Abstract
Background
Periodontitis is a chronic infective disease of the gums caused by bacteria present in dental plaque. This condition induces the breakdown of the tooth supporting apparatus until teeth are lost. Surgery may be indicated to arrest disease progression and regenerate lost tissues. Several surgical techniques have been developed to regenerate periodontal tissues including guided tissue regeneration (GTR), bone grafting (BG) and the use of enamel matrix derivative (EMD). EMD is an extract of enamel matrix and contains amelogenins of various molecular weights. Amelogenins are involved in the formation of enamel and periodontal attachment formation during tooth development.
Objectives
To test whether EMD is effective, and to compare EMD versus GTR, and various BG procedures for the treatment of intrabony defects.
Search methods
We searched the Cochrane Oral Health Group Trials Register, CENTRAL, MEDLINE and EMBASE. Several journals were handsearched. No language restrictions were applied. Authors of randomised controlled trials (RCTs) identified, personal contacts and the manufacturer were contacted to identify unpublished trials. Most recent search: February 2009.
Selection criteria
RCTs on patients affected by periodontitis having intrabony defects of at least 3 mm treated with EMD compared with open flap debridement, GTR and various BG procedures with at least 1 year follow up. The outcome measures considered were: tooth loss, changes in probing attachment levels (PAL), pocket depths (PPD), gingival recessions (REC), bone levels from the bottom of the defects on intraoral radiographs, aesthetics and adverse events. The following time‐points were to be evaluated: 1, 5 and 10 years.
Data collection and analysis
Screening of eligible studies, assessment of the methodological quality of the trials and data extraction were conducted in duplicate and independently by two authors. Results were expressed as random‐effects models using mean differences for continuous outcomes and risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CI). It was decided not to investigate heterogeneity, but a sensitivity analysis for the risk of bias of the trials was performed.
Main results
Thirteen trials were included out of 35 potentially eligible trials. No included trial presented data after 5 years of follow up, therefore all data refer to the 1‐year time point. A meta‐analysis including nine trials showed that EMD treated sites displayed statistically significant PAL improvements (mean difference 1.1 mm, 95% CI 0.61 to 1.55) and PPD reduction (0.9 mm, 95% CI 0.44 to 1.31) when compared to placebo or control treated sites, though a high degree of heterogeneity was found. Significantly more sites had < 2 mm PAL gain in the control group, with RR 0.53 (95% CI 0.34 to 0.82). Approximately nine patients needed to be treated (NNT) to have one patient gaining 2 mm or more PAL over the control group, based on a prevalence in the control group of 25%. No differences in tooth loss or aesthetic appearance as judged by the patients were observed. When evaluating only trials at a low risk of bias in a sensitivity analysis (four trials), the effect size for PAL was 0.62 mm (95% CI 0.28 to 0.96), which was less than 1.1 mm for the overall result. Comparing EMD with GTR (five trials), GTR showed statistically significant more postoperative complications (three trials, RR 0.12, 95% CI 0.02 to 0.85) and more REC (0.4 mm 95% CI 0.15 to 0.66). The only trial comparing EMD with a bioactive ceramic filler found statistically significant more REC (‐1.60 mm, 95% CI ‐2.74 to ‐0.46) at the EMG treated sites.
Authors' conclusions
One year after its application, EMD significantly improved PAL levels (1.1 mm) and PPD reduction (0.9 mm) when compared to a placebo or control, however, the high degree of heterogeneity observed among trials suggests that results have to be interpreted with great caution. In addition, a sensitivity analysis indicated that the overall treatment effect might be overestimated. The actual clinical advantages of using EMD are unknown. With the exception of significantly more postoperative complications in the GTR group, there was no evidence of clinically important differences between GTR and EMD. Bone substitutes may be associated with less REC than EMD.
Plain language summary
Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects
Emdogain might have some advantages over other methods of regenerating the tissue supporting teeth lost by gum disease, such as less postoperative complications, but has not been shown to save more compromised teeth or that patients noticed any aesthetic improvement 1 year after its application. Bacteria in plaque can cause gum disease (periodontitis) that breaks down tissue supporting teeth. Surgical cleaning tries to stop the disease to save loose teeth. Bone grafting, guided tissue regeneration and enamel matrix derivatives (such as Emdogain) aim to regenerate support tissues. Emdogain contains proteins (derived from developing pig teeth) believed to regenerate tooth attachment. The review found that adjunctive application of Emdogain regenerates about 1 mm more tissue than surgical cleaning alone, although it is unclear to which extent such improvement is noticeable since patients did not find any difference in the aesthetic results. Emdogain showed similar clinical results to guided tissue regeneration, but is simpler to use and determines less complications. Bone substitutes may induce less gum retraction than Emdogain. No serious adverse reactions to Emdogain were reported in trials.
Summary of findings
Background
Periodontitis is a chronic infective disease of the gums with severe forms affecting 10% to 30% of the adult population. Periodontitis rarely affects children and young adults but its prevalence increases steadily with advancing age. Periodontitis is caused by bacteria present in the dental plaque that induce an inflammatory response of the periodontal tissues. In susceptible individuals, this chronic inflammation will induce the breakdown of the periodontal ligament and the surrounding alveolar bone resulting in the formation of periodontal pockets around the roots. Such pockets constitute an ideal protected environment for bacteria and allow the proliferation of more aggressive anaerobic species. The symptoms of periodontitis are often underestimated and may include bleeding and recession of the gums. Painful periodontal abscesses may also form. At a more advanced stage teeth may drift and become increasingly mobile. The end result of the disease is tooth loss.
The treatment of periodontitis is cause‐related. The role of the patient's home plaque control is crucial for the success of the therapy, since pockets can be re‐colonised by bacteria in a few weeks. Periodontal pockets and root surfaces have to be mechanically cleaned from bacteria (debridement). In the presence of deep pockets surgery may also be indicated to get access to the deepest parts of the pockets to properly clean them and to reduce the depth of the pockets (pocket elimination). The goal of this treatment approach is to stop the progression of periodontal disease. Following treatment, healing occurs by repair without the formation of new periodontal attachment (Bowers 1989a). One of the main concerns for many patients is that after periodontal treatment, the gum recession is increased and may cause aesthetic problems.
The ideal treatment would be to recover the periodontal tissues that have been lost (periodontal tissue regeneration). Several surgical techniques have been developed in an attempt to regenerate periodontal tissues including guided tissue regeneration (GTR), bone grafting (BG) and the use of enamel matrix derivative (EMD). All these treatments have been shown to have the potential to regenerate at least some periodontal attachment in humans (Bosshardt 2005; Bowers 1989b; Sculean 1999). With GTR, a biocompatible barrier (either resorbable or non‐resorbable) is surgically positioned around the root to seal the bone defect and protect the blood clot. A Cochrane review (Needleman 2006) has shown that GTR is a little more effective than open flap debridement (1.2 mm in probing attachment levels (PAL) gain and 1.2 mm in probing pocket depths (PPD) reduction), however it was also observed that there was a marked variability of results (heterogeneity) with GTR among various randomised clinical trials. Grafting techniques may include autogenous bone grafting, demineralised freeze‐dried bone allografts (DFDBA), animal derived graft materials (xenografts) and synthetic bone graft materials (alloplasts such as hydroxyapatite). The effectiveness of bone grafting for periodontal regeneration in intrabony defects was assessed in two systematic reviews (Reynolds 2003; Trombelli 2002). Both reviews showed improved probing attachment levels when grafts were used when compared to open flap debridement. However, in one review the gain varied considerably with respect to the different materials used (Trombelli 2002). The authors remarked that due to a significant heterogeneity in results between studies, general conclusions need to be drawn with caution (Trombelli 2002). The other review (Reynolds 2003) concluded that there were no differences in clinical outcome measures among various graft types. The results of both these reviews have to be carefully evaluated since the methodological standards were not similar, therefore further research is needed to confirm these findings. Both GTR and grafting procedures are based on the concept of selective exclusion of epithelial cells from colonizing the wound and space maintaining for the blood clot to regenerate the periodontal tissues. In addition, bone grafts may possess osteoinductive and osteoconductive properties.
Periodontal regeneration mediated by EMD is based on a different concept. It is believed that EMD used in periodontal lesions mimics the development of the tooth supporting apparatus during tooth formation (Hammarström 1997a). The enamel matrix is composed of a number of proteins, 90% of which are amelogenins. Such proteins are thought to induce the formation of the periodontal attachment during tooth formation. The only commercially available product using EMD is called Emdogain® and is produced by Biora (Malmö, Sweden). The company has been incorporated into Straumann Biologics Division since 1 April 2004. Originally the product consisted of EMD and a vehicle solution (propylene glycol alginate) that had to be mixed before use. In order to save time and simplify the procedures a ready‐to‐use Emdogain gel was developed. A large multicentre randomised controlled trial (RCT) showed no differences between the original EMD and the new ready‐to‐use Emdogain gel formulation (Bratthall 2001). EMD is derived from the developing teeth germs of 6‐month old piglets (Hammarström 1997b). Since EMD is a porcine‐derived material, it might have the potential of stimulating immune reactions in humans. However, EMDs are quite similar among mammalian species (Brookes 1995), thus are less likely to be antigenic. Multiple exposures to EMD during periodontal therapy have been shown to be safe for the patient (Froum 2004; Heard 2000; Zetterström 1997). It is of interest to note that the vehicle solution (propylene glycol alginate abbreviated in PGA) of the EMD has significant antimicrobial effects on periodontal pathogens (Arweiler 2002; Sculean 2001c; Spahr 2002). However, these authors interpreted their findings as Emdogain having antimicrobial properties.
Another issue was whether EMD could improve periodontal wound healing. Despite that EMD was not marketed or approved for non‐surgical use, an RCT of 3‐week duration suggested that EMD treated sites healed better than contralateral sites treated with the vehicle‐control after non surgical root‐planing and curettage (Wennström 2002). However, such findings were not confirmed by two non‐placebo controlled RCTs using masked examiners for evaluating the early postsurgical healing events (Hagenaars 2004; Wachtel 2003). A third placebo‐controlled RCT (Grusovin 2009) also failed to show any improved healing at the EMD treated sites.
Two RCTs compared the effect of postoperative antibiotics and no antibiotics in combination with EMD (Mombelli 2005; Sculean 2001d). Results were contradictory: while one study suggested no advantages in using postoperative antibiotics (Sculean 2001d), the other suggested that additional benefits may be expected using systemic antibiotics (Mombelli 2005). However, patients of the latter trial were subjected to non‐surgical interventions for which EMD is not marketed or approved.
Prior to the application of EMD, most authors 'condition' the root surface after mechanical debridement for gently removing the 'smear layer' (the residual of the debridement procedure). Various 'conditioning agents' have been used and the manufacturer of EMD produces one root conditioner called PrefGel® composed of 24% ethylenediaminetetra‐acetic acid (EDTA) at neutral pH. There is no evidence that this procedure is effective (Sculean 2006). Traditionally such root conditioners were used to chemically modify the root surface in order to stimulate periodontal regeneration. A systematic review (Mariotti 2003) failed to show the efficacy of such procedures.
EMD is also currently used in many other clinical situations such as the treatment of furcation defects of periodontally compromised teeth, recession, in combinations with GTR, BG, etc. A new recent application, for which EMD was not marketed or approved for, is to promote periodontal attachment regeneration around reimplanted traumatically avulsed teeth or reimplanted ankylotic teeth. However, contradictory results were reported (Filippi 2001; Filippi 2002; Schjøtt 2005).
In conclusion, there is conflicting evidence on the efficacy of EMD, and a comprehensive high‐quality systematic review could be one way to investigate whether EMD is effective or not, and whether there are relevant clinical advantages for the patients in the treatment of intrabony defects.
After the publication of the first version of the present review, four different systematic reviews were published on the efficacy of EMD in the treatment of intrabony defects (Giannobile 2003; Kalpidis 2002; Trombelli 2002; Venezia 2004), reaching, in some cases, rather different conclusions. Many more systematic reviews were published from 2006.
Objectives
Primary
To test the null hypothesis of no difference in outcomes using enamel matrix derivative (EMD) versus a placebo or not for the treatment of intrabony defects.
Secondary
To test the null hypothesis of no difference in outcomes between EMD versus guided tissue regeneration (GTR) for the treatment of intrabony defects. To test the null hypothesis of no difference in outcomes between EMD versus various 'bone' grafting procedures (BG) for the treatment of intrabony defects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (RCTs) testing the efficacy of EMD with at least 1 year follow up. The following time‐points were to be evaluated: 1, 5 and 10 years.
Types of participants
Patients affected by chronic, aggressive, or early onset periodontitis with intrabony defects having an intrabony component of at least 3 mm to be treated. The depths of intrabony component could be assessed on intraoral radiographs, but intrasurgical measurements were preferred. Trials clearly including patients with shallower intrabony defects were excluded.
Types of interventions
(1) Interventions comparing the use of EMD versus a placebo or not. Both the test and the control sites had to undergo the same intervention, surgical or not, the only difference being the use of EMD for the treatment of intrabony defects. (2) Interventions comparing the use of EMD versus GTR with barriers for the treatment of intrabony defects. (3) Interventions comparing the use of EMD versus various types of BG, including animal‐derived and synthetic bone, for the treatment of intrabony defects. Trials describing the combined used of EMD, GTR, BG or other growth factors were not included in the present review.
Types of outcome measures
Primary
(1) Tooth loss (2) Changes in probing attachment level (PAL) (3) Aesthetics (better, no change or worse according to patient opinion) (4) Postoperative complications and other adverse events.
Secondary
(1) PAL gain < 2 mm (dichotomous outcome only for Emdogain versus control) (2) Changes in probing pocket depth (PPD) (3) Changes in gingival recession (REC) (4) Changes in bone level from the bottom of the defect (BD) in relation to cemento‐enamel junction (CEJ) on intraoral radiographs taken with a parallel technique.
Search methods for identification of studies
For the identification of studies included or considered for this review we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE via OVID but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms. The subject search for MEDLINE was combined with the Cochrane Highly Sensitive Search Strategy for identifying reports of randomised controlled trials (RCTs) (as published in Box 6.4.c in the Cochrane Handbook for Systematic Reviews of Interventions version 5.0.1 updated September 2008 (Higgins 2008)).
Databases searched
The Cochrane Oral Health Group Trials Register (to 4 February 2009) (seeAppendix 2)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 1) (seeAppendix 3)
MEDLINE (1966 to 4 February 2009) (seeAppendix 1)
EMBASE (1980 to 4 February 2009) (seeAppendix 4).
The most recent electronic search was carried out 4 February 2009.
Handsearching
We identified the following journals as being important to be handsearched for this review: European Journal of Oral Implantology, International Journal of Periodontics and Restorative Dentistry, Journal of Clinical Periodontology, Journal of Dental Research, Journal of Periodontal Research, Journal of Periodontology. For further information about the journals being handsearched consult the Cochrane Oral Health Group website www.ohg.cochrane.org. Where these journals had not already been searched as part of the Cochrane Journal Handsearching Programme, the journals were handsearched by one of the review authors.
Language
Non‐English papers were included. The Cochrane Oral Health Group had non‐English language trials translated.
Unpublished trials
The bibliographies of papers and review articles were checked for studies outside the handsearched journals. Authors of RCTs identified, personal contacts, the old and the new manufacturers were written to in an attempt to identify unpublished or ongoing trials.
Data collection and analysis
The titles and abstracts (when available) of all reports identified were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained and was assessed independently by two review authors to establish whether the studies met the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third author was consulted. All studies meeting the inclusion criteria then underwent validity assessment and data were extracted. Studies rejected at this or subsequent stages were recorded in the table of excluded studies, and reasons for exclusion recorded.
Data extraction
Data were extracted by two review authors independently using specially designed data extraction forms. Any disagreement was discussed and a third review author consulted where necessary. Authors of the RCTs were contacted for clarification or missing information. Data were excluded until further clarification was available if agreement could not be reached. For each trial the following data were recorded.
Year of publication, country of origin, setting and source of study funding.
Details of the participants including demographic characteristics and criteria for inclusion.
Details on the study design (parallel group or split mouth).
Details on the type of intervention.
Details of the outcomes reported, including method of assessment and time intervals.
Risk of bias in included studies
An assessment of the risk of bias in included studies was undertaken following the recommendations as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 (Higgins 2008). Two review authors independently and in duplicate assessed the risk of bias of all included studies. Any disagreement was discussed and where necessary a third review author was consulted to achieve consensus. Authors were contacted directly for clarification.
A specific tool for assessing risk of bias in each included study was adopted. This comprised a description and a judgement for each entry in a risk of bias table, where each entry addressed a specific feature of the study:
Adequate sequence generation
Allocation concealment
Blinding (of outcome assessor)
Incomplete outcome data addressed
Free of selective reporting
Free of other bias.
The judgement for each entry involved answering a question, with answers 'Yes' indicating low risk of bias, 'No' indicating high risk of bias, and 'Unclear' indicating either lack of information or uncertainty over the potential for bias.
Allocation concealment was considered adequate if it was centralised (e.g. allocation by a central office unaware of subject characteristics); pharmacy‐controlled randomisation; pre‐numbered or coded identical containers which were administered serially to participants; on‐site computer system combined with allocation kept in a locked unreadable computer file that can be accessed only after the characteristics of an enrolled patient have been entered; sequentially numbered, sealed, opaque envelopes; and other approaches similar to those listed above, along with the reassurance that the person who generated the allocation scheme did not administer it. Some schemes may be innovative and not fit any of the approaches above, but still provide adequate concealment. Approaches to allocation concealment which were considered clearly inadequate included: alternation, use of case record numbers, dates of birth or day of the week, and any procedure that was entirely transparent before allocation, such as an open list of random numbers. Ideally the surgeon should have known the group allocation only after having elevated the flap and debrided the root surface. Those articles or authors stating that allocation concealment procedures were implemented but did not provide details on how this was accomplished, were coded as 'unclear'.
After taking into account the additional information provided by the authors of the trials, the overall risk of bias in included studies was assessed using three key domains: allocation concealment, blinding of outcome assessor (where applicable) and completeness of follow up. Studies were graded into the following categories.
Low risk of bias (plausible bias unlikely to seriously alter the results) if all three key domains were met.
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more key domains were not met.
Data synthesis
For dichotomous outcomes, the estimates of effects of an intervention were expressed as risk ratios together with 95% confidence intervals. For continuous outcomes, mean differences and 95% confidence intervals were used to summarise the data for each group. The statistical unit was the patient and not the treated sites. Numbers needed to treat (NNT) were calculated for PAL gain < 2 mm.
Meta‐analyses were done only with studies of similar comparisons reporting the same outcome measures. Risk ratios were combined for dichotomous data, and mean differences for continuous data, using random‐effects models. Data from split‐mouth and parallel group studies were combined using the procedures outlined in Elbourne 2002. It was necessary to estimate the appropriate standard errors where these were not presented in the trial reports using the methods presented by Follmann 1992. We did not have the paired standard deviations for one split‐mouth study and we imputed this from the standard deviations of the two groups assuming an intraclass correlation coefficient (icc) of 0.25 as this was the median icc found in a review using the same outcomes from similar studies (Needleman 2006). The generic inverse variance procedure in Review Manager (RevMan) 5 was used to combine these two subgroups in the analyses.
The significance of any discrepancies in the estimates of the treatment effects from the different trials was assessed by means of Cochran's test for heterogeneity and the I2 statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance. However, it was decided not to try to explain the heterogeneity. The motivation for this choice is the following: in general, subgroup analyses are exploratory investigations to generate hypotheses to be tested in future studies. The results from these are only tentative and need to be confirmed in studies designed specifically for this purpose. Unfortunately, too much weight is often put on the results from subgroup analyses in this area, and too often such tentatively explanations are misused. We have therefore decided not to undertake any subgroup analyses apart from for study design, with subgroups for split‐mouth and parallel group studies. Random‐effects metaregression analysis was used to investigate whether the effect of study design (post hoc comparison) could explain heterogeneity for PAL, PPD and REC changes in the various comparisons.
Sensitivity analyses were undertaken to examine the effect size in PAL, PPD and REC changes, excluding trials at high risk of bias on the assessment of the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was to be examined.
Results
Description of studies
Of the 35 potentially eligible trials, 13 were included in this review (Crea 2008; Francetti 2004; Grusovin 2009; Heijl 1997; Leknes 2009; Okuda 2000; Pontoriero 1999; Rösing 2005; Sanz 2004; Silvestri 2000; Silvestri 2003; Tonetti 2002; Zucchelli 2002) and 22 trials (Bokan 2006; Chambrone 2007; Doertbudak 2000; Eger 1998; Francetti 2005; Froum 2001; Ghaffar 2001; Hagenaars 2004; Lombardo 2000; Martinez 2001; Martu 2000a; Martu 2000b; Minabe 2002; Mombelli 2005; Ozcelik 2007; Parashis 2004; Sculean 1999; Sculean 2001a; Sculean 2001b; Vandana 2004; Wachtel 2003; Windisch 2002) were excluded for the following reasons: not an RCT (Doertbudak 2000; Eger 1998; Lombardo 2000; Martu 2000a; Martu 2000b; Parashis 2004), teeth extracted after 6 months (Sculean 1999; Windisch 2002), insufficient data presented (Ghaffar 2001; Martinez 2001), data in an inappropriate form (Francetti 2005), data presented in a way that we could not use (Froum 2001; Minabe 2002; Wachtel 2003), too short follow up (Hagenaars 2004; Ozcelik 2007), included intrabony defects less than 3 mm deep (Chambrone 2007; Mombelli 2005; Sculean 2001a; Sculean 2001b; Vandana 2004) and different flap techniques were used (Bokan 2006).
Characteristics of the trial setting and investigators
Nine trials had a parallel group design (Crea 2008; Francetti 2004; Grusovin 2009; Pontoriero 1999; Sanz 2004; Silvestri 2000; Silvestri 2003; Tonetti 2002; Zucchelli 2002) and five studies were designed as split‐mouth trials (Heijl 1997; Leknes 2009; Okuda 2000; Pontoriero 1999; Rösing 2005). The comparisons made in one trial (Pontoriero 1999) were both within patients and between patients. Seven trials were conducted in Italy (Crea 2008; Francetti 2004; Grusovin 2009; Pontoriero 1999; Silvestri 2000; Silvestri 2003; Zucchelli 2002), two in Norway (Leknes 2009; Rösing 2005), one in Japan (Okuda 2000), one in Sweden (Heijl 1997), and two trials were conducted in several countries (Sanz 2004; Tonetti 2002). Six trials were multicentre (Heijl 1997; Sanz 2004; Silvestri 2000; Silvestri 2003; Tonetti 2002; Zucchelli 2002). Five trials were conducted in university dental clinics (Crea 2008; Francetti 2004; Leknes 2009; Okuda 2000; Rösing 2005), five were conducted both in university dental clinics and private practices (Sanz 2004; Silvestri 2000; Silvestri 2003; Tonetti 2002; Zucchelli 2002), two studies in private practices (Grusovin 2009; Pontoriero 1999) and one trial in a public specialist clinic of periodontology (Heijl 1997). Nine trials were funded or partially supported by manufacturers (Francetti 2004; Grusovin 2009; Heijl 1997; Pontoriero 1999; Rösing 2005; Sanz 2004; Silvestri 2000; Silvestri 2003; Tonetti 2002), such information was explicit only in four trials (Grusovin 2009; Heijl 1997; Sanz 2004; Tonetti 2002). Four trials were not supported by manufacturers (Crea 2008; Leknes 2009; Okuda 2000; Zucchelli 2002).
In total 653 patients were treated in the 13 included trials.
Characteristics of the interventions
Nine trials (Francetti 2004; Grusovin 2009; Heijl 1997; Okuda 2000; Pontoriero 1999; Rösing 2005; Silvestri 2000; Tonetti 2002; Zucchelli 2002) compared EMD versus control flap surgery. The surgical techniques for the control flaps were: the modified Widman flap in four trials (Heijl 1997; Okuda 2000; Pontoriero 1999; Silvestri 2000) whereas in the other five trials (Francetti 2004; Grusovin 2009; Rösing 2005; Tonetti 2002; Zucchelli 2002) the simplified or the modified papilla preservation techniques were used. In five trials (Grusovin 2009; Heijl 1997; Okuda 2000; Pontoriero 1999; Rösing 2005) a placebo (the propylene glycol alginate vehicle gel solution) was used in the control flaps.
Six trials (Crea 2008; Pontoriero 1999; Sanz 2004; Silvestri 2000; Silvestri 2003; Zucchelli 2002) compared EMD versus guided tissue regeneration (GTR). In four trials non‐resorbable barriers were used (Crea 2008; Silvestri 2000; Silvestri 2003; Zucchelli 2002), in one trial resorbable barriers were used (Sanz 2004), and in one trial (Pontoriero 1999) both resorbable and non‐resorbable barriers were used, however we used data only from the non‐resorbable barrier group since defects shallower than 3 mm were included in the two groups in which resorbable barriers were used. Non‐resorbable barriers were removed 6 weeks after their insertion with the exception of one trial (Pontoriero 1999) in which they were removed after 4 weeks. For one trial it is unclear when the barriers were removed (Sanz 2004). In one study connective tissue grafts were placed in six patients after barrier removal (Silvestri 2000).
One trial (Leknes 2009) compared EMD versus a bone graft (BG). A bone substitute made of granulated ceramic (PerioGlas, US Biomaterials, Alachua, FL, USA) was used (Leknes 2009).
The following root‐conditioning procedures before EMD application were implemented in all trials.
36% ortho‐phosphoric acid for 15 seconds, also to the controls (Heijl 1997; Okuda 2000).
24% ethylenediaminetetra‐acetic acid (EDTA) gel for 2 minutes only in the EMD treated sites (Crea 2008; Francetti 2004; Leknes 2009; Sanz 2004) and also to the open flap debridement control sites (Grusovin 2009; Pontoriero 1999; Rösing 2005; Tonetti 2002; Zucchelli 2002) and the GTR sites (Silvestri 2003; Zucchelli 2002).
17% EDTA solution for 20 seconds only for the EMD group (Silvestri 2000).
The following postoperative systemic antibiotics and hygiene procedures were prescribed.
Doxycycline (Vibramycin, Pfizer) 200 mg day 1 and 100 mg for 3 weeks; 0.2% chlorhexidine rinsing for 4 to 6 weeks and no mechanical cleaning in operated areas for 6 weeks (Heijl 1997).
Amoxicillin 3 grams 1 hour before surgery; 0.12% chlorhexidine rinsing twice a day for 6 weeks (Pontoriero 1999).
Cefaclor 750 mg per day for 5 days; 0.12% chlorhexidine rinsing three times a day for 6 weeks and no mechanical cleaning for the first postoperative week (Okuda 2000).
Amoxicillin and clavulanic acid (Augmentin, Smith Klein Beecham) 2 grams per day for 6 days; 0.2% chlorhexidine rinsing twice a day for 8 weeks and no mechanical cleaning in operated areas for 2 months (Silvestri 2000; Silvestri 2003).
Amoxicillin 500 mg three per day for 10 days; chlorhexidine rinsing twice a day for the initial healing period (Rösing 2005).
In the published article the use of antibiotics was not mentioned but the authors informed us that antibiotics were used in five patients of the Emdogain group and seven control patients; 0.12% chlorhexidine rinsing twice a day for 4 weeks and gentle sweeping of operated areas with a postsurgical toothbrush starting from the third postoperative day without interdental cleaning for 4 weeks (Tonetti 2002).
Amoxicillin and clavulanic acid (Augmentin, Smith Klein Beecham) 1 gram per day starting 1 day before surgery for 6 days thereafter; 0.2% chlorhexidine rinsing twice a day for 11 weeks without interdental cleaning in the operated areas (Zucchelli 2002).
Amoxicillin and clavulanic acid (Augmentin, Smith Klein Beecham) 1 gram per day for 7 days; 0.2% chlorhexidine rinsing twice a day for 6 weeks without mechanical cleaning in the operated areas (Francetti 2004).
In the published article the use of antibiotics was not mentioned but the authors informed us that amoxicillin 500 mg for 4 days was prescribed; 0.12% chlorhexidine rinsing twice a day for 4 weeks and gentle sweeping of operated areas with a postsurgical toothbrush starting from the third postoperative day without interdental cleaning for 4 weeks (Sanz 2004).
Amoxicillin 500 grams twice daily starting 1 day before surgery for 6 days; 1% chlorhexidine gel twice daily for 4 weeks (Crea 2008).
No antibiotics; 0.12% chlorhexidine rinsing twice a day for 3 weeks and gentle sweeping of operated areas with a postsurgical toothbrush starting from the second postoperative week without interdental cleaning for 4 weeks (Grusovin 2009).
No antibiotics; 0.2% chlorhexidine rinsing twice a day for 2 weeks (Leknes 2009).
Characteristics of outcome measures
After contacting the authors, postoperative complications (infection) were available for all trials.
Tooth loss was not described in one trial (Sanz 2004).
Changes in PAL and PPD were described in all trials.
PAL gain < 2 mm was described in six trials (Francetti 2004; Grusovin 2009; Heijl 1997; Silvestri 2000; Tonetti 2002; Zucchelli 2002).
Four trials did not describe changes in REC (Francetti 2004; Heijl 1997; Rösing 2005; Silvestri 2003).
Bone level measurements from the bottom of the defect to the CEJ on intraoral radiographs taken with a paralleling technique were performed in six trials (Crea 2008; Francetti 2004; Grusovin 2009; Heijl 1997; Okuda 2000; Rösing 2005). Radiographic data from two studies were not used (Francetti 2004; Okuda 2000) because of data presented as per cent relative area of bone density and not as linear measurements (Okuda 2000) and for not having used a fixed reference mark to assess changes over time (Francetti 2004).
Aesthetics according to the patient's opinion was measured in two trials (Grusovin 2009; Tonetti 2002). Data could not be combined in a meta‐analysis because were presented as continuous data (Tonetti 2002) or ordinal data (Grusovin 2009). Patients' opinion from one trial (Grusovin 2009) was dichotomised into patients not satisfied or patients moderately and highly satisfied with the aesthetics outcome.
Baseline characteristics
Specific exclusion criteria
None in particular (Heijl 1997; Leknes 2009; Pontoriero 1999).
Smokers (Crea 2008; Okuda 2000; Silvestri 2000).
Medium smokers, i.e. more than 10 cigarettes per day (Silvestri 2003).
Heavy smokers, i.e. more than 20 cigarettes per day (Sanz 2004; Tonetti 2002; Zucchelli 2002).
Any periodontal treatment in the previous 2 years (Okuda 2000).
Any periodontal treatment in the previous 3 years (Francetti 2004).
Antibiotics in the previous 6 months (Okuda 2000; Rösing 2005; Zucchelli 2002) or 3 months (Grusovin 2009).
Less than 2 mm of attached gingiva (Francetti 2004; Okuda 2000; Tonetti 2002).
Teeth with crowns or supporting fixed partial bridges (Crea 2008).
Endodontically treated teeth (Crea 2008).
In all trials defects did not extend into furcations (in one study, Grusovin 2009, only teeth with furcation degree 3 were excluded) and patients were selected because they were motivated and had good oral hygiene.
Presurgical treatments
All patients treated with repeated mechanical debridement and some with antimicrobials and surgical interventions over long time periods (Heijl 1997).
All patients treated with mechanical debridement and antiseptics and/or antibiotics when indicated (Tonetti 2002).
All patients treated with mechanical debridement (Crea 2008; Francetti 2004; Leknes 2009; Okuda 2000; Pontoriero 1999; Rösing 2005; Sanz 2004; Silvestri 2000; Silvestri 2003; Zucchelli 2002).
All patients treated with mechanical debridement and, when indicated, with surgery (Grusovin 2009).
Characteristics of the defects
PPD greater or equal to 6 mm and intrabony defects with a depth greater or equal to 4 mm (Francetti 2004; Heijl 1997; Okuda 2000; Silvestri 2000).
PPD greater or equal to 6 mm and intrabony defects with a depth greater or equal to 3 mm (Pontoriero 1999).
PPD greater or equal to 7 mm and intrabony defects with a depth greater or equal to 3 mm (Leknes 2009; Zucchelli 2002).
Intrabony defects with a depth greater or equal to 3 mm (Rösing 2005; Sanz 2004; Tonetti 2002).
Intrabony defects with a depth greater or equal to 4 mm (Crea 2008; Grusovin 2009; Silvestri 2003) and wider than 2 mm (Grusovin 2009).
Baseline comparisons among groups
No statistically significant differences among test and control groups for PAL, PPD and radiographic bone levels (Heijl 1997; Rösing 2005).
No statistically significant differences among test and control groups for full mouth plaque score (FMPS), full mouth bleeding score (FMBS), PAL, PPD, REC and intrabony components (Okuda 2000; Pontoriero 1999; Sculean 2001a; Zucchelli 2002) and distribution of number of walls of the bony defects (Tonetti 2002) and smokers (Sanz 2004).
No statistically significant differences among test and control groups for FMPS, PAL, PPD, REC and intrabony components (Sculean 2001b).
No statistically significant differences among test and control groups for PAL, PPD, REC and intrabony components (Silvestri 2003).
No statistically significant differences among test and control groups for intrabony components (Francetti 2004; Silvestri 2000).
Slightly more compromised periodontal situation in the group treated with GTR than in the EMD group (Crea 2008).
1 mm deeper and wider circumferential defects in the EMD group than in the placebo group (Grusovin 2009).
More recession (1.3 mm) in the BG group than in the EMD group (Leknes 2009), no data provided on the depth of the infrabony defect component.
Type of maintenance and frequency during the postoperative phase and the follow up of the trials
Supragingival professional tooth cleaning at weeks 2, 4, 6 and thereafter, depending on the level of plaque control, at 3, 6, 9 and 12 months or at 4, 8 and 12 months. At 1 year an individual recall programme was decided and patients were recalled at least every 6 months (Heijl 1997).
Supragingival professional tooth cleaning every 15 days; 1 year (Pontoriero 1999).
Supragingival professional cleaning weekly for the first 6 weeks and thereafter once a month; 1 year (Okuda 2000).
Supragingival professional cleaning weekly for the first month and thereafter every 3 months; 1 year (Leknes 2009).
Supragingival professional cleaning weekly for the first 6 weeks and thereafter every 3 months; 3 years (Crea 2008).
Supragingival professional cleaning weekly for the first 8 weeks and thereafter every 3 months; 1 year (Silvestri 2000; Silvestri 2003).
Supragingival professional tooth cleaning at weeks 1, 2, 3, 4, 6 and thereafter every 3 months; 1 year (Grusovin 2009; Sanz 2004; Tonetti 2002).
Supragingival professional tooth cleaning once a month; 1 year (Francetti 2004; Zucchelli 2002).
Supragingival professional tooth cleaning once every 2 weeks for 8 weeks and thereafter every 3 months (Rösing 2005).
Duration of follow up
3 years (Crea 2008; Grusovin 2009; Heijl 1997). Data analysed only at 1 year in one study (Grusovin 2009).
2 years (Francetti 2004).
1 year (Leknes 2009; Okuda 2000; Pontoriero 1999; Rösing 2005; Sanz 2004; Silvestri 2000; Silvestri 2003; Tonetti 2002; Zucchelli 2002).
In the present review only 1‐year data were used with the exception of one trial (Heijl 1997) for which 16‐month data were used.
Risk of bias in included studies
Allocation concealment
Six papers described clearly the procedure for allocation concealment (Crea 2008; Grusovin 2009; Heijl 1997; Leknes 2009; Rösing 2005; Sanz 2004). All the other trials were marked as unclear. All authors replied to our request for additional clarification. With three exceptions, they replied that allocation was concealed without providing any description of the concealment procedures. Thus all those trials were still scored as 'unclear' (Pontoriero 1999; Zucchelli 2002), as additional information on the method of allocation concealment was not provided. The authors of four trials (Francetti 2004; Okuda 2000; Silvestri 2003; Tonetti 2002) described the allocation concealment procedure which was then judged to be adequate. Allocation was not concealed and was scored as 'No' for one trial (Silvestri 2000).
Blinding
Outcome assessors were considered to be blinded in seven trials (Crea 2008; Grusovin 2009; Heijl 1997; Leknes 2009; Okuda 2000; Rösing 2005; Zucchelli 2002), unclear in three cases (Pontoriero 1999; Silvestri 2000; Silvestri 2003) and not blinded in three cases (Francetti 2004; Sanz 2004; Tonetti 2002). After contacting the authors one trial was considered blinded (Pontoriero 1999), and two were not (Silvestri 2000; Silvestri 2003).
Withdrawals
The reporting and explanation of withdrawals and drop outs were clear in 11 trials (Crea 2008; Francetti 2004; Grusovin 2009; Heijl 1997; Leknes 2009; Okuda 2000; Rösing 2005; Silvestri 2000; Silvestri 2003; Tonetti 2002; Zucchelli 2002). After correspondence with authors all trials with only one exception (Sanz 2004) were considered to have clear explanations for withdrawals and drop outs.
Sample size
Sample size calculations were performed in six studies (Grusovin 2009; Heijl 1997; Leknes 2009; Rösing 2005; Sanz 2004; Tonetti 2002). In one trial (Heijl 1997), the sample size was calculated to detect 1 mm difference (assuming standard deviation (SD) of 1 mm) of PAL and radiographic bone gain between test and control with a power (one minus beta) of at least 90% 8 months after surgery. For Tonetti 2002, the size of the sample required to detect a true difference of 0.5 mm for PAL between test and control with 90% power and with an alpha error of 0.05 was 150 patients completing the trial. Rösing 2005 was designed to have sufficient power to detect a 2 mm difference in PAL gain, adopting an alpha set at 0.05 and a power of 80%. It was calculated that a paired sample of nine individuals was sufficient. In those studies more patients than needed to detect the assumed differences completed the trials. Sanz 2004 was designed to have sufficient power to detect a true difference of 1 mm of PAL gain with alpha set at 0.05 and a power of 0.8. However, the authors concluded that the trial had insufficient power to detect potentially clinically relevant differences. Grusovin 2009 was designed to have sufficient power to detect a true difference of 1 mm difference in mean values between the two groups (49 subjects in each group) with a 90% power, assuming that the common SD was 1.500 using a two‐group t‐test with a 0.050 two‐sided significance level. It was planned to include 50 patients per group. However, the calculated sample size could not be obtained because the Emdogain manufacturer stopped supplying the placebos after the delivery of a first batch of 15 placebos. Leknes 2009, which included 13 patients in a split‐mouth study, was powered to detect a difference of 0.5 mm in PAL or PPD assuming a standard deviation of 0.7 mm with the level of significance set at 0.05 and 73% power. This calculation is obviously post hoc, i.e. it was made after the results were known and not a priori to correctly calculate the sample size needed to detect a 0.5 mm difference.
Agreement in methodological assessment
The agreed quality of the included trials after having incorporated the information provided by the authors of the trials is summarized in Additional Table 6. Six trials where considered to be at low risk of bias (Crea 2008; Grusovin 2009; Heijl 1997; Leknes 2009; Okuda 2000; Rösing 2005), and the remaining trials at high risk of bias.
1. Results of quality assessment after correspondence with authors.
| Study | Concealment of allocation | Blinding of assessor | Reasons for drop outs | Risk of bias |
| Heijl 1997 | Yes | Yes | Reasons given | Low |
| Pontoriero 1999 | Unclear | Yes | No drop outs | High |
| Okuda 2000 | Yes | Yes | No drop outs | Low |
| Silvestri 2000 | No | No | No drop outs | High |
| Tonetti 2002 | Yes | No | Reasons given | High |
| Zucchelli 2002 | Unclear | Yes | No drop outs | High |
| Silvestri 2003 | Yes | No | Reasons given | High |
| Francetti 2004 | Yes | No | No drop outs | High |
| Sanz 2004 | Yes | No | No reasons given | High |
| Rösing 2005 | Yes | Yes | Reasons given | Low |
| Crea 2008 | Yes | Yes | Reasons given | Low |
| Grusovin 2009 | Yes | Yes | Reasons given | Low |
| Leknes 2009 | Yes | Yes | No drop outs | Low |
Effects of interventions
for the main comparison.
| Emdogain compared with Control for periodontal tissue regeneration in intrabony defects | ||||||
|
Patient or population:patients with intrabony defects Settings: practice Intervention: Emdogain Comparison: Control flap surgery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control flap surgery | Emdogain | |||||
| Tooth loss | See comment | See comment | 371 [9] | See comment | too few teeth lost to undertake analysis | |
| PAL1 mm gain from baseline 1 year |
The mean PAL gain ranged across control groups from 0.8 to 2.2 | The mean PAL gain in the intervention groups was 1.1 higher (0.6 to 1.6 higher) | 371 [9] | ++OO low | ||
| Aesthetics | The mean VAS score for the control group was 62 | The mean VAS gain in the intervention groups was 1.0 higher (‐5.4 to 7.4) | 166 [1] | ++OO low | ||
| PPD2 mm reduction from baseline 1 year |
The mean PPD reduction ranged across control groups from 1.4 to 4.5 | The mean PPD reduction in the intervention groups was 0.7 higher (0.5 to 1.0 higher) | 371 [9] | ++OO low | ||
| REC3 mm change from baseline 1 year |
The mean REC ranged across control groups from ‐1.7 to ‐0.2 | The mean REC in the intervention groups was 0.02 higher (‐0.3 to 0.3 higher)(less recession) | 302 [6] | ++OO low | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; GRADE: GRADE Working Group grades of evidence (see explanations) | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
1 probing attachment level
2 probing pocket depth
3 gingival recession
2.
| Emdogain compared with GTR for periodontal tissue regeneration in intrabony defects | ||||||
|
Patient or population:patients with intrabony defects Settings: practice Intervention: Emdogain Comparison: GTR | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| GTR4 | Emdogain | |||||
| Tooth loss | See comment | See comment | 237 [5] | See comment | too few teeth lost to undertake analysis | |
| PAL1 mm gain from baseline 1 year |
The mean PAL gain ranged across GTR groups from 2.5 to 4.9 | The mean PAL gain in the intervention groups was 0.2 lower (‐0.20 to 0.55 lower) | 304 [6] | ++OO low | ||
| PPD2 mm reduction from baseline 1 year |
The mean PPD reduction ranged across GTR groups from 3.3 to 6.5 | The mean PPD reduction in the intervention groups was 0.4 lower (‐0.2 to 1.1 lower) | 304 [6] | ++OO low | ||
| Aesthetics | See comment | See comment | 0 [0] |
See comment | No studies reported this | |
| REC3 mm change from baseline 1 year |
The mean REC change ranged across GTR groups from ‐1.8 to 1.0 | The mean REC change in the intervention groups was 0.4 higher (0.2 to 0.7 higher)(less recession) | 206 [5] | ++OO low | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; GRADE: GRADE Working Group grades of evidence (see explanations) | ||||||
GRADE Working Group grades of evidence High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
1 probing attachment level
2 probing pocket depth
3 gingival recession
4 Guided Tissue Regeneration
Data from parallel and split‐mouth trials are analysed as separate subgroups, then combined using the generic inverse variance procedure in RevMan. No trial with a follow up of 5 years was included. It should be remembered that trials combining the use of Emdogain (EMD), guided tissue regeneration (GTR) and bone grafting (BG) as well as other regenerative procedures (e.g. BG plus GTR or EMD plus GTR) were not included in the present review.
Emdogain versus control/placebo at 1 year (Comparison 1, Outcomes 1.1 to 1.7)
Nine trials provided data for this comparison between EMD and control or placebo interventions (Francetti 2004; Grusovin 2009; Heijl 1997; Okuda 2000; Pontoriero 1999; Rösing 2005; Silvestri 2000; Tonetti 2002; Zucchelli 2002), four of which were split‐mouth placebo‐controlled trials (Heijl 1997; Okuda 2000; Pontoriero 1999; Rösing 2005). The raw data for each trial for PAL, PPD and REC is given in Additional Table 7; Table 8; and Table 9.
2. Control versus Emdogain: PAL at 1 year.
| Study | Parallel group/Split mouth |
EMD n mean (SD) |
Control n mean (SD) |
Difference n mean (SE) |
| Silvestri 2000 | P | 10 4.5 (1.58) | 10 1.20 (1.03) | 20 3.30 (0.60) |
| Tonetti 2002 | P | 83 3.1 (1.5) | 83 2.5 (1.5) | 166 0.60 (0.23) |
| Zucchelli 2002 | P | 30 4.2 (0.9) | 30 2.6 (0.8) | 60 1.6 (0.22) |
| Francetti 2004 | P | 12 4.14 (1.35) | 12 2.29 (0.95) | 24 1.85 (0.48) |
| Grusovin 2009 | P | 15 3.4 (1.1) | 15 3.3 (1.2) | 30 0.1 (0.42) |
| Heijl 1997 | S | 31 2.3 (1.6) | 31 1.7 (1.2) | 31 0.6 (0.22) |
| Pontoriero 1999 | S | 10 3.0 | 10 1.8 | 10 1.1 (0.43) |
| Okuda 2000 | S | 16 1.72 (1.07) | 16 0.83 (0.86) | 16 0.89 (0.22) |
| Rosing 2005 | S | 14 2.01 (1.76) | 14 2.16 (1.87) | 14 ‐0.15 (0.69) (0.90)* |
*authors' value from e‐mail PAL = probing attachment level SD = standard deviation SE = standard error
3. Control versus Emdogain: PPD at 1 year.
| Study | Parallel group/Split mouth |
EMD n mean (SD) |
Control n mean (SD) |
Difference n mean (SE) |
| Silvestri 2000 | P | 10 4.9 (1.79) | 10 1.40 (1.26) | 20 3.5 (0.69) |
| Tonetti 2002 | P | 83 3.9 (1.7) | 83 3.3 (1.7) | 166 0.60 (0.26) |
| Zucchelli 2002 | P | 30 5.1 (0.7) | 30 4.5 (1.0) | 60 0.60 (0.22) |
| Francetti 2004 | P | 12 4.71 (1.60) | 12 2.57 (1.27) | 24 2.14 (0.59) |
| Grusovin 2009 | P | 15 3.9 (1.0) | 15 4.2 (1.6) | 30 0.3 (0.49) |
| Heijl 1997 | S | 31 3.3 (1.4) | 31 2.6 (1.2) | 31 0.70 (0.25) |
| Pontoriero 1999 | S | 10 4.4 | 10 3.5 | 10 0.7 (0.47) |
| Okuda 2000 | S | 16 3.0 (0.97) | 16 2.22 (0.81) | 16 0.78 (0.32) |
| Rosing 2005 | S | 14 4.17 (1.80) | 14 4.39 (1.14) | 14 ‐0.22 (0.57) (0.64)* |
*authors' value from e‐mail PPD = probing pocket depth SD = standard deviation SE = standard error
4. Control versus Emdogain: REC at 1 year.
| Study | Parallel group/Split mouth |
EMD n mean (SD) |
Control n mean (SD) |
Difference n mean (SE) |
| Silvestri 2000 | P | 10 ‐0.5 (0.97) | 10 ‐0.20 (0.63) | 20 ‐0.30 (0.37) |
| Tonetti 2002 | P | 83 ‐0.8 (1.2) | 83 ‐0.8 (1.2) | 166 0 (0.19) |
| Zucchelli 2002 | P | 30 ‐1.0 (0.5) | 30 ‐1.6 (1.0) | 60 0.60 (0.20) |
| Grusovin 2009 | P | 15 ‐0.8 (1.0) | 15 ‐0.6 (1.1) | 30 ‐0.2 (0.38) |
| Pontoriero 1999 | S | 10 ‐1.7 | 10 ‐1.7 | 10 0 (0.34) |
| Okuda 2000 | S | 16 ‐1.22 (0.16) | 16 ‐1.22 (0.88) | 16 0 (0.27) |
REC = gingival recession SD = standard deviation SE = standard error
Tooth loss: there were insufficient numbers of teeth lost to undertake an analysis of these. All teeth were extracted for prosthetic reasons. Four EMD treated teeth removed: two in Heijl 1997 and two in Rösing 2005 versus two control teeth removed in Heijl 1997. In another trial (Grusovin 2009) after 3 years two teeth were judged in need of a second surgical intervention. At the time of judgement the clinician was blinded. Both teeth belonged to the EMD group.
PAL: The meta‐analysis of nine trials showed a significant gain in mean PAL for EMD compared with control sites with mean difference of 1.08 mm (95% confidence interval (CI) 0.61 to 1.55, Chi2 = 38.10, 8 degrees of freedom (df), Pheterogeneity < 0.00001, I2 = 79%) (Figure 1).
Aesthetics: there were two trials reporting this (Grusovin 2009; Tonetti 2002). The trials could not be combined in a meta‐analysis but no statistically significant difference between EMD and control treatment was found (Figure 2; Figure 3).
Complications and other adverse events: no particular adverse events or infection attributable to EMD were recorded in any of the trials with the exception of few problems attributable to the use of postoperative antibiotics. There were no differences in postoperative frequency of subjects reporting pain, intensity of pain recorded on a visual analogue scale (VAS), duration of pain, use of analgesic tablets, edema, hematoma, wound dehiscence, and root sensitivity (Tonetti 2002).
PAL gain < 2 mm: there were significantly more sites with less than 2 mm PAL gain in the control group risk ratio (RR) 0.53 (95% CI 0.34 to 0.82; Chi2 = 5.3, 5 df, P = 0.39, I2 = 5%) (six trials) (Figure 4). The number of patients needed to treat (NNT) in the control group to help one patient gain > 2 mm is 9 (95% CI 6 to 22) based on a prevalence of 25% of patients having < 2 mm gain in PAL. The NNT increases to 14 for a prevalence of 15%, and reduces to 4 with a prevalence of 50%.
PPD: The meta‐analysis of nine trials showed a significant reduction in mean PPD for EMD compared with control sites with mean difference of 0.88 mm (95% CI 0.44 to 1.31; Chi2 = 25.43, 8 df, P = 0.001, I2 = 69%) (Figure 5).
REC: there was no statistically significant difference between the EMD and the control in REC (six trials; Peffect = 0.56, Pheterogeneity = 0.13; I2 = 41%) (Figure 6).
Radiographic bone level: there was no statistically significant difference between the EMD and the control for radiographic bone gain (three trials; Peffect = 0.27, Pheterogeneity = 0.01; I2 = 78%) (Figure 7).
1.
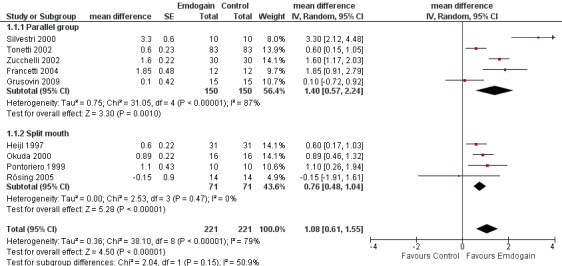
Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.1 PAL.
2.

Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.6 Aesthetics (continuous data).
3.
Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.7 Aesthetics (dichotomous data).
4.
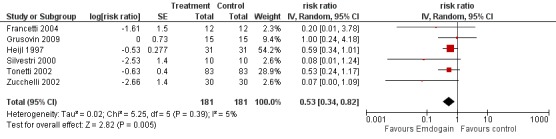
Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.2 PAL < 2 mm.
5.
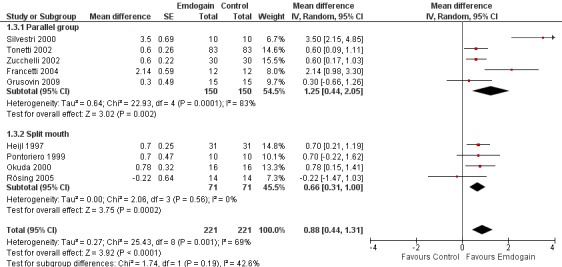
Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.3 PPD.
6.
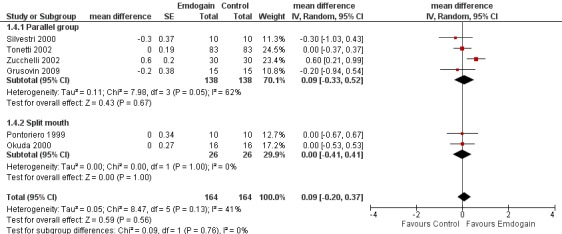
Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.4 REC.
7.
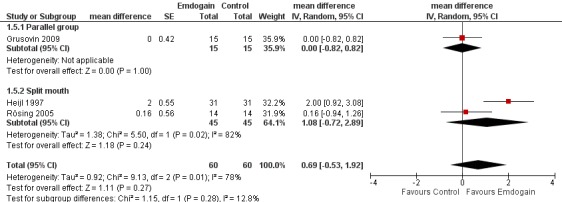
Forest plot of Comparison 1 Emdogain versus control: 1 year; Outcome 1.5 Marginal bone level.
Heterogeneity
There was substantial heterogeneity for PAL (P < 0.00001; I2 = 79%), PPD (P = 0.001; I2 = 69%), REC (P = 0.13; I2 = 41%) and radiographic bone levels (P = 0.01; I2 = 78%). However, we decided to only investigate this for study design, comparing split‐mouth with parallel group studies between EMD and the control group. The results are given in Additional Table 10 and none of these were significant.
5. Random‐effects metaregression analysis of outcomes PAL, PPD, REC.
| Characteristic | Outcome | Studies | Slope estimate (SE) | 95% CI | Slope | P value |
| Parallel versus split mouth | PAL | 9 | 0.68 (0.63) | (‐0.81, 2.19) | Emdogain in parallel group trials has higher effect | 0.31 |
| Parallel versus split mouth | PPD | 9 | 0.71 (0.66) | (‐0.87, 2.28) | Emdogain in parallel group trials has higher effect | 0.32 |
| Parallel versus split mouth | REC | 6 | 0.28 (0.36) | (‐0.72, 1.28) | Emdogain in parallel group trials has higher effect | 0.48 |
CI = confidence interval PAL = probing attachment level PPD = probing pocket depth REC = gingival recession
Sensitivity analysis
Only four studies were judged as at low risk of bias (Grusovin 2009; Heijl 1997; Okuda 2000; Rösing 2005). From the sensitivity analysis including only these four trials, the effect size for PAL was 0.62 mm (95% CI RE 0.28 to 0.96), which was less than 1.08 mm for the overall result, and for PPD was 0.60 mm (95% CI (Random Effects) 0.26 to 0.95) compared with 0.88 mm of the overall result.
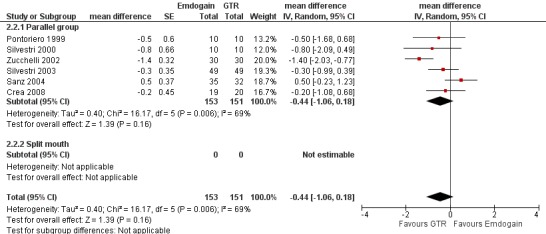
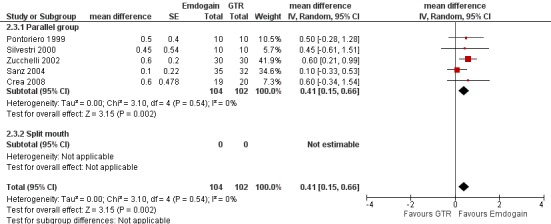
Emdogain versus GTR at 1 year (Comparison 2, Outcomes 2.1 to 2.5)
Six trials provided data for this comparison between EMD and GTR (Crea 2008; Pontoriero 1999; Sanz 2004; Silvestri 2000; Silvestri 2003; Zucchelli 2002), none of which was a split‐mouth trial. The comparison for another split‐mouth trial (Pontoriero 1999) was between patients randomly allocated to the study groups, not using the split‐mouth data. The raw data for each trial for PAL, PPD and REC is given in Additional Table 11; Table 12;and Table 13.
6. GTR versus Emdogain: PAL at 1 year.
| Study | Parallel group/Split mouth |
EMD n mean (SD) |
Control n mean (SD) |
Difference n mean (SE) |
| Pontoriero 1999 | P | 10 2.9 (1.5) | 10 2.9 (1.1) | 20 0 (0.59) |
| Silvestri 2000 | P | 10 4.5 (1.58) | 10 4.80 (2.10) | 20 ‐0.30 (0.83) |
| Zucchelli 2002 | P | 30 4.2 (0.9) | 30 4.9 (1.6) | 60 ‐0.70 (0.34) |
| Silvestri 2003 | P | 49 4.1 (1.8) | 49 4.3 (1.9) | 98 ‐0.20 (0.38) |
| Sanz 2004 | P | 35 3.1 (1.8) | 32 2.5 (1.9) | 67 0.60 (0.45) |
| Crea 2008 | P | 19 2.7 | 20 2.8 | 39 0.1 (0.66) |
GTR = guided tissue regeneration PAL = probing attachment level SD = standard deviation SE = standard error
7. GTR versus Emdogain: PPD at 1 year.
| Study | Parallel group/Split mouth |
EMD n mean (SD) |
Control n mean (SD) |
Difference n mean (SE) |
| Pontoriero 1999 | P | 10 4.2 (1.3) | 10 4.7 (1.4) | 20 ‐0.50 (0.60) |
| Silvestri 2000 | P | 10 4.9 (1.79) | 10 5.7 (1.06) | 20 ‐0.80 (0.66) |
| Zucchelli 2002 | P | 30 5.1 (0.7) | 30 6.5 (1.6) | 60 ‐1.40 (0.32) |
| Silvestri 2003 | P | 49 5.3 (1.9) | 49 5.6 (1.5) | 98 ‐0.30 (0.35) |
| Sanz 2004 | P | 35 3.8 (1.5) | 32 3.3 (1.5) | 67 0.50 (0.37) |
| Crea 2008 | P | 19 3.4 | 20 3.6 | 39 ‐0.2 (0.45) |
GTR = guided tissue regeneration PPD = probing pocket depth SD = standard deviation SE = standard error
8. GTR versus Emdogain: REC at 1 year.
| Study | Parallel group/Split mouth |
EMD n mean (SD) |
Control n mean (SD) |
Difference n mean (SE) |
| Pontoriero 1999 | P | 10 ‐1.3 (0.9) | 10 ‐1.8 (0.9) | 20 0.50 (0.40) |
| Silvestri 2000 | P | 10 ‐0.5 (0.97) | 10 ‐0.95 (1.40) | 20 0.45 (0.54) |
| Zucchelli 2002 | P | 30 ‐1.0 (0.5) | 30 ‐1.6 (1.0) | 60 0.60 (0.20) |
| Sanz 2004 | P | 35 ‐0.6 (0.9) | 32 ‐0.7 (0.9) | 67 0.1 (0.22) |
| Crea 2008 | P | 19 ‐0.6 | 20 1.0 | 39 0.6 (0.478) |
GTR = guided tissue regeneration REC = gingival recession SD = standard deviation SE = standard error
Tooth loss: there were no teeth lost in either group in any of these trials.
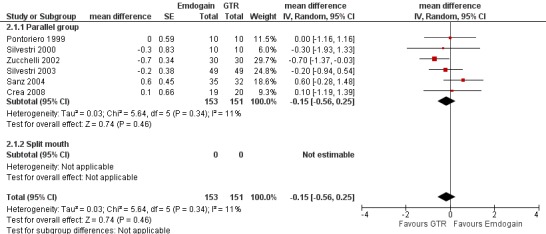
PAL: there were no statistically significant differences (six trials) (Figure 8).
Aesthetics: no trial evaluated this.
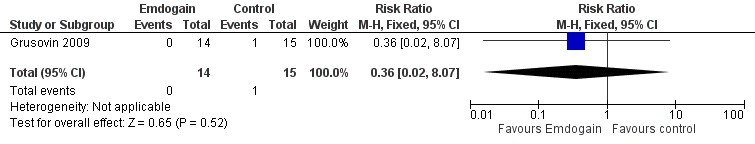
Complications and other adverse events: there were statistically significant more postoperative complications in the GTR group (three trials; P = 0.03), RR 0.12 (95% CI 0.02 to 0.85) (Figure 9).
PPD: there were no statistically significant differences (six trials) (Figure 10).
REC: there were significant differences between EMD and GTR for change from baseline in REC (five trials), with a significant increase in recession for GTR with mean difference 0.41 mm (95% CI 0.15 to 0.66; Chi2 = 3.10, 4 df, P = 0.54) (Figure 11).
Radiographic bone level: there were no statistically significant differences (one trial) (Figure 12).
8.
Forest plot of Comparison 2 Emdogain versus GTR: 1 year; Outcome 2.1 PAL.
9.
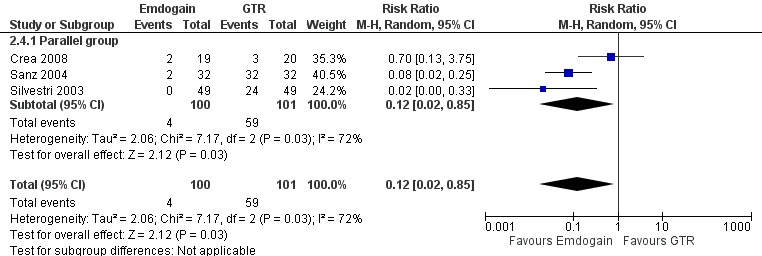
Forest plot of Comparison 2 Emdogain versus GTR: 1 year; Outcome 2.4 Postoperative complications.
10.
Forest plot of Comparison 2 Emdogain versus GTR: 1 year; Outcome 2.2 PPD.
11.
Forest plot of Comparison 2 Emdogain versus GTR: 1 year; Outcome 2.3 REC.
12.

Forest plot of Comparison 2 Emdogain versus GTR: 1 year; Outcome 2.5 Marginal bone level.
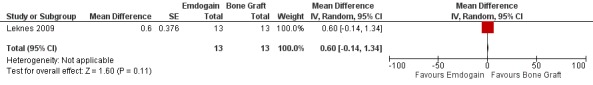
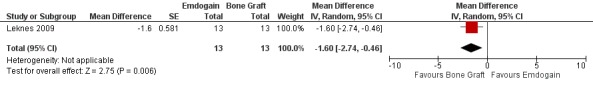
Emdogain versus BG (Comparison 3, Outcomes 3.1 to 3.3)
One trial comparing the use of EMD alone to BG alone was identified (Leknes 2009). The standard deviations of the differences were not given for PAL, PPD and REC. These had to be estimated as described in the methods section. Only data at proximal sites were used.
Tooth loss: no teeth was lost in either group.
PAL: there were no statistically significant differences (Figure 13).
Aesthetics: no trial evaluated this.
Complications and other adverse events: none occurred.
PPD: there were no statistically significant differences (Figure 14).
REC: there was significantly more REC in the EMD group: ‐1.60 mm (95% CI ‐2.74 to ‐0.46; P = 0.006) (Figure 15). A sensitivity analysis putting an intraclass correlation coefficient of zero in, to estimate the standard error also confirmed this statistically significant difference between the groups (P = 0.02).
Radiographic bone level: no trial evaluated this.
13.
Forest plot of Comparison 3 Emdogain versus bone graft; Outcome 3.1 PAL.
14.

Forest plot of Comparison 3 Emdogain versus bone graft; Outcome 3.2 PPD.
15.
Forest plot of Comparison 3 Emdogain versus bone graft; Outcome 3.3 REC.
Discussion
The meta‐analysis of nine trials showed that the use of EMD led to a statistically significant improvement in average PAL (1.1 mm) and PPD (0.9 mm) over control flap surgery when used in the treatment of intrabony defects after 1 year. However, the high degree of heterogeneity found (I2 = 79% for PAL and I2 = 69% for PPD) prevents us from assuming average values as a demonstration of the extent of the difference between the therapies (mean values in the included trials varied from ‐0.15 to 3.3 mm for PAL gain; from ‐0.22 to 3.5 mm for PPD reduction). From the sensitivity analysis (i.e. a meta‐analysis including only those trials at low risk of bias), the effect size for PAL was reduced to 0.62 mm and for PPD to 0.60 mm. This may indicate that the overall treatment effects of EMD are actually overestimated in the present meta‐analysis, and may go someway to explain the heterogeneity.
The number needed to treat (NNT) was calculated to help clinicians understand how many patients would need to be treated with Emdogain to have one more patient gaining 2 mm or more PAL than would have done so in the control group. NNT depends on the prevalence of gaining less than 2 mm PAL in the control group. The mean prevalence was calculated across six studies and NNTs for a range of prevalences considered. For example the mean prevalence in the control group was 25% and the NNT was 9, and this increased to 14 for a reduced prevalence of 15% and reduced to 4 for an increased prevalence of 50%.
Only two trials (Grusovin 2009; Tonetti 2002) investigated patient‐centred outcomes and aesthetics as perceived by the patients themselves. After 1 year, there were no statistically differences among the EMD and the control groups. In Tonetti 2002 a general statistically significant improvement in patient‐centred outcomes was reported. The observation that both groups perceived an improvement in aesthetics despite that in reality some degree of gingival recession had occurred, emphasizes how the patient's judgement may be influenced simply by having received the therapy which they expected to improve their status (Hawthorne effect).
It is interesting to observe that in the multicentre trial in which a multivariate analysis was used to investigate whether the treating centre had an influence on PAL gain (Tonetti 2002), it was found that the centre effect (worse versus better) was statistically significant (‐2.6 mm (SD 0.6)), while the overall treatment effect recalculated in the present review, was of 0.6 mm (SD 0.2). There could be several explanations for this: for instance, the technique is extremely sensitive to the operators, the characteristics of the patients were different, the measurements were differently biased in the various centres, since outcomes assessors were not blinded, or a combination of the various explanations.
While the improvements in PAL and PPD levels are without any doubt positive findings, the real clinical utility of EMD may be debated. In particular, there is no evidence that more compromised teeth could be saved using EMD, that the amount of tissue regeneration was clinically significant, or that patients preferred the EMD treatment for aesthetic reasons. It may be argued that only short‐term follow‐up studies on EMD are available, therefore it is unlikely that a difference in tooth loss could become apparent. Since the decision to remove a periodontally compromised tooth is generally driven by the dentist, it is imperative that the person who takes this decision is unaware of the precise nature of the treatment that the patient has received (i.e. EMD versus control flap surgery or EMD versus GTR). In fact, the knowledge of the type of therapy administered might influence the decision‐making process of the dentist, who might systematically decide to remove more teeth from a certain patient group, according to personal belief, introducing bias in the results. In one trial with a 3‐year follow up (Grusovin 2009), the clinician was still unaware whether patients received EMD or placebo and judged two teeth needing an additional surgical intervention, curiously both teeth were in the group treated with EMD.
When comparing EMD with GTR (five trials), we found that GTR produced a statistically significant increase in REC (0.41 mm) after 1 year. This statistical difference may not be of clinical significance. However, there were statistically significant more postoperative complications in the GTR treated group. Complications were reported in three trials (Crea 2008; Sanz 2004; Silvestri 2003) and more specifically four patients in the EMD group experienced complications versus 59 patients treated with GTR. The great majority of these complications were small flap dehiscences over the barriers but we were also informed that two abscesses occurred at GTR treated sites in one study (Silvestri 2003). In one study (Sanz 2004), 100% of the sites treated with GTR had at least one complication versus only 6% of the sites treated with EMD. It is known that postoperative complications are common when using the GTR technique, but a 100% complication rate looks rather high. It could be hypothesized that the antibiotic coverage used (500 mg of Amoxicillin for 4 days) was insufficient to prevent infection of the barriers.
Only few minor postoperative complications occurred at EMD treated sites (Crea 2008; Sanz 2004). This suggests that EMD is a safe treatment procedure. In the literature there is only one report (St George 2006) of two cases describing inflammatory external root resorption in association with EMD treatment dictating tooth extraction. However, it is impossible to say whether the root resorption was triggered by EMD or it would have occurred independently of EMD application. No adverse reactions were reported for patients in the EMD or control groups with the exception of a few problems attributed to the use of antibiotics. While antibiotics may be useful when placing a barrier around teeth, they may not be necessary with EMD (Sculean 2001d), though this matter needs additional investigations in view of more recent findings (Mombelli 2005). It may also be useful to emphasize that the vehicle of EMD has shown antibacterial properties in vitro (Sculean 2001c; Spahr 2002). In addition, if non‐resorbable barriers are used a second operation is needed for their removal. Taken together, all these aspects suggest that EMD might be a preferable choice over GTR.
It is unclear whether patients treated with EMD may benefit from postoperative antibiotics since conflicting results were published (Mombelli 2005; Sculean 2001d). Postoperative antibiotics were prescribed in all but two trials (Grusovin 2009; Leknes 2009). In one trial (Tonetti 2002) the operators were free to decide when to use systemic antibiotics. While the administration of antibiotics may be understandable for methodological reasons in trials comparing EMD with GTR, it should be considered whether it is appropriate to use antibiotics in those trials comparing EMD with flap surgery alone, since a generalized use of antibiotics is associated with some risk. The only trial evaluating the efficacy of antibiotics after surgical application of EMD, failed to disclose any advantages by using antibiotics (Sculean 2001d).
When comparing the efficacy of EMD with a bone grafting procedure, only one RCT (Leknes 2009) could be found. Just 13 patients were included, therefore, only limited and provisional conclusions can be made. It appeared that less recessions (1.6 mm on average) occurred at proximal sites (papillae) when using a bone substitute. This might be tentatively explained by the presence of the filler which having physically occupied the space in the intrabony defect prevented the complete collapse of the papilla. If these findings are confirmed by other trials, a bone substitute could be a more interesting treatment alternative than EMD at least from an aesthetic point of view.
We intentionally did not include RCTs describing the use of EMD in conjunction with other treatments such as GTR, BG, etc. This was done because we wanted to know whether EMD was effective, and whether there were some differences when compared to other regenerative techniques. This can only be done by reducing the number of confounding factors.
The manufacturer suggests root‐conditioning prior to the application of EMD and in all the included RCTs this was done. However, the clinical efficacy of such a procedure has not been validated (Sculean 2006).
The quality of reporting of the trials (Crea 2008; Grusovin 2009; Leknes 2009) included in the present update of this review has improved, and all trials were considered to be at low risk of bias. An improvement in trial design and reporting is a positive finding since it will increase the reliability of results and conclusions. With respect to the generalization of the findings of this review to a more general population, we have to be very cautious since treatments were administered, in many cases, by experienced clinicians, in some trials smokers were excluded and, moreover, very strict maintenance regimens were employed that are not generally used in routine clinical situations. In addition, the high degree of heterogeneity indicates that even within these 'optimal' conditions, the results of treatments were highly variable. Therefore, defining optimal patient selection, aspects of treatment delivery or maintenance is not possible from this review and this was not one the aims.
Authors' conclusions
Implications for practice.
One year after treatment, the application of EMD during surgery showed statistically significant improvements in PAL (1.1 mm) and PPD reduction (0.9 mm) when compared to a placebo or a control. However, the high degree of heterogeneity observed among trials, and the fact that trials judged to be at a lower risk of bias showed less benefit of the use of EMD, suggests that results have to be interpreted with great caution and that the overall PAL gain may represent an overestimation of the actual treatment effect. Approximately nine patients needed to be treated with Emdogain to help one gain at least 2 mm of PAL. It is therefore the patient's and clinician's decision whether the clinical gain of periodontal attachment found in the present review is of clinical relevance.
No evidence of major differences between EMD and GTR could be found with the exception of slightly increased REC (0.4 mm) and significantly more postoperative complications in the GTR treated sites. EMD seems simpler to use, may not need antibiotic coverage and does not need a second surgical intervention (if compared with non‐resorbable barriers). Therefore if patients and clinicians decide to attempt a regeneration of the lost periodontal tissues, they have to consider risk‐benefits and, when comparing EMD with GTR, the EMD treatment might be preferable in light of the above issues.
The only trial comparing EMD with a ceramic filler suggested that more recession (1.6 mm) may occur at EMD treated sites.
Implications for research.
The main implications for research are. (1) More information is needed on whether EMD can actually save more teeth with a questionable prognosis. Teeth with questionable prognosis should be included in trials and followed for at least 5 years. Ideally those responsible to take the decision whether to extract or not a tooth should be unaware whether the tooth was treated with EMD or without.
(2) An independent and large multicentre placebo‐controlled trial evaluating the efficacy of Emdogain would be useful. Ideally also the effect of the placebo per se (the EMD carrier) should be tested having as control the identical operations without the placebo.
(3) The advantages and disadvantages of bone substitutes should be compared with the use of EMD in intrabony defects. Aesthetic outcomes should also be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2019 | Review declared as stable | This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 30 November 2010 | Amended | Minor edits to figures to ensure greater clarity. |
| 5 November 2009 | Amended | Minor edit. |
| 27 May 2009 | New search has been performed | Searches updated February 2009. |
| 27 May 2009 | New citation required but conclusions have not changed | Change in review authors. Three new included studies. |
| 20 June 2008 | Amended | Converted to new review format. |
| 5 August 2005 | New citation required and major changes | Substantive amendment. Changes from the first version: Two additional trials were included, and two previously included studies were excluded, but no significant changes in the results and conclusions occurred. Numerous pending and new trials were excluded. Quality assessment was slightly simplified. Data from split‐mouth trials were entered in the MetaView. Heterogeneity is now also assessed by I2. One additional post hoc subgroup analysis evaluating the effects of study design (parallel group versus split‐mouth trials) was evaluated. Several previous post hoc subgroup analyses were excluded. Outcome endpoints are now measured at 1, 5 and 10 years. We have added the dichotomous outcome PAL < 2 mm, and calculated NNT. |
Notes
This Cochrane Review is currently not a priority for updating. However, following the results of Cochrane Oral Health's latest priority setting exercise and if a substantial body of evidence on the topic becomes available, the review would be updated in the future.
Acknowledgements
We wish to thank Sylvia Bickley and Anne Littlewood (Cochrane Oral Health Group) for their assistance with literature searching; Emma Tavender and Luisa Fernandez Mauleffinch (Cochrane Oral Health Group) for their help with the preparation of this review; Regina Mitezki for translating a German article and Pierpaolo Cortellini, Orhun Dörtbudak, Luca Francetti, Stuart J Froum, Lars Heijl, Oliver Hoffmann, Jan Lindhe, Onur Ozcelik, Kazuhiro Okuda, Andreas Parashis, Cassiano Rösing, Mariano Sanz, Anton Sculean, Maurizio Silvestri, Maurizio Tonetti, K Vandana, Fridus Van der Weijden and Giovanni Zucchelli for providing us with information on their trials. We would also like to thank the following referees: Olaf F Alvarez, Jan Clarkson, Anne‐Marie Glenny, Lars Heijl, Oliver Hoffmann, Lee Hooper, Anne Littlewood, David Moles, Ian Needleman, Michele Nieri, Anton Sculean and Leonardo Trombelli.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Periodontal Diseases/ 2. periodont$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 3. intra bony defect$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 4. infra bony defect$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 5. intrabony defect$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 6. infrabony defect$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 7. or/1‐6 8. Emdogain$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 9. (enamel matrix derivative$ or enamel matrix protein$ or dental enamel protein$ or (teeth and enamel protein$) or (tooth and enamel protein$)).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 10. or/8‐9 11. 7 and 10
Appendix 2. Cochrane Oral Health Group Trials Register search strategy
((periodont* or "intra bony defect*" or "intra‐bony defect*" or "intrabony defect*" or "infra bony defect*" or "infra‐bony defect*" or "infrabony defect*") AND (emdogain* OR "enamel matrix derivative*" OR "enamel‐matrix derivative*"OR "enamel matrix protein*" OR "enamel‐matrix protein*" OR "dental enamel protein*" OR (teeth AND "enamel protein*") OR (tooth AND "enamel protein*")))
Appendix 3. CENTRAL search strategy
#1 PERIODONTAL DISEASES (Explode MeSH) #2 periodont* #3 (intra next bony next defect*) OR (intrabony NEXT defect*) #4 (infra next bony next defect*) OR (infrabony NEXT defect*) #5 (#1 or #2 or #3 or #4) #6 emdogain* #7 (enamel next matrix next derivative*) #8 (enamel next matrix next protein*) #9 (dental next matrix next protein*) #10 (teeth and (enamel next protein*)) #11 (tooth and (enamel next protein*)) #12 (#6 or #7 or #8 or #9 or #10 or #11) #13 (#5 and #12)
Appendix 4. EMBASE (OVID) search strategy
1. exp Periodontal Disease/ 2. periodont$.mp. 3. intra bony defect$.mp. 4. infra bony defect$.mp. 5. intrabony defect$.mp. 6. infrabony defect$.mp. 7. or/1‐6 8. Emdogain$.mp. 9. (enamel matrix derivative$ or enamel matrix protein$ or dental enamel protein$ or (teeth and enamel protein$) or (tooth and enamel protein$)).mp. 10. or/8‐9 11. 7 and 10
Filter for EMBASE via OVID
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Data and analyses
Comparison 1. Emdogain versus control: 1 year.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PAL | 9 | 442 | mean difference (Random, 95% CI) | 1.08 [0.61, 1.55] |
| 1.1 Parallel group | 5 | 300 | mean difference (Random, 95% CI) | 1.40 [0.57, 2.24] |
| 1.2 Split mouth | 4 | 142 | mean difference (Random, 95% CI) | 0.76 [0.48, 1.04] |
| 2 PAL < 2 mm | 6 | 362 | risk ratio (Random, 95% CI) | 0.53 [0.34, 0.82] |
| 3 PPD | 9 | 442 | Mean difference (Random, 95% CI) | 0.88 [0.44, 1.31] |
| 3.1 Parallel group | 5 | 300 | Mean difference (Random, 95% CI) | 1.25 [0.44, 2.05] |
| 3.2 Split mouth | 4 | 142 | Mean difference (Random, 95% CI) | 0.66 [0.31, 1.00] |
| 4 REC | 6 | 328 | mean difference (Random, 95% CI) | 0.09 [‐0.20, 0.37] |
| 4.1 Parallel group | 4 | 276 | mean difference (Random, 95% CI) | 0.09 [‐0.33, 0.52] |
| 4.2 Split mouth | 2 | 52 | mean difference (Random, 95% CI) | 0.0 [‐0.41, 0.41] |
| 5 Marginal bone level | 3 | 120 | mean difference (Random, 95% CI) | 0.69 [‐0.53, 1.92] |
| 5.1 Parallel group | 1 | 30 | mean difference (Random, 95% CI) | 0.0 [‐0.82, 0.82] |
| 5.2 Split mouth | 2 | 90 | mean difference (Random, 95% CI) | 1.08 [‐0.72, 2.89] |
| 6 Aesthetics (continuous data) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Parallel group | 1 | 166 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐5.42, 7.42] |
| 7 Aesthetics (dichotomous data) | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.07] |
1.1. Analysis.
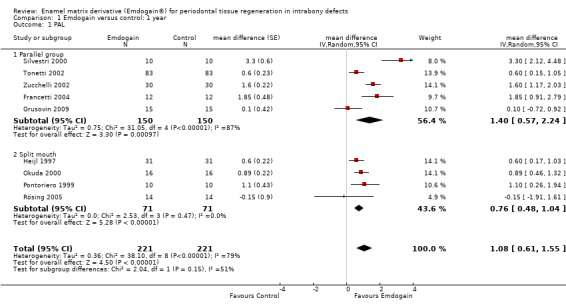
Comparison 1 Emdogain versus control: 1 year, Outcome 1 PAL.
1.2. Analysis.
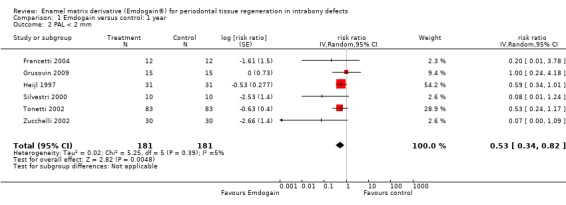
Comparison 1 Emdogain versus control: 1 year, Outcome 2 PAL < 2 mm.
1.3. Analysis.
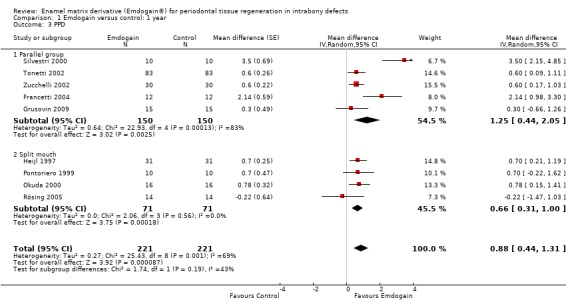
Comparison 1 Emdogain versus control: 1 year, Outcome 3 PPD.
1.4. Analysis.
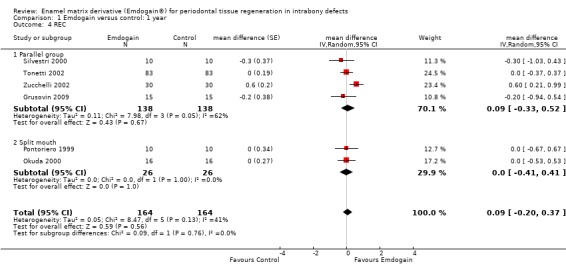
Comparison 1 Emdogain versus control: 1 year, Outcome 4 REC.
1.5. Analysis.
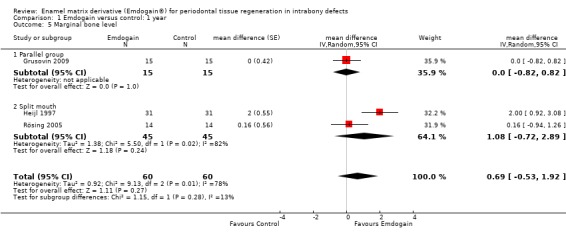
Comparison 1 Emdogain versus control: 1 year, Outcome 5 Marginal bone level.
1.6. Analysis.
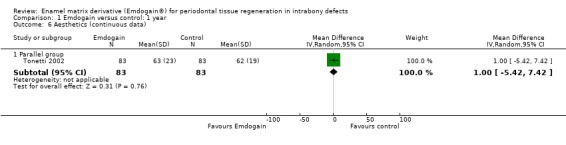
Comparison 1 Emdogain versus control: 1 year, Outcome 6 Aesthetics (continuous data).
1.7. Analysis.
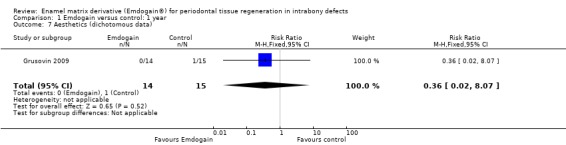
Comparison 1 Emdogain versus control: 1 year, Outcome 7 Aesthetics (dichotomous data).
Comparison 2. Emdogain versus GTR: 1 year.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PAL | 6 | 304 | mean difference (Random, 95% CI) | ‐0.15 [‐0.56, 0.25] |
| 1.1 Parallel group | 6 | 304 | mean difference (Random, 95% CI) | ‐0.15 [‐0.56, 0.25] |
| 1.2 Split mouth | 0 | 0 | mean difference (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 PPD | 6 | 304 | mean difference (Random, 95% CI) | ‐0.44 [‐1.06, 0.18] |
| 2.1 Parallel group | 6 | 304 | mean difference (Random, 95% CI) | ‐0.44 [‐1.06, 0.18] |
| 2.2 Split mouth | 0 | 0 | mean difference (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 REC | 5 | 206 | mean difference (Random, 95% CI) | 0.41 [0.15, 0.66] |
| 3.1 Parallel group | 5 | 206 | mean difference (Random, 95% CI) | 0.41 [0.15, 0.66] |
| 3.2 Split mouth | 0 | 0 | mean difference (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Postoperative complications | 3 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.85] |
| 4.1 Parallel group | 3 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.85] |
| 5 Marginal bone level | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.34, 0.14] |
2.1. Analysis.
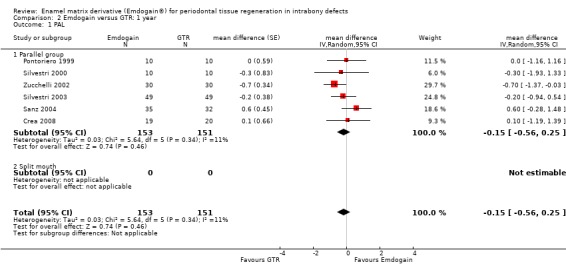
Comparison 2 Emdogain versus GTR: 1 year, Outcome 1 PAL.
2.2. Analysis.
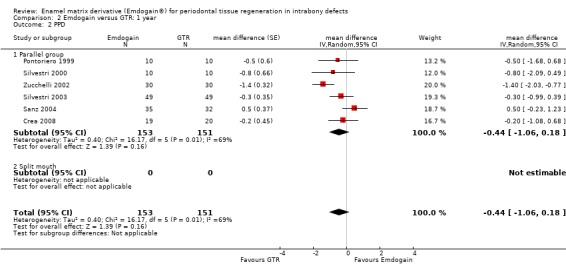
Comparison 2 Emdogain versus GTR: 1 year, Outcome 2 PPD.
2.3. Analysis.

Comparison 2 Emdogain versus GTR: 1 year, Outcome 3 REC.
2.4. Analysis.
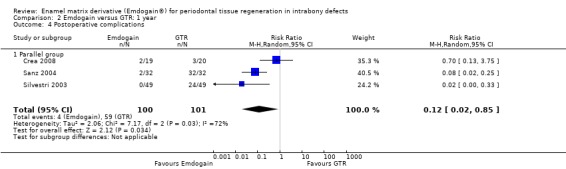
Comparison 2 Emdogain versus GTR: 1 year, Outcome 4 Postoperative complications.
2.5. Analysis.
Comparison 2 Emdogain versus GTR: 1 year, Outcome 5 Marginal bone level.
Comparison 3. Emdogain versus bone graft.
3.1. Analysis.
Comparison 3 Emdogain versus bone graft, Outcome 1 PAL.
3.2. Analysis.
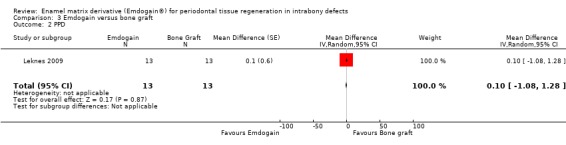
Comparison 3 Emdogain versus bone graft, Outcome 2 PPD.
3.3. Analysis.
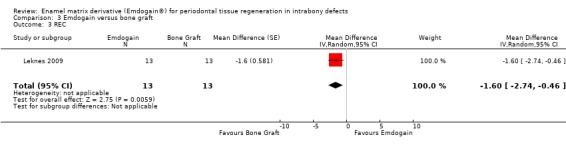
Comparison 3 Emdogain versus bone graft, Outcome 3 REC.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Crea 2008.
| Methods | 3‐year follow‐up parallel‐group study including 2 groups with 40 patients in total. 1 drop out from the Emdogain group since the patient failed to attend the scheduled appointments following surgery. | |
| Participants | Patients in good general health and with good oral hygiene. Teeth with IBD deeper than or equal to 4 mm with 3‐wall defects were included. Endodontically treated teeth, teeth with crowns or fixed partial dentures were excluded. Smokers were excluded. All patients had received non‐surgical periodontal treatment without antibiotic therapy. Age ranging between 35 and 66; 18 males and 21 females recruited at Department of Periodontology, Catholic University of Sacred Heart, Rome, Italy, and treated by the same clinician. | |
| Interventions | Emdogain versus GTR with Gore‐Tex non‐resorbable barriers. In case of postoperative wound dehiscence in both groups the intervention was repeated. | |
| Outcomes | PAL, PPD, REC, IBD on standardised intraoral radiographs at baseline, 1 and 3 years. Tooth loss, postoperative complications and adverse events. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the beginning of the study, the 40 recruited patients were randomly assigned to one of the two treatment groups (n = 20 per group). Study statisticians prepared a randomized, numbered (1 to 40) list with the technique as variable, and forms with the chosen treatment modality were put into envelopes with the corresponding number on the outside". |
| Allocation concealment (selection bias) | Low risk | Quote: "The sealed envelopes were placed into the custody of a surgeon (LD) who was not involved in diagnosis or treatment delivery. After the defect was degranulated, surgeon LD entered the surgical room, opened an envelope bearing the number by which the patient would subsequently be identified, and informed the surgeon which randomly assigned treatment was to be performed". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The researchers who performed the measurements (GD) and the randomization (GGZ) did not include the periodontist who performed the initial treatment or the surgeon who provided the surgical treatment. Hence, the examiner was masked to the treatment designations and was not involved in the delivery of treatment or maintenance care". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are presented in Table 2: Changes in clinical parameters over time. Comment: No missing outcome data. Drop out is explained adequately. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified clinical outcomes are properly presented in Table 2: Changes in clinical parameters over time. Adverse events are reported in the Results section. No teeth were extracted. This trial has not evaluated aesthetics. |
| Other bias | Low risk | Quote: "The authors report no conflicts of interest related to this study. No financial or material support was provided by any company to the authors or the patients involved in this study". Comment: The GTR group had slightly more advanced periodontal disease than the EMD group for all outcomes on baseline. Nevertheless there is no indication of extreme baseline imbalance. |
Francetti 2004.
| Methods | 2‐year follow‐up parallel group study including 2 groups with 24 patients in total. No drop outs at 1 year. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 4 mm. 1‐, 2‐ and 3‐wall defects were included. Teeth with degree III mobility, necrotic, with incongruous reconstructions or under occlusal trauma were excluded. Patients should not have been treated for periodontitis in the last 3 years. Age ranging between 30 and 66; 11 males and 13 females recruited at 1 university dental clinic. | |
| Interventions | Emdogain versus flap surgery. | |
| Outcomes | FMPS, FMBS. For experimental teeth only: PAL, PPD, IBD on standardised intraoral radiographs at baseline, 1 and 2 years. Tooth loss, postoperative infections and adverse events. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were subsequently allocated to either test or control group in accordance with a 1:1 computer‐generated randomization list". |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation to treatment group was concealed from clinicians until the patients received the treatment". Comment: Author informed us that the allocation to the intervention groups was concealed. During surgery, after debridement a sequentially numbered sealed opaque envelope containing the randomisation code was opened. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quotes: "It was conducted according to an open‐label, randomized parallel study protocol". "Patients were blinded as to treatment assignment throughout the study". "All radiographs were evaluated by a single examiner blind to treatment". Comment: Assessor was not blinded for the clinical outcomes due to open‐label procedure. He was blinded only for the radiographic evaluation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are presented in Table 1: Mean values of the parameters at baseline and after 12 months and 24 months. Comment: No missing outcome data. No drop outs. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes are properly presented in Table 1: Mean values of the parameters at baseline and after 12 months and 24 months. No adverse events for 1‐year data. No teeth were extracted. REC and aesthetics were not evaluated as treatment outcomes. |
| Other bias | Low risk | No other source of bias can be identified. No fixed reference points were used in the radiographic assessment and therefore we decided not to use those data. |
Grusovin 2009.
| Methods | 3‐year follow‐up parallel‐group study including 2 groups with 30 patients in total, however most of the data were presented at 1 year. 1 drop out from the placebo group at 1 year though the 6‐month data were evaluated instead. | |
| Participants | Patients in good general health and with good oral hygiene (full mouth plaque, bleeding and bleeding on probing score less than 20%). Teeth with IBD deeper than or equal to 4 mm and larger than or equal to 2 mm. 1‐, 2‐ and 3‐wall defects were included. Teeth with vertical tooth mobility, endo‐perio lesions and overhangs were excluded. All patients had received systematic periodontal treatments (repeated debridement in some cases supplemented with surgical treatment). Age ranging between 25 and 68; 16 males and 14 females recruited at 2 private practices but treated by the same clinician. | |
| Interventions | Emdogain versus flap surgery and placebo. | |
| Outcomes | PAL, PPD, REC, IBD on standardised intraoral radiographs at baseline, 6 months, 1 and 3 years but only 1‐year data presented. Patient evaluation of treatment and aesthetics at 1 year. Tooth loss, any complications and adverse events. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A manual restricted randomisation list was generated by a person not involved in the study and stored in a password‐protected computer. The randomisation codes were associated to the sequential numbers given to the patients and applied to identical packages by the same person". |
| Allocation concealment (selection bias) | Low risk | Quote: "At the time of EDTA conditioning the package was opened according to the sequential number. Division in two groups according to the code was done at the time of statistical analysis. The code assigned to the treatment was known only by the person not involved in the study that generated the codes and was disclosed after data processing (3 years after the last patient was treated)". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Since the same packing was used for Emdogain and placebo the treatment was blind to the operator who also acted as outcome assessor". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for 30 patients are presented in Tables 3, 4 and 5. Comment: No missing outcome data. Drop outs are explained adequately. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified clinical outcomes are properly presented in Tables 3, 4 and 5. The aesthetic evaluation is reported in Table 6. No adverse events are reported and no teeth were extracted. |
| Other bias | Low risk | Quote: "It was planned to include 50 patients per group; however, the trial had to be stopped after the first 30 patients were included owing to lack of placebo". Comment: Early termination of trial precluded the achievement of the planned sample size. Comment: Although the manufacturer provided the placebos, this trial has been conducted independently. Comment: The average baseline intrabony component was 1 mm deeper and 1.1 mm wider in EMD group than placebo group. Nonetheless this slight imbalance is not considered significant enough to increase selection bias. |
Heijl 1997.
| Methods | 3‐year follow‐up split‐mouth study including 33 patients. 3 drop outs at 16 months (tooth extractions in 2 cases and accident for 1 patient). | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 4 mm. 1‐, 2‐ and 3‐wall defects were included. All patients had received systematic periodontal treatments (repeated debridement in some cases supplemented with antimicrobial and surgical treatment over long periods of time). Age ranging between 33 and 68; 7 males and 26 females recruited at 3 specialist clinics. | |
| Interventions | Emdogain versus flap surgery and placebo. | |
| Outcomes | FMPS and for experimental teeth only: BOP, PAL, PPD, IBD on standardised intraoral radiographs at baseline, 8, 16 months and 3 years. Tooth loss, postoperative infections and adverse events. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sites were distinguished by their tooth number (18 through 48) and the randomization code specified the treatment assignment for the site with the lowest as well as highest tooth number. The randomization process targeted one of the sites for test treatment and the other site for control treatment. Patient numbers were assigned in chronological order as patients were enrolled in the trial". Author's reply: "Randomization codes were computer generated in blocks". |
| Allocation concealment (selection bias) | Low risk | Quote: "At the time of periodontal surgery, and only after the first surgical site was fully prepared, the envelope containing the randomisation code was opened to expose treatment assignments". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quotes: "Readings of all radiographs were performed by a separate, blinded examiner and in a randomised fashion". "All re‐examination measurements were made by the same blinded investigator who made the initial measurements". Comment: Assessors were blinded both for the clinical and radiographic outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are presented in Table 3: Mean values for pocket depth, clinical attachment level and radiographic bone level. Comment: No missing outcome data. Drop outs are explained adequately. |
| Selective reporting (reporting bias) | Low risk | All the primary pre‐specified clinical outcomes are properly presented in Table 3: Mean values for pocket depth, clinical attachment level and radiographic bone level. Adversed events are reported in Safety (AEs) section. 4 teeth were extracted, 2 for each group. REC and aesthetics were not evaluated as treatment outcomes. |
| Other bias | Unclear risk | The trial was supported by the manufacturer. |
Leknes 2009.
| Methods | 1‐year follow‐up split‐mouth study including 13 patients. No drop outs at 1 year. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 3 mm. 2‐ and 3‐wall defects were included. All patients had received 2‐4 weeks of subgingival debridement. Age ranging between 41 and 74; 5 males and 8 females recruited at a university clinic. | |
| Interventions | Emdogain versus a granular ceramic filler (PerioGlas, US Biomaterials, Alachua, FL, USA). | |
| Outcomes | PAL, PPD, REC, tooth mobility at baseline and 1 year. Tooth loss and postoperative complications. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...assigned randomly (by flipping a coin) to EMD or BCF treatment using a split‐mouth design". |
| Allocation concealment (selection bias) | Low risk | Quote: "A mucoperiosteal flap was elevated using a sulcular incision under local anaesthesia. Vertical release incisions were used as necessary. The defects were evaluated and, if meeting the inclusion criteria with regard to defect configuration, they were assigned randomly (by flipping a coin) to EMD or BCF treatment using a split‐mouth design". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The clinical examinations were performed by one examiner who was not involved in the surgical procedure and was masked with regard to the treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clinical outcome data for PPD, PAL and REC are presented in Tables 2, 3 and 4. Comment: No missing outcome data. No drop outs. |
| Selective reporting (reporting bias) | Unclear risk | All primary pre‐specified outcomes are reported in Tables 2, 3 and 4. Comment: Infrabony defects were recorded at baseline on periapical radiographs but were not reported. No adverse complications were seen or reported. No teeth were extracted. Aesthetics were not evaluated. |
| Other bias | Low risk | No other source of bias can be identified. |
Okuda 2000.
| Methods | 1‐year follow‐up split‐mouth study including 16 patients. No drop outs at 1 year. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 4 mm in presence of 2 mm of keratinized gingiva on the buccal aspect. Patients should not have been treated for periodontitis in the last 2 years. No antibiotics in the previous 6 months. Smokers were excluded. Age ranging between 45 and 67; 8 males and 8 females recruited at 1 university dental clinic. | |
| Interventions | Emdogain versus flap surgery and placebo. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: vertical relative attachment gain, tooth mobility, PAL, PPD, REC, IBD on standardised intraoral radiographs measured as radiographic bone density at baseline and 1 year. Tooth loss, postoperative infections and adverse events. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The paired intrabony defects selected for treatment were randomly assigned to receive either the EMD treatment or the placebo treatment by a flip of a coin". |
| Allocation concealment (selection bias) | Low risk | Author's reply: "At first a surgeon operated open flap and debridement at both sites. After these procedures were finished, the surgeon was put a blindfold condition. At next stage, another person who was not involved in the surgery, applied EMD or placebo to the site determined by a flip of a coin. The surgeon again open eyes, sutured the flap". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The clinical examinations were performed by a single examiner (author KO), who was not involved in the surgical procedures". Also the author made it clear that the trial was triple blinded, i.e. patient, clinicians and evaluators had no information regarding the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are presented in Table 3: Mean clinical and radiographical (RBD) changes at 12 months (mean ± SD). Comment: No missing data. No drop outs. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes for PPD, PAL, REC and IBD are reported in Table 3: Mean clinical and radiographical (RBD) changes at 12 months (mean ± SD). No teeth were extracted and no adverse complications were reported. Aesthetics were not evaluated. |
| Other bias | Low risk | No other source of bias can be identified. |
Pontoriero 1999.
| Methods | 1‐year follow‐up split‐mouth study including 4 parallel arms with 40 patients in total. Only 2 parallel arms evaluated. No drop outs at 1 year. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 3 mm. In 2 groups, however, defects shallower than 3 mm were included and therefore were excluded from the present review. Age ranging between 32‐61; 15 males and 25 females recruited in 1 private practice. | |
| Interventions | 4 split‐mouth groups were included: (1) GTR with Guidor resorbable barriers versus flap surgery; (2) GTR with Resolut resorbable barriers versus flap surgery; (3) GTR with Gore‐Tex non‐resorbable barriers versus flap surgery; (4) Emdogain versus flap surgery and placebo. We analysed only groups (3) and (4) since in the other 2 groups defects shallower than 3 mm were included. | |
| Outcomes | FMPS, BOP and for experimental teeth only: PAL, PPD, REC at baseline and 1 year. Tooth loss and postoperative infections. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 40 subjects were randomly divided into 4 treatment groups including 10 subjects each: 3 membrane groups and one Emdogain® group". |
| Allocation concealment (selection bias) | Unclear risk | Author informed us that allocation to intervention group was concealed, but did not explain how. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Author informed us that both the outcome assessor and the patients were blinded to which site received which treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data on PAL, PPD and REC are presented in Table 2: Result of GTR and Emdogain® therapy. Comment: No missing data. No drop outs. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes are reported in Tables 2 and 4. Comment: Only data from the Gore‐Tex® and Emdogain® groups (3 and 4) are included in this review. Data from the other 2 groups had to be excluded on the basis of not meeting the 3 mm intrabony defect criterion. The author informed us that no teeth were extracted and no postoperative complication was reported. Changes in bone level and aesthetics were not evaluated as treatment outcomes. |
| Other bias | Low risk | No other source of bias can be identified. |
Rösing 2005.
| Methods | 1‐year follow‐up split‐mouth study including 16 patients. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with IBD greater or equal to 3 mm and wider than 2 mm on intraoral radiographs. Age ranging between 29‐54; patients recruited in 1 university dental clinic. | |
| Interventions | Emdogain versus flap surgery and placebo. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: BOP, PAL, PPD, IBD on standardised intraoral radiographs at baseline, 6 months, and 1 year. Tooth loss, postoperative infections and adverse events. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Then, by means of the flip of a coin, the experimental (EMD) and the placebo (both provided by the manufacturer) solutions were applied in accordance to the instructions". |
| Allocation concealment (selection bias) | Low risk | Quote: "The present study was carried out according to a typical double‐masked, split‐mouth design, with the codes kept by the manufacturer until the data had been collected and organised in the computer program for statistical analysis". Author's reply: "Randomization of the site was decided with the flip of a coin after debridement of both sites and application of the EDTA solution". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Analysis of the radiographic outcomes were performed using computerized linear measurements from the cemento‐enamel junction (CEJ) to the bone crest (BC), CEJ to the bottom of the defect (BD), and BC to BD by an examiner masked to time and treatment. All clinical and radiographic measurements were performed according to a double‐masked protocol". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are presented in Tables 1 and 3. Comment: No missing data. Drop outs are explained adequately. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes for PPD, PAL and IBD are reported in Tables 1 and 3. 2 teeth were extracted. No adverse events were noted. REC and aesthetics were not evaluated as treatment outcomes. |
| Other bias | Low risk | Although EMD and placebo materials were provided by the manufacturer, this was an independently conducted study. |
Sanz 2004.
| Methods | 1‐year follow‐up parallel group study including 2 groups with 72 patients in total. 5 drop outs for unknown reasons and from unspecified groups. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with IBD greater or equal to 3 mm in presence of 2 to 3 mm of keratinized gingiva on the buccal aspect. Heavy smokers (> 20 cigarettes per day) were excluded. 1‐, 2‐ and 3‐wall defects were included. Age ranging between 43 to 61; females were 54.3% in the test and 53.1% in the control groups. Patients were recruited both from university dental clinics and private practices. | |
| Interventions | Emdogain versus GTR with Resolut resorbable barriers. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: PAL, PPD, REC at baseline and 1 year. Postoperative infections. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | 100% of postoperative complications (flap dehiscence, suppuration) in the GTR group versus 6% in the Emdogain group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All subjects were assigned a patient number and were assigned to one of the two treatment regimens using a random number table". |
| Allocation concealment (selection bias) | Low risk | Quote: "Clinicians were not aware of treatment allocation until after root debridement". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "In each center a single clinician served as examiner and surgeon". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcome data for PAL, PPD and REC are presented in Clinical Outcomes part of the Results section of the paper. Comment: No missing data regarding PAL, PPD and REC. Unclear explanation for the 5 drop outs and withdrawals. |
| Selective reporting (reporting bias) | High risk | All the pre‐specified outcomes are reported in the Results section of the paper. Tooth loss was not described. Postoperative complications are discussed in the Results and Discussion parts of the paper but not clearly described, not even after requesting the data. Changes in bone level and aesthetics were not evaluated. |
| Other bias | Low risk | The study received a research grant from the manufacturer Biora AB. |
Silvestri 2000.
| Methods | 1‐year follow‐up parallel‐group study including 3 groups with 30 patients in total. No drop outs at 1 year. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 4 mm. Smokers were excluded. Age ranging between 37 and 59; 11 males and 19 females recruited in 1 university dental clinic and several private practices. | |
| Interventions | Emdogain versus GTR with Gore‐Tex non‐resorbable barriers versus flap surgery. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: PAL, PPD, REC at baseline and 1 year. Tooth loss and postoperative infections. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Once the patients met on all entry criteria, they were randomly (Fleiss 1992) assigned to 1 of 3 surgical procedures". |
| Allocation concealment (selection bias) | High risk | Author informed us that group allocation was not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Author informed us that no blinding method was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for PAL, PPD and REC are presented in Table 1. Comment: No missing data. No drop outs. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes are reported in Table 1. No teeth were extracted. Aesthetics and changes in bone level were not evaluated. |
| Other bias | Unclear risk | Manufacturers partially supported the trial by offering free materials. We do not think that this has affected the outcome of the trial. Connective tissue grafts were placed in 6 patients after membrane removal. We are unsure whether this affected the outcome of the trial. |
Silvestri 2003.
| Methods | 1‐year follow‐up parallel‐group study including 2 groups with 100 patients in total. 2 drop outs at 1 year. 2 patients (1 from each group) did not show up at the 1‐year examination for personal reasons. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 6 mm and IBD greater or equal to 4 mm. Smokers (> 10 cigarettes per day) were excluded. 1‐, 2‐ and 3‐wall defects were included. Age ranging between 39 and 58; 45 males and 53 females recruited in 1 university dental clinic and several private practices. | |
| Interventions | Emdogain versus GTR with Gore‐Tex non‐resorbable barriers. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: PAL, PPD, REC at baseline and 1 year. Tooth loss and postoperative infections. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment was determined by central randomization using balanced random permuted blocks". |
| Allocation concealment (selection bias) | Low risk | Author's reply: "The clinicians learned the treatment during the surgery after defect debridement by a code inside an envelope". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Author informed us that no blinding method was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for PAL and PPD are reported in Table 3: PAL gain and PD reduction for the two groups 1 year postop. Comment: The reason for 2 drop outs (1 for each group) was explained. |
| Selective reporting (reporting bias) | High risk | Only PAL and PPD outcomes are reported in Table 3: PAL gain and PD reduction for the two groups 1 year postop. Comment: No teeth were extracted. No report of REC in 1‐year data. Aesthetics and changes in bone level were not evaluated. |
| Other bias | Low risk | The manufacturer partially supported the trial. We do not think that this has affected the outcome of the trial. |
Tonetti 2002.
| Methods | 1‐year follow‐up parallel‐group study including 2 groups with 172 patients in total. 6 drop outs at 1 year. 3 patients withdrew consent before surgery. 3 patients (2 from the test and 1 from the control group) were unable to comply with the follow up for reasons independent from the treatments. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with IBD greater or equal to 3 mm in presence of 2 to 3 mm of keratinized gingiva on the buccal aspect. Heavy smokers (> 20 cigarettes per day) were excluded. 1‐, 2‐ and 3‐wall defects were included. Age ranging between 39 and 57; females were 54.2% in the test and 60.2% in the control groups. Patients were recruited both from university dental clinics and private practices. | |
| Interventions | Emdogain versus flap surgery. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: PAL, PPD, REC at baseline and 1 year. Tooth loss and postoperative infections. Additional intrasurgical measurements were taken. 1‐year data used. Postoperative morbidity, patient satisfaction, aesthetics and several other patient‐centred outcomes were evaluated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All subjects were assigned a patient number, and were randomly assigned to one of the two treatment regiments. Assignment was performed by a central randomization facility using a custom‐made program based on balanced random permuted blocks". |
| Allocation concealment (selection bias) | Low risk | Author informed us that the allocation to the intervention groups was concealed. During surgery, after debridement, a sealed opaque envelope containing the randomisation code was opened. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "In each center, the examiner and the therapist were identical". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data are presented in Table 2: Clinical outcomes at 1 year. Comment: No missing data. Drop outs are explained adequately. |
| Selective reporting (reporting bias) | Low risk | All the pre‐specified outcomes are reported in Table 2: Clinical outcomes at 1 year. No teeth were extracted. No infectious complications were observed. The aesthetics evaluation was reported in Tonetti et al 2004 JCP 31:1092‐8. Changes in bone level were not assessed. |
| Other bias | Low risk | The trial was partially supported with a research grant from the manufacturer. We do not think that this has affected the outcome of the trial. |
Zucchelli 2002.
| Methods | 1‐year follow‐up parallel‐group study including 3 groups with 90 patients in total. No drop outs at 1 year. | |
| Participants | Patients in good general health and motivated for good oral hygiene. Teeth with PPD greater or equal to 7 mm and IBD greater or equal to 3 mm. Heavy smokers (more than 20 cigarettes per day) were excluded. No antibiotics in the previous 6 months. Age ranging between 39 and 57; 30 males and 61 females. Patients were recruited from 1 university dental clinic and several private practices. | |
| Interventions | Emdogain versus GTR with Gore‐Tex titanium‐reinforced non‐resorbable barriers versus flap surgery. | |
| Outcomes | FMPS and FMBS. For experimental teeth only: PAL, PPD, REC at baseline and 1 year. Tooth loss and postoperative infections. Additional intrasurgical measurements were taken. 1‐year data used. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before surgery, assignment to the 3 treatment regimens (30 patients/group) was performed using a custom‐made program based on balanced permuted blocks". |
| Allocation concealment (selection bias) | Unclear risk | Author informed us that allocation to intervention group was concealed, but did not explain how. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "A single investigator blinded with respect to the treatments, performed the clinical measurements at baseline and at 1 year". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data are presented in Table 2: Clinical parameters at 1 year. Comment: No missing data. No drop outs. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes are reported in Table 2: Clinical parameters at 1 year. Postoperative infections are reported in Early Healing Event part of the Results section of the paper. No teeth were extracted. Aesthetics and changes in bone level were not evaluated. |
| Other bias | Low risk | No other source of bias can be identified. |
BOP = bleeding on probing FMBS = full mouth bleeding score FMPS = full mouth plaque score GTR = guided tissue regeneration IBD = intrabony depth PAL = probing attachment level PPD = probing pocket depth REC = gingival recession
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bokan 2006 | Different flap designs used in test and control sites that made the comparison inappropriate to answer the question of this review. |
| Chambrone 2007 | Included patients with less than 3 mm intrabony defect component and follow up to 6 months. |
| Doertbudak 2000 | Authors informed us that trial was a CCT. |
| Eger 1998 | Not a RCT. |
| Francetti 2005 | Multicentre study comparing Emdogain versus control, with data presented on site not patient basis. The authors have replied to a request for further information however have not supplied this. |
| Froum 2001 | Trial comparing Emdogain versus control. This study was designed as a split‐mouth study, and the data are presented for 53 defects in Emdogain group and 31 defects in control, in 23 subjects. The presentation of the data does not include an estimate of the standard error for the paired data and cannot therefore be included in the meta‐analyses for this review. The authors have replied to a request for further information however they have not supplied the required standard errors, or variance estimates, despite repeated requests as suggested by one of the referees. |
| Ghaffar 2001 | Insufficient data presented. Written to author and sponsor but no reply to letters. |
| Hagenaars 2004 | Trial designed to evaluate the early postoperative phase (up to 8 weeks). Written to authors asking whether longer follow up was planned, but they replied that this was not their intention. |
| Lombardo 2000 | Judged to be a CCT. No reply to letter. |
| Martinez 2001 | Insufficient data presented. No reply to letter. |
| Martu 2000a | Judged to be a CCT. No reply to letter. Possibly same trial as Marthu 2000b. |
| Martu 2000b | Judged to be a CCT. No reply to letter. Possibly same trial as Marthu 2000a. |
| Minabe 2002 | Parallel‐group study with more than 1 site per patient treated in the Emdogain group. We are unable to extract data at a patient level. Authors did not respond to our request for further data. |
| Mombelli 2005 | Included patients with less than 3 mm intrabony defect component. |
| Ozcelik 2007 | No outcomes of interest and follow up of only 1 week. |
| Parashis 2004 | Authors informed us that trial was a CCT. |
| Sculean 1999 | Study designed so that teeth are extracted after 6 months. Unclear if this is the same study as Windisch 2002. |
| Sculean 2001a | Included patients with less than 3 mm intrabony defect component. |
| Sculean 2001b | Included patients with less than 3 mm intrabony defect component. |
| Vandana 2004 | Unclear whether RCT or CCT. Authors replied it was a RCT. Trial excluded since the follow up was 9 months instead of 1 year and the intrabony components of some defects were less than 3 mm. |
| Wachtel 2003 | Split‐mouth study with more than 1 site per quadrant treated with 1 intervention. We were unable to extract simple 'paired data' for each patient and the authors did not respond to our request for further data. |
| Windisch 2002 | 6‐month study designed so that teeth are extracted after 6 months. Unclear if this is the same study as Sculean 1999. |
CCT = controlled clinical trial RCT = randomised controlled trial
Differences between protocol and review
Changes from the protocol. We investigated heterogeneity using post hoc factors found in the trial reports as follows: placebo or control group, antibiotics given, surgical technique used in control group, funded by manufacturer, depth of baseline intrabony defects, whether the trial was conducted in Italy or not. We have added adverse effects to the list of outcomes, however none were found in the included trials.
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME)). Developing search strategy and undertaking searches (ME, Paul Coulthard (PC)). Screening search results and retrieved papers against inclusion criteria (ME, Gabriella Grusovin (GG), Nikolaos Papanikolaou (NP)). Appraising quality (ME, PC, GG, NP, Helen Worthington (HW)). Extracting data from papers (ME, HW). Writing to authors for additional information (ME, HW, NP, GG). Data management for the review and entering data into RevMan (HW, ME). Analysis and interpretation of data (HW, ME). Writing the review (ME, HW). Providing general advice on the review (PC, GG). Performing previous work that was the foundation of current study (ME, HW, PC).
Sources of support
Internal sources
Division of Dentistry, The University of Manchester, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed are those of the authors and not necessarily those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Declarations of interest
None known. Maria Gabriella Grusovin and Marco Esposito were authors of one of the included trials. However, they were not involved in the quality assessment of this trial.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Crea 2008 {published and unpublished data}
- Crea A, Dassatti L, Hoffmann O, Zafiropoulos GG, Deli G. Treatment of intrabony defects using guided tissue regeneration or enamel matrix derivative: a 3‐year prospective randomized clinical study. Journal of Periodontology 2008;79(12):2281‐9. [DOI] [PubMed] [Google Scholar]
Francetti 2004 {published and unpublished data}
- Francetti L, Fabbro M, Basso M, Testori R, Weinstein R. Enamel matrix proteins in the treatment of intra‐bony defects. A prospective 24‐month clinical trial. Journal of Clinical Periodontology 2004;31(1):52‐9. [DOI] [PubMed] [Google Scholar]
Grusovin 2009 {published and unpublished data}
- Grusovin MG, Esposito M. The efficacy of enamel matrix derivates (Emdogain) for the treatment of infrabony defects. A placebo‐controlled randomised clinical trial. European Journal of Oral Implantology 2009;2:43‐54. [PubMed] [Google Scholar]
Heijl 1997 {published and unpublished data}
- Heijl L, Heden G, Svardstrom G, Ostgren A. EMDOGAIN in the treatment of intrabony periodontal defects. Journal of Dental Research 1997;76 (Special Abstract Issue 1):292. [DOI] [PubMed] [Google Scholar]
- Heijl L, Heden G, Svardstrom G, Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. Journal of Clinical Periodontology 1997;24(9 Pt 2):705‐14. [DOI] [PubMed] [Google Scholar]
Leknes 2009 {published data only (unpublished sought but not used)}
- Leknes KN, Andersen KM, Boe OE, Skavland RJ, Albandar JM. Enamel matrix derivative versus bioactive ceramic filler in the treatment of intrabony defects: 12‐month results. Journal of Periodontology 2009;80(2):219‐27. [DOI] [PubMed] [Google Scholar]
Okuda 2000 {published and unpublished data}
- Okuda K, Miyazaki A, Momose M, Murata M, Yokoyama S, Yonezawa Y. Enamel matrix derivative (EMD) in the treatment of human intrabony periodontal osseous defects. Journal of Periodontology 2000;71:1913. [DOI] [PubMed] [Google Scholar]
- Okuda K, Momose M, Miyazaki A, Murata M, Yokoyama S, Yonezawa Y, et al. Enamel matrix derivative in the treatment of human intrabony osseous defects. Journal of Periodontology 2000;71(12):1821‐8. [DOI] [PubMed] [Google Scholar]
Pontoriero 1999 {published and unpublished data}
- Pontoriero R, Wennstrom J, Lindhe J. The use of barrier membranes and enamel matrix proteins in the treatment of angular bone defects. A prospective controlled clinical study. Journal of Clinical Periodontology 1999;26(12):833‐40. [DOI] [PubMed] [Google Scholar]
Rösing 2005 {published and unpublished data}
- Rösing CK, Aass AM, Mavropoulos A, Gjermo P. Clinical and radiographic effects of enamel matrix derivative in the treatment of intrabony periodontal defects: a 12‐month longitudinal placebo‐controlled clinical trial in adult periodontitis patients. Journal of Periodontology 2005;76(1):129‐33. [DOI] [PubMed] [Google Scholar]
Sanz 2004 {published data only}
- Sanz M, Tonetti M, Zabalegui I, Blanco J, Rebelo H, Sicilia A, et al. Treatment of infrabony defects with enamel matrix proteins. A multicentre practice‐based study. Conference Proceedings of the Pan European Federation of the International Association for Dental Research. Newcastle: Pattinson and Sons, 2002:Abstract No 164.
- Sanz M, Tonetti MS, Zabalegui I, Sicilia A, Blanco J, Rebelo H, et al. Treatment of intrabony defects with enamel matrix proteins or barrier membranes. Results from a multicentre practice‐based clinical trial. Journal of Periodontology 2004;75:726‐33. [DOI] [PubMed] [Google Scholar]
Silvestri 2000 {published and unpublished data}
- Silvestri M, Ricci G, Rasperini G, Sartori S, Cattaneo V. Comparison of treatments of infrabony defects with enamel matrix derivative, guided tissue regeneration with a nonresorbable membrane and Widman modified flap. A pilot study. Journal of Clinical Periodontology 2000;27(8):603‐10. [DOI] [PubMed] [Google Scholar]
Silvestri 2003 {published and unpublished data}
- Silvestri M, Sartori S, Rasperini G, Ricci G, Rota C, Cattaneo V. Comparison of infrabony defects treated with enamel matrix derivative versus guided tissue regeneration with a non‐resorbable membrane. Journal of Clinical Periodontology 2003;30(5):386‐93. [DOI] [PubMed] [Google Scholar]
- Silvestri M, Sartori S, Rasperini G, Ricci G, Rota C, Cattaneo V. The treatment of infrabony defects. Clinical/statistical comparison in cases treated with enamel matrix derivative (Emdogain) versus GTR procedure and Widman modified flap. Journal of Clinical Periodontoloy 2001;27 (Supplement 1 July):60. [Google Scholar]
- Silvestri M, Sartori S, Rasperini G, Ricci G, Rota C, Cattaneo V. Treatment of infrabony defects with enamel matrix derivative (EMD) or non‐resorbable membrane: a randomized controlled multicenter clinical trial. Journal of Periodontology 2002;73:1402. [Google Scholar]
Tonetti 2002 {published and unpublished data}
- Tonetti MS, Fourmousis I, Suvan J, Cortellini P, Brägger U, Lang NP. Healing, post‐operative morbidity and patient perception of outcomes following regenerative therapy of intrabony defects. Journal of Clinical Periodontology 2004;31(12):1092‐8. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Lang NP, Cortellini P, Suvan, JE, Adriaens P, Dubravec D, et al. Enamel matrix proteins in the regenerative therapy of deep intrabony defects. Journal of Clinical Periodontology 2002;29(4):317‐25. [DOI] [PubMed] [Google Scholar]
Zucchelli 2002 {published and unpublished data}
- Zucchelli G, Bernardi F, Montebugnoli L, De M. Enamel matrix proteins and guided tissue regeneration with titanium‐reinforced expanded polytetrafluoroethylene membranes in the treatment of infrabony defects: a comparative controlled clinical trial. Journal of Periodontology 2002;73(1):3‐12. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bokan 2006 {published data only}
- Bokan I, Bill JS, Schlagenhauf U. Primary flap closure combined with Emdogain alone or Emdogain and Cerasorb in the treatment of intra‐bony defects. Journal of Clinical Periodontology 2006;33:885‐93. [DOI] [PubMed] [Google Scholar]
Chambrone 2007 {published data only}
- Chambrone D, Pasin IM, Conde MC, Panutti C, Carneiro S, Lima LA. Effect of enamel matrix proteins on the treatment of intrabony defects: a split‐mouth randomized controlled trial study. Brazilian Oral Research 2007;21(3):241‐6. [DOI] [PubMed] [Google Scholar]
Doertbudak 2000 {published data only}
- Doertbudak O, Durstberger G, Bernhart T, Haas R. Treatment of periodontal defects with an enamel matrix derivative (Emdogain). Journal of Clinical Periodontology 2000;27 (Supplement 1 July):61. [Google Scholar]
Eger 1998 {published data only}
- Eger T, Muller H‐P. Periodontal regeneration in vertical bone defects with resorbable barriers and enamel‐matrix proteins [Parodontale regeneration in vertikalen knochendefecten mit resorbierbaren membranen und schmelz‐matrix‐proteinen]. Deutsche Zahnrztliche Zeitschrift 1998;53:590‐4. [Google Scholar]
Francetti 2005 {published data only (unpublished sought but not used)}
- Francetti L. A multicenter study to evaluate the clinical eligibility to periodontal treatment with enamel matrix derivative. Preliminary data. Journal of Clinical Periodontology 2000;27 (Supplement 1 July):60. [Google Scholar]
- Francetti L, Trombelli L, Lombardo G, Guida L, Cafiero C, Roccuzzo M, et al. Evaluation of efficacy of enamel matrix derivative in the treatment of intrabony defects: a 24‐month multicenter study. International Journal of Periodontics and Restorative Dentistry 2005;25:461‐73. [PubMed] [Google Scholar]
Froum 2001 {published data only (unpublished sought but not used)}
- Froum SJ, Weinberg MA, Rosenberg E, Tarnow D. A comparative study utilizing open flap debridement with and without enamel matrix derivative in the treatment of periodontal intrabony defects: a 12‐month re‐entry study. Journal of Periodontology 2001;72(1):25‐34. [DOI] [PubMed] [Google Scholar]
Ghaffar 2001 {published data only}
- Ghaffar KA, Hosny MM, Garrett S. Enamel matrix proteins and bioresorbable membranes in the treatment of early onset periodontitis. Journal of Dental Education 2001;80 (January 2001 Special Issue AADR Abstracts):82. [Google Scholar]
Hagenaars 2004 {published and unpublished data}
- Hagenaars S, Louwerse PH, Timmerman MF, Velden U, Weijden GA. Soft‐tissue wound healing following periodontal surgery and Emdogain application. Journal of Clinical Periodontology 2004;31(10):850‐6. [DOI] [PubMed] [Google Scholar]
Lombardo 2000 {published data only}
- Lombardo G, Bernini R, Urbani G. Treatment of intrabony periodontal defects using enamel matrix derivative (EMDOGAIN). Journal of Clinical Periodontology 2000;27 (Supplement 1 July):61. [Google Scholar]
Martinez 2001 {published data only}
- Martinez GA, Rodriguez F, Sanz M. Efficacy of enamel matrix proteins derivate (EMDOGAIN) in intra‐osseous defects. Journal of Dental Research 2001;80:1213. [Google Scholar]
Martu 2000a {published data only}
- Martu S, Burlui V, Mocanu C, Forna N. Periodontal regeneration with enamel derivate proteins (Emdogain) ‐ clinical evaluation. Revista Medico‐Chirurgicala a Societatii de Medici si Naturalisti din Iasi 2000;104(4):147‐51. [PubMed] [Google Scholar]
Martu 2000b {published data only}
- Martu S, Burlui V, Forna N, Mocanu C. Preliminary account on the use of enamel matrix derivate (Emdogain) in intra‐osseous defects. Journal of Clinical Periodontology 2000;27 (Supplement 1 July):61. [Google Scholar]
Minabe 2002 {published data only}
- Minabe M, Kodama T, Kogou T, Takeuchi K, Fushimi H, Sugiyama T, et al. A comparative study of combined treatment with a collagen membrane and enamel matrix proteins for the regeneration of intraosseous defects. The International Journal of Periodontics and Restorative Dentistry 2002;22(6):595‐605. [PubMed] [Google Scholar]
Mombelli 2005 {published data only}
- Mombelli A, Brochut P, Plagnat D, Casagni F, Giannopoulou C. Enamel matrix proteins and systemic antibiotics as adjuncts to non‐surgical periodontal treatment: clinical effects. Journal of Clinical Periodontology 2005;32(3):225‐30. [DOI] [PubMed] [Google Scholar]
Ozcelik 2007 {published data only (unpublished sought but not used)}
- Ozcelik O, Haytac MC, Seydaoglu G. Immediate post‐operative effects of different periodontal treatment modalities on oral health‐related quality of life: a randomized clinical trial. Journal of Clinical Periodontology 2007;34:788‐96. [DOI] [PubMed] [Google Scholar]
Parashis 2004 {published data only}
- Parashis A, Andronikaki‐Faldami A, Tsiklakis K. Clinical and radiographic comparison of three regenerative procedures in the treatment of intrabony defects. International Journal of Periodontics and Restorative Dentistry 2004;24(1):81‐90. [PubMed] [Google Scholar]
- Parashis A, Andronikaki‐Faldami A, Tsiklakis K. Comparison of three regenerative procedures in the treatment of intrabony defects: a clinical & radiographic study. Journal of Clinical Periodontology 2000;27 (Supplement 1 July):29. [Google Scholar]
Sculean 1999 {published data only}
- Sculean A, Donos N, Windisch P, Brecx M, Gera I, Reich E, et al. Healing of human intrabony defects following treatment with enamel matrix proteins or guided tissue regeneration. Journal of Periodontal Research 1999;34(6):310‐22. [DOI] [PubMed] [Google Scholar]
Sculean 2001a {published and unpublished data}
- Sculean A, Donos N, Blaes A, Lauermann M, Reich E, Brecx M. Comparison of enamel matrix proteins and bioabsorbable membranes in the treatment of intrabony periodontal defects. A split‐mouth study. Journal of Periodontology 1999;70(3):255‐62. [DOI] [PubMed] [Google Scholar]
- Sculean A, Donos N, Blaes A, Reich E, Brecx M. Enamel matrix proteins (Emdogain) and guided tissue regeneration in the treatment of intrabony periodontal defects. A split‐mouth clinical study. Journal of Dental Research 1998;77 (June Special Abstract Issue B):924. [Google Scholar]
- Sculean A, Donos N, Blaes A, Reich E, Brecx M. Enamel matrix proteins and GTR in the treatment of intrabony defects. Three year results of a split‐mouth study. Journal of Clinical Periodontology 2000;27 (Supplement 1 July):62. [Google Scholar]
- Sculean A, Donos N, Miliauskaite A, Arweiler N, Brecx M. Treatment of intrabony defects with enamel matrix proteins or bioabsorbable membranes. A 4‐year follow‐up split‐mouth study. Journal of Periodontology 2001;72(12):1695‐701. [DOI] [PubMed] [Google Scholar]
- Sculean A, Schwarz, F, Miliauskaite A, Kiss A, Arweiler N, Becker J, et al. Treatment of intrabony defects with an enamel matrix protein derivative or bioabsorbable membrane: an 8‐year follow‐up split‐mouth study. Journal of Periodontology 2006;77:1879‐86. [DOI] [PubMed] [Google Scholar]
Sculean 2001b {published and unpublished data}
- Sculean A, Donos N, Schwarz F, Becker J, Brecx M, Arweiler NB. Five‐year results following treatment of intrabony defects with enamel matrix proteins and guided tissue regeneration. Journal of Clinical Periodontology 2004;31(7):545‐9. [DOI] [PubMed] [Google Scholar]
- Sculean A, Kiss A, Miliauskaite A, Schwarz F, Arweiler NB, Hannig M. Ten‐year results following treatment of intra‐bony defects with enamel matrix proteins and guided tissue regeneration. Journal of Clinical Periodontology 2008;35:817‐24. [DOI] [PubMed] [Google Scholar]
- Sculean A, Windisch P, Blaes A, Gera I, Brecx M, Donos N. Treatment of intrabony defects with enamel matrix proteins and GTR. Journal of dental Research 2000;79 (Special Abstract Issue 1):171. [Google Scholar]
- Sculean A, Windisch P, Chiantella GC, Donos N, Brecx M, Reich E. Treatment of intrabony defects with enamel matrix proteins and guided tissue regeneration. A prospective controlled clinical study. Journal of Clinical Periodontology 2001;28(5):397‐403. [DOI] [PubMed] [Google Scholar]
Vandana 2004 {published data only}
- Vandana KL, Shah K, Prakash S. Clinical and radiographic evaluation of Emdogain as a regenerative material in the treatment of interproximal vertical defects in chronic and aggressive periodontitis patients. The International Journal of Periodontics and Restorative Dentistry 2004;24(2):185‐91. [PubMed] [Google Scholar]
Wachtel 2003 {published data only}
- Wachtel H, Schenk G, Böhm S, Weng D, Zuhr O, Hürzeler MB. Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: a controlled clinical study. Journal of Clinical Periodontology 2003;30(6):496‐504. [DOI] [PubMed] [Google Scholar]
Windisch 2002 {published data only}
- Windisch P, Sculean A, Klein F, Toth V, Gera I, Reich E, et al. Comparison of clinical, radiographic, and histometric measurements following treatment with guided tissue regeneration or enamel matrix proteins in human periodontal defects. Journal of Periodontology 2002;73(4):409‐17. [DOI] [PubMed] [Google Scholar]
Additional references
Arweiler 2002
- Arweiler NB, Auschill TM, Donos N, Sculean A. Antibacterial effect of an enamel matrix protein derivative on in vivo dental biofilm vitality. Clinical Oral Investigations 2002;6(4):205‐9. [DOI] [PubMed] [Google Scholar]
Bosshardt 2005
- Bosshardt DD, Sculean A, Windisch P, Pjetursson BE, Lang NP. Effects of enamel matrix proteins on tissue formation along the roots of human teeth. Journal of Periodontal Research 2005;40(2):158‐67. [DOI] [PubMed] [Google Scholar]
Bowers 1989a
- Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. Histologic evaluation of new attachment apparatus formation in humans. Part I. Journal of Periodontology 1989;60(12):664‐74. [DOI] [PubMed] [Google Scholar]
Bowers 1989b
- Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. Histologic evaluation of new attachment apparatus formation in humans. Part II. Journal of Periodontology 1989;60(12):675‐82. [DOI] [PubMed] [Google Scholar]
Bratthall 2001
- Bratthall G, Lindberg P, Havemose‐Poulsen A, Holmstrup P, Bay L, Soderholm G, et al. Comparison of ready‐to‐use Emdogain‐gel and Emdogain in patients with chronic adult periodontitis. A multicenter clinical trial. Journal of Clinical Periodontology 2001;28(10):923‐9. [DOI] [PubMed] [Google Scholar]
Brookes 1995
- Brookes SJ, Robinson C, Kirkham J, Bonass WA. Biochemistry and molecular biology of amelogenin proteins of developing dental enamel. Archives of Oral Biology 1995;40(1):1‐14. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Filippi 2001
- Filippi A, Pohl Y, Arx T. Treatment of replacement resorption with Emdogain. Preliminary results after 10 months. Dental Traumatology 2001;17(3):134‐8. [DOI] [PubMed] [Google Scholar]
Filippi 2002
- Filippi A, Pohl Y, Arx T. Treatment of resorption with Emdogain. A prospective clinical study. Dental Traumatology 2002;18(3):138‐43. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Froum 2004
- Froum S, Weinberg M, Novak J, Mailhot J, Mellonig J, Dyke T, et al. A multicenter study evaluating the sensitization potential of enamel matrix derivative after treatment of two infrabony defects. Journal of Periodontology 2004;75(7):1001‐8. [DOI] [PubMed] [Google Scholar]
Giannobile 2003
- Giannobile WV, Somerman MJ. Growth and amelogenin‐like factors in periodontal wound healing. A systematic review. Annals of Periodontology 2003;8(1):193‐204. [DOI] [PubMed] [Google Scholar]
Hammarström 1997a
- Hammarström L. Enamel matrix, cementum development and regeneration. Journal of Clinical Periodontology 1997;24(9 Pt 2):658‐68. [DOI] [PubMed] [Google Scholar]
Hammarström 1997b
- Hammarström L, Heijl L, Gestrelius S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. Journal of Clinical Periodontology 1997;24(9 Pt 2):669‐77. [DOI] [PubMed] [Google Scholar]
Heard 2000
- Heard RH, Mellonig JT, Brunsvold MA, Lasho DJ, Meffert RM, Cochran DL. Clinical evaluation of wound healing following multiple exposures to enamel matrix protein derivative in the treatment of intrabony periodontal defects. Journal of Periodontology 2000;71(11):1715‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from: www.cochrane‐handbook.org.
Kalpidis 2002
- Kalpidis CD, Ruben MP. Treatment of intrabony periodontal defects with enamel matrix derivative: a literature review. Journal of Periodontology 2002;73(1):1360‐76. [DOI] [PubMed] [Google Scholar]
Mariotti 2003
- Mariotti A. Efficacy of chemical root surface modifiers in the treatment of periodontal disease. Annals of Periodontology 2003;8(1):205‐26. [DOI] [PubMed] [Google Scholar]
Needleman 2006
- Needleman IG, Worthington HV, Giedrys‐Leeper E, Tucker R. Guided tissue regeneration for periodontal infra‐bony defects. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001724] [DOI] [PubMed] [Google Scholar]
Reynolds 2003
- Reynolds MA, Aichelmann‐Reidy ME, Branch‐Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Annals of Periodontology 2003;8(1):227‐65. [DOI] [PubMed] [Google Scholar]
Schjøtt 2005
- Schjøtt M, Andreasen JO. Emdogain does not prevent progressive root resorption after replantation of avulsed teeth: a clinical study. Dental Traumatology 2005;21(1):46‐50. [DOI] [PubMed] [Google Scholar]
Sculean 2001c
- Sculean A, Auschill TM, Donos N, Brecx M, Arweiler NB. Effect of an enamel matrix protein derivative (Emdogain) on ex vivo dental plaque vitality. Journal of Clinical Periodontology 2001;28(11):1074‐8. [DOI] [PubMed] [Google Scholar]
Sculean 2001d
- Sculean A, Blaes A, Arweiler N, Reich E, Donos N, Brecx M. The effect of postsurgical antibiotics on the healing of intrabony defects following treatment with enamel matrix proteins. Journal of Periodontology 2001;72(2):190‐5. [DOI] [PubMed] [Google Scholar]
Sculean 2006
- Sculean A, Berakdar M, Willershausen B, Arweiler NB, Becker J, Schwarz F. Effect of EDTA root conditioning on the healing of intrabony defects treated with an enamel matrix protein derivative. Journal of Periodontology 2006;77:1167‐72. [DOI] [PubMed] [Google Scholar]
Spahr 2002
- Spahr A, Lyngstadaas SP, Boeckh C, Andersson C, Podbielski A, Haller B. Effect of the enamel matrix derivative Emdogain on the growth of periodontal pathogens in vitro. Journal of Clinical Periodontology 2002;29(1):62‐72. [DOI] [PubMed] [Google Scholar]
St George 2006
- George G, Darbar U, Thomas G. Inflammatory external root resorption following surgical treatment for intra‐bony defects: a report of two cases involving Emdogain and a review of the literature. Journal of Clinical Periodontology 2006;33:449‐54. [DOI] [PubMed] [Google Scholar]
Trombelli 2002
- Trombelli L, Heitz‐Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. Journal of Clinical Periodontology 2002;29 Suppl 3:117‐35. [DOI] [PubMed] [Google Scholar]
Venezia 2004
- Venezia E, Goldstein M, Boyan BD, Schwartz Z. The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta‐analysis. Critical Reviews in Oral Biology and Medicine 2004;15(6):382‐402. [DOI] [PubMed] [Google Scholar]
Wennström 2002
- Wennström JL, Lindhe J. Some effects of enamel matrix proteins on wound healing in the dento‐gingival region. Journal of Clinical Periodontology 2002;29(1):9‐14. [DOI] [PubMed] [Google Scholar]
Zetterström 1997
- Zetterström O, Andersson C, Eriksson L, Fredriksson A, Friskopp J, Heden G, et al. Clinical safety of enamel matrix derivative (EMDOGAIN) in the treatment of periodontal defects. Journal of Clinical Periodontology 1997;24(9 Pt 2):697‐704. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Esposito 2003
- Esposito M, Coulthard P, Worthington HV. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003875] [DOI] [PubMed] [Google Scholar]
Esposito 2004
- Esposito M, Coulthard P, Thomsen P, Worthington HV. Enamel matrix derivative for periodontal tissue regeneration in treatment of intrabony defects: a Cochrane systematic review. Journal of Dental Education 2004;68(8):834‐44. [PubMed] [Google Scholar]
Esposito 2005
- Esposito M, Grusovin MG, Coulthard P, Worthington HV. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003875.pub2] [DOI] [PubMed] [Google Scholar]


