Abstract
Background:
To determine if serum pigment epithelium-derived factor (PEDF) levels predict cardiovascular events, renal dysfunction and mortality in the Veterans Affairs Diabetes Study (VADT).
Methods:
PEDF was evaluated in relation to subsequent cardiovascular outcomes, mortality, and renal dysfunction (defined as urinary albumin creatinine ratio (ACR) ≥300 mg/g), or chronic kidney disease (CKD) stages 3 (eGFR<60 ml/min) or 4 (eGFR<60 and <30 ml/min respectively). PEDF was measured by ELISA on sera from 881 participants collected a median (range) of 1.7 (0–5.0) years post-baseline, and later, from 832 participants 4.0 (1.5–6.9) years post-baseline.
Results:
In 743 participants, PEDF was measured at both time-points. PEDF increased over time from (mean±SD) 10.5±4.03 to 11.0±4.86 ng/ml (paired t-test p=0.0092). Lower eGFR (p<0.01), higher serum creatinine (p<0.01) and urinary ACR (p<0.01) were associated with increasing PEDF. Multivariate event time models included either one or two follow-up windows (i.e., between first and second PEDF measures; and, when available, from second PEDF measure until study-end). PEDF tertiles were not associated with cardiovascular events, but were significantly associated with all-cause mortality [HR=2.00 (1.03, 3.89) comparing first to third tertile] in models adjusted for age, minority status, VADT treatment arm and prior cardiovascular event status. Higher PEDF levels also associated with development of kidney dysfunction with adjusted HRs (95% CI comparing third to first PEDF tertiles: 2.74 (1.71, 4.39) for stage 3 CKD; and 3.84 (95% CI: 1.17, 12.5) for stage 4 CKD.
Conclusions:
Over 2-years, higher serum PEDF levels predicted advanced nephropathy in patients with type 2 diabetes.
Keywords: PEDF, cardiovascular, renal, cohort
INTRODUCTION
Inflammation, neovascularization, cell death, and fibrosis are common pathological features in diabetic vascular complications[1–5]; all are promoted by growth factor imbalance. For example, complications of diabetes are associated with elevated circulating levels of the anti-angiogenic factor, pigment epithelium-derived factor (PEDF)[6, 7], which is produced by many tissues, including liver, adipocytes, retina, kidneys, and vascular tissue, and is abundant in plasma and serum. PEDF is an adipokine with anti-oxidant, anti-inflammatory, anti-fibrotic[8–11], and insulin-sensitizing effects[12, 13]. Circulating levels have been associated with insulin resistance, diabetes and diabetic vascular complications. Hence, altered levels may be associated with, predict or mediate vascular damage, and PEDF could constitute a therapeutic target and / or agent[14–17].
In a prior report, using samples from a single time-point in the Veterans Affairs Diabetes Trial (VADT), we found that serum PEDF levels were positively associated with body habitus (reflected by waist-to-hip ratio and body mass index (BMI)), and with renal dysfunction (i.e. high PEDF concentrations associated with lower estimated glomerular filtration rate (eGFR) and higher serum creatinine levels)[18]. Higher serum PEDF was also associated with adverse lipid profiles (higher triglyceride, lower HDL-cholesterol levels)[18]. For the present study, serum PEDF has been measured at a second time point, a median of 2.02 years (range: 0.97 to 3.98 years) after the first time-point, and using both measures, we now assess PEDF as a predictor of incident cardiovascular outcomes, renal disease (macroalbuminuria, stages 3 and 4 chronic kidney disease (CKD)), and mortality. Moreover, because serum PEDF levels have been measured at two time-points approximately 2-years apart in most study participants, we are able to examine the stability of PEDF levels over time.
METHODS
The VADT design and population
The details of the VADT design have been reported previously[1]. Briefly, 1791 veterans with type 2 diabetes and suboptimal glucose control were randomized at 20 participating sites to receive either intensive or standard glycemic management. The goal was an absolute reduction in HbA1c of 1.5% (16.4 mmol/mol IFCC) in the intensive- vs. standard-therapy group. All other modifiable cardiovascular risk factors were treated aggressively and uniformly in both study arms, as per American Diabetes Association (ADA) guidelines for blood pressure, diet, exercise, and diabetes education at the time of study entry[2]. Unless contraindicated, all patients were prescribed aspirin, and all patients with elevated lipid levels were prescribed HMG-CoA reductase inhibitors (i.e., statins). The study was approved by the Institutional IRB at each participating site, and was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
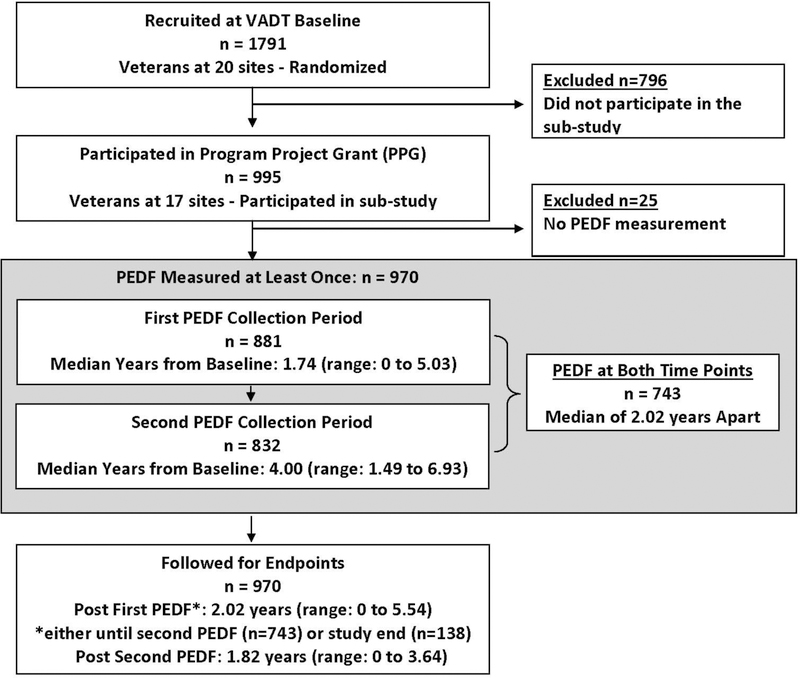
Of the 1791 VADT study participants, 995 (from 17 sites and about equally distributed between randomization groups), agreed to participate in a sub-study to investigate associations between novel biomarkers and vascular disease. The biochemical, physical, and demographic profiles of this group did not differ significantly from the 796 who did not participate in the sub-study, apart from having slightly lower age and LDL-cholesterol, slightly higher triglycerides, and a higher prevalence of aspirin use at baseline, as previously described [19]. For the current report, the study cohort comprised 970 participants, in whom PEDF was measured at either one or both time points, with 743 patients having PEDF measured twice.
Enrollment for the VADT lasted from December 2000 to May 2003. The standardized baseline VADT cohort examination included interviews, blood pressure and anthropometric measurements and fasting venipuncture[20, 21]. Due to the time required for ethics approvals (at 17 sites) for the PEDF substudy samples for PEDF measurement were not available at VADT baseline, nor were detailed demographics data available at the post-baseline visit at which blood for PEDF measurement was collected. PEDF was measured on 881 serum samples collected a median of 1.74 years (range: 0 to 5.03 years), and 832 serum samples collected a median of 4.00 years (range: 1.49 to 6.93 years), after baseline; 743 participants had PEDF measured at both time points a median of 2.02 years apart (range: 0.97 to 3.98 years). Blood was collected after an overnight fast, and serum was isolated and stored (−80ºC) until assayed. Study participants were followed until May 2008, unless they were lost to follow-up or died. Median follow-up time subsequent to the first sampling for PEDF quantification was 2.02 years (range: 0 to 5.54) and following the second, 1.82 years (range: 0 to 3.64 years). Figure 1 describes the PEDF related study design.
Figure 1.
VADT PEDF Sub-study Consort Diagram
Measurement of PEDF
Serum PEDF was quantified by ELISA (Chemicon Int., Inc., Temecula, CA) as previously described[22], with intra- and inter-assay coefficients of variation of 3.4% and 12.0% respectively. The mean of duplicate measures was used in data analyses.
Cardiovascular Endpoints
The primary endpoint for the VADT study was time to first occurrence of any one of a composite of cardiovascular events. Each event was adjudicated by an end-point committee that employed strict algorithms to define and document the event. The composite endpoint included documented myocardial infarction (MI); stroke; death from cardiovascular disease (CVD); new or worsening congestive heart failure; surgical intervention for cardiac, cerebrovascular, or peripheral vascular disease; inoperable coronary artery disease (CAD); and amputation for ischemic gangrene. Secondary outcomes included MI, CAD, death from CVD, and death from any cause. CAD included MI, coronary revascularization procedures and clinically identified inoperable CAD.
Renal Endpoints
As renal function includes two aspects, albuminuria and creatinine clearance, several renal endpoints, all beyond the stages of early renal dysfunction, were used: incident macroalbuminuria and incident stage 3 or stage 4 CKD. Urinary albumin:creatinine ratio (ACR: mg/g) was measured annually, and eGFR was calculated at least annually, and often quarterly, using the MDRD formula[23]. Macroalbuminuria was defined in two ways: as the presence of (a) one or (b) two (not necessarily consecutive) urinary ACR measures ≥300 mg/g. Time to macroalbuminuria was defined using the date of the second qualifying ACR measure. Stage 3 CKD was defined as any two eGFR measures <60 ml/min/1.73 m2, and time to stage 3 CKD was defined using the date of the second eGFR value <60 ml/min/1.73 m2. Two definitions of stage 4 CKD were used: either (a) one or (b) two estimated eGFR measures <30 ml /min/1.73 m2; time to stage 4 CKD was defined using the date of the second eGFR value <30 ml/min/1.73 m2.
Statistical analyses
Prospective analyses were performed to assess the utility of serum PEDF levels as a biomarker for incident CVD or renal dysfunction (as defined above) or (all-cause) mortality. For summary presentation, clinical and demographic characteristics of the cohort were stratified by tertile of PEDF at first measurement for that individual, be it during the first or second collection period. Differences across PEDF tertiles were tested using chi-square for categorical characteristics and F test for continuous variables. Linear regression was used to examine the relationship between individual clinical and demographic characteristics, measured either at baseline or at the time of the first PEDF measurement, and their ability to predict changes in PEDF level (i.e., second minus first PEDF measurement). Initial models were unadjusted, and second models adjusted, for PEDF levels at the first measurement.
For cardiovascular, renal and all-cause mortality time-to-event outcomes, marginal multivariate Cox proportional hazard models were used to calculate hazard ratios (HRs) for endpoints of interest in relation to serum PEDF measured at the first and second time-points. These models included one or two follow-up windows (i.e., time between first and second PEDF measurement; and time from second PEDF measurement until study end (Figure 1)) for each participant; adjusted for correlation within participant. They also included left-truncation to account for differences in time at risk because sera for the first measurement of PEDF was not available at the VADT baseline examination, but rather a median of 1.74 years later. Serum PEDF was modeled both as a continuous variable and by tertiles. Initial models to address associations of PEDF with cardiovascular events controlled for age, ethnicity, VADT treatment arm, and prior cardiovascular event. Secondary models additionally adjusted for diabetes duration and smoking status at VADT randomization, and systolic blood pressure (SBP), HDL-cholesterol, use of ACE inhibitors and statin use at time of initial PEDF measurement. Initial models to address associations of PEDF with renal outcomes controlled for age, ethnicity, VADT treatment arm, diabetes duration and use of ACE inhibitors at time of first PEDF measurement. Secondary models additionally adjusted for ACR or eGFR levels, as appropriate, at time of initial PEDF measurement. These covariates were chosen a priori since they represent either study design variables or established cardiovascular or renal disease risk factors. Appropriate interaction terms were used to determine whether treatment arm or ethnicity modified the relationship between PEDF and the outcomes of interest. The assumption of proportional hazards was evaluated by testing for interaction between the two PEDF index variables and continuous time variables. Reported p-values are two-sided with a type-I error rate significance level of α=0.05. Hazard ratios and their 95% confidence intervals (CI) are displayed with the format: HR, (95% CI). All analyses were performed using SAS v. 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
At VADT (pre-randomization) baseline, mean age of the PEDF study population was 59.7 years, and mean known duration of diabetes was 11.4 years. Of the 970 participants studied, 96.9% were males, 60.8 % were non-Hispanic white, 38.7% had experienced a prior vascular event, and 49.6% were assigned to the VADT intensive (glucose control) treatment group. At VADT baseline, participants in the higher PEDF tertiles were more likely to be younger, Non-Hispanic White, and adherent to diet (Table 1). Body mass index (BMI) and fasting triglyceride levels increased as serum PEDF tertile increased, while HDL-cholesterol levels declined. Moreover, eGFR declined, while serum creatinine, urinary ACR, and use of ARBs increased as serum PEDF tertile increased. In cross-sectional analyses diabetes duration, HbA1c levels, use of ACE inhibitors and blood pressure levels were not associated with serum PEDF tertiles.
Table 1.
| Low Tertile (N=293) ( 1.7 to 8.5 ng/ml) |
Middle Tertile (N=293) ( 8.6 to 11.9 ng/ml) |
High Tertile (N=293) (12.0 to 27.0 ng/ml) |
P‡ | |
|---|---|---|---|---|
| At VADT Baseline | ||||
| Age (years) | 60.9 (59.9, 61.9) | 60.4 (59.4, 61.4) | 59.0 (58.1, 60.0) | 0.03 |
| Diabetes Duration (years) | 11.5 (10.5, 12.4) | 11.1 (10.3, 11.9) | 11.4 (10.6, 12.2) | 0.86 |
| Male (%) | 96.3 | 98.0 | 95.9 | 0.33 |
| Non-Hispanic White (%) | 59.0 | 63.8 | 71.3 | 0.01 |
| Intensive Treatment (%) | 49.8 | 51.2 | 50.2 | 0.94 |
| Prior Vascular Event (%) | 39.6 | 38.2 | 42.7 | 0.53 |
| Current Smoker (%) | 17.4 | 16.8 | 16.8 | 0.97 |
| Hypertension (%) | 71.6 | 72.3 | 76.7 | 0.31 |
| Exercise (%) | 48.5 | 46.7 | 41.8 | 0.24 |
| Adherent to Diet (%) | 52.2 | 47.3 | 42.1 | 0.05 |
| At time of first PEDF Measure | ||||
| Aspirin (%) | 93.1 | 91.1 | 89.0 | 0.22 |
| Statin (%) | 74.9 | 73.6 | 81.2 | 0.07 |
| ACE § (%) | 70.1 | 65.8 | 67.1 | 0.52 |
| ARB § (%) | 5.5 | 13.7 | 14.7 | <0.01 |
| eGFR (ml/min/1.73m2) | 86 (84, 89) | 80 (78, 83) | 73 (70, 75) | <0.01 |
| Serum Creatinine (mg/dl) | 1.0 (0.99, 1.05) | 1.1 (1.07, 1.13) | 1.2 (1.16, 1.23) | <0.01 |
| Hemoglobin A1c (%) | 8.0 (7.8, 8.1) | 7.8 (7.7. 8.0) | 8.1 (7.9, 8.3) | 0.13 |
| Urine ACR (mg/g)** | 13 (11, 16) | 20 (16, 24) | 31 (25, 38) | <0.01 |
| Body Mass Index (kg/m2) | 30.9 (30.3, 31.5) | 32.4 (31.8, 32.9) | 33.8 (33.3, 34.4) | <0.01 |
| Systolic BP (mmHg) | 127 (125, 129) | 128 (126, 129) | 129 (127, 130) | 0.53 |
| Diastolic BP (mmHg) | 73 (72, 75) | 73 (72, 74) | 73 (72, 74) | 0.82 |
| HDL–Cholesterol (mg/dl) | 42 (41, 44) | 37 (36, 38) | 34 (33, 36) | <0.01 |
| LDL–Cholesterol (mg/dl) | 98 (94, 101) | 99 (95, 103) | 96 (93, 99) | 0.42 |
| Triglycerides (mg/dl)** | 125 (118, 132) | 156 (146, 166) | 190 (178, 203) | <0.01 |
Continuous characteristics are shown as means with associated 95% confidence intervals while categorical characteristics are shown as percentages.
Limited to first measurements which occurred within 4 years of study enrollment.
Chi-square for categorical variables and F test for continuous variables.
Non-normal distributions are presented as geometric means and 95% confidence intervals
Table 2 examines patient characteristics measured at VADT baseline and at the time of the first serum PEDF measurement in relationship to changes in serum PEDF between the first and second measurement. For the 743 participants with two PEDF measures, mean PEDF level (±SD) at the first measure was 10.5 ng/ml (±4.03) and at second measurement was 11.0 ng/ml (±4.86), with a mean difference between measures of 0.41 ng/ml (±4.31) (paired t-test p=0.0092). PEDF levels increased by over 10% in 42.7% of participants; decreased by more than 10% in 35.1% of participants; and remained within 10% of baseline levels in 22.2% of participants. After adjustment for serum PEDF at the initial measurement, higher age at baseline was associated with declining serum PEDF between the two measures (p<0.05). Moreover, at the time of the initial serum PEDF measurement lower eGFR (p<0.01), higher serum creatinine (p<0.01) and urinary ACR (p<0.01) were associated with increasing serum PEDF. Higher BMI and triglycerides at the time of the first serum PEDF measurement were also associated with increasing serum PEDF levels between the first and second measurement (p<0.01). HbA1c levels at the time of the first serum PEDF measurement were not associated with changes in serum PEDF levels (p=0.90). Interestingly, lower eGFR (p<0.01) and higher urinary ACR (p<0.01) remained associated with increasing serum PEDF even after adjustment for PEDF, age, BMI and triglycerides at time of first PEDF measurement (additional data to that provided in Table 2).
Table 2.
Univariate analysis with PEDF difference between pass2 and pass1 (pass2 - pass1). For each clinical characteristic PEDF difference is modeled as the dependent variable with the clinical characteristic being measured either at VADT baseline or at the time of the first PEDF measurement.
| Clinical Characteristic | N | Unadjusted Regression Coefficient Estimates* |
P-value | Adjusted* Regression Coefficient Estimates |
P-value |
|---|---|---|---|---|---|
| At VADT Baseline | |||||
| Age (years) | 743 | −0.02 | 0.28 | −0.04 | 0.04 |
| Diabetes Duration (years) | 735 | 0.04 | 0.09 | 0.03 | 0.19 |
| Non-Hispanic White (%) | 743 | −0.21 | 0.52 | −0.42 | 0.18 |
| Intensive Treatment (%) | 743 | −0.24 | 0.45 | −0.36 | 0.23 |
| Prior Vascular Event (%) | 743 | 0.18 | 0.57 | 0.31 | 0.32 |
| Current Smoker (%) | 743 | −0.06 | 0.90 | −0.07 | 0.86 |
| Hypertension† (%) | 740 | 0.27 | 0.45 | 0.40 | 0.23 |
| Exercise (%) | 742 | −0.21 | 0.50 | −0.39 | 0.19 |
| Adherent to Diet (%) | 742 | −0.29 | 0.37 | −0.57 | 0.06 |
| At time of first PEDF Measure | |||||
| Aspirin (%) | 741 | 0.11 | 0.86 | −0.02 | 0.97 |
| Statin (%) | 741 | 0.20 | 0.59 | −0.39 | 0.29 |
| ACE (%) | 741 | 0.01 | 0.97 | −0.04 | 0.90 |
| ARB (%) | 741 | 0.06 | 0.88 | 0.66 | 0.16 |
| eGFR | 741 | −0.01 | 0.37 | −0.02 | <0.01 |
| Serum Creatinine | 741 | 0.91 | 0.11 | 2.33 | <0.01 |
| Hemoglobin A1c (%) | 741 | −0.07 | 0.52 | 0.01 | 0.90 |
| Ln Urine ACR (mg/g) | 741 | 0.19 | 0.04 | 0.39 | <0.01 |
| Body Mass Index (kg/m2) | 741 | 0.06 | 0.05 | 0.13 | <0.01 |
| Systolic BP (mmHg) | 741 | 0.01 | 0.21 | 0.01 | 0.15 |
| Diastolic BP (mmHg) | 741 | 0.004 | 0.78 | 0.002 | 0.88 |
| HDL–Cholesterol (mg/dl) | 741 | 0.03 | 0.09 | −0.02 | 0.28 |
| LDL–Cholesterol (mg/dl) | 740 | 0.01 | 0.26 | 0.004 | 0.46 |
| Ln Triglycerides (mg/dl) | 741 | 0.44 | 0.12 | 1.37 | <0.01 |
Adjusted for PEDF at pass1
History of hypertension
Marginal multivariate Cox proportional hazard models were used to examine the ability of PEDF to predict macrovascular endpoints and (all-cause) death (Table 3). These models allowed us to incorporate one or, if available, both PEDF measurements per person and therefore to focus on PEDF measured a median of 2-years prior to an event of interest. After adjusting for age, ethnicity, VADT treatment arm, and prior cardiovascular event, the tertile of PEDF was not associated with MI, CHD, or the composite endpoint, but was positively associated with all-cause mortality. Specifically, hazard ratios for all-cause mortality, comparing the second and third tertiles of PEDF to the first tertile were 2.03 (95% CI: 1.06, 3.88) and 2.00 (95% CI: 1.03, 3.89), respectively. However, after adjusting in addition for diabetes duration, smoking status, systolic blood pressure, HDL-cholesterol, eGFR, ACR, use of ACE inhibitors and statin use, mortality no longer differed between PEDF tertiles [HR=1.40 (95% CI: 0.68, 2.88) for third versus first PEDF tertile. There was no evidence that study treatment arm, ethnicity, or prior history of a cardiovascular event modified the association between PEDF level and any of the macrovascular endpoints or total mortality.
Table 3.
Adjusted hazard ratios (and 95% confidence intervals) from Cox proportional hazard regression models for cardiovascular outcomes versus tertile of PEDF. Uses PEDF data measured at two time points.
| Event (n) | PEDF Tertile | Initial Model HR (95% CI)* |
Second Model HR (95% CI)† |
|---|---|---|---|
| MI (n=46) |
Lowest | 1.00 | 1.00 |
| Second | 0.58 (0.26, 1.28) | 0.56 (0.24, 1.30) | |
| Third | 1.06 (0.56, 2.02) | 1.03 (0.48, 2.19) | |
| MI, procedure or inoperable disease (n=104) |
Lowest | 1.00 | 1.00 |
| Second | 0.73 (0.44, 1.21) | 0.70 (0.41, 1.17) | |
| Third | 1.03 (0.66, 1.60) | 0.91 (0.54, 1.51) | |
| MI or CV Death (n=67) |
Lowest | 1.00 | 1.00 |
| Second | 0.72 (0.38, 1.36) | 0.58 (0.29, 1.14) | |
| Third | 1.26 (0.73, 2.18) | 1.02 (0.55, 1.89) | |
| Composite Endpoint (n=165) |
Lowest | 1.00 | 1.00 |
| Second | 1.02 (0.69, 1.50) | 0.97 (0.64, 1.46) | |
| Third | 1.21 (0.84, 1.74) | 1.01 (0.66, 1.55) | |
| All Death (n=66) |
Lowest | 1.00 | 1.00 |
| Second | 2.03 (1.06, 3.88) | 1.65 (0.82, 3.31) | |
| Third | 2.00 (1.03, 3.89) | 1.40 (0.68, 2.88) |
Adjusted for age, minority status, treatment arm and prior cardiovascular event. Moreover, utilization of left-truncation in our marginal multivariate Cox proportional hazard models enables us to account for age at VADT baseline and at first PEDF measurement. These models also allow us to include PEDF measurements at two time points for a single individual when data is available.
Additionally adjusted for smoking status and diabetes duration at date of randomization as well as systolic blood pressure, HDL, use of ACE inhibitors and statins at time of biomarker measurement.
We also used marginal multivariate Cox proportional hazard models to examine the ability of PEDF to predict development of macro-albuminuria and incident stage 3 and stage 4 CKD. Of the 136 participants who developed stage 3 CKD defined by eGFR<60 ml/min/1.73 m2 twice during follow-up, 25 had an ACR >300 mg/g at baseline, 21 developed macroalbuminuria based on at least a single ACR >300 mg/g during follow-up, two did not have a subsequent measurement of ACR and 88 remained free of macroalbuminuria. Of the 87 participants who developed macroalbuminuria, 23 had an eGFR<60 ml/min/1.73 m2 at baseline, 21 developed stage 3 CKD during follow-up, two did not have a subsequent eGFR measure and 41 remained free of stage 3 CKD. In Table 4, PEDF was modeled as a continuous variable, and risks associated with an increase of PEDF by one standard deviation were assessed. After adjusting for age, VADT treatment arm, diabetes duration, ethnicity, and use of ACE inhibitors, a one standard deviation increase in PEDF was associated with a 50% increased hazard [HR=1.50 (95% CI: 1.25, 1.80)] for macroalbuminuria defined as ACR ≥ 300 µg/mg on at least one occasion. Moreover, a one standard deviation increase in PEDF was associated with increased hazard of developing stage 3 CKD (i.e., eGFR <60 ml/min/1.73 m2 on two occasions) [HR=1.76 (95% CI: 1.54, 2.01)] or stage 4 CKD (i.e., eGFR <30 ml/min/1.73 m2 on two occasions) [HR=2.20 (95% CI: 1.52, 3.19)]. Adjusting for ACR and eGFR at the first PEDF measurement when macroalbuminuria was the outcome of interest attenuated the results moderately; nevertheless PEDF remained significantly associated with development of macroalbuminuria [HR=1.27 (95% CI: 1.02, 1.59)]. Similarly, adjusting for ACR and eGFR at the first PEDF measurement when either stage 3 or 4 CKD was the outcome of interest attenuated the results moderately, but they remained significant [HR 1.48 (95% CI: 1.28, 1.73) and 1.80 (95% CI: 1.06, 3.07), respectively].
Table 4.
Cox Proportional Hazard models for renal outcomes for a one standard deviation increase in PEDF. Uses PEDF data measured at two time points.
| Event | N* | Initial Model† | Second Model‡ | ||
|---|---|---|---|---|---|
| HR(95% CI) | p-value | HR(95% CI) | p-value | ||
| Macroalbuminuria (ACR ≥300 µg/mg once) |
87 | 1.50 (1.25, 1.80) | <0.0001 | 1.27 (1.02, 1.59) | 0.0320 |
| Macroalbuminuria (ACR ≥300 µg/mg twice) |
34 | 1.45 (1.11, 1.91) | 0.0071 | 1.00 (0.72, 1.37) | 0.9737 |
| CKD Stage 3 (eGFR < 60 twice) |
135 | 1.76 (1.54, 2.01) | <0.0001 | 1.48 (1.28, 1.73) | <0.0001 |
| CKD Stage 4 (eGFR < 30 ml/min once) |
31 | 1.75 (1.44, 2.12) | <0.0001 | 1.43 (1.13, 1.80) | 0.0028 |
| CKD Stage 4 (eGFR < 30 ml/min twice) |
14 | 2.20 (1.52, 3.19) | <0.0001 | 1.80 (1.06, 3.07) | 0.0308 |
Number of events.
Adjusted for age, minority status, treatment arm, diabetes duration and use of ACE inhibitors at initial measurement of PEDF. Moreover, utilization of left-truncation in our marginal multivariate Cox proportional hazard models enables us to account for age and diabetes duration at VADT baseline and at first PEDF measurement. These models also allow us to include PEDF measurements at two time points for a single individual when data is available.
Additionally adjusted for ACR levels and eGFR levels at time of first PEDF measurement.
In Table 5, PEDF tertiles were modeled in relationship to the risk of development of renal dysfunction. Two models were employed: the first adjusted for age, minority status, treatment arm, diabetes duration and use of ACE inhibitors at the time of initial measurement of PEDF; the second adjusted, in addition, for corresponding measures of renal function at the time of the first PEDF measurement. As shown (Table 5), by the second model, hazard ratios comparing the third to first PEDF tertile for incident macro-albuminuria were 1.58 (95% CI: 0.89, 2.81); for incident stage 3 CKD: 2.74 (95% CI: 1.71, 4.39); for incident stage 4 CKD based on eGFR <30 ml/min/1.73 m2 on one occasion: 3.84 (95% CI: 1.17, 12.5); and for incident stage 4 CKD based on eGFR <30 ml/min/1.73 m2 on two occasions: 3.27 (95% CI: 0.38, 28.1). There was no evidence that study treatment arm or minority status modified the association between PEDF level and any of the renal endpoints examined.
Table 5.
Cox Proportional Hazard models for renal outcomes versus tertile of PEDF. Uses PEDF data measured at two time points.
| Event | PEDF Tertile | Initial Model* HR (95% CI) |
Second Model† HR (95% CI) |
|---|---|---|---|
| Macroalbuminuria (ACR ≥300 µg/mg once) |
Lowest | 1.00 | 1.00 |
| Second | 1.37 (0.77, 2.44) | 1.09 (0.61, 1.96) | |
| Third | 2.48 (1.44, 4.26) | 1.58 (0.89, 2.81) | |
| Macroalbuminuria (ACR ≥300 µg/mg twice) |
Lowest | 1.00 | 1.00 |
| Second | 1.32 (0.50, 3.45) | 0.93 (0.37, 2.30) | |
| Third | 2.44 (1.01, 5.89) | 0.99 (0.40, 2.44) | |
| CKD Stage 3 (eGFR < 60 ml/min twice) |
Lowest | 1.00 | 1.00 |
| Second | 1.74 (1.08, 2.81) | 1.49 (0.91, 2.42) | |
| Third | 4.07 (2.63, 6.31) | 2.74 (1.71, 4.39) | |
| CKD Stage 4 (eGFR < 30ml/min once) |
Lowest | 1.00 | 1.00 |
| Second | 2.49 (0.63, 9.83) | 1.85 (0.47, 7.28) | |
| Third | 7.62 (2.21, 26.3) | 3.84 (1.17, 12.5) | |
| CKD Stage 4 (eGFR < 30ml/min twice) |
Lowest | 1.00 | 1.00 |
| Second | 3.08 (0.32, 29.5) | 1.80 (0.19, 17.0) | |
| Third | 10.3 (1.35, 78.6) | 3.27 0.38, 28.1) |
Adjusted for age, minority status, treatment arm, diabetes duration and use of ACE inhibitors at initial measurement of PEDF. Moreover, utilization of left-truncation in our marginal multivariate Cox proportional hazard models enables us to account for age and diabetes duration at VADT baseline and at first PEDF measurement. These models also allow us to include PEDF measurements at two time points for a single individual when data is available.
Additionally adjusted for ACR levels and eGFR levels at time of first PEDF measurement.
DISCUSSION:
Higher serum PEDF levels were significantly associated with the development, within two years, of renal dysfunction, assessed as macroalbuminuria or as stages 3 or 4 CKD. PEDF levels were also associated with increased all-cause mortality in initial models, but did not predict cardiovascular outcomes. With regard to renal function, the current findings are consistent with our previous cross-sectional observation that higher serum PEDF levels were associated with lower eGFR and higher ACR in the VADT[18]. In that earlier study, we also reported a short-term prospective analysis of 216 study participants who had PEDF measured on a single occasion within a narrow window around two-years post-randomization: we concluded then that despite a cross-sectional relationship, PEDF levels were not prospectively associated with worsening of albuminuria or changes in renal function[18]. At the time, study limitations related to small sample size, short follow-up and little change in renal function, were recognized[14]. The current analysis refutes our earlier conclusion regarding lack of predictive significance of PEDF for renal dysfunction. It is based on a much larger sample size and employs two serum PEDF measurements, a median of 2.02 years apart. As a result, our study is now fully in concert with another recent prospective study by Hui et al.[24], who found a strong positive correlation between a single baseline measurement of PEDF and the subsequent progression of CKD and albuminuria in a cohort of 1,136 Chinese Type 2 diabetic patients in Hong Kong, studied over 4-years.
It is important to note that although many studies have now documented increased circulating levels of PEDF in patients with cardiometabolic diseases, including atherosclerosis[25, 26], type 2 diabetes[27] and metabolic syndrome[27, 28], these associations may reflect a potentially beneficial response to reduced tissue PEDF levels at sites of disease. Low PEDF levels in ocular aqueous fluid from people with diabetes[11, 29] and in rats with ischemic retinopathy[30] have been demonstrated, and we have demonstrated increased circulating PEDF levels in patients with versus without diabetic microvascular complications[31]. There are no studies of PEDF levels in human diabetic renal tissue, but in rats, diabetes was associated with decreased PEDF expression, particularly in the glomeruli[32]; furthermore, in mesangial cell culture, PEDF was found to inhibit expression of TGFβ, a mediator of over-production of extracellular matrix[32]. The latter is usually increased in diabetes and in cells exposed to elevated glucose concentrations[31]. Also in rats, exogenous recombinant PEDF, raising both serum and glomerular levels, ameliorated proteinuria[33], while in human mesangial cells, purified PEDF can block injury mediated by advanced glycation end-products[34, 35]. PEDF can also mitigate high-glucose-induced oxidative stress[36] and activation of pathways leading to TGFβ production[37]. Thus, elevated serum PEDF in people with type 2 diabetes compared with non-diabetic subjects may represent a compensatory response[22]: the beneficial effects of PEDF observed in animal and cell culture work support this idea. Consistent with it, small peptide derivatives of PEDF hold promise as treatments for diabetic nephropathy[15, 34], and for angiogenesis involved in diabetic retinopathy[14, 15, 17].
It must be noted that PEDF is not decreased in all tissues in diabetes: we have shown elevated hepatic levels in diabetic rats, coincident with the low renal (and high serum) concentrations[32]. As the liver is a major source of circulating PEDF, increased serum levels could reflect a hepatic response to lower tissue levels, and indeed serum PEDF levels are reduced in patients with cirrhosis[38]. In brief, a dichotomy exists between serum and tissue PEDF levels, which is analogous, although opposite, to that observed for VEGF[39]. In the current analysis, we found serum PEDF levels differed depending upon age, minority status, BMI, lipid levels, and kidney function, but not blood pressure levels. Moreover, changes in serum PEDF levels over a two-year period were influenced by age, BMI, fasting triglyceride levels, and renal function; and trended upwards. They were not related to changes in HbA1c over time.
Diabetes is a leading cause of renal failure and CVD[40–42]. In both cases, disturbed angiogenesis is implicated, yet longitudinal biomarker studies in major cohorts are lacking. Even though, in recent years, the effects of the complications of diabetes have been mitigated to some extent by improved control of traditional risk factors, very substantial “residual risk” remains[21, 43–46]. Therefore, there is a great need for new biomarkers to stratify complication risk, and to develop and monitor new therapies. Our current data suggest, in agreement with Hui et al.[24], that circulating PEDF levels may have a useful role as a biomarker for renal dysfunction in type 2 diabetes, and have a future role as a therapeutic agent[14–17].
The strengths of our study include its use of the VADT cohort: the VADT was a rigorously conducted prospective study of the vascular complications of diabetes, with detailed recording of clinical and especially vascular complication status. In addition, we measured serum PEDF on two occasions in a relatively large number of subjects. Limitations include a lack of generalizability, since patients were almost all male, and concerns about selection bias (not all VADT study subjects volunteered for our sub-study). Another limitation is that we were unable to evaluate individuals who developed both CKD and macroalbuminuria, or different combinations of these outcomes due to limited size of the cohort and the extent of disease at baseline. Not all participants provided two samples for PEDF assay, and no ‘pre-randomization’ samples were available. Finally, because the VADT design included tight control of LDL-cholesterol levels and hypertension for all participants we are not able to examine the influence of high LDL-cholesterol or uncontrolled hypertension on associations of interest.
In summary, higher serum PEDF levels were significantly associated with subsequent development of renal dysfunction and of (all-cause) mortality in patients with type 2 diabetes. Serum PEDF levels are reasonably stable over ~2 years, with small but significant changes being related to renal dysfunction. In concert with the findings of Hui et al.,[24] we conclude that over two-years, serum PEDF may serve as a predictive biomarker for advanced nephropathy in people with type 2 diabetes. The data complement our prior human and animal studies, and add to the body of work suggesting that PEDF, or a derivative, may serve as a potential therapeutic target and / or agent.
Highlights:
Pigment epithelium-derived factor (PEDF) is an adipokine that also possesses anti-oxidant, anti-inflammatory, anti-fibrotic, and insulin-sensitizing effect.
Higher serum PEDF was significantly associated with subsequent development of renal dysfunction and of (all-cause) mortality in patients with type 2 diabetes.
Serum PEDF levels are reasonably stable over ~2 years, with small but significant changes being related to renal dysfunction.
The data complement our prior human and animal studies, and add to the body of work suggesting that PEDF, or a derivative, may serve as a potential therapeutic target and / or agent.
ACKNOWLEDGEMENTS
Supported by National Heart Lung and Blood Institute Research Grant P01 HL55782; National Institute for Diabetes, Digestive, and Kidney Diseases Grants R01DK080043 and R21 HL80921; American Diabetes Association Research Grants (1-09-CR-38 and 7-12-CT-46); the Medical University of South Carolina General Clinical Research Center (Grant M01-RR-1070); the University of Oklahoma General Clinical Research Center (Grant MO1-RR-14467); National Center for Advancing Translational Sciences (NCATS) Grants TL1 TR001451 and UL1 TR001450; and GlaxoSmithKline, which provided logistic support. The VA Diabetes Trial was supported by the Veterans Affairs Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development; the American Diabetes Association; and the National Eye Institute. Pharmaceutical and other supplies and financial assistance for VADT were provided by GlaxoSmithKline, Novo Nordisk, Roche Diagnostics, Sanofi-Aventis, Amylin, and Kos Pharmaceuticals. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no relevant conflicts of interest.
References
- 1.Wang JJ, Zhang SX, Mott R, Knapp RR, Cao W, Lau K, Ma JX: Salutary effect of pigment epithelium-derived factor in diabetic nephropathy: evidence for antifibrogenic activities. Diabetes 2006, 55(6):1678–1685. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX: Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 2006, 20(2):323–325. [DOI] [PubMed] [Google Scholar]
- 3.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Nakamura M: New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther 2000, 2(4):601–608. [DOI] [PubMed] [Google Scholar]
- 4.Breyer MD, Bottinger E, Brosius FC, Coffman TM, Fogo A, Harris RC, Heilig CW, Sharma K: Diabetic nephropathy: of mice and men. Adv Chronic Kidney Dis 2005, 12(2):128–145. [DOI] [PubMed] [Google Scholar]
- 5.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B et al. : A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004, 18(12):1450–1452. [DOI] [PubMed] [Google Scholar]
- 6.King GL, Suzuma K: Pigment-epithelium-derived factor--a key coordinator of retinal neuronal and vascular functions. N Engl J Med 2000, 342(5):349–351. [DOI] [PubMed] [Google Scholar]
- 7.Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J: Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett 2001, 489(2–3):270–276. [DOI] [PubMed] [Google Scholar]
- 8.Wang JJ, Zhang SX, Mott R, Chen Y, Knapp RR, Cao W, Ma JX: Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol 2008, 294(5):F1166–1173. [DOI] [PubMed] [Google Scholar]
- 9.Takenaka K, Yamagishi S, Matsui T, Nakamura K, Jinnouchi Y, Yoshida Y, Ueda S, Katsuki Y, Katsuda Y, Imaizumi T: Pigment epithelium-derived factor (PEDF) administration inhibits occlusive thrombus formation in rats: a possible participation of reduced intraplatelet PEDF in thrombosis of acute coronary syndromes. Atherosclerosis 2008, 197(1):25–33. [DOI] [PubMed] [Google Scholar]
- 10.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP: Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 1999, 285(5425):245–248. [DOI] [PubMed] [Google Scholar]
- 11.Boehm BO, Lang G, Volpert O, Jehle PM, Kurkhaus A, Rosinger S, Lang GK, Bouck N: Low content of the natural ocular anti-angiogenic agent pigment epithelium-derived factor (PEDF) in aqueous humor predicts progression of diabetic retinopathy. Diabetologia 2003, 46(3):394–400. [DOI] [PubMed] [Google Scholar]
- 12.Zvonic S, Lefevre M, Kilroy G, Floyd ZE, DeLany JP, Kheterpal I, Gravois A, Dow R, White A, Wu X et al. : Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics 2007, 6(1):18–28. [DOI] [PubMed] [Google Scholar]
- 13.Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H, Bickel PE, Andersen JS, Fernandez MM, Bunkenborg J et al. : A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics 2002, 1(3):213–222. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Chen HH, Zhang LW: Potential therapeutic effects of pigment epithelium-derived factor for treatment of diabetic retinopathy. Int J Ophthalmol 2013, 6(2):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awad AS, Gao T, Gvritishvili A, You H, Liu Y, Cooper TK, Reeves WB, Tombran-Tink J: Protective role of small pigment epithelium-derived factor (PEDF) peptide in diabetic renal injury. Am J Physiol Renal Physiol 2013, 305(6):F891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim AS, Tawfik AM, Hussein KA, Elshafey S, Markand S, Rizk N, Duh EJ, Smith SB, Al-Shabrawey M: Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids. Biochim Biophys Acta 2015, 1851(3):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Zhai G, Shi W, Wang Y, Zhu L, Dai Y, Chen C: Pigment Epithelium-Derived Factor Inhibits Oxygen-Induced Retinal Neovascularization in a Murine Model. Fetal Pediatr Pathol 2016, 35(3):173–185. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins AJ, Fu D, Azar M, Stoner JA, Kaufman DG, Zhang S, Klein RL, Lopes-Virella MF, Ma JX, Lyons TJ et al. : Clinical correlates of serum pigment epithelium-derived factor in type 2 diabetes patients. J Diabetes Complications 2014, 28(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopes-Virella MF, Hunt KJ, Baker NL, Virella G, Moritz T: The levels of MDA-LDL in circulating immune complexes predict myocardial infarction in the VADT study. Atherosclerosis 2012, 224(2):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duckworth WC, McCarren M, Abraira C: Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care 2001, 24(5):942–945. [DOI] [PubMed] [Google Scholar]
- 21.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R et al. : Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009, 360(2):129–139. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins A, Zhang SX, Gosmanova A, Aston C, Dashti A, Baker MZ, Lyons T, Ma JX: Increased serum pigment epithelium derived factor levels in Type 2 diabetes patients. Diabetes Res Clin Pract 2008, 82(1):e5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006, 145(4):247–254. [DOI] [PubMed] [Google Scholar]
- 24.Hui E, Yeung CY, Lee PC, Woo YC, Fong CH, Chow WS, Xu A, Lam KS: Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metab 2014, 99(11):E2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Ma X, Zhou M, Pan X, Ni J, Gao M, Lu Z, Hang J, Bao Y, Jia W: Serum pigment epithelium-derived factor levels are independently correlated with the presence of coronary artery disease. Cardiovasc Diabetol 2013, 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tahara N, Yamagishi S, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Mohar D, Ishibashi M, Hayabuchi N, Imaizumi T: Serum level of pigment epithelium-derived factor is a marker of atherosclerosis in humans. Atherosclerosis 2011, 219(1):311–315. [DOI] [PubMed] [Google Scholar]
- 27.Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, Lee KW, Nam MS, Park YS, Woo JT et al. : C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes 2012, 61(11):2932–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arimura T, Miura S, Sugihara M, Iwata A, Yamagishi S, Saku K: Association between plasma levels of pigment epithelium-derived factor and renal dysfunction in patients with coronary artery disease. Cardiol J 2011, 18(5):515–520. [DOI] [PubMed] [Google Scholar]
- 29.Bakri SJ, Patel SP: Retinal pigment epithelial tear following intravitreal bevacizumab. Eye (Lond) 2007, 21(3):424–425. [DOI] [PubMed] [Google Scholar]
- 30.Gao X, Dinkova-Kostova AT, Talalay P: Powerful and prolonged protection of human retinal pigment epithelial cells, keratinocytes, and mouse leukemia cells against oxidative damage: the indirect antioxidant effects of sulforaphane. Proc Natl Acad Sci U S A 2001, 98(26):15221–15226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins AJ, Zhang SX, Rowley KG, Karschimkus CS, Nelson CL, Chung JS, O’Neal DN, Januszewski AS, Croft KD, Mori TA et al. : Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med 2007, 24(12):1345–1351. [DOI] [PubMed] [Google Scholar]
- 32.Wang JJ, Zhang SX, Lu K, Chen Y, Mott R, Sato S, Ma JX: Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. Diabetes 2005, 54(1):243–250. [DOI] [PubMed] [Google Scholar]
- 33.Fujimura T, Yamagishi S, Ueda S, Fukami K, Shibata R, Matsumoto Y, Kaida Y, Hayashida A, Koike K, Matsui T et al. : Administration of pigment epithelium-derived factor (PEDF) reduces proteinuria by suppressing decreased nephrin and increased VEGF expression in the glomeruli of adriamycin-injected rats. Nephrol Dial Transplant 2009, 24(5):1397–1406. [DOI] [PubMed] [Google Scholar]
- 34.Ishibashi Y, Matsui T, Taira J, Higashimoto Y, Yamagishi S: Protective Role of PEDF-Derived Synthetic Peptide Against Experimental Diabetic Nephropathy. Horm Metab Res 2016, 48(9):613–619. [DOI] [PubMed] [Google Scholar]
- 35.Ide Y, Matsui T, Ishibashi Y, Takeuchi M, Yamagishi S: Pigment epithelium-derived factor inhibits advanced glycation end product-elicited mesangial cell damage by blocking NF-kappaB activation. Microvasc Res 2010, 80(2):227–232. [DOI] [PubMed] [Google Scholar]
- 36.Mao T, Gao L, Li H, Li J: Pigment epithelium-derived factor inhibits high glucose induced oxidative stress and fibrosis of cultured human glomerular mesangial cells. Saudi Med J 2011, 32(8):769–777. [PubMed] [Google Scholar]
- 37.Mao T, Chen H, Hong L, Li J: Pigment epithelium-derived factor inhibits high glucose-induced JAK/STAT signalling pathway activation in human glomerular mesangial cells. Saudi Med J 2013, 34(8):793–800. [PubMed] [Google Scholar]
- 38.Matsumoto K, Ishikawa H, Nishimura D, Hamasaki K, Nakao K, Eguchi K: Antiangiogenic property of pigment epithelium-derived factor in hepatocellular carcinoma. Hepatology 2004, 40(1):252–259. [DOI] [PubMed] [Google Scholar]
- 39.Burgos R, Simo R, Audi L, Mateo C, Mesa J, Garcia-Ramirez M, Carrascosa A: Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia 1997, 40(9):1107–1109. [DOI] [PubMed] [Google Scholar]
- 40.Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E et al. : Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375(9733):2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414(6865):813–820. [DOI] [PubMed] [Google Scholar]
- 42.Cheung N, Mitchell P, Wong TY: Diabetic retinopathy. Lancet 2010, 376(9735):124–136. [DOI] [PubMed] [Google Scholar]
- 43.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005, 353(25):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Research G, Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, Orchard TJ: Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med 2009, 169(14):1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White NH, Sun W, Cleary PA, Danis RP, Davis MD, Hainsworth DP, Hubbard LD, Lachin JM, Nathan DM: Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol 2008, 126(12):1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993, 329(14):977–986. [DOI] [PubMed] [Google Scholar]