Abstract
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency causes elevated androgen levels, which can lead to virilization of female external genitalia. Prenatal dexamethasone treatment has been shown to be effective in preventing virilization of external genitalia when started prior to 7–9 weeks of gestation in females with classic CAH. However, CAH cannot be diagnosed prenatally until the end of the first trimester. Treating pregnant women with a fetus at risk of developing classic CAH exposes a significant proportion of fetuses unnecessarily, because only 1 in 8 would benefit from treatment. Consequently, prenatal dexamethasone treatment has been met with much controversy due to the potential adverse outcomes when exposed to high-dose steroids in utero. Here, we review the short- and long-term outcomes for fetuses and pregnant women exposed to dexamethasone treatment, the ethical considerations that must be taken into account, and current practice recommendations.
Keywords: Congenital Adrenal Hyperplasia, Treatment, Prenatal, Ethics
Introduction
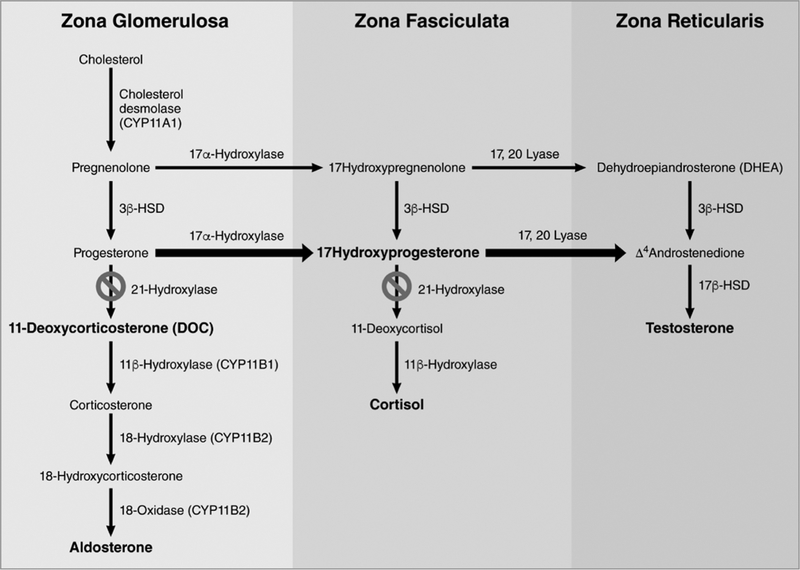
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders most commonly caused by mutations in the 21-hydroxylase gene, CYP21A2. The resulting deficiency in 21-hydroxylase production impairs cortisol synthesis with subsequent accumulation of their steroid precursors, which are shunted toward the adrenal androgen synthesis pathway (figure 1). Excess adrenal androgens cause virilization of the external genitalia of affected female fetuses during the 9th to13th weeks of gestation. Virilization includes clitoromegaly, labioscrotal fold fusion, and formation of a common urogenital sinus (figure 2). Genital ambiguity in females can lead to difficulties in sex assignment after delivery, significant parental distress, and potentially complex reconstructive genital surgery. Current recommendations are to perform clitoral and perineal reconstruction in females with severe virilization (Prader stage of ≥ 3), within 2–6 months postnatally (1). However, deferring surgery to adolescence reduces the risk of post-operative complications, including vaginal stenosis and the need for vaginal dilation. In addition, adolescent patients can become more involved and provide input in surgical decisions. Adolescents with previous genital surgery may develop long-term issues, including cosmetic appearance concerns, urinary incontinence, vaginal stenosis, sexual function, and deficient clitoral sensation.
Figure 1.
Adrenal Biosynthesis Defect in 21 hydroxylase Deficiency
Figure 2.
Process of female sexual differentiation
In addition to physical complications, excessive prenatal androgen exposure can have a masculinizing effect on behavior in females with CAH. Thus, young girls with classic CAH have shown increased aggressive behavior and activity levels during childhood (2), more masculine playmate selections, and play styles typical of boys (3). Roger Gorski’s research was influential in establishing the concept of hormone-dependent sexual differentiation of brain structures and functions (4). Studies have suggested that early androgen exposure might have an imprinting effect on neuronal pathways in females.
Since the 1980s, pregnant women at risk of carrying a fetus with CAH have been offered prenatal dexamethasone treatment to prevent potential virilization of the female fetus. Dexamethasone suppresses excessive ACTH and inhibits adrenal androgen overproduction (5). Unlike lower potency glucocorticoids, such as cortisol or prednisone, dexamethasone can cross the placenta into the fetal circulation. The current recommended dexamethasone dose for pregnant women is 20 mcg/kg/day, based on pre-pregnancy weight, with a maximum dose of 1.5 mg/day. This dose is designed to provide continuous ACTH suppression in the fetus, which prevents external virilization. However, this dose is approximately 6-fold higher than the physiologic cortisol levels typically produced in a pregnant woman; thus, fetal cortisol levels are approximately 60-fold higher than typical in utero levels (6). Moreover, to prevent virilization in a female fetus at risk for CAH, dexamethasone is most effective when it is initiated before the 7th week of gestation (5). Unfortunately, CAH cannot be diagnosed until approximately 12 weeks gestation. Thus, timely treatment would unnecessarily expose a significant proportion of unaffected fetuses to high doses of glucocorticoids. Figure 3 shows the algorithm for prenatal dexamethasone treatment for a fetus potentially affected by CAH. Because CAH is an autosomal recessive condition, the risk of having an affected fetus is 1 in 4, but only half of these fetuses will be female; therefore, treatment might potentially benefit only 1 in 8 fetuses. Dexamethasone treatment is highly controversial, given the limited data on the long-term effects on both fetuses and mothers. However, recent advances in early fetal sex determination, CAH molecular genetics, fetal adrenal physiology, and glucocorticoid treatments have necessitated re-evaluation of dexamethasone. Here, we explore the targeted outcomes for treating a fetus at risk of CAH and the medical and ethical considerations of treating both the pregnant woman and her fetus.
Figure 3.
Algorithm for Decisions Pertaining to the Prenatal Diagnosis and Treatment of 21-Hydroxylase Deficiency
Outcomes of Prenatal Dexamethasone Treatment
Virilization Outcomes
Data are limited on virilization outcomes. Forest et al. described nine affected females with CAH who were treated prenatally with dexamethasone (7). Six patients showed full or partial responses to treatment, based on no virilization or only mild virilization of external genitalia, respectively. All had two separate openings of the urethra and vagina, including patients with partial responses. However, some patients did not respond to treatment, and showed severe virilization. Those authors attributed treatment failures to late therapy initiation, inadequate dexamethasone dosing, or poor maternal tolerance causing a need to reduce dexamethasone to an insufficient dose or early discontinuation of treatment. Similar results were reported by New et al. They described outcomes for 25 female fetuses affected by CAH that were treated prenatally with dexamethasone. Eleven of those patients had female genitalia medically classified as normal, and 11 patients were significantly less virilized than those who were untreated based on Prader score (8). In addition, the Prader scores indicated that the virilization of external genitalia was less severe in 16 patients that received treatment, compared to the female siblings affected by CAH that remained untreated. The average Prader score for those treated at or before 9 weeks gestation was 0.96, compared to a score of 3.75 from those with no prenatal treatment (p<< 0.003). Successful prevention of virilization was also reported by Lajic et al., who noted that successful treatment was dependent on starting before 7 weeks of gestation, continuing the treatment to term, and maternal compliance (9). That retrospective study compared the Prader stages between 6 CAH-affected females that had received treatment and their elder, untreated siblings with CAH. In the former group, 5 patients had severe CAH; of those 4 patients showed significantly reduced virilization of the external genitalia compared to the elder siblings. In one case the female infant was more virilized at birth than her older well-treated sibling which was due to poor compliance of the mother. A 2010 meta-analysis reported a reduction in virilization as reported by Prader score in affected females treated early in pregnancy (weighted mean difference −2.33, 95% CI −3.38 to −1.27 using units of Prader score) (10).
Short Term Outcomes and Animal Studies
The safety of prenatal dexamethasone treatment for the fetus and pregnant woman has been poorly evaluated. Animal studies have shown that high dose prenatal administration of dexamethasone is anatomically and metabolically teratogenic to fetuses. Reported issues include cleft palate, impaired kidney and thyroid function, low birthweight, glucose intolerance, anxiety, and negative effects on brain development (11–18).
Some studies that evaluated short-term outcomes in newborns treated prenatally have not shown significant effects on birth weight, birth length, or head circumference (7–9). In contrast, other studies showed reductions in newborn growth parameters among infants whose mothers were treated with antenatal steroids; however, in those studies, the treatment had aimed to enhance fetal lung maturity prior to a preterm birth, rather than prevent virilization of a female potentially affected by CAH (19–21). However, the risk of stillbirths, spontaneous abortions, or congenital malformations were not significantly increased in newborns affected by CAH that received prenatal dexamethasone treatments (9,10).
In pregnant women, the short-term side effects of dexamethasone treatment include increased weight gain, edema, and striae (7–10). There has been no confirmed association with hypertension or gestational diabetes (8). Overall, maternal side effects seem to be mild, and treatment is generally well tolerated by the mother. However, Lajic et al. reported that, among all the women that received dexamethasone in their study, approximately one-third stated that they would not undergo treatment again (9).
Cognitive and Behavioral Outcomes
The long-term neurocognitive and behavioral effects of prenatal dexamethasone treatment is an area of much interest, but studies have produced mixed results. An early pilot study by Trautman et al. did not show any negative effects of dexamethasone in terms of developmental milestones or cognitive development among 26 children treated prenatally compared to untreated controls. This finding was based on an analysis of standardized questionnaires that mothers completed about their children’s emotional and physical development. However, that study found that the prenatally treated patients showed increased shyness (p <0.004), which included difficulty in making friends, reduced sociability, and a lack of friendliness or taking a long time to warm up to strangers (22). A larger study by the same group showed no significant differences in terms of cognitive, motor, or social development in 174 children that were prenatally exposed to dexamethasone compared to 313 unexposed controls (23). Those data were based on standardized developmental questionnaires mailed to mothers. A Swedish study was the first to assess long-term cognitive development, based on direct neuropsychological tests in children treated prenatally with dexamethasone. They found that patients unaffected by CAH that received short-term prenatal treatment performed worse on verbal working memory tests than controls (p=0.003) (24). On a questionnaire that assessed self-perception of scholastic competence, these same patients rated lower (p=0.003), and they showed higher self-rated social anxiety (p=0.026), compared to controls. There were no differences in other cognitive measures, including IQ, learning, and long-term memory. The same Swedish group evaluated whether prenatal exposure to dexamethasone had long-term effects on temperament and behavioral problems. They examined school-aged children affected or unaffected by CAH treated with dexamethasone, and an age-matched group of untreated controls (25). They found no significant differences were noted between 26 children exposed to dexamethasone and 35 matched controls, regarding parental ratings of behavioral problems or psychopathology. The behavior problems investigated included social withdrawal, somatic complaints, anxiety/depression, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior. Children exposed to dexamethasone were reported to be more sociable than controls based on parental questionnaires. However, when all three groups were compared (CAH-affected, CAH-unaffected, and controls), the difference was no longer significant. This result could most likely be explained by the small sample size. Although an increase in social anxiety was noted in the children’s self-ratings, this result was not confirmed by their parents. A follow-up to that study examined only children unaffected by CAH that were identified as at-risk for CAH and treated only during the first trimester. That study compared 34 patients treated with dexamethasone (16 females, 18 males, aged 7–17 years) and 66 untreated controls (36 females, 30 males). They did not find any significant differences between groups regarding parental ratings of behavioral problems, psychopathology, social anxiety, adaptive functioning, or school performance. In addition, self-reported social anxiety was not increased in patients exposed to dexamethasone, compared to controls. That finding was consistent with the findings from a previous study conducted at the same center, which included children affected and children unaffected by CAH (26). More recently, the Swedish group conducted a study that showed that healthy girls unaffected by CAH that had received prenatal dexamethasone scored lower than control girls on measures of verbal and nonverbal intelligence and verbal working memory tasks (27). The patients also performed worse than controls on visual spatial working memory. All children included in that study were evaluated by trained psychologists that administered standardized and normed neuropsychological tests. The authors concluded that prenatal dexamethasone treatment in the context of CAH was unsafe and should not be performed.
A pilot study was conducted to investigate the effects of prenatal dexamethasone exposure on gender role behavior in boys and girls. They found that boys unaffected by CAH that were treated with dexamethasone presented more gender-neutral behaviors than controls (p=0.04). They also observed that boys treated with dexamethasone tended to show less masculine behavior than controls, but the difference did not reach significance (p=0.13) (28). In girls unaffected by CAH, dexamethasone treatment did not alter gender role behavior compared to age-matched untreated controls. This same pattern was observed when both boys and girls unaffected by CAH were analyzed; however, patients affected by CAH were not analyzed separately due to the small sample size.
Metabolic and Cardiovascular Outcomes
Long-term data are lacking on metabolic and cardiovascular outcomes in children that were exposed to dexamethasone during pregnancy. These outcomes are just starting to be addressed due to the fact that the oldest children are now reaching early adulthood. A cross-sectional study evaluated the long-term effects of antenatal glucocorticoid exposure, given for fetal lung maturation, in 6 to 11-year-olds. The children participated in a public speaking task and a mental arithmetic task carried out in front of an audience, and cortisol responses were measured after completing the task. That study showed that children exposed to antenatal steroids exhibited significantly elevated cortisol reactivity after acute psychosocial stress, compared to controls (p<0.001) (29). The authors noted that elevated HPA-axis reactivity has been previously linked to the development of metabolic syndrome and depression; thus, they proposed that further longitudinal research was needed to determine whether dysregulation of the HPA axis was a causal factor in the high cortisol response to stress. Overall, the accumulated evidence has suggested that prenatal steroid treatment may adversely affect metabolic, cognitive, and behavioral functions. However, larger, controlled, prospective long-term follow-up studies are needed to elucidate this hypothesis.
Ethical Considerations
A 2012 historical review of the literature with a focus on the ethics of using prenatal dexamethasone for CAH identified several ethical issues. For example, some pharmaceutical company representatives have used misleading promotional materials to describe dexamethasone treatment to physicians and families affected by CAH, without fully disclosing the potential risks. Additionally, in some cases, de facto experimentation was performed without the necessary protections of approved research. Moreover, there are troubling parallels with the history of prenatal use of diethylstilbestrol. This synthetic estrogen was prescribed to women to prevent miscarriage with insufficient data to support this use or to document outcomes (30). Research later not only found it to be ineffective in preventing miscarriage, but that it led to a marked increase in malignancies and fertility issues in their exposed offspring (30). Finally, it is controversial whether medicine and public monies should be used for attempts to prevent benign behavioral sex variations (31). The authors of the review stated that weak and unsupported conclusions have left major gaps in the systems that are meant to protect subjects of high-risk medical research, particularly pregnant women and their fetuses.
Determining the ethical obligations in these cases can be difficult. McCullough and Chervenak developed an ethical model designed to balance beneficence- and autonomy-based obligations to pregnant woman with beneficence-based obligations to the fetus, while also considering the pregnant woman’s beneficence-based obligations to her fetus (32). In any assessment of beneficence-based obligations, evidence-based reasoning is essential.
Ethical Obligations to the Woman
The principle of beneficence obligates clinicians to endeavor to provide a greater therapeutic benefit (clinical good) than the therapeutic burden (clinical harm), determined by patient feedback (32). Autonomy is the patient’s right to determine what treatment he/she receives; it obligates clinicians to provide patients and their surrogates the information necessary for making an informed choice between therapeutically appropriate options (including the choice of no therapeutic intervention) (32).
Ethical Obligations to the Fetus
It is hoped that a pregnant woman would be willing to accept reasonable risk to her own health for the benefit of her fetus. This hope is realized when the pregnant woman presents her fetus to clinicians as a patient; in so doing, she assumes a beneficence-based obligations to her fetus (32). The fetus has no autonomy-based right to self-determination; thus, the physician has a professional obligation to provide only therapies that uphold the principle of beneficence to the fetus, when the pregnant woman presents her fetus as a patient (33).
According to the ethical paradigm presented by Chervenak and McCullough (33), a previable fetus is considered a patient when a number of conditions are met. First, the pregnant woman presents her fetus to the physician or other healthcare professional as a patient. Second, medical interventions exist that are reliably expected to result in a greater balance of clinical good over clinical harm for the child. However, the pregnant woman has the ethical and legal right to withdrawal the status of patient from her previable fetus at any time; thus, not all fetuses are patients.
Now we consider the balance of competing obligations associated with dexamethasone treatment. In light of the side-effect profile of dexamethasone, beneficence-based obligations to the pregnant woman do not support the use of prenatal dexamethasone for treating CAH. Autonomy-based obligations must be given to the pregnant woman, and treatment will vary, depending on her autonomous choice. The biopsychosocial well-being of her fetus and the burden on her own health will likely be the determining factors; therefore, the medical team must provide thorough evidence-based counseling. Beneficence-based obligations to the fetus are lacking, both on the part of the medical team and on the part of the pregnant woman, due to the high risk of neurodevelopmental consequences and the low likelihood of benefit for any particular fetus. Thus, there is no ethical obligation to provide antenatal dexamethasone for treating CAH. In fact, based on this analysis, we must conclude that the obligation is to remove antenatal dexamethasone treatment from IRB-approved protocols, unless new technologies emerge that allow earlier identification of female fetuses that are affected by CAH.
Current Practice Recommendations
All medical societies concerned with prenatal treatment of CAH, including the 2000 Technical Report of The American Academy of Pediatrics, the 2002 statement by the Lawson Wilkins Pediatric Endocrine Society, and the Endocrine Society’s 2010 Clinical Practice Guidelines for CAH, agree that treatment should only be performed with IRB-approved prospective research protocols after written informed consent is obtained from the parents (1,34,35). These recommendations are based on concerns about the efficacy and safety of this treatment. Exposing fetuses unaffected by CAH to this treatment poses significant risks, particularly in terms of long-term cognitive and behavioral problems.
Noninvasive Prenatal Diagnosis
New approaches for earlier fetal sex determination have been developed. Previously, the prenatal CAH diagnosis was based on genetic testing of fetal DNA obtained from either chorionic villus samples, collected at ~11 weeks gestation, or amniocentesis samples, collected at ~14–16 weeks gestation. These methods are invasive; they increase the risk of bleeding and infection, and to a lesser extent, the chance of miscarriage. Lo et al. found that maternal serum contains circulating cell-free fetal DNA. This finding allowed for fetal sex determination by detecting the Y chromosome present in males (SRY) (36). A French retrospective study performed in 2014 showed that the SRY test was sensitive for identifying Y-chromosome material at 4 weeks and 5 days of gestation (37). That finding led to avoiding unnecessary dexamethasone treatment in 68% of males included in the study, and this percentage decreased over time. New et al. evaluated the effectiveness of performing targeted, massive, parallel sequencing of fetal cell-free DNA isolated from maternal plasma for use as a prenatal diagnosis of CAH (38). They could provide the correct fetal genotype of 14 patients as early as 5 weeks 6 days gestation. Figure 3 describes the timeline of events of noninvasive fetal DNA testing for CAH. This noninvasive strategy represents a method for targeting treatment to only female fetuses affected with CAH. However, larger studies are needed to evaluate the sensitivity and specificity of this method. Potentially, it might be necessary to impose a moratorium on IRB-approved protocols, until this technique can be confirmed as a safe method for avoiding unwarranted treatment in fetuses affected by CAH. Ideally, we must all work in this era under protocols to ensure that we control the side effects and collect valid data. On the clinical side, it is not always feasible to follow the protocols. Hence, trained specialists must, to a certain extent, use what seems to be anachronistic methods, follow their own judgement in a case-by-case approach, and eventually collect and evaluate the outcomes.
Conclusions/Future Directions
It remains highly controversial whether prenatal dexamethasone treatment should be used in pregnant women that may be carrying a female fetus affected with CAH. Currently, this treatment can only be provided under an IRB-approved investigational study protocol. Accumulated evidence has shown that dexamethasone was effective in suppressing virilization in female fetuses affected by CAH, but it had no impact on the long-term treatment outcome of CAH postnatally, in terms of whether daily glucocorticoid and mineralocorticoid replacement was necessary. In general, maternal side effects of the treatment are tolerable, but in treated fetuses, findings have been mixed in terms of cognitive and behavioral outcomes.
Data from in vitro, animal, and clinical studies have shown that prenatal dexamethasone had substantial long-term negative effects on the individual’s metabolism, future health risks, intellectual function, and behavioral adaptation. Therefore, this treatment should only be used in the context of IRB-approved research protocols, and families must be made aware of potential risks before providing written consent. Newer approaches to prenatal diagnosis, including preimplantation genetic testing, fetal sex determination, testing maternal blood for CAH mutations in fetal DNA, may minimize the treatment of fetuses unaffected by CAH, but larger prospective studies are needed.
Acknowledgements
We appreciate working with patients who have the condition of CAH as they taught us the challenges of the disease. We also want to dedicate this paper to Dr. Maria New who was a pioneer in the area of prenatal diagnosis and treatment of CAH.
References
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC, Endocrine S. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95(9):4133–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasterski V, Hindmarsh P, Geffner M, Brook C, Brain C, Hines M. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH). Horm Behav 2007;52(3):368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and childhood sex segregation: playmate and play style preferences in girls with congenital adrenal hyperplasia. Horm Behav 2011;59(4):549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorski RA. Sexual differentiation of the brain: a model for drug-induced alterations of the reproductive system. Environ Health Perspect 1986;70:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David M, Forest MG. Prenatal treatment of congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency. J Pediatr 1984;105(5):799–803 [DOI] [PubMed] [Google Scholar]
- 6.Witchel SF, Miller WL. Prenatal treatment of congenital adrenal hyperplasia-not standard of care. J Genet Couns 2012;21(5):615–624 [DOI] [PubMed] [Google Scholar]
- 7.Forest MG, David M, Morel Y. Prenatal diagnosis and treatment of 21-hydroxylase deficiency. J Steroid Biochem Mol Biol 1993;45(1–3):75–82 [DOI] [PubMed] [Google Scholar]
- 8.New MI, Carlson A, Obeid J, Marshall I, Cabrera MS, Goseco A, Lin-Su K, Putnam AS, Wei JQ, Wilson RC. Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 2001;86(12):5651–5657 [DOI] [PubMed] [Google Scholar]
- 9.Lajic S, Wedell A, Bui TH, Ritzen EM, Holst M. Long-term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 1998;83(11):3872–3880 [DOI] [PubMed] [Google Scholar]
- 10.Merce Fernandez-Balsells M, Muthusamy K, Smushkin G, Lampropulos JF, Elamin MB, Abu Elnour NO, Elamin KB, Agrwal N, Gallegos-Orozco JF, Lane MA, Erwin PJ, Montori VM, Murad MH. Prenatal dexamethasone use for the prevention of virilization in pregnancies at risk for classical congenital adrenal hyperplasia because of 21-hydroxylase (CYP21A2) deficiency: a systematic review and meta-analyses. Clin Endocrinol (Oxf) 2010;73(4):436–444 [DOI] [PubMed] [Google Scholar]
- 11.Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res 1990;53(2):157–167 [DOI] [PubMed] [Google Scholar]
- 12.Celsi G, Kistner A, Aizman R, Eklof AC, Ceccatelli S, de Santiago A, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 1998;44(3):317–322 [DOI] [PubMed] [Google Scholar]
- 13.Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol 2007;292(1):R453–R461 [DOI] [PubMed] [Google Scholar]
- 14.de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest 2007;117(4):1058–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews SG. Antenatal glucocorticoids and the developing brain: mechanisms of action. Semin Neonatol 2001;6(4):309–317 [DOI] [PubMed] [Google Scholar]
- 16.Sloboda DM, Challis JR, Moss TJ, Newnham JP. Synthetic glucocorticoids: antenatal administration and long-term implications. Curr Pharm Des 2005;11(11):1459–1472 [DOI] [PubMed] [Google Scholar]
- 17.Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol 1999;94(2):213–218 [DOI] [PubMed] [Google Scholar]
- 18.Moss TJ, Doherty DA, Nitsos I, Sloboda DM, Harding R, Newnham JP. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol 2005;192(1):146–152 [DOI] [PubMed] [Google Scholar]
- 19.Davis EP, Waffarn F, Uy C, Hobel CJ, Glynn LM, Sandman CA. Effect of prenatal glucocorticoid treatment on size at birth among infants born at term gestation. J Perinatol 2009;29(11):731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun T, Husar A, Challis JR, Dudenhausen JW, Henrich W, Plagemann A, Sloboda DM. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta 2013;34(5):407–415 [DOI] [PubMed] [Google Scholar]
- 21.Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S, Delisle MF, Amankwah K, Guselle P, Gafni A, Lee SK, Armson BA, Group MC. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet 2008;372(9656):2143–2151 [DOI] [PubMed] [Google Scholar]
- 22.Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: results of a pilot study. Psychoneuroendocrinology 1995;20(4):439–449 [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI. Cognitive and motor development of children with and without congenital adrenal hyperplasia after early-prenatal dexamethasone. J Clin Endocrinol Metab 2004;89(2):610–614 [DOI] [PubMed] [Google Scholar]
- 24.Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Wedell A, Lajic S. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab 2007;92(2):542–548 [DOI] [PubMed] [Google Scholar]
- 25.Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Lajic S. Long-term follow-up of prenatally treated children at risk for congenital adrenal hyperplasia: does dexamethasone cause behavioural problems? Eur J Endocrinol 2008;159(3):309–316 [DOI] [PubMed] [Google Scholar]
- 26.Wallensteen L, Zimmermann M, Sandberg MT, Gezelius A, Nordenstrom A, Hirvikoski T, Lajic S. Evaluation of behavioral problems after prenatal dexamethasone treatment in Swedish adolescents at risk of CAH. Horm Behav 2016;85:5–11 [DOI] [PubMed] [Google Scholar]
- 27.Wallensteen L, Zimmermann M, Thomsen Sandberg M, Gezelius A, Nordenstrom A, Hirvikoski T, Lajic S. Sex-Dimorphic Effects of Prenatal Treatment With Dexamethasone. J Clin Endocrinol Metab 2016;101(10):3838–3846 [DOI] [PubMed] [Google Scholar]
- 28.Hirvikoski T, Lindholm T, Lajic S, Nordenstrom A. Gender role behaviour in prenatally dexamethasone-treated children at risk for congenital adrenal hyperplasia--a pilot study. Acta Paediatr 2011;100(9):e112–e119 [DOI] [PubMed] [Google Scholar]
- 29.Alexander N, Rosenlocher F, Stalder T, Linke J, Distler W, Morgner J, Kirschbaum C. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab 2012;97(10):3538–3544 [DOI] [PubMed] [Google Scholar]
- 30.Newbold R Prenatal exposure to diethylstilbestrol (DES). Fertility and Sterility 2008;89(2):e55–e56 [DOI] [PubMed] [Google Scholar]
- 31.Dreger A, Feder EK, Tamar-Mattis A. Prenatal Dexamethasone for Congenital Adrenal Hyperplasia: An Ethics Canary in the Modern Medical Mine. J Bioeth Inq 2012;9(3):277–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chervenak FA, McCullough LB. Perinatal ethics: a practical method of analysis of obligations to mother and fetus. Obstet Gynecol 1985;66(3):442–446 [PubMed] [Google Scholar]
- 33.Chervenak FA, McCullough LB. Ethical issues in recommending and offering fetal therapy. West J Med 1993;159(3):369–399 [PubMed] [Google Scholar]
- 34.Technical report: congenital adrenal hyperplasia. Section on Endocrinology and Committee on Genetics. Pediatrics 2000;106(6):1511–1518 [PubMed] [Google Scholar]
- 35.Joint LECAHWG. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 2002;87(9):4048–4053 [DOI] [PubMed] [Google Scholar]
- 36.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350(9076):485–487 [DOI] [PubMed] [Google Scholar]
- 37.Tardy-Guidollet V, Menassa R, Costa JM, David M, Bouvattier-Morel C, Baumann C, Houang M, Lorenzini F, Philip N, Odent S, Guichet A, Morel Y. New management strategy of pregnancies at risk of congenital adrenal hyperplasia using fetal sex determination in maternal serum: French cohort of 258 cases (2002–2011). J Clin Endocrinol Metab 2014;99(4):1180–1188 [DOI] [PubMed] [Google Scholar]
- 38.New MI, Tong YK, Yuen T, Jiang P, Pina C, Chan KC, Khattab A, Liao GJ, Yau M, Kim SM, Chiu RW, Sun L, Zaidi M, Lo YM. Noninvasive prenatal diagnosis of congenital adrenal hyperplasia using cell-free fetal DNA in maternal plasma. J Clin Endocrinol Metab 2014;99(6):E1022–E1030 [DOI] [PMC free article] [PubMed] [Google Scholar]