Abstract
Spaceflight Associated Neuro-ocular Syndrome (SANS) describes a series of morphological and functional ocular changes in astronauts first reported by Mader et al. in 2011. SANS is currently clinically defined by development of optic disc edema during prolonged exposure to the weightless (microgravity) environment which currently occurs on International Space Station (ISS). However, as improvements in our understanding of the ocular changes emerge, the definition of SANS is expected to evolve. Other ocular SANS signs that arise during and after ISS missions include hyperopic shifts, globe flattening, choroidal/retinal folds, and cotton wool spots. Over the last 10 years, approximately one in three astronauts flying long-duration ISS missions have presented with one or more of these ocular findings. Commensurate with research that combines disparate specialties (vision biology and spaceflight medicine), lessons from SANS investigations may also yield insight into ground-based ocular disorders, such as glaucomatous optic neuropathy that may have the potential to lessen the burden of this irreversible cause of vision loss on Earth.
Spaceflight Associated Neuro-ocular Syndrome (SANS) describes a series of morphological and functional ocular changes in astronauts first reported by Mader et al. in 20111. This was previously referred to as Visual Impairment and Intracranial Pressure (VIIP) syndrome because the presentation of available data was similar to terrestrial intracranial hypertension. However, because astronauts do not have documented irreversible central visual acuity loss (hyperopic shift is correctable) and because pathologically elevated intracranial pressure (ICP) remains unclear, the National Aeronautics and Space Administration (NASA) decided that SANS was a better descriptor of the changes in astronauts. Commensurate with research that combines disparate specialties (vision biology and spaceflight medicine), lessons from SANS investigations may also yield insight into ground-based ocular disorders such as glaucomatous optic neuropathy that may have the potential to lessen the burden of this irreversible cause of vision loss on Earth.
Spaceflight-Associated Neuro-ocular Syndrome (SANS)
SANS is currently clinically defined by development of optic disc edema during prolonged exposure to the weightless (microgravity) environment which currently occurs on International Space Station (ISS) (Fig. 1). However, as improvements in our understanding of the ocular changes emerge, the definition of SANS is expected to evolve. Other ocular SANS signs that arise during and after ISS missions include hyperopic shifts, globe flattening, choroidal/retinal folds, and cotton wool spots (Fig. 1). Over the last 10 years, approximately one in three astronauts flying long-duration ISS missions have presented with one or more of these ocular findings. However, it is important to note that no irreversible functional change has been documented during long duration spaceflight (up to 6-months). Thus far visual acuity is correctable with corrective lenses during spaceflight. Visual field testing has not shown reproducible visual field defects in returning astronauts. However visual fields may not detect subtle changes present during post-flight testing, and therefore long term follow-up of astronauts is warranted2. Again, this was one of the reasons that motivated the name change from VIIP to SANS. To gain more insight, electrophysiological testing targeting the pattern electroretinogram3 and photopic negative response4 of the ganglion cell are planned as part of future NASA-funded research studies. Thus, since NASA’s has limited experience with long duration space missions, it may take some time to understand if there are any long-term functional changes to astronaut vision.
Figure 1.

A) Photo of disc edema after spaceflight. This finding defines SANS. B) Ultrasound showing optic nerve sheath dilatations and posterior globe flattening (arrows). C) Photo showing choroidal folds (black arrow) and cotton wool spot (white arrow). D) Total number of US Astronauts tested for each ocular variable (blue) and the number demonstrating the finding through 2017. Optic disc edema defined as Frisèn Scale grade ≥ 1 from postflight fundoscopy images; Choroidal folds visualized on fundoscopy images and/or optical coherence tomography B-scans when available; Globe flattening based on a subjective call from either MRI or ocular ultrasound images; Refractive error shift based on pre to postflight change in cycloplegic refraction ≥0.75 diopters; cotton wool spot observed on fundoscopy images during or after spaceflight. Images adapted from Mader et al., 2011 and 20131,70.
Multiple mechanisms have been hypothesized for causing SANS, including increased ICP, cephalad fluid shifts, radiation exposure retinopathy, and hypercapnia. Among these, increased ICP is a well-known cause of disc edema on Earth, but there have been no direct measures of ICP during spaceflight, and preflight comparator lumbar puncture ICP measures have not been conducted5. A handful of lumbar punctures have been performed during the postflight period when astronauts were re-adapting to a gravitational environment.1 These were on the higher end of normal or only moderately elevated (22, 21, 28.5, and 28 cm H2O). Consequently, interpretation of these data are challenging. Future studies plan to directly measure lumbar puncture ICP before and after spaceflight and to determine if a relationship exists with the manifestation of ocular changes in-flight (https://taskbook.nasaprs.com). Thus, among the other hypotheses, the focus of several research investigations thus far has been on the fluid shift hypothesis.
Fluid Shift Hypothesis and Intraocular Pressure (IOP)
Lack of gravity causes fluids shifts. On Earth, body fluids are exposed to gravity and thus the upright posture has associated hydrostatic pressure from head to toe, with higher pressures at the lower extremities during upright posture, as compared to the level of the eye. In space and during weightlessness, the removal of the gravitational gradient leads to a headward shift of fluids, resulting in altered local tissue fluid pressures (Fig. 2)6. Given that astronauts are unable to “stand up” during spaceflight, the headward fluid shift creates a constant and chronic stimulus that has been hypothesized to be a primary contributing factor to the development of optic disc edema. Thornton used a stocking plethysmograph to document approximately two liters of fluid movement from the legs to the thorax during spaceflight7. Upon entering weightlessness astronauts reported symptoms of “Space Adaptation Syndrome,” including facial edema, headaches, and dizziness8. Furthermore, lack of a gravitational gradient not only shifts fluid toward the head, but also decreases fluid volume in the limbs7. On Earth, this concept is consistent with limb elevation above heart level as a method to reduce limb edema for various medical conditions9. Ultrasound images of the internal jugular vein of astronauts during short-duration spaceflight demonstrated a 40% increase in cross sectional area relative to the seated posture on Earth10. Similarly, during long-duration ISS missions, internal jugular vein volume increased by as much 200% compared to preflight supine values11. Taken together, these results demonstrate a robust spaceflight induced cephalad fluid shift, but what role this plays in the pathophysiology of SANS remains an active part of multiple investigations.
Figure 2.
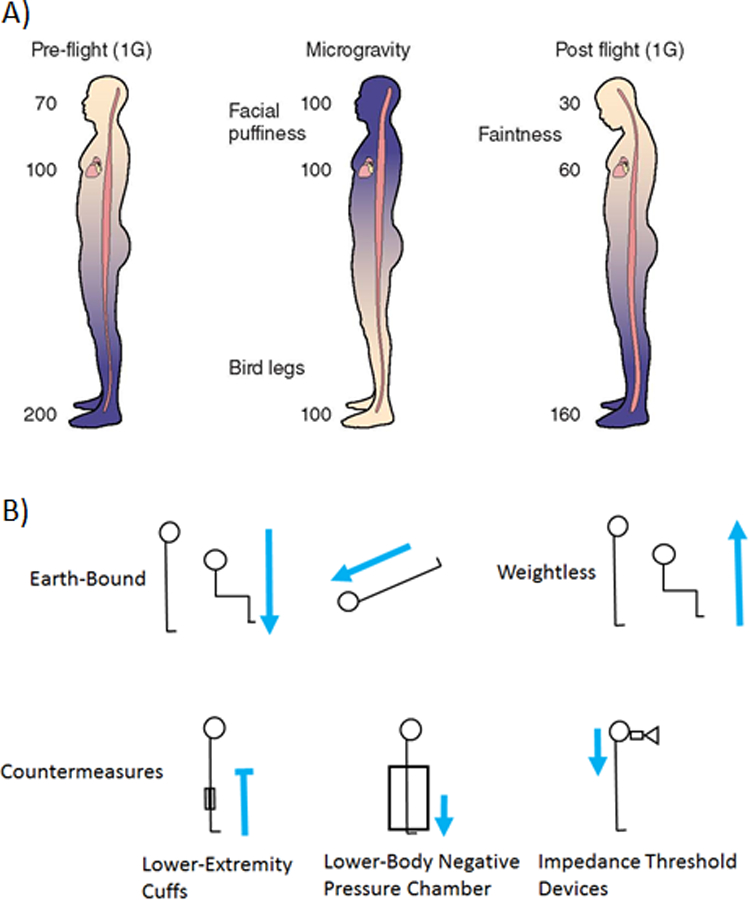
A) Preflight on Earth (1G) arterial blood pressure at the head are approximately 70 mmHg, however the pressure at the feet is 200 mmHg. During microgravity it is hypothesized that arterial blood pressure from head to foot are similar because of the absence of the hydrostatic pressure gradient. Post flight (1G), without the use of countermeasures blood volume is reduced from preflight levels resulting in reduced blood pressure at head level (figure adapted from Zhang and Hargens 2017)71. B) On Earth, gravity directs body fluid towards the lower extremities in upright positions but toward the head in head-down-tilt position. During weightlessness, in the microgravity environment of space, cephalad fluid shift is seen even in upright positions. Proposed countermeasures theoretically combat this. Lower-extremities cuffs block cephalad fluid flow under weightlessness (blue line with blunted head). Lower-body negative pressure chambers draw fluid to the lower-extremities. Impedance threshold devices draw fluid in the head and into the thoracic cavity. Blue arrows denote overall body fluid flow.
Theoretically, the cephalad fluid shift that develops in weightlessness from microgravity should cause IOP to increase as a result of increased episcleral venous pressure as dictated by the Goldman equation (see below; Relevance to Earth-Bound Eye Disease). Acute weightlessness induced for ~20–30 seconds of parabolic flight does cause ~7 mmHg increase in IOP12. Similarly, after 25 minutes of weightlessness during the 8-day manned German Spacelab Mission, IOP increased by ~ 5 mmHg ,13 which was similar to the 4–7 mmHg increase observed during the first day on 6 Space Shuttle missions5. However, IOP then normalized after the first 4 days of spaceflight to values observed before flight in the seated posture5. During the German-Russian MIR 10-day Spacelab D2 mission, IOP measurements were performed 15 minutes after launch and also demonstrated that IOP increased from baseline values of 10 mmHg (OD) and 10 mmHg (OS), to 23 mmHg (OD) and 22 (OS) within the first 24 hours of the mission14. Again, IOP values returned to preflight baseline values by the 4th day of spaceflight14. IOP measured during long-duration spaceflight suggests it remains similar to preflight values throughout the duration of the mission5. After return from long-duration spaceflight, IOP values were similar to preflight measures (10–14 mmHg versus 10–16 mmHg, respectively)1. Thus, currently published data suggest that there is an immediate increase in IOP upon entering weightlessness, but that it normalizes to ground-based levels after a few days of flight. Chronically elevated IOP is not observed in astronauts during long-duration ISS missions. However, this normalization of IOP during spaceflight appears to occur despite a sustained cephalad venous fluid shift. These data suggest that a compensatory mechanism normalizes IOP, but this process is not yet fully understood. Moreover, because spaceflight induced IOP changes appear to be on the order of approximately 5 mmHg, future systematic investigation of IOP on ISS should employ a IOP device that has high sensitivity.
On Earth the effect of an acute cephalad fluid-shift on IOP is well-documented15–20, reproducible, and investigations regarding IOP in SANS research may provide insights into Earth-bound diseases. Indeed, inducing a cephalad fluid shift on Earth by assuming a supine or head-down tilt (HDT; ~6 – 15 degrees) body position shifts fluids towards the head as the head moves below the hydrostatic indifference point, causing an increase in IOP. It is believed that this acute posture change causes an increase in episcleral venous pressure (EVP), and this has been measured in the supine position21. The magnitude of IOP increase can be remarkable (~40 mmHg in vertical head-down suspension)22 with associated diminished retinal electrophysiological responses23,22. Thus, IOP change during supine sleep is likely explained in-part by alteration in body position,16 and spaceflight data suggests that if this increase were sustained the normal healthy response would be to normalize IOP. Understanding this normalization mechanism may prove useful for better treating glaucoma patients with chronically elevated IOP. Understanding the regulation of IOP during posture changes and in healthy astronauts during prolonged weightlessness may provide new insight into the long-debated role of body position IOP elevation in glaucoma.
SANS Studies using Head-Down Tilt (Chronic and Acute)
On Earth, HDT (chronic and acute) has been used to study fluids shifts and to replicate, study, and develop countermeasures against many of the physiologic changes that occur in weightlessness24. However, until recently, SANS findings have not been observed using this spaceflight analog.25 Previous bed rest studies allowed subjects to lift their head and upper torso during meals, and prop their head up using a pillow throughout the day and night.25–27 This may have led to differences in the headward fluid shift compared to that which occurs during weightlessness. Further, since the environment failed to fully replicate the mild elevation in CO2 levels that exist on ISS, a recent bed rest study was designed to limit lifting the head and to include a mild CO2 environment. For the first time, use of strict HDT bed rest for 30 days in a mild hypercapnic environment (3.8 mmHg ambient PCO2) induced Frisèn grade28 1 or 2 optic disc edema in 5 of 11 healthy test subjects.29 OCT images were used to quantify an average increase in total retinal thickness of 39 microns around the nerve after 30 days of HDT bed rest29. This was a ~4–5 times greater change than what has been observed after 70-day HDT bed rest with a standard pillow in a normal ambient environment25. Six-months after completion of the trial, Frisèn grade optic disc edema resolved in all cases (personal communication, Steven Laurie). Thus, this study was critically important because it created the first model of “reversible” human optic disc edema that could be used to study SANS and develop potential countermeasures for use during spaceflight. The incidence of optic disc edema during spaceflight is 16% and frequently presents with folds; however, during bedrest 50% of the subjects presented with optic disc edema but none of the subjects developed folds (personal communication, Steven Laurie). Therefore, it remains to be determined if the mechanism underlying optic disc edema during bed rest is exactly the same as that which occurs during spaceflight. Future research remains necessary to understand multiple outstanding questions, including intravenous retinal angiography to look for disc leakage, optical coherence tomography angiography (OCTA) to investigate microvascular alternations, and electrophysiological assessment of ganglion cells to determine if functional changes develop concurrent with the observed structural changes.
Since SANS-like changes have only begun to be observed in chronic HDT, acute (short-term) HDT has been useful to evaluate various SANS countermeasures. One countermeasure is lower body negative pressure (LBNP) which is a technique that applies a vacuum to the lower-extremities to shift fluid away from the central circulation17. While effective at translocating fluid, LBNP tethers the crew to a single location in a spacecraft and requires continuous cardiovascular medical monitoring to ensure syncope does not develop, making this countermeasure operationally challenging to implement.
Venoconstrictive thigh cuffs (VTC) are another countermeasure that applies pressure over the femoral vein to trap venous blood in the legs as a means of shifting fluid out of the upper body (Fig. 3). VTCs have been used by Russian Cosmonauts to combat Space Adaptation Syndrome and reduce symptoms of “head congestion”30. While these alone may not be as successful at moving as much fluid as LBNP, they do allow crew to move freely about the spacecraft while completing other activities for extended periods of time and do not require continuous cardiovascular monitoring, making them more operationally desirable.
Figure 3.
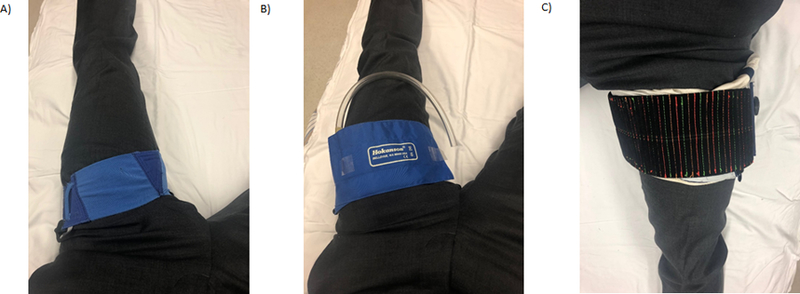
A) Photo of Russian Bralset venoconstrictive thigh cuff device. B) Photo of pneumatic venoconstrictive thigh cuff (pVTC) device. C) Photo of mobile venoconstrictive thigh cuff (mVTC) device with modified width and variable pressure adjustment mechanism.
At an average systemic blood pressure of 120/80 mmHg, mean arterial pressure (MAP) is approximately ~90 mmHg. Thus, with single leg VTCs compressing at 50–60 mmHg, there is not enough pressure to block arterial blood flow into the lower extremities, but there is enough pressure to compress venous beds to diminish venous return to the upper body. In support of these approaches, the “Medical Flight” 1994–1995 experiments on the Mir space-station demonstrated that use of VTCs reduced jugular vein cross-section area by 12–20%31, 32. Use of VTCs in ground-based studies have demonstrated similar results of decreased internal jugular vein (IJV) diameter as well effects on the femoral vein33.
Currently, two additional types of VTCs are under investigation. A pneumatic VTC (pVTC) uses an electrically driven pump to inflate a bladder to set the pressure that is automatically maintained (Fig. 3). The advantage is the ability to deliver precise and constant pressure, and the disadvantage is an electrical requirement tethering astronauts to a power source. Mobile VTCs (mVTC) also exist and compress the upper thigh using micro- and macro-manipulators which tighten and lock the cuff (Fig. 3). Theoretically, this should provide even greater mobility, however a disadvantage is that it currently lacks automatic feedback mechanisms to maintain a steady compression.
Because the goal of VTCs or LBNP is to translocate fluid away from the head through the venous system and thereby reduce venous pressure at the level of the eye, the effectiveness of these countermeasures can be quantified by studying alterations to the eye that come from acute HDT. The application of LBNP during HDT has been shown to significantly lower IOP by ~2 mmHg,17 and we have shown the ability of both VTCs to reverse the increased IOP and subfoveal choroid thickmess that develops during HDT.18 Preliminarily, OCTA has also documented foveal avascular zone shrinkage in HDT position that was partially reversed with VTCs34. Therefore, ocular endpoints like IOP exist for body position alteration-induced acute fluid shifts, and they have been useful to assess potential countermeasures on Earth. However, it is important to state again that acute HDT differs from the presumably mild chronic cephalad fluid shift experienced by astronauts on the ISS. Thus, the real purpose of these studies was to identify candidate countermeasures on Earth that could effectively reverse a headward fluid shift at the level of the eye before future studies are conducted during spaceflight.
Relevance to Eye Disease on Earth
While these studies are illuminating for understanding and preventing SANS, they also offer potential insight into Earth-bound diseases. For example, the observation that VTCs partially reversed acute HDT-induced IOP elevation18 may have relevance for glaucoma by raising the complex relationship between ICP, IOP, ocular blood flow, and body position.
There may be a role for ICP in the pathophysiology of SANS and glaucoma, however the narrative is complex. Again, initially the signs and symptoms of SANS suggested that ICP may be the initiating factor. However, with ground based and ISS research studies complete or near completion, well controlled and quantitative data suggest that elevated ICP may not be significant or at most just one of the potential pathophysiological mechanism. This encouraged the name change from VIIP to SANS35. In glaucoma, the interplay between IOP and ICP has also gained recent attention due to both retrospective36 and prospective 37 observations that glaucoma patients have decreased ICP36. Thus, decreased ICP (or increased IOP) to cause an increased translaminar pressure gradient has been postulated as an additional risk factor in glaucoma. Of course the difference between glaucoma and SANS is the opposite speculated direction for ICP change. However, if research in either field can yield improved and potentially non-invasive ways to measure ICP or translaminar pressure gradient, this becomes an example of how investigation in one discipline could help the other.
Considered alone, many pivotal studies have shown that elevated mean IOP is a very important risk factor for glaucomatous optic nerve progression38, 39, 40, 41, 42. However, the mechanism behind IOP elevation damaging the nerve is not fully understood. Mechanical mechanisms have been proposed related to the pressure that is exerted on the nerve in addition to the support (or lack thereof) of nerve fibers traversing the lamina cribosa43, 44. Additionally, blood flow theories hold that elevated IOP diminishes the ocular perfusion pressure (OPP) gradient45, 46. OPP is the difference between the arterial pressure entering the eye and the IOP that pushes back against this. With no method to directly measure OPP, it is assessed during clinical research as the difference between adjusted mean arterial pressure (MAP) and IOP (OPP = adjusted MAP- IOP) (Table 1). Adjusted MAP is obtained from blood pressure taken at the upper arm followed by mathematical correction to try to account for the distance between the arm and eye (see below). Thus, one hypothesis is that decreased OPP may cause glaucomatous optic nerve progression due either to high IOP or low arterial blood flow entering the eye.
Table 1.
BP = blood pressure and IOP = intraocular pressure. Adapted from Liu et al., 2003 J. Ocu. Pharm. Ther72 and Zhang and Hargens, 2017 Physiol Rev71.
| Parameter and Body Position | Equation |
|---|---|
| Mean Arterial Pressure (MAP) | Diastolic BP + (Systolic BP – Diastolic BP)/3 |
| Adjusted MAP for Arterial Pressure at Eye (Seated) | (0.66 X MAP) |
| Adjusted MAP for Arterial Pressure at Eye (Supine) | (0.88 X MAP) |
| Adjusted MAP for Arterial Pressure at Eye (Head-Down Tilt) | MAP + 7 |
The true importance of OPP is unclear, but the concept is tantalizing because of its constituent components. The risk factor of IOP in glaucoma is well-known. Low blood-pressure has also been associated with glaucomatous optic neuropathy47, 48, 45. However, attempts to directly link calculated OPP to glaucoma have been very difficult. First, adjusted MAP is obtained from routine systemic blood-pressure measurements at the arm. As the eye is greater than 1 foot away from the arm, equations have been devised to estimate the arterial pressure entering the eye (adjusted MAP) (Table 1). These equations are nuanced by body position because in a vertical position the eye is above the arm, and when supine the arm and eye are at equal levels.
With all of this in mind, the weaknesses of this overall OPP approach are significant. Imagine for a moment this scenario. A theoretical eye drop medication entered the eye, traversed the vitreous, and actually altered the retinal or optic nerve vasculature to bring additional blood flow into the eye. The drug improved arterial perfusion and thus improved OPP without directly altering IOP. In this case, there is only one way to experimentally observe this finding using above methods. The eye drop has to increase systemic blood pressure measured at the arm. This is obviously nonsensical, difficult to achieve, and not the actual goal49. Therefore, improving OPP through the arterial component of the equation is hard because the proper tools don’t yet exist to demonstrate it at the level of the eye. In the future, new vasculature assessment methods such as OCTA may be useful50, 51, 52.
Improving OPP by altering IOP is more straight-forward. It can be argued that this is exactly what is done every day in glaucoma clinics through the use of eye drops (prostaglandins and aqueous suppressants) to lower IOP. This is where HDT studies offer additional insights18. As discussed above, acute HDT led to increased IOP which could be considered deleterious. This risk is unclear though because HDT also increases passive arterial blood flow into the eye based on gravity (see above adjusted MAP equations). Therefore, gravity’s simultaneous elevation of IOP and ocular arterial blood flow could theoretically negate each other and result in minimal to no net alteration to OPP. This is also why it is unclear if solely taking a supine position at night is necessarily harmful in glaucoma despite increased IOP. However, VTCs (the SANS countermeasure discussed above) lowered IOP irrespective of body position (HDT-VTC IOP < HDT IOP) effectively de-coupling IOP (and EVP) from blood flow despite body position change.
It is important to appreciate that VTCs in SANS countermeasures research were not designed to treat glaucoma. For many patients on Earth, they are not only Earth-bound but literally bed-bound due to the inability to regulate total body volume as a result of diseases such as cardiomyopathy. Regardless of cause (dilatory, hypertrophic, or restrictive)53, lack of cardiac forward flow can lead to pulmonary and lower extremity edema. In such cases, passive limb elevation or simple compressive stockings are used to facilitate venous return to the central circulation. This is opposite for what VTCs are intended for. Further, by slowing down venous blood flow the theoretical risk of lower-extremity thrombus formation exists. Thus, the goal should not be to give every glaucoma patient VTCs but instead to understand the actual mechanisms through how VTCs lower IOP. Here it appears that VTCs work by modulating EVP, and now new innovative ways can be considered to achieve the same end-point.
The import of EVP in IOP regulation is gaining traction. IOP is determined by a balance between aqueous humor production (flow into the eye) and aqueous humor outflow (AHO) via the conventional and unconventional pathways. This process is best modeled by the modified Goldman equation54 where IOP = (Fin - Fout)(R) + EVP (IOP = intraocular pressure [mm Hg]; Fin = aqueous production [μl/min]; Fout = unconventional outflow [μl/min]; R = conventional outflow resistance [mm Hg*min/μl]; and EVP = episcleral venous pressure [mm Hg]). Using aqueous angiography, real-time AHO has been visualized and noted to be segmental around the limbus in multiple species including humans in both laboratory eyes and living subjects55, 56, 57, 58, 59, 60. Aqueous angiography has demonstrated the novel finding of dynamic AHO where regions with and without baseline episcleral vein AHO can actually gain or lose flow (Fig. 4)61, 62. This means that there may be local regulation of AHO at episcleral veins which in part reflects the growing appreciation that the distal AHO pathways (including episcleral veins) play a role in IOP and possibly glaucoma. Moreover, future technology advances may enable the measurement of EVP in various body postures.
Figure 4.
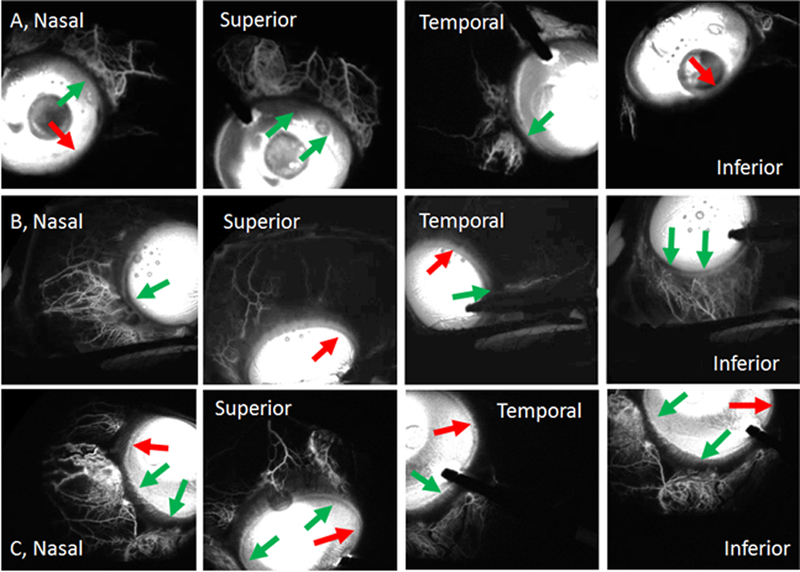
Episcleral Veins. Aqueous angiography introduces fluorescent tracers into the eye followed by angiographic imaging in live human subjects, here undergoing routine clinically-indicated cataract surgery. Rows (A-C) show episcleral venous patterns from three eyes from three live patients. Each row is one subject. Segmental patterns are seen showing regions with (green arrows) and without (red arrows) angiographic signal. It is these episcleral veins which may be impacted by body position to alter IOP or be a target for IOP lowering using cytoskeletal relaxing agents. Figure reproduced from (Huang et al., 2018 JOG) with permission from author59.
Today, new FDA-approved treatments exist that may already work by lowering EVP. Cytoskeletal relaxing agents are a new drug class including rho-kinase (ROCK) inhibitors and nitric-oxide (NO) donors63, 64. Initially, laboratory evaluation demonstrated that cytoskeletal relaxing agents lowered AHO resistance65, 66. Focused on the trabecular meshwork (TM), these drugs mechanistically impacted TM cytoskeleton, contractility, and extracellular matrix to promote easier AHO. However, long-studied in other systems, NO-derivatives are also well known to vasodilate and are first-line treatments for acute coronary syndrome and vasospastic angina67. Decades-old animal studies have shown that NO-derivatives lower EVP in some species as an TM-independent mechanism to lower IOP68. During ROCK inhibitor clinical trials, up to 50% of patients demonstrated ocular surface vessel dilation64. Recent aqueous humor dynamic measurements under ROCK inhibitor treatment also showed statistically significant EVP reduction in humans69. Therefore, while undoubtedly impacting the TM, the suggestion has also been raised as to whether cytoskeletal relaxing agent also lower IOP through mechanisms outside or past the TM at aqueous and episcleral veins. It is here that SANS research supports these concepts because fluid-shift altering VTCs lowered HDT-induced IOP elevation. Now, future innovation could be directed toward attempting to lower IOP by re-distributing fluid balance in and around the eye.
Conclusion
Human exploration, for example during the early sea voyages, identified the previously unknown nutritional requirement of vitamin C to prevent scurvy. Here, long duration spaceflight on the ISS has revealed SANS, a unique syndrome without an Earth-based correlate and therefore may offer new insights into the fields of vision science and ocular biology. Potentially involving several ocular anatomical compartments acutely or chronically (for example, the optic nerve, IOP, retina, blood flow, and refraction) a multi-disciplinary approach is required to understand and mitigate SANS. Therefore, SANS represents a major astronaut safety hurdle for exploration-class space missions and further research will be needed to understand the pathophysiology, identify additional risk-factors, create preventative countermeasures, and possibly develop treatments. NASA has funded several investigations that are ongoing or being implemented that employ the chronic HDT model and one-year spaceflight mission on the ISS. In addition, NASA has funded several ground and flight rodent experiments to obtain data that are difficult or impossible to obtain in human research studies. At this point, what is clear is that SANS is a unique disease entity and not the same as similar Earth-based optic neuropathies such as idiopathic intracranial hypertension. Further research into SANS has the potential to yield new insights into Earth-based diseases such as glaucoma and various optic neuropathies.
Acknowledgements
Funding for this work came from NASA Human Research Program #NNJ11ZSA002NA [MBS and BRM]; NASA Ocular Health Study [MBS and BRM]; 80JSC017N0001-BPBA [BRM]; National Institutes of Health, Bethesda, MD (Grant Numbers K08EY024674 [ASH]; Research to Prevent Blindness Career Development Award 2016 [ASH]; an unrestricted grant from Research to Prevent Blindness [UCLA] (New York, NY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We also thank Steven Laurie, PhD for this assistance revising this manuscript.
References
- 1.Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118(10):2058–2069. doi: 10.1016/j.ophtha.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 2.Mader TH, Gibson CR, Otto CA, et al. Persistent Asymmetric Optic Disc Swelling After Long-Duration Space Flight: Implications for Pathogenesis. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc. 2017;37(2):133–139. doi: 10.1097/WNO.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 3.Moss HE. Objective Measures of Visual Function in Papilledema. Adv Ophthalmol Optom. 2016;1(1):231–247. doi: 10.1016/j.yaoo.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karanjia R, Berezovsky A, Sacai PY, et al. The Photopic Negative Response: An Objective Measure of Retinal Ganglion Cell Function in Patients With Leber’s Hereditary Optic Neuropathy. Invest Ophthalmol Vis Sci. 2017;58(6):BIO300–BIO306. doi: 10.1167/iovs.17-21773 [DOI] [PubMed] [Google Scholar]
- 5.Stenger MB, Tarver WJ, Brunstetter T, et al. NASA Human Research Program Evidence Report: Risk of Spaceflight Associated Neuro-Ocular Syndrome (SANS). NASA Johnson Space Center; 2017. https://humanresearchroadmap.nasa.gov/evidence/reports/SANS.pdf?rnd=0.434276635495143. Accessed February 15, 2018.
- 6.Watenpaugh DE, Hargens AR. The Cardiovascular System in Microgravity. In: Handbook of Physiology. Vol 1 Envirnomental Physiolgy. Bethesda, Maryland: American Physiological Society; 1996:631–674. [Google Scholar]
- 7.Moore TP, Thornton WE. Space shuttle inflight and postflight fluid shifts measured by leg volume changes. Aviat Space Environ Med. 1987;58(9 Pt 2):A91–96. [PubMed] [Google Scholar]
- 8.Kirsch KA, Baartz FJ, Gunga HC, Röcker L. Fluid shifts into and out of superficial tissues under microgravity and terrestrial conditions. Clin Investig. 1993;71(9):687–689. [DOI] [PubMed] [Google Scholar]
- 9.Hargens AR. Fluid shifts in vascular and extravascular spaces during and after simulated weightlessness. Med Sci Sports Exerc. 1983;15(5):421–427. [PubMed] [Google Scholar]
- 10.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol. 2001;86(2):157–168. [DOI] [PubMed] [Google Scholar]
- 11.Arbeille P, Provost R, Zuj K, Vincent N. Measurements of jugular, portal, femoral, and calf vein cross-sectional area for the assessment of venous blood redistribution with long duration spaceflight (Vessel Imaging Experiment). Eur J Appl Physiol. 2015;115(10):2099–2106. doi: 10.1007/s00421-015-3189-6 [DOI] [PubMed] [Google Scholar]
- 12.Mader TH, Gibson CR, Caputo M, et al. Intraocular pressure and retinal vascular changes during transient exposure to microgravity. Am J Ophthalmol. 1993;115(3):347–350. [DOI] [PubMed] [Google Scholar]
- 13.Draeger J, Schwartz R, Groenhoff S, Stern C. Self-tonometry under microgravity conditions. Aviat Space Environ Med. 1995;66(6):568–570. [PubMed] [Google Scholar]
- 14.Draeger J, Schwartz R, Groenhoff S, Stern C. [Self tonometry during the German 1993 Spacelab D2 mission]. Ophthalmol Z Dtsch Ophthalmol Ges. 1994;91(5):697–699. [PubMed] [Google Scholar]
- 15.Weinreb RN, Cook J, Friberg TR. Effect of inverted body position on intraocular pressure. Am J Ophthalmol. 1984;98(6):784–787. [DOI] [PubMed] [Google Scholar]
- 16.Liu JHK, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44(10):4439–4442. [DOI] [PubMed] [Google Scholar]
- 17.Macias BR, Liu JHK, Grande-Gutierrez N, Hargens AR. Intraocular and intracranial pressures during head-down tilt with lower body negative pressure. Aerosp Med Hum Perform. 2015;86(1):3–7. doi: 10.3357/AMHP.4044.2015 [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanian S, Tepelus T, Stenger MB, et al. Thigh Cuffs as a Countermeasure for Ocular Changes in Simulated Weightlessness. Ophthalmology. 2018;125(3):459–460. doi: 10.1016/j.ophtha.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 19.Laurie SS, Vizzeri G, Taibbi G, et al. Effects of short-term mild hypercapnia during head-down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol Rep. 2017;5(11):e13302. doi: 10.14814/phy2.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson AP, Swan JG, Phillips SD, et al. Acute effects of changes to the gravitational vector on the eye. J Appl Physiol. 2016;120(8):939–946. doi: 10.1152/japplphysiol.00730.2015 [DOI] [PubMed] [Google Scholar]
- 21.Arora N, McLaren JW, Hodge DO, Sit AJ. Effect of Body Position on Epsicleral Venous Pressure in Healthy Subjects. Invest Ophthalmol Vis Sci. 2017;58(12):5151–5156. doi: 10.1167/iovs.17-22154 [DOI] [PubMed] [Google Scholar]
- 22.Linder BJ, Trick GL, Wolf ML. Altering body position affects intraocular pressure and visual function. Invest Ophthalmol Vis Sci. 1988;29(10):1492–1497. [PubMed] [Google Scholar]
- 23.Ferreri G, Buceti R, Ferreri FMB, Roszkowska AM. Postural modifications of the oscillatory potentials of the electroretinogram in primary open-angle glaucoma. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd. 2002;216(1):22–26. doi: 10.1159/000048292 [DOI] [PubMed] [Google Scholar]
- 24.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol. 2007;101(2):143–194. doi: 10.1007/s00421-007-0474-z [DOI] [PubMed] [Google Scholar]
- 25.Taibbi G, Cromwell RL, Zanello SB, et al. Ocular outcomes comparison between 14- and 70-day head-down-tilt bed rest. Invest Ophthalmol Vis Sci. 2016;57(2):495–501. doi: 10.1167/iovs.15-18530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taibbi G, Kaplowitz K, Cromwell RL, Godley BF, Zanello SB, Vizzeri G. Effects of 30-Day head-down bed rest on ocular structures and visual function in a healthy subject. Aviat Space Environ Med. 2013;84(2):148–154. doi: 10.3357/ASEM.3520.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taibbi G, Cromwell RL, Zanello SB, et al. Ocular outcomes evaluation in a 14-day head-down bed rest study. Aviat Space Environ Med. 2014;85(10):983–992. doi: 10.3357/ASEM.4055.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisén L Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45(1):13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurie SS, Macias BR, Dunn JT, et al. Optic Disc Edema after 30 Days of Strict Head-down Tilt Bed Rest. Ophthalmology. October 2018. doi: 10.1016/j.ophtha.2018.09.042 [DOI] [PubMed] [Google Scholar]
- 30.Gorgiladze GI, Brianov II. [Space motion sickness]. Kosm Biol Aviakosm Med. 1989;23(3):4–14. [PubMed] [Google Scholar]
- 31.Arbeille P h, Fomina G, Pottier J, et al. Hemodynamic response to LBNP during the 14 month MIR spaceflight (94–95). J Gravitational Physiol J Int Soc Gravitational Physiol. 1996;3(2):95–96. [PubMed] [Google Scholar]
- 32.Herault S, Fomina G, Alferova I, Kotovskaya A, Poliakov V, Arbeille P. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets). Eur J Appl Physiol. 2000;81(5):384–390. [DOI] [PubMed] [Google Scholar]
- 33.Arbeille P, Herault S, Fomina G, Roumy J, Alferova I, Gharib C. Influences of thigh cuffs on the cardiovascular system during 7-day head-down bed rest. J Appl Physiol. 1999;87(6):2168–2176. [DOI] [PubMed] [Google Scholar]
- 34.Huang A, Balasubramanian S, Stenger M, et al. Simulated weightlessness alters retinal foveal structure and imcrovasculature and is reversed by a venoconstrictive thigh cuff countermeasure. Assoc Res Vis Ophthalmol Conf. 2018;(Abstract). [Google Scholar]
- 35.Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595(6):2115–2127. doi: 10.1113/JP273557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008;49(12):5412–5418. doi: 10.1167/iovs.08-2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010;117(2):259–266. doi: 10.1016/j.ophtha.2009.06.058 [DOI] [PubMed] [Google Scholar]
- 38.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. [DOI] [PubMed] [Google Scholar]
- 39.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol Chic Ill 1960. 2002;120(6):701–713; discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 40.Ederer F, Gaasterland DE, Sullivan EK, AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15(4):299–325. [DOI] [PubMed] [Google Scholar]
- 41.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol Chic Ill 1960. 2002;120(10):1268–1279. [DOI] [PubMed] [Google Scholar]
- 42.Anderson DR, Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14(2):86–90. [DOI] [PubMed] [Google Scholar]
- 43.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol Chic Ill 1960. 1981;99(1):137–143. [DOI] [PubMed] [Google Scholar]
- 44.Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci Off Publ Am Acad Optom. 2008;85(6):425–435. doi: 10.1097/OPX.0b013e31817841cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neefe JR, Glass J. Abrogation of interferon-induced resistance to interferon-activated major histocompatibility complex-unrestricted killers by treatment of a melanoma cell line with 5-fluorouracil. Cancer Res. 1991;51(12):3159–3163. [PubMed] [Google Scholar]
- 46.Costa VP, Arcieri ES, Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93(10):1276–1282. doi: 10.1136/bjo.2008.149047 [DOI] [PubMed] [Google Scholar]
- 47.Okumura Y, Yuki K, Tsubota K. Low diastolic blood pressure is associated with the progression of normal-tension glaucoma. Ophthalmol J Int Ophtalmol Int J Ophthalmol Z Augenheilkd. 2012;228(1):36–41. doi: 10.1159/000335978 [DOI] [PubMed] [Google Scholar]
- 48.Charlson ME, de Moraes CG, Link A, et al. Nocturnal systemic hypotension increases the risk of glaucoma progression. Ophthalmology. 2014;121(10):2004–2012. doi: 10.1016/j.ophtha.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khawaja AP, Crabb DP, Jansonius NM. The role of ocular perfusion pressure in glaucoma cannot be studied with multivariable regression analysis applied to surrogates. Invest Ophthalmol Vis Sci. 2013;54(7):4619–4620. doi: 10.1167/iovs.13-12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akagi T, Uji A, Huang AS, et al. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am J Ophthalmol. 2018;196:1–9. doi: 10.1016/j.ajo.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akil H, Chopra V, Al-Sheikh M, et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br J Ophthalmol. August 2017. doi: 10.1136/bjophthalmol-2016-309816 [DOI] [PubMed] [Google Scholar]
- 52.Akil H, Huang AS, Francis BA, Sadda SR, Chopra V. Retinal vessel density from optical coherence tomography angiography to differentiate early glaucoma, pre-perimetric glaucoma and normal eyes. PloS One. 2017;12(2):e0170476. doi: 10.1371/journal.pone.0170476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wexler RK, Elton T, Pleister A, Feldman D. Cardiomyopathy: an overview. Am Fam Physician. 2009;79(9):778–784. [PMC free article] [PubMed] [Google Scholar]
- 54.Alm A, Nilsson SFE. Uveoscleral outflow--a review. Exp Eye Res. 2009;88(4):760–768. doi: 10.1016/j.exer.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 55.Saraswathy S, Tan JCH, Yu F, et al. Aqueous Angiography: Real-Time and Physiologic Aqueous Humor Outflow Imaging. PloS One. 2016;11(1):e0147176. doi: 10.1371/journal.pone.0147176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang AS, Saraswathy S, Dastiridou A, et al. Aqueous Angiography-Mediated Guidance of Trabecular Bypass Improves Angiographic Outflow in Human Enucleated Eyes. Invest Ophthalmol Vis Sci. 2016;57(11):4558–4565. doi: 10.1167/iovs.16-19644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang AS, Mohindroo C, Weinreb RN. Aqueous Humor Outflow Structure and Function Imaging At the Bench and Bedside: A Review. J Clin Exp Ophthalmol. 2016;7(4). doi: 10.4172/2155-9570.1000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang AS, Saraswathy S, Dastiridou A, et al. Aqueous Angiography with Fluorescein and Indocyanine Green in Bovine Eyes. Transl Vis Sci Technol. 2016;5(6):5. doi: 10.1167/tvst.5.6.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang AS, Penteado RC, Saha SK, et al. Fluorescein Aqueous Angiography in Live Normal Human Eyes. J Glaucoma. 2018;27(11):957–964. doi: 10.1097/IJG.0000000000001042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang AS, Francis BA, Weinreb RN. Structural and functional imaging of aqueous humour outflow: a review. Clin Experiment Ophthalmol. 2018;46(2):158–168. doi: 10.1111/ceo.13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang AS, Li M, Yang D, Wang H, Wang N, Weinreb RN. Aqueous Angiography in Living Nonhuman Primates Shows Segmental, Pulsatile, and Dynamic Angiographic Aqueous Humor Outflow. Ophthalmology. 2017;124(6):793–803. doi: 10.1016/j.ophtha.2017.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang AS, Camp A, Xu BY, Penteado RC, Weinreb RN. Aqueous Angiography: Aqueous Humor Outflow Imaging in Live Human Subjects. Ophthalmology. 2017;124(8):1249–1251. doi: 10.1016/j.ophtha.2017.03.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinreb RN, Liebmann JM, Martin KR, Kaufman PL, Vittitow JL. Latanoprostene Bunod 0.024% in Subjects With Open-angle Glaucoma or Ocular Hypertension: Pooled Phase 3 Study Findings. J Glaucoma. 2018;27(1):7–15. doi: 10.1097/IJG.0000000000000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serle JB, Katz LJ, McLaurin E, et al. Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients With Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am J Ophthalmol. 2018;186:116–127. doi: 10.1016/j.ajo.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 65.Chang JYH, Stamer WD, Bertrand J, et al. Role of nitric oxide in murine conventional outflow physiology. Am J Physiol Cell Physiol. 2015;309(4):C205–214. doi: 10.1152/ajpcell.00347.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42(5):1029–1037. [PubMed] [Google Scholar]
- 67.Curfman GD, Heinsimer JA, Lozner EC, Fung HL. Intravenous nitroglycerin in the treatment of spontaneous angina pectoris: a prospective, randomized trial. Circulation. 1983;67(2):276–282. [DOI] [PubMed] [Google Scholar]
- 68.Nathanson JA. Nitrovasodilators as a new class of ocular hypotensive agents. J Pharmacol Exp Ther. 1992;260(3):956–965. [PubMed] [Google Scholar]
- 69.Kazemi A, McLaren JW, Kopczynski CC, Heah TG, Novack GD, Sit AJ. The Effects of Netarsudil Ophthalmic Solution on Aqueous Humor Dynamics in a Randomized Study in Humans. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther. 2018;34(5):380–386. doi: 10.1089/jop.2017.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mader TH, Gibson CR, Pass AF, et al. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol. 2013;33(3):249–255. doi: 10.1097/WNO.0b013e31829b41a6 [DOI] [PubMed] [Google Scholar]
- 71.Zhang L-F, Hargens AR. Spaceflight-Induced Intracranial Hypertension and Visual Impairment: Pathophysiology and Countermeasures. Physiol Rev. 2017;98(1):59–87. doi: 10.1152/physrev.00017.2016 [DOI] [PubMed] [Google Scholar]
- 72.Liu JHK, Gokhale PA, Loving RT, Kripke DF, Weinreb RN. Laboratory Assessment of Diurnal and Nocturnal Ocular Perfusion Pressures in Humans. J Ocul Pharmacol Ther. 2003;19(4):291–297. doi: 10.1089/108076803322279354 [DOI] [PubMed] [Google Scholar]


