Abstract
Introduction
Frequent premature ventricular contractions (PVCs) can cause cardiomyopathy (CM). Post-extrasystolic potentiation and irregularity have been in implicated as triggers of PVC-CM. Since both phenomena can also be found in premature atrial contractions (PACs), it is speculated that frequent PACs have similar consequences.
Methods and Results
A single center, retrospective study included all consecutive patients undergoing a 14-day Holter monitors (November 2014 to October 2016). Patients were divided into 4 groups by ectopy burden: group 1 (<1%) and remaining by tertiles (group 2–4). Echocardiographic and arrhythmic data were compared between PAC and PVC burdens. In addition, a translational PAC animal model was used to assess the chronic effects of frequent PACs. A total 846 patients were reviewed. In contrast to PVCs, we found no difference in LV ejection fraction (LVEF), end-systolic and end-diastolic dimensions and presence of CM (LVEF <50%) between different PAC groups. Multivariate regression analysis demonstrated that only PVC burden predicted low EF (odds ratio: 1.1, CI-1.03–1.13. p= 0.001). While there was a weak correlation between PAC burden and SVT episodes and AF burden (r= 0.19, p <0.001), there was no correlation between PAC burden and LVEF or cardiomyopathy. Lastly, atrial bigeminy in our animal model did not significantly decrease LVEF after 3 months.
Conclusion
PAC burden is associated with increased AF and SVT episodes. In contrast to high PVC burden, high PAC burden is not associated with cardiomyopathy. Our findings suggest that heart rate irregularity and/or post-extrasystolic potentiation may play a minimal role on the pathophysiology of PVC-cardiomyopathy.
Keywords: Premature ventricular contractions, Premature atrial contractions, Cardiomyopathy, Left ventricular dysfunction, Ventricular tachycardia, Supraventricular tachycardia, Atrial fibrillation, ambulatory Holter monitor
Condensed abstract
Frequent premature ventricular contractions (PVCs) can cause cardiomyopathy (CM). Post-extrasystolic potentiation and irregularity have been in implicated as triggers of PVC-CM. Since both phenomena can be found in PVCs and premature atrial contractions (PACs), it is speculated that frequent PACs have similar consequences. In this single center, retrospective, cross-sectional study including patients undergoing a 14-day ambulatory ECG Holter monitor enrolling 846 consecutive patients, high PAC burden is not associated with cardiomyopathy. Our findings suggest that heart rate irregularity and/or post-extrasystolic potentiation may play a minimal role on the pathophysiology of PVC-cardiomyopathy.
Introduction
Frequent ventricular ectopy is known to cause non-ischemic cardiomyopathy, often referred to as premature ventricular contraction (PVC)-cardiomyopathy.1–4 Similar to PVCs, atrial fibrillation 5, 6 and frequent premature atrial contractions (PACs) 7, 8 are believed to cause cardiomyopathy due to increased heart rate, heart rate (HR) irregularity and/or associated post-extrasystolic potentiation. These phenomena are not unique to PVCs as they also occur in PACs. However, definitive data linking frequent PACs with cardiomyopathy or LV dysfunction remain unclear with only a single case report of this potential association.8 Ziopatch™ is a novel, leadless electrocardiographic monitoring device that allows for extended 14-days monitoring with high patient compliance providing a percentage of PACs, referred as PAC burden. We present a retrospective study of consecutive patients wearing a Holter Ziopatch™ monitor to characterize the relationship between PACs and LV dysfunction and other arrhythmias, as well as better understand the mechanistic implications of heart rate irregularity and post-extrasystolic potentiation on the development of PVC-Cardiomyopathy. Finally, we used an established chronic large animal model to confirm findings of the retrospective clinical database.
Methods
This was a single center, retrospective, cross-sectional study of 846 consecutive patients with a 14-day ambulatory ECG Holter Ziopatch™ monitor (iRhythm Technologies, San Francisco, CA) at Hunter Holmes McGuire VA Medical Center from November 2014 through October 2016. Ambulatory Holter monitor report generated by iRhythm included mean heart rate (HR), PVC and PAC burden, atrial fibrillation (AF) burden, supraventricular tachycardia (SVT) and ventricular tachycardia (VT) episodes.
PAC burden (%) was defined as the total number of PACs over the total beats in a period of 14 days. Similarly, PVC burden was defined as the total number of PVCs over the total beats in a period of 14 days. SVT was defined as 4 or more consecutive narrow complex beats. All 846 ambulatory ECG monitors were grouped based on PAC and PVC burden. Ziopatch reports either atrial or ventricular ectopic burden only when is above 1%. Thus, all subjects with a PAC burden <1% were assigned to “PAC Group 1”. Grouping patients arbitrarily based on PAC or PVC burden (e.g. 1 – 4.9%, 5–9.9%, >10% burden) provided a small proportion of patients in the PAC or PVC burden greater than 10%. To have equal number of patients within PAC groups, the remaining patients with PACs >1% were subsequently divided into tertiles and grouped as follows: a) “PAC group 2” (PAC burden range: 1%−2.1%), b) “PAC group 3” (range: 2.2%−4.9%), and c) “PAC group 4” (range: 5%−24%) 9. Similarly, patients with PVC burden <1% were grouped together as “PVC group 1”, whereas the remaining were divided into tertiles as follows: a) “PVC group 2” (PVC burden range: 1%−2.4%), b) “PVC group 3” (range: 2.5%−6%), and c) “PVC group 4” (range: 6.1%−44.7%).
Echocardiographic data was reviewed on all subjects and considered for analysis only if performed within 6 months prior or after the ambulatory ECG recoding. All echocardiographic parameters were independently assessed by a cardiologist board-certified in echocardiography. LV dysfunction or cardiomyopathy was defined as left ventricular ejection fraction (LVEF%) below 50%. All patients with LVEF >50% were grouped as “LVEF group 1” (50–81.40%). In order to have equal number of patients in different groups with LVEF <50%, the remaining population were divided into tertiles as follows: a) “LVEF group 2” (LVEF range: 42.5%−49.6%), b) “LVEF group 3” (range: 30%−40%), and c) “LVEF group 4” (range: 12.5%−27.50%). Basic demographic data, comorbidities, echocardiographic parameters and Holter monitor data were collected for all patients and compared between the groups based on PACs, PVCs and LVEF.
Animal model
The effect of frequent PAC was studied experimentally using a highly reproducible and validated premature pacing algorithm (St. Jude Medical, Inc; Sylamar, CA) as previously described 10. These pacemakers allow animals to be exposed to 12 weeks of atrial bigeminy (50% burden). Seven canines had an endocardial lead implanted in RA appendage connected to a pacemaker in order to induce atrial bigeminy with a 200-ms coupling interval, while 9 animals with the same lead and pacemaker implantation were used as sham controls. Echocardiograms were obtained at baseline and every 4 weeks during the 12-week protocol to assess changes in LV function. A 24-hour ambulatory ECG monitor was obtained to assess PAC burden and mean HR. All procedures were approved by the McGuire Institutional Animal Care and Use Committee (IACUC) in accordance with USDA Animal Welfare Act Regulations and Standards.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD). Categorical variables were presented as proportions (%) and analyzed using Chi-square (χ2) test and/or Fisher’s exact test when appropriate. Normally distributed continuous variables were analyzed using independent sample t-test. When more than two groups were present, analysis of variance (ANOVA) was used with Bonferroni correction if indicated. Non-parametric data was represented using the median and inter-quartile range and analyzed using the Mann-Whitney U test. When more than two groups (ex: AF) were present, Kruskal-Wallis test was used. Association of PAC and PVC burden with low EF was analyzed using the Pearson correlation. Univariate and multivariate predictors of low EF were identified using the logistic regression analysis (hypertension, diabetes, coronary artery disease, gender, PACs and PVCs). A p value of ≤0.05 was considered as statistically significant. P-values for categorical variables with more than 2×2 tables were reported with linear-by-linear association. All statistical analysis was performed using IBM SPSS Statistics, Version 24.0. (Armonk, NY: IBM Corp).
Results
A total of 846 consecutive 14-day ambulatory Holters were reviewed. Male gender constituted 88% (745) consistent with the VA patient population, while hypertension (HTN, 71%), diabetes (32%) and coronary artery disease (CAD, 28%) were prevalent comorbidities. The average HR was 74+/−11 bpm and the mean AF burden was 1.2 ± 8.5%. The mean PAC and PVC burden for the entire population was 1.7 ± 2.6 (range: 0.9 % to 24%) and 2.6 ± 4.7 (range: 0.4% to 44.7%) respectively. A total of 435 (51%) patients underwent 2D echocardiogram with a mean LVEF of 51 ± 10 (range: 12.50% – 81.40%). LV dysfunction (LVEF <50%) was observed in 24% (103 patients) of the study population.
PAC analysis
Patients with PAC burden <1% (Group 1) constituted 78% (659) of study population. Table 1 compares the demographics, arrhythmias and echocardiographic parameters between all 4 PAC groups. Male gender, HTN, CAD, SVT episodes and AF burden demonstrated a statistical significance between PAC groups. While there was a weak correlation between PAC burden and SVT episodes (r= 0.19, p <0.001) and AF burden (r= 0.19, p<0.001), no significant correlation was found between SVT episodes and AF (r=0.52, p=0.13). Nonetheless, PAC Group 4 (highest PAC group) had a significantly higher prevalence of HTN, CAD and number of SVT episodes compared to lowest PAC group 1 (burden < 1%).
Table 1.
Comparison of demographics, arrhythmias and echocardiographic parameters between PAC groups.
| Variable |
PAC Grp 1 n= 659 |
PAC Grp 2 n= 62 |
PAC Grp 3 n= 63 |
PAC Grp 4 n= 62 |
P value |
| Demographics | |||||
| Males | 568 (86%) | 57 (92%) | 61(97%) | 59 (95%) | 0.01 |
| Hypertension | 456 (69%)‡ | 50 (81%) | 49 (78%) | 52 (84%)* | 0.01 |
| Diabetes | 202 (31%) | 20 (32%) | 23 (36%) | 28 (45%) | 0.1 |
| CAD | 172 (26%)‡ | 23 (37%) | 15 (24%) | 25 (40%)* | 0.03 |
| Ambulatory ECG | |||||
| Heart rate (bpm) | 74±10 | 74±14 | 76±11 | 74±11 | 0.65 |
| AF burden (%) | 0.6±4.8† | 4.8±15.3‡*~ | 1.1±3.1† | 1.9±6.9† | 0.001 |
| SVT episodes (No.) | 13±80‡ | 244±1422 | 825±4203 | 946±2961* | 0.001 |
| PVC burden (%) | 2.6±4.9 | 3.0±4.4 | 3.0±4.8 | 2.1±2.4 | 0.62 |
| Total PVC (No.) | 32094±166899 | 34901±67505 | 36001±76420 | 23468±48220 | 0.96 |
| VT Episodes (No.) | 1.9±21.5 | 7.0±48 | 1.4±9.1 | 4.5±26.5 | 0.39 |
| Echocardiograph Parameters | (n=332) | (n=38) | (n=26) | (n=39) | P value |
| LVEF | 51.8±10.0 | 49.2±12.8 | 50.2±11.1 | 47.7±13.3 | 0.08 |
| LVEF < 50% | 71 (22%) | 11 (29%) | 7 (27%) | 14 (36%) | 0.18 |
| LVEDD (cm) | 4.8±1.3 | 4.9±0.82 | 4.8±0.73 | 4.9±0.72 | 0.97 |
| LVESD (cm) | 3.4±0.81 | 3.7±0.83 | 3.6±0.95 | 3.7±0.85 | 0.15 |
| LA in cm | 3.8±0.64 | 4.0±0.6 | 3.8±0.56 | 4.0±0.79 | 0.16 |
PAC group 1 (range< 1%, median (IQR)-0.9 (0.9-0.9), n 659), PAC group 2 (range: 1.1 - 2.1%, median (IQR)-1.4 (1.1-1.7), n=62), PAC group 3 (range: 2.2 - 4.9%, median (IQR)-3.4 (2.6-4.1), n=63), PAC group 4 (range: 5 - 24%, median (IQR)-8.5 (6.1-12.1), n=62). Data is presented as mean and SD. Note that echocardiographic data includes a lower number of patients since not all had associated 2-D echocardiogram. LV dysfunction (LVEF <50%). P value using one-way ANOVA. Significant Bonferroni correction (<0.05) for PAC
group 1
group 2
group 3
and group 4.
Grp-Group, LVEF-Left ventricular ejection fraction, LVESD-Left ventricular end systolic diameter, PAC-premature atrial contraction, PVC-Premature ventricular contraction, SVT-Supraventricular tachycardia, VT-Ventricular tachycardia.
No difference in presence of cardiomyopathy (LVEF <50%), LVEDD and LVESD was found between different PAC groups (p=0.18). Although a trend was present, LVEF among different PAC groups had no significant difference (p=0.08). Furthermore, no correlation between the PAC burden and LVEF was found (r=0.03, p=0.46). Nevertheless, there were more patients with cardiomyopathy in PAC group 4 compared to other PAC groups, and a weak correlation between the PAC burden and cardiomyopathy seen only in this group (highest PAC burden, r=0.32, p=0.04). Whereas univariate logistic regression analysis demonstrated that the group with highest PAC burden (group 4: 5–24% burden) predicted the presence of cardiomyopathy (OR: 2.64, CI-1.22–5.68. p=0.01), multivariate regression analysis demonstrated that the predictive power was lost after adjusting for PVC burden among other variables (OR: 1.02, CI-0.95–1.11. p= 0.49).
PVC analysis
Similar to PAC analysis, patients with PVC burden <1% (PVC Group 1) constituted the majority (71%) of the study population. Demographics, arrhythmias and echocardiographic parameters between PVCs groups are summarized in Table 2. No significant differences in comorbidities, HR and AF burden were found between different PVC groups. Only PAC burden and VT episodes were significantly different between PVC groups. Yet, only VT episodes had a weak correlation with PVC burden (r=0.39, p<0.001). In addition, VT episodes were significantly more frequent in PVC group 4 compared to all other PVC groups. Conversely, PAC burden was significantly lower in patients with highest burden of PVCs (PVC group 4) compared to PVC group 2.
Table 2.
Comparison of demographics, arrhythmias and echocardiographic parameters between PVC groups.
| Variable |
PVC Grp 1 (n=599) |
PVC Grp 2 (n=82) |
PVC Grp 3 (n=81) |
PVC Grp 4 (n=83) |
P value |
| Demographics | |||||
| Males | 516 (86%)† | 75 (93%) | 75 (90%) | 79 (96%)* | 0.02 |
| Hypertension | 428 (71%) | 62 (76%) | 61 (73%) | 56 (68%) | 0.66 |
| Diabetes | 185 (31%) | 32 (39%) | 27(32%) | 29 (35%) | 0.41 |
| CAD | 155 (26%) | 22 (27%) | 30 (36%) | 28 (34%) | 0.12 |
| Ambulatory ECG | |||||
| Heart rate (bpm) | 75±11 | 72±11 | 72±10 | 74±10 | 0.08 |
| PAC burden (%) | 1.6±2.4 | 2.5±3.6‡ | 2.3±3.6 | 1.4±1.2† | 0.03 |
| AF burden (%) | 1.2±6.6 | 1.4±7.8 | 1.1±6 | 0.1±0.7 | 0.55 |
| SVT episodes (No.) | 138±1416 | 331±1869 | 279±2071 | 18±62 | 0.03 |
| Total PVC (No.) | 702±2191 | 20799±9182 | 48394±20367 | 252199±41943 | 0.000 |
| VT episodes (No.) | 0.43±5.9‡ | 0.49±1.4‡ | 0.69±1.3‡ | 20±74*†~ | 0.000 |
| Echocardiographic parameters | (n=296) | (n=49) | (n=48) | (n=40) | P value |
| LVEF (%) | 52.7±9.4 | 50.2±9.8 | 47.1±13.5* | 44.9±13.9* | 0.0001 |
| LVEF < 50% | 53 (18%)~‡ | 14 (29%) | 18 (37%)* | 17 (42%)* | 0.0001 |
| LVEDD (cm) | 4.8±1.3 | 5.0±0.8 | 5.0±0.8 | 5.3±0.7* | 0.03 |
| LVESD (cm) | 3.3±0.7†~‡ | 3.7±0.9* | 3.8±0.9* | 4.1±0.9* | 0.000 |
| LA (cm) | 3.8±0.61† | 4.1±0.6* | 3.9±0.7 | 4.1±0.70 | 0.008 |
PVC group 1 (< 1%, median (IQR)-0.9 (0.9-0.9), n 600), PVC group 2 (range: 1.1-2.4%, median (IQR)-1.4 (1.1-2.0), n=81), PVC group 3 (range: 2.5-5.8%, median (IQR)-4.1 (3.3-4.9), n=83), PVC group 4 (range: 5.9-44.7%, median (IQR)-14.1 (8.8-19.9), n=82). Data is presented as mean and SD. Note that echocardiographic data includes a lower number of patients since not all had associated 2-D echocardiogram. P value using one-way ANOVA. Significant Bonferroni correction (<0.05) for PVC
group 1
group 2
group 3
and group 4.
Grp-Group, LVEF-Left ventricular ejection fraction, LVESD-Left ventricular end systolic diameter, PAC-premature atrial contraction, PVC-Premature ventricular contraction, SVT-Supraventricular tachycardia, VT-Ventricular tachycardia.
The presence of cardiomyopathy (LVEF <50%) was significantly between different PVC groups (p=0.0001). LVEF, LVEDD, LVESD and LA dimension were significantly different between different PVC groups (Table 2 and Figure 1). LVEDD and LVESD were significantly higher, while LVEF was significantly lower in the highest burden PVC group compared with PVC group 1 (<1% burden). We also found a weak but significant inverse relationship between PVC burden and LVEF (r −0.27, p=0.001). Univariate regression analysis demonstrated that PVC group 3 (PVC burden 2.5%−6.0%) and group 4 (PVC burden 6.1%−44.7%) predicted the presence of cardiomyopathy (OR: 2.75, CI-1.42–5.29. P<0.001 and OR: 3.38, CI-1.69–6.78. P<0.001, respectively). As anticipated, multivariate regression analysis demonstrated that high PVC burden (PVC group 3 and 4) independently predicted the presence of cardiomyopathy (odds ratio: 1.1, CI-1.03–1.13. p= 0.001).
Figure 1:
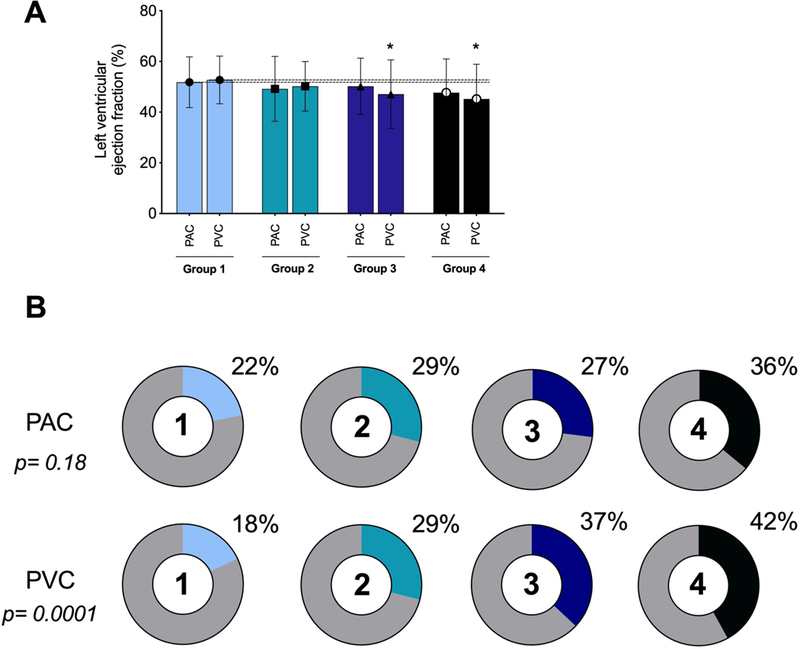
Left ventricular ejection fraction (panel A) and percentage of patients with cardiomyopathy (LVEF <50%) based on different PACs and PVCs groups (panel B).
(* p value is statistically significant between PVC group 1 and PVC group 3, 4).
LV function and ectopy
Table 3 summarizes the co-morbidities and different arrhythmias between different LVEF groups. LVEF greater than 50% was observed in 76% of patients (group 1). Whereas there was a significant difference in PVC burden between LVEF groups, no significant difference in PAC burden was found between different LVEF groups. More specifically, the group with the lowest LVEF (Group 4) had a significantly higher PVC burden compared to the those with a normal LVEF (Group 1) (p<0.0001). Correlation analysis demonstrated a significant association between LVEF and PVC burden (r=0.27, p=0.01), but not with PAC or AF burden. The relationship between LVEF and CM, PACs and PVCs are showed in Figure 1.
Table 3.
Comparison of demographics and arrhythmias between different LVEF groups.
| Variable | LVEF Grp 1 n=331 |
LVEF Grp 2 n=38 |
LVEF Grp 3 n=32 |
LVEF Grp 4 n=34 |
P value |
|---|---|---|---|---|---|
| Demographics | |||||
| Males | 292 (88%) | 37 (97%) | 28 (88%) | 34 (100%) | 0.61 |
| Hypertension | 246 (74%) | 31 (82%) | 26 (81%) | 28 (82%) | 0.49 |
| Diabetes | 113 (34%) | 14 (37%) | 16 (50%) | 16 (47%) | 0.17 |
| CAD | 90 (27%) | 16 (42%) | 19 (59%) | 18 (53%) | <0.001 |
| Ambulatory ECG | |||||
| Heart rate (bpm) | 75±11 | 72±10 | 75±10 | 75±12 | 0.64 |
| PAC burden (%) | 1.8±2.8 | 1.6±1.6 | 2.2±3.5 | 2.1±2.2 | 0.72 |
| AF burden (%) | 1.5±10 | 1.9±6.5 | <0.1 | 2.6±14.2 | 0.73 |
| SVT episodes (No.) | 240±1294 | 326±1737 | 11±17 | 183±1839 | 0.56 |
| PVC burden (%) | 1.5±3.8 | 2.0±3.8 | 3.3±6.3 | 6.3±10.2 | <0.001 |
| Total PVC (No.) | 20970±53968 | 25438±56339 | 44786±89163 | 188977±663996 | <0.001 |
| VT episodes (No.) | 0.7±8 | 0.4±0.9 | 19±75 | 13.6±64 | 0.01 |
LVEF group 1 (range: 50%−81.4%, n=331), LVEF group 2 (range: 42.5-49.6%, n=38), LVEF group 3 (range: 30-40%, n=32), LVEF group 4 (range: 12.5-27.5%, n=34). Data is presented as mean and SD. P value using one-way ANOVA. Significant Bonferroni correction (<0.05) for LVEF
group 1
group 2
group 3
and group 4.
Grp-Group, LVEF-Left ventricular ejection fraction, LVESD-Left ventricular end systolic diameter, PAC-premature atrial contraction, PVC-Premature ventricular contraction, SVT-Supraventricular tachycardia, VT-Ventricular tachycardia.
Animal model of atrial bigeminy
Ambulatory 24-hour ECG monitor confirmed a significantly higher PAC burden in the PAC group when compared to sham group; (PAC group 64865 ± 21205 (40%) vs. sham 4770 ± 4342 (3.5%), p <0.005). Atrial bigeminy (50% burden) in the canine model showed no significant change in LV function after 12-weeks of PAC exposure (LVEF 63.3±5.3% at baseline to 61±4.8% after 12-week, p=NS) and no significant difference compared to sham group (LVEF 60.4±6.4% at baseline to 59.1±5.1%; p=NS) despite increase in mean HR (119.1 ± 8.5 bpm PAC group vs. 99 ± 4.2 sham, p=0.007) (Figure 2).
Figure 2.
Changes in LV ejection fraction by sham and PAC group. PAC group exposed to 3-month PAC 50% PAC burden in a canine model.
Discussion
Persistent heart rate irregularity and post-extrasystolic potentiation generated by frequent ectopy have been proposed as a potential mechanism of PVC-cardiomyopathy. This hypothesis arose in part from the development of LV systolic dysfunction in subjects with AF despite appropriate rate control and from a single report of an experimental canine model of chronic PAC-induced CM 7, 11. Heart rate irregularity and post-extrasystolic potentiation occur with both PACs and PVCs 12. Thus, we hypothesized that patients with frequent PACs would develop a similar non-ischemic reversible cardiomyopathy as frequent PVCs if irregularity and/or post-extrasystolic potentiation are major triggers responsible for the development of cardiomyopathy. With that premise, we sought to investigate the association of frequent PACs detected by ambulatory ECG monitors and LV dysfunction, and the effects of frequent PACs using a translational animal PAC model on LV function.
To our best knowledge this is the first study to assess the effects of high PAC burden in LV function and arrhythmias. Our key findings are: a) high PAC burden is associated with a higher SVT and atrial fibrillation burden (Table 1); b) in contrast to PVCs, PACs are not associated with LV dysfunction; c) LV dysfunction was not observed in an animal model despite 50% PAC burden and a significant increase in HR after a 3-month period. This is in contrast to findings in experimental PVC models where 50% and 100% of animals developed CM when exposed to 33 and 55% PVC burden, respectively 10, 13–15. Ambulatory ECGs in our atrial bigeminy (50% PACs) animal model resulted on a 40% prematurity of R-R (conducted PAC) which in theory should have caused CM in at least half of all animals exposed to PACs. Findings from our translational PAC canine model are supported by a recent PAC and PVC swine model, where atrial bigeminy was not associated with a significant change in LV systolic function16 These studies are inconsistent with the results of Pacchia et.al. 7, where 4 of 5 animals exposed to 50% PAC burden (using a dual chamber pacemaker) developed LV dysfunction (LVEF<50%). Unfortunately, the authors did not obtain holters to document mean HR, mean PAC or R-R coupling interval, only assessing atrial bigeminy during intermittent device interrogation. Thus, it is possible that inappropriate device function triggered episodes of persistent tachycardia resulting on a tachycardia-induced rather than a PAC-induced CM.
While overall there was a small number of patients with PAC burden >5% (PAC group 4) and LVEF assessment (n=39 patients), the number of patients with PVC >6.1% (PVC group 4) and LVEF assessment was also small (n=42). Despite relatively low number of patients with high PVC and PAC burdens, only high PVC burden demonstrated a significant difference in LVEF compared to patients with low PVC burden (p=0.0001 between groups 1 vs group 4, and p=0.003 between groups 1 and 3). Furthermore, a multivariate regression analysis including PAC and PVC burden demonstrated that PVC burden, but not PACs, was an independent predictor for CM. Thus, despite having a low number of patients with high PAC burden, the data is clinically relevant.
The mechanism by which frequent PVCs result in cardiomyopathy has not been definitively identified 17–19. Heart rate irregularity and post-extrasystolic potentiation have been implicated as potential mechanisms. Similar to results from the atrial bigeminy animal model (also characterized by heart rate irregularity and post-extrasystolic potentiation), modest PAC burden (>10%) in our patients was not associated with LV dysfunction. Moreover, an elegant PVCs swine study demonstrate that LV systolic dysfunction was found in PVCs associated with higher degree of LV dyssynchrony (LV epicardial and RV free wall PVCs) rather than those without dyssynchrony (PAC and control groups)16. Altogether, our findings suggest that heart rate irregularity and post-extrasystolic potentiation may not have significant implications in the development of PVC-cardiomyopathy.
Surprisingly, high PAC burden was associated with lower PVC burden. We speculate that PVCs are less prevalent in patients with high PACs states due to conduction of PACs to the ventricle to overdrive the PVC focus. Moreover, we did find a weak association between PAC burden and incidence of AF (r-0.19, p<0.001) based on a single Holter monitor, which is in-line with the results of Dewland et al20.
Incidence and prevalence of PVC-CM remains unclear and underestimated. A recent large population-based study reported an incidence of systolic HF in patients with PVCs of 62.8 per 1,000 patient-years without a significant gender difference 21. We found that a PVC burden of 1–2.1%, 2.2–4.9% and >5% had an associated CM (LVEF<50%) in 30%, 40% and 40% of patients with LVEF assessment, respectively. Nevertheless, the diagnosis of PVC-CM in this veteran population cannot be validated since not all patients went on to receive a PVC suppression strategy. Our PVC database analysis compliments prior publications since the correlation between PVC burden and CM persists after introducing PAC burden in to the regression analysis, which was never performed on prior studies. Our findings support that while patients with LV dysfunction should be screened with a continuous cardiac monitor to quantify the PVC burden and possible PVC-cardiomyopathy, patients with high PAC burden are unlikely to develop LV dysfunction.
Limitations
The single center, retrospective, cross-sectional nature of the study has its inherent limitations, lacking medication history, previous interventions/ablations or follow up echocardiographic parameters. The cross-sectional nature as well as the lack of intended treatment and follow up do not allow us to assess for a possible correlation between PACs and cardiomyopathy. Sample selection was based on patients with a Holter indication (ex: palpitations) which preselects specific patient population. Identification of PACs and PVCs is limited to proprietary Ziopatch automated algorithm (iRhythm, Inc.). Determination of PAC burden by Ziopatch (IRhythm Inc. algorithm) relies in part by R-R interval variability, thus it excludes PACs that lack AV node conduction with subsequent QRS. Also, it is possible that some SVT episodes could have been categorized as AF or vice versa. Moreover, ambulatory ECG holters do not provide of the origin and coupling interval of PAC. Lastly, the average PAC burden in patients with burden greater than 1% was smaller when compared to the equivalent PVC burden in our population (median (IQR) PAC and PVC burden, 3.4 (1.7–6.1) and 4.1 (2.0– 8.9) respectively). We therefore cannot exclude the possibility that heart rate irregularity and/or post-extrasystolic potentiation may have played a role in the development of PVC-cardiomyopathy. Nevertheless, results from our and other animal PAC bigeminal model indicate that their contribution may be limited at best.
Conclusions
In contrast to high burden PVCs, high PAC burden is not associated with LV dysfunction (LVEF <50%). PAC burden only demonstrated a weak correlation with AF and SVT episodes. Thus, heart rate irregularity and/or post-extrasystolic potentiation may play a minimal role on the pathophysiology of PVC-cardiomyopathy.
Clinical competencies and translational outlook
Frequent premature ventricular contractions (PVCs) are known to cause a reversible non-ischemic cardiomyopathy (PVC-Cardiomyopathy). In contrast to PVCs, frequent premature atrial contractions (PACs) are not associated with cardiomyopathy despite similar heart rate irregularity, increased heart rate and associated post-extrasystolic potentiation. This suggests that heart rate irregularity and post-extrasystolic potentiation play a minimal role as a trigger of PVC-cardiomyopathy. Future clinical and translational studies are needed to better understand the mechanism and triggers of PVC-Cardiomyopathy. Identifying the triggers and mechanism of PVC-Cardiomyopathy are key to better recognize patients at risk and identify alternative treatments to PVC-Cardiomyopathy in patients that do not respond to antiarrhythmics and/or radiofrequency ablation.
Acknowledgments
NIH Funding: 1R01HL139874-01 (PI: Huizar), 1R56HL133182-01 (PI: Huizar).
Abbreviations
- CM
Cardiomyopathy
- EF
Ejection fraction
- PAC
Premature atrial contraction
- PVC
Premature ventricular contraction
- PESP
Post-extra systolic potentiation
- SVT
Supra ventricular tachycardia
Footnotes
Relationship with Industry Policy: None
References
- 1.Deyell MW, Park KM, Han Y, Frankel DS, Dixit S, Cooper JM, et al. Predictors of recovery of left ventricular dysfunction after ablation of frequent ventricular premature depolarizations. Heart Rhythm September 2012;9:1465–1472. [DOI] [PubMed] [Google Scholar]
- 2.Yokokawa M, Good E, Crawford T, Chugh A, Pelosi F Jr., Latchamsetty R, et al. Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm February 2013;10:172–175. [DOI] [PubMed] [Google Scholar]
- 3.Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm July 2007;4:863–867. [DOI] [PubMed] [Google Scholar]
- 4.Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol May 14 2019;73:2328–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation June 15 2004;109:2839–2843. [DOI] [PubMed] [Google Scholar]
- 6.Phillips E, Levine SA. Auricular fibrillation without other evidence of heart disease; a cause of reversible heart failure. Am J Med October 1949;7:478–489. [DOI] [PubMed] [Google Scholar]
- 7.Pacchia CF, Akoum NW, Wasmund S, Hamdan MH. Atrial bigeminy results in decreased left ventricular function: an insight into the mechanism of PVC-induced cardiomyopathy. Pacing Clin Electrophysiol October 2012;35:1232–1235. [DOI] [PubMed] [Google Scholar]
- 8.Hasdemir C, Simsek E, Yuksel A. Premature atrial contraction-induced cardiomyopathy. Europace December 2013;15:1790. [DOI] [PubMed] [Google Scholar]
- 9.Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. J Am Coll Cardiol July 14 2015;66:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, et al. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol August 2011;4:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling LH, Khammy O, Byrne M, Amirahmadi F, Foster A, Li G, et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium: implications for the interaction between heart failure and atrial fibrillation. Circ Heart Fail November 2012;5:786–793. [DOI] [PubMed] [Google Scholar]
- 12.Takada H, Takeuchi S, Ando K, Kaito A, Yoshida S. Experimental studies on myocardial contractility and hemodynamics in extrasystoles. Jpn Circ J May 1970;34:419–430. [DOI] [PubMed] [Google Scholar]
- 13.Akoum NW, Daccarett M, Wasmund SL, Hamdan MH. An animal model for ectopy-induced cardiomyopathy. Pacing Clin Electrophysiol March 2011;34:291–295. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Rahmutula D, Duggirala S, Nazer B, Fang Q, Olgin J, et al. Diffuse fibrosis leads to a decrease in unipolar voltage: Validation in a swine model of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm February 2016;13:547–554. [DOI] [PubMed] [Google Scholar]
- 15.Tan AY, Hu YL, Potfay J, Kaszala K, Howren M, Sima AP, et al. Impact of ventricular ectopic burden in a premature ventricular contraction-induced cardiomyopathy animal model. Heart Rhythm March 2016;13:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters TE, Rahmutula D, Szilagyi J, Alhede C, Sievers R, Fang Q, et al. Left Ventricular Dyssynchrony Predicts the Cardiomyopathy Associated With Premature Ventricular Contractions. J Am Coll Cardiol December 11 2018;72:2870–2882. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Eltit JM, Kaszala K, Tan A, Jiang M, Zhang M, et al. Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm November 2014;11:2064–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamon D, Rajendran PS, Chui RW, Ajijola OA, Irie T, Talebi R, et al. Premature Ventricular Contraction Coupling Interval Variability Destabilizes Cardiac Neuronal and Electrophysiological Control: Insights From Simultaneous Cardioneural Mapping. Circ Arrhythm Electrophysiol April 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang M, Zhang M, Howren M, Wang Y, Tan A, Balijepalli RC, et al. JPH-2 interacts with Cai-handling proteins and ion channels in dyads: Contribution to premature ventricular contraction-induced cardiomyopathy. Heart Rhythm March 2016;13:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med December 3 2013;159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal V, Vittinghoff E, Whitman IR, Dewland TA, Dukes JW, Marcus GM. Relation Between Ventricular Premature Complexes and Incident Heart Failure. Am J Cardiol April 15 2017;119:1238–1242. [DOI] [PubMed] [Google Scholar]


