp. 3. ((osseointegrated adj implant$) and (dental or oral)).mp. 4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. 5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. 6. "implant supported dental prosthesis".mp. 7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. 8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. 9. ((dental or oral or tooth or teeth) and implant$).mp. 10. or/1‐9
The above search was run with the Cochrane Oral Health Group's search strategy for isolating RCTs in EMBASE:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Data and analyses
Comparison 1. Antibiotics versus placebo/no antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Implant failures | 6 | 1162 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.16, 0.67] |
| 2 Prosthesis failures | 5 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.19, 1.00] |
| 3 Postoperative infections | 6 | 1162 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.36, 1.35] |
| 4 Adverse events | 6 | 1162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.85] |
1.1. Analysis.
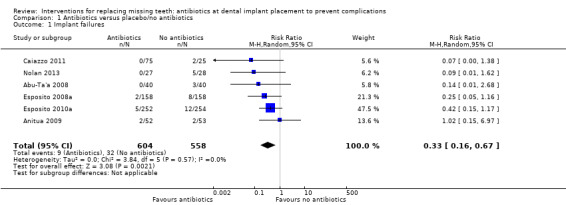
Comparison 1 Antibiotics versus placebo/no antibiotics, Outcome 1 Implant failures.
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Esposito 2008a.
| Methods | Multicentre, placebo‐controlled, parallel RCT of 4 months' duration. 7 exclusions from each group for various explained reasons | |
| Participants | Partially‐ and fully‐edentulous participants. Adults treated in 11 private Italian dental practices. Participants excluded if they were: allergic to penicillins, needing prophylaxis for endocarditis, immunodeficient, diabetic, had implanted prostheses, required bone augmentation at implant placement with infections in the vicinity of the implant site(s), had been irradiated in the head and neck area, were already receiving antibiotic treatment, had been treated or receiving treatment with intravenous amino‐bisphosphonates, were pregnant or lactating. 165 participants included in each group and results given for 158 | |
| Interventions | 2 g of amoxicillin given 1 h preoperatively versus identical placebo tablets. Operators were allowed to place and restore the implants according to their routine procedures. 1 week prior to implant placement, all participants underwent at least 1 session of oral hygiene instruction and professionally‐delivered debridement, if required. All participants rinsed with chlorhexidine digluconate 0.2% for 1 minute just prior to surgery and postoperatively twice a day for at least 1 week. Operators were allowed to place and restore the implants according to their routine procedures. Postoperative complications were assessed at 1 and 2 weeks, and implant success at 4 months. Various implant systems brands were used (Zimmer Dental, Dentsply Friadent, Nobel Biocare, Intra‐Lock, Camlog, Dyna, Biomet 3i, and Endopore) | |
| Outcomes | Prosthesis and implant failures, postoperative complications, adverse events. Postoperative complications assessed 1 and 2 weeks after placement, and implant stability at 4 months | |
| Notes | Antibiotics and placebo donated by a drug company manufacturing generic drug; the company was not involved in the design of the study, in the data evaluation, or in commenting on the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from the article: "Twelve computer generated restricted randomization lists with equal groups of participants were made." |
| Allocation concealment (selection bias) | Low risk | Quoted from the article: "Only one of the investigators (Dr Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomization sequence and could have access to the randomization lists stored in his password protected portable computer. The randomized codes (1 or 2) were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially 1 hour prior to implant placement and patients assumed 2 tablets taken from identical white plastic containers labelled with the same code of the envelopes (1 or 2), containing the antibiotic or identical placebo tablets. Therefore treatment allocation was concealed to the investigators in charge of enrolling and treating the patients . . . " |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quoted from the article: " . . . and both patients and operators/outcome assessors were blinded to the tested intervention. Also the statistician was kept blind and performed all analyses without knowing to which group the patients were allocated." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions and missing data reported and explained |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other bias identified |
Abu‐Ta'a 2008.
| Methods | Single centre parallel RCT of 5 months duration. No drop‐outs | |
| Participants | Partially‐ and fully‐edentulous participants. Adults treated at the Department of Periodontology of the University Hospital of the Catholic University Leuven, Belgium. Participants were excluded if they were: allergic to penicillins, needing prophylaxis for endocarditis, immunodeficient, with uncontrolled diabetes mellitus, or irradiated in the head and neck area. 40 participants included in each group and results given for 40 | |
| Interventions | 1 g of amoxicillin given 1 h preoperatively plus 500 mg of amoxicillin 4 times a day for 2 days versus no antibiotics. All participants rinsed with chlorhexidine digluconate for 1 minute just prior to surgery and postoperatively twice a day for 7 to 10 days. The perioral skin was disinfected for 30 s with cetrimonium bromide 0.5% and chlorhexidine 0.05% in water. Measures of asepsis included use of sterile drapes around the participant's mouth, head, and over the supine body of the participant, a meshed nose guard, and 2 suction tips (1 only for the mouth and 1 only for the wound). Postoperative complications were assessed at 7 to 10 days and implant success at 5 months. An unknown type of dental implant was used | |
| Outcomes | Implant failures, postoperative infections, adverse events, microbiological evaluation. Postoperative infections were assessed 7 to 10 days after placement, and implant success at 5 months | |
| Notes | No external funding was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from the article: "These patients were randomly assigned into one of two groups (with and without antibiotics = AB) of 40 patients each using random sampling with masking of the person performing the randomization." Author's reply: "After verification of the inclusion criteria, 80 patients were enrolled into the study. All patients were assigned a patient number, and were randomly assigned to one of the two treatment regimens. Assignment was performed by one of our department's nurses using a randomization table and by applying the simple randomization method." |
| Allocation concealment (selection bias) | Unclear risk | No information provided in the article. Author's reply: "Masking: It was maintained up to the day of implant installation. Afterwards, of course, it was difficult to maintain the masking since patients were asked about their postoperative experiences and any side effect of the antibiotic when removing the stitches." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quoted from the article: "Both the surgical team and the patients were blinded to the groups." Author's reply: "No, see above." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data or excluded patients |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other bias identified |
Anitua 2009.
| Methods | Placebo‐controlled parallel RCT of 3 months' duration. No drop‐outs | |
| Participants | Only included people needing single implants in medium bone quality and all implants were inserted after flap elevation. Adults treated in 8 private Spanish dental practices. Participants were excluded if: they were allergic to beta‐lactam antibiotics; had concurrent local or systemic infections requiring antibiotic treatment; had systemic diseases that contraindicated the surgery including cardiovascular diseases, respiratory diseases, haematological and metabolic disorders, bone diseases, collagenosis, immunodeficiencies and renal insufficiency; or had received irradiation to the head and neck (> 5000 rads). 52 participants included in the antibiotic group and 53 in the placebo group with results given for 52 and 53 participants, respectively | |
| Interventions | 2 g of amoxicillin given 1 h preoperatively versus identical placebo tablets. During the days prior to the intervention participants received appropriate prophylaxis and adequate oral hygiene instructions. Antibiotics and other medications were not allowed 15 days before the surgery. All participants rinsed with 0.2% chlorhexidine digluconate for one minute just prior to surgery. Only single implants in medium bone quality were included and all implants were inserted after flap elevation. Before installation, implants were carefully humidified with liquid plasma rich in growth factors (PRGF). Peripheral blood (20 ml to 30 ml) from each participant was taken by venipuncture before surgery and placed directly into 9 ml tubes containing 3.8% (wt/vol) sodium citrate as anticoagulant. Liquid PRGF was prepared by centrifugation (PRGF System®, BTI) at 460 × g for 8 minutes at room temperature; 1 ml plasma fraction was collected and deposited in a glass dish. In order to initiate clotting, PRGF activator (calcium chloride) was added to the liquid PRGF preparation (50 μl PRGF activator per ml of preparation). Postoperative infections were assessed at days 3, 10, 30 and 60. Implant stability was also evaluated at 3 months using Osstell. BTI dental implants were used | |
| Outcomes | Implant failures (assessed with Ostell at 3 months), postoperative infections, adverse events, microbiological evaluation. Postoperative infections assessed at days 3, 10, 30 and 90 after implant placement | |
| Notes | Trial supported by the implant manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from the article: "The randomisation was performed using a random numbers table, assigning each patient to one of two treatment groups (active or placebo). Each of the enrolled patients had a patient number and, according to the randomisation table, was assigned to each treatment group." |
| Allocation concealment (selection bias) | Low risk | Quoted from the article: "Both researchers and patients remained blinded to the received treatment group. For this purpose, the tablets corresponding to each patient were included in a package identified only by the study number and the patient code. Researchers had a sealed envelope for each patient to establish the randomly assigned treatment if necessary. The envelope was opened at the end of the study. Only in those situations in which the clinician observed any side‐effect was the envelope opened before." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data or excluded participants |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other bias identified |
Esposito 2010a.
| Methods | Multicentre, placebo‐controlled, parallel RCT of 4 months' duration. 2 exclusions from the antibiotic group and 1 from the placebo group for various, explained reasons | |
| Participants | Partially‐ and fully‐edentulous participants. Adults treated in 10 private Italian dental practices. Participants excluded if: they were allergic to penicillins, immunodeficient, diabetic, needing prophylaxis for endocarditis, had implanted prostheses, required bone augmentation at implant placement and had infections in the vicinity of the implant site(s), irradiated in the head and neck area, already receiving antibiotic treatment, treated or receiving treatment with intravenous amino‐bisphosphonates, were pregnant or lactating. 254 participants included in the antibiotic group and 255 in the placebo group; results given for 242 and 254 participants, respectively | |
| Interventions | 2 g of amoxicillin given 1 h preoperatively versus identical placebo tablets. Operators were allowed to place and restore the implants according to their routine procedures. 1 week prior to implant placement, all participants underwent at least 1 session of oral hygiene instruction and professionally‐delivered debridement, if required. All participants rinsed with chlorhexidine digluconate 0.2% for 1 minute just prior to surgery and postoperatively twice a day for at least 1 week. Operators were allowed to place and restore the implants according to their routine procedures. Postoperative complications were assessed at 1 and 2 weeks, and implant success at 4 months. Various implant systems brands were used (Zimmer Dental, Dentsply Friadent, Nobel Biocare, Intra‐Lock, Camlog, Dyna, Biomet 3i, Endopore, Z‐system, PF Tecom, Ghimas, Silpo, MegaGen and Geass) | |
| Outcomes | Prosthesis and implant failures, postoperative complications, adverse events. Postoperative complications assessed 1 and 2 weeks after placement, and implant stability at 4 months | |
| Notes | Antibiotics and placebo donated by a drug company manufacturing generic drug; the company was not involved in the design of the study, in the data evaluation, or in commenting on the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from the article: "Thirteen computer generated restricted randomization lists with equal groups of participants were made." |
| Allocation concealment (selection bias) | Low risk | Quoted from the article: "Only one of the investigators (Dr Marco Esposito), not involved in the selection and treatment of the patients, was aware of the randomization sequence and could have access to the randomization lists stored in his password protected portable computer. The randomized codes (1 or 2) were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially 1 hour prior to implant placement and patients assumed 2 tablets taken from identical white plastic containers labelled with the same code of the envelopes (1 or 2), containing the antibiotic or identical placebo tablets. Therefore treatment allocation was concealed to the investigators in charge of enrolling and treating the patients . . . " |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quoted from the article: ". . . and both patients and operators/outcome assessors were blinded to the tested intervention. Also the statistician was kept blind and performed all analyses without knowing to which group the patients were allocated." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions and missing data reported and explained |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other bias identified |
Caiazzo 2011.
| Methods | Multicentre parallel RCT with 4 arms, of 3 months' duration. No drop‐outs | |
| Participants | Adult treated in 2 private Italian dental practices. Participants were excluded if they had: a history of systemic diseases contraindicating surgical treatment, long‐term nonsteroidal anti‐inflammatory drug therapy, medically necessary antibiotic therapy, a history of antibiotic therapy 6 months prior to the study, a history of allergic reactions to penicillins or related drugs, or were pregnant. 25 participants included in each group and results given for 100 | |
| Interventions | 4 interventions compared: 2 g of amoxicillin given 1 h preoperatively; 2 g amoxicillin given 1 h preoperatively + 1 g twice a day for 7 days; 1 g of amoxicillin given immediately after implantation twice a day for 7 days; and no antibiotic. All participants had at least 1 session of oral hygiene instruction and professionally‐delivered debridement 1 week prior to implant placement. All participants rinsed with chlorhexidine digluconate 0.2% for 1 minute just prior to surgery and postoperatively twice a day for 15 days. Clinicians placed implants according to their routine procedures. Postoperative complications were assessed at 1, 2, 4 and 8 weeks, and implant success at 3 months. Used Biomet 3i Osseotite implants with external connection | |
| Outcomes | Implant failures, postoperative complications, adverse events. Postoperative complications assessed 1, 2, 4 and 8 weeks after placement, and implant stability at 3 months | |
| Notes | No external funding was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from the article: "The patients were randomly allocated ‐ using a computer‐generated randomisation list ‐ to 4 different groups: . . . " The authors also informed us that they used the Random Allocation Software to prepare the randomisation schedule |
| Allocation concealment (selection bias) | High risk | No information provided in the article The authors kindly informed us that no allocation concealment procedures were implemented |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information provided in the article The authors kindly informed us that the operators recorded the outcome measures, so they were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data or excluded patients |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other bias identified |
Nolan 2013.
| Methods | Single centre placebo‐controlled parallel RCT of 3‐4 months' duration | |
| Participants | Partially‐ and fully‐edentulous participants. Adults treated in the Dublin Dental University Hospital, Department of Restorative Dentistry and Periodontology, Dublin, Ireland. Participants were excluded if they had medical conditions that required antibiotic premedication such as prosthetic heart valve replacement, skeletal or joint replacement, previous history of infective endocarditis and a history of rheumatic fever; metabolic diseases such as type I or II diabetes mellitus; past or present neoplastic disease; previous radiotherapy in the head and neck area; were immunosuppressed; had blood coagulation impairment; had a history of systemic steroid medication or recent systemic antibiotic therapy; were pregnant or lactating; or had an allergy to the antibiotic chosen. 83 participants randomised but results given for only 27 of the antibiotic group and 28 of the placebo group | |
| Interventions | 3 g of amoxicillin given 1 h preoperatively versus identical placebo tablets. All participants rinsed with 0.2% chlorhexidine digluconate for 1 minute just prior to surgery. Implants were placed according to the manufacturers guidelines using standard surgical procedures. Participants were instructed to use a chlorhexidine 0.2% mouthwash 4 to 5 times daily for the first postoperative week. Postoperative complications and adverse events were assessed at 2 and 7 days, and implant success at 3 to 4 months. Various implant brands were used (Biomet 3i, Nobel Biocare, Ankylos, Dentsply Friadent, Germany, Straumann SLA Implants) | |
| Outcomes | Implant failures, postoperative complications, adverse events, pain and interference with daily activities. Postoperative complications were assessed 2 and 7 days after implant placement, and implant stability at 3‐4 months | |
| Notes | No external funding was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quoted from the article: "Every patient that agreed to participate was randomly assigned to one of the following two groups" The authors kindly informed us that envelopes and random computer generated numerical codes were prepared Stickers with these codes were placed outside sealed opaque envelopes |
| Allocation concealment (selection bias) | Low risk | Quoted from the article: "Consecutively treated patients received consecutive numbers correlating with the number of an envelope. This envelope contained either the 3 g amoxicillin, or similar placebo capsules. A master file held the key to whether an envelope contains the 3 g amoxicillin or the placebo capsules. An independent person administered the antibiotic or the placebo. Neither the surgeon nor the patient knew which has been taken to ensure a double‐blind approach" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The article reported that two independent examiners, performed all assessment The authors kindly informed us that assessors were masked to the interventions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Partcipants who did not attend the 2 and 7 day postoperative examinations (16 patients) were excluded from the study, so the fate of their implants is unknown |
| Selective reporting (reporting bias) | High risk | The authors could not supply information about whether some of the prostheses could not be placed after implant failures without placing additional implants for replacing the failed ones. This was due to the fact that participants were assigned a unique number to identify their sample at the beginning of the study and the participants' names were not be used after assignment of this number (as per ethics committee requirements). The list identifying participants had been deleted recently, so their records could not be identified to see what happened with their prostheses |
| Other bias | Low risk | No other bias identified |
> = more than; h = hour(s); RCT = randomised controlled trial
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Tan 2013 | Follow‐up too short (8 weeks post‐implantation) |
Differences between protocol and review
None.
Contributions of authors
Conceiving, designing and co‐ordinating the review: Marco Esposito (ME). Developing search strategy and undertaking searches: ME. Screening search results and retrieved papers against inclusion criteria: ME, Maria Gabriella Grusovin (GG). Writing to authors for additional information: ME. Appraising quality: GG, ME, Helen Worthington (HW). Data extraction: ME, GG, HW. Analysis and interpretation of the data: ME, HW. Writing the review: ME.
Sources of support
Internal sources
Division of Dentistry, The University of Manchester, UK.
-
Manchester Academic Health Sciences Centre (MAHSC), UK.
Cochrane Oral Health is supported by MAHSC and the NIHR Manchester Biomedical Research Centre.
External sources
Swedish Medical Research Council (9495), Sweden.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
Marco Esposito is the first author of two of the included studies, however, he was not involved in the quality assessment of these trials. Maria Bariella Grusovin: no interests to declare. Helen Worthington: no interests to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abu‐Ta'a 2008 {published data only}
- Abu‐Ta'a M, Quirynen M, Teughels W, Steenberghe D. Asepsis during periodontal surgery involving oral implants and the usefulness of peri‐operative antibiotics: a prospective, randomized, controlled clinical trial. Journal of Clinical Periodontology 2008;35(1):58‐63. [DOI] [PubMed] [Google Scholar]
Anitua 2009 {published data only}
- Anitua E, Aguirre JJ, Gorosabel A, Barrio P, Errazquin JM, Román P, et al. A multicentre placebo‐controlled randomised clinical trial of antibiotic prophylaxis for placement of single dental implants. European Journal of Oral Implantology 2009;2(4):283‐92. [PubMed] [Google Scholar]
Caiazzo 2011 {published and unpublished data}
- Caiazzo A, Casavecchia P, Barone A, Brugnami F. A pilot study to determine the effectiveness of different amoxicillin regimens in implant surgery. Journal of Oral Implantology 2011;37:691‐6. [DOI] [PubMed] [Google Scholar]
Esposito 2008a {published data only}
- Esposito M, Cannizzaro G, Bozzoli P, Consolo U, Felice P, Ferri V, et al. Efficacy of prophylactic antibiotics for dental implants: a multicentre placebo‐controlled randomised clinical trial. European Journal of Oral Implantology 2008;1(1):23‐31. [PubMed] [Google Scholar]
Esposito 2010a {published data only}
- Esposito M, Cannizzaro G, Bozzoli P, Checchi L, Ferri V, Landriani S, et al. Effectiveness of prophylactic antibiotics at placement of dental implants: a pragmatic multicenter placebo‐controlled randomized clinical trial. European Journal of Oral Implantology in press. [PubMed]
Nolan 2013 {published and unpublished data}
- Nolan R, Kemmoona M, Polyzois I, Claffey N. The influence of prophylactic antibiotic administration on post‐operative morbidity in dental implant surgery. A prospective double blind randomized controlled clinical trial. Clinical Oral Implants Research 2013 Feb 13 [Epub ahead of print]. [DOI] [PubMed]
References to studies excluded from this review
Tan 2013 {published data only}
Additional references
Adell 1985
- Adell R, Lekholm U, Branemark PI. Surgical procedures. In: Branemark PI, Zarb GA, Albrektsson T editor(s). Tissue‐integrated prostheses. Chicago: Quintessence Publishing Co, Inc, 1985:211‐32. [Google Scholar]
Binahmed 2005
- Binahmed A, Stoykewych A, Peterson L. Single preoperative dose versus long‐term prophylactic antibiotic regimens in dental implant surgery. The International Journal of Oral and Maxillofacial Implants 2005;20(1):115‐7. [PubMed] [Google Scholar]
Dent 1997
- Dent CD, Olson JW, Farish SE, Bellome J, Casino AJ, Morris HF, et al. The influence of preoperative antibiotics on success of endosseous implants up to and including stage II surgery: a study of 2,641 implants. Journal of Oral and Maxillofacial Surgery 1997;55(12 Suppl 5):19‐24. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 1998a
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I) Success criteria and epidemiology. European Journal of Oral Sciences 1998;106(1):527‐51. [DOI] [PubMed] [Google Scholar]
Esposito 1998b
- Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II) Etiopathogenesis. European Journal of Oral Sciences 1998;106(3):721‐64. [DOI] [PubMed] [Google Scholar]
Esposito 1999
- Esposito M, Thomsen P, Ericson LE, Lekholm U. Histopathologic observations on early oral implant failures. The International Journal of Oral and Maxillofacial Implants 1999;14(6):798‐810. [PubMed] [Google Scholar]
Flemmig 1990
- Flemmig TF, Newman MG. Antimicrobials in implant dentistry. In: Newman MG, Kornman K editor(s). Antibiotics/antimicrobial use in dental practice. Chicago: Quintessence Publishing Co, Inc, 1990:187‐200. [Google Scholar]
Gynther 1998
- Gynther GW, Kondell PA, Moberg LE, Heimdahl A. Dental implant installation without antibiotic prophylaxis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 1998;85(5):509‐11. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Laskin 2000
- Laskin DM, Dent CD, Morris HF, Ochi S, Olson JW. The influence of preoperative antibiotics on success of endosseous implants at 36 months. Annals of Periodontology 2000;5(1):166‐74. [DOI] [PubMed] [Google Scholar]
Lindeboom 2003
- Lindeboom JA, Akker HP. A prospective placebo‐controlled double‐blind trial of antibiotic prophylaxis in intraoral bone grafting procedures: a pilot study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2003;96(6):669‐72. [DOI] [PubMed] [Google Scholar]
Lindeboom 2005
- Lindeboom JA, Tuk JG, Kroon FH, Akker HP. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone grafting procedures: single‐dose clindamycin versus 24‐hour clindamycin prophylaxis. Mund‐, Kiefer‐ und Gesichtschirurgie 2005;9(6):384‐8. [DOI] [PubMed] [Google Scholar]
Lindeboom 2006
- Lindeboom JA, Frenken JW, Tuk JG, Kroon FH. A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone‐grafting procedures: preoperative single‐dose penicillin versus preoperative single‐dose clindamycin. International Journal of Oral and Maxillofacial Surgery 2006;35(5):433‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2013 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013.
Schwartz 2007
- Schwartz AB, Larson EL. Antibiotic prophylaxis and postoperative complications after tooth extraction and implant placement: a review of the literature. Journal of Dentistry 2007;35(12):881‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Esposito 2003
- Esposito M, Coulthard P, Oliver R, Thomsen P, Worthington HV. Antibiotics to prevent complications following dental implant treatment. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004152] [DOI] [PubMed] [Google Scholar]
Esposito 2008b
- Esposito M, Grusovin MG, Talati M, Coulthard P, Oliver R, Worthington HV. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004152.pub2] [DOI] [PubMed] [Google Scholar]
Esposito 2008c
- Esposito M, Grusovin MG, Coulthard P, Oliver R, Worthington HV. The efficacy of antibiotic prophylaxis at placement of dental implants: a Cochrane systematic review of randomized controlled clinical trials. European Journal of Oral Implantology 2008;1(2):95‐103. [PubMed] [Google Scholar]
Esposito 2010b
- Esposito M, Grusovin MG, Loli V, Coulthard P, Worthington HV. Effectiveness of prophylactic antibiotics at placement of dental implants: a Cochrane systematic review. European Journal of Oral Implantology 2010;3:101‐10. [PubMed] [Google Scholar]
Esposito 2010c
- Esposito E, Worthington HW, Loli V, Coulthard P, Grusovin MG. Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD004152.pub3] [DOI] [PubMed] [Google Scholar]
Esposito 2011
- Esposito M, Worthington HW, Loli V, Coulthard P, Grusovin MG. Effectiveness of prophylactic antibiotics at placement of dental implants: a Cochrane systematic review [La profilassi antibiotica all’inserimento implantare può ridurre i fallimenti precoci? Una revisione sistematica Cochrane]. Rivista Italiana di Stomatologia 2011;79(1):18‐28. [Google Scholar]


