Abstract
Ovarian cancer is the eighth most common cancer in women worldwide and incidence rates vary markedly by world region. This study provides a comprehensive overview of ovarian cancer incidence trends globally, examining the influence of birth cohort and period of diagnosis on changing risk. We presented current patterns and trends of ovarian cancer incidence until 2012 using data from successive volumes of Cancer Incidence in Five Contents. The incidence of ovarian cancer is highest in northern and eastern European countries, and in northern America. Declining trends were observed in most countries with the exception of a few central and eastern Asian countries. Marked declines were seen in Europe and North America for women aged 50–74 where rates have declined up to 2.4% (95% CI: −3.9, ‒0.9) annually in Denmark over the last decade. Additionally, declines in the incidence rate ratio (IRR) were observed for generations born after the 1930s, with an additional strong period effect seen around 2000 in United States and Denmark. In contrast, IRRs increased among younger generations born after the 1950s in Japan and Belarus. Overall, the favourable trends in ovarian cancer incidence is likely due to the increase use of oral contraceptive pills, and changes in the prevalence of other reproductive risk and protective factors for ovarian cancer over the years studied. Changes in disease classifications and cancer registry practices may also partially contribute to the variation in ovarian cancer incidence rates. Thus, continuous cancer surveillance is essential to detect the shifting patterns of ovarian cancer.
Keywords: ovarian cancer, global incidence trends, age-period-cohort
Introduction
There were an estimated 300,000 new cases of ovarian cancer diagnosed worldwide in 20181, corresponding to 3.4% of all cancer cases among women. Although there is substantial geographic variation in the burden of ovarian cancer (rates varying from 5.0 per 100,000 person-years in Africa to 9.5 per 100,000 person-years in Europe1), gradual declines in incidence have been observed in most countries in Europe (e.g. Denmark, Norway, and France) and in North America (e.g. United States and Canada), where incidence has been historically higher compared to less developed regions2. Nevertheless, many countries in these regions, such as Belarus, Poland and Czech Republic, continue to have a high incidence of ovarian cancer compared to other regions of the world3. Recently, however, a steady increase in ovarian cancer has been observed in some Asian countries that previously had low rates, such as Japan or India3.
The reasons for differences in ovarian cancer rates between countries is likely multifactorial. Family history and reproductive factors, including a lower number of children and the use of menopausal hormone therapy, increase a woman’s risk of ovarian cancer4. In contrast, the use of oral contraceptives (OC), breastfeeding, and tubal ligation may be protective4. In particular, the widespread use of OC has played an important role in the decline of ovarian cancer incidence over the past few decades5, 6.
The main purpose of this article is to provide an overview of the latest patterns and trends of ovarian cancer worldwide. We examined the incidence of ovarian cancer globally using national estimates and population-based cancer registry data recorded in 27 different countries. We also assessed the influence of birth cohort and period of diagnosis on the secular trends to better identify factors underlying the changing trends.
Materials and Methods
Data source
Incidence data on primary malignant ovarian cancer (International Classification o f Disease for Oncology, 3rd edition (ICD-O-3): C56) was obtained from Cancer Incidence in Five Continents (CI5) plus database (http://ci5.iarc.fr/CI5plus), which contains high-quality global cancer incidence data provided by national and subnational population-based cancer registries (PBCR) worldwide7. PBCRs with 25 or more consecutive years of incidence data were included, yielding a total of 53 PBCRs from 30 countries eligible for analysis. Multiple subnational cancer registries in the same countries were combined, and countries with population coverage of less than 500,000 females were excluded in the analysis, namely, Austria (Tyrol), Germany (Saarland) and Iceland. Hong Kong, China was also excluded due to the inconsistency of the data from 1983–2000. In addition, a few high quality registries had to be excluded due to the unavailability of the data in the public CI5 database for the last 5-year period. After all the exclusions, a total of 27 countries remained in the analysis.
Statistical analysis
Truncated age-standardized incidence rates (ASR, per 100,000 person-years) for two broad age groups (25–49 and 50–74 years) were calculated per calendar year using the World Standard population8, 9. A separate analysis was performed to calculate the truncated age-standardized incidence rates for women 75+ years. Incidence rates were plotted on a log-scale, and trend lines were smoothed with a smoothing coefficient of 0.5. The estimated annual percent change (EAPC) and corresponding 95% confidence intervals were computed for the latest 15-year period (1998–2012) to summarize the change in incidence for the most recent years.
To examine the effects of birth cohorts and period by 5-year interval, age-period-cohort (APC) analysis was performed for five selected countries representing different world regions, namely, Australia, Belarus, Japan, Denmark and United States. Age-specific incidence rates were plotted against the estimated year of birth and year of diagnosis. Due to the linear dependency of age, period and cohort, the effects of these three factors cannot be analysed simultaneously. In this analysis, a method developed by Holford10 was applied to calculate the incidence rate ratio (IRR) using the 1998–2002 period of diagnosis as the reference period and 1950 as the reference birth cohort. To examine the cohort effect, the period effect was constrained to zero on average with an assumption that the linear change in rates is due to the influence of birth cohort. A similar method was applied to examine the influence of period to the secular trend. The apcfit command in Stata was used, a restricted cubic splines model was fitted with age, period and cohort as continuous variables11. The goodness-of-fit of the models was assessed using the analysis of deviance of nested models as described by Clayton and Schifflers12, 13, and the importance of non-linear cohort and period effects were examined using the likelihood ratio test. Findings were considered statistically significant at a p-value of <0.05. All analyses were performed using Stata/IC version 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Incidence trends of ovarian cancer
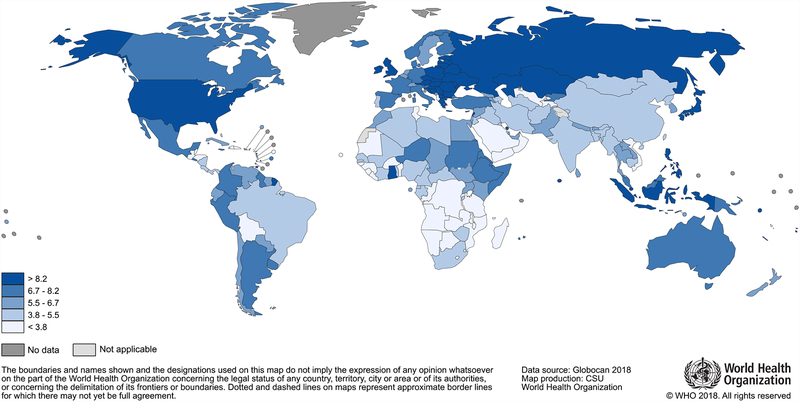
Figure 1 illustrates the estimated ovarian cancer incidence rates by country worldwide from GLOBOCAN 2018. Highest rates in 2018 (ranging from 8.2 to 16.6 per 100,000 person-years) were estimated mostly in Eastern Europe (e.g. Serbia, Belarus, and Poland). Table 1 presents the total number of observed cases and ASRs in countries with long-term ovarian cancer data for the last 5-year period (2008–2012) for pre- and post-menopausal age groups: 25–49 years and 50–74 years. Overall, European countries had the highest incidence of ovarian cancer among women ages 50–74 years, ranging from 27.1 per 100,000 person-years in Spain to 44.9 in Lithuania. In the more recent 5–year period, Belarus (ASR=14.8) had the highest incidence in the younger age group (25–49 years), followed by Lithuania (ASR=13.7). Switzerland (ASR=4.2) had the lowest incidence of ovarian cancer, followed by black women in the United States (ASR=4.5).
Figure 1.
Estimated age-standardized incidence rates (world standard, per 100,000 person-years) for ovarian cancer in 2018.
Table 1.
Age-standardized incidence rates (per 100,000 person-years) of ovarian cancer for 2008–2012¶, by age groups
| Country | Population coverage (million) | Period | 24–49 years | 50–74 years | |||
|---|---|---|---|---|---|---|---|
| Start | End | Cases | ASR | Cases | ASR | ||
| Asia | |||||||
| China (Shanghai) | 3.1 | 1988 | 2012 | 353 | 6.2 | 1143 | 19.2 |
| India (Chennai) | 2.3 | 1983 | 2012 | 303 | 6.9 | 498 | 25.7 |
| Israel† | 3.9 | 1963 | 2012 | 313 | 5.4 | 1093 | 28.0 |
| Japan‡ | 6.6 | 1978 | 2010 | 577 | 8.8 | 1454 | 23.2 |
| Philippines (Manila) | 3.3 | 1983 | 2012 | 631 | 11.4 | 838 | 37.6 |
| Thailand (Chiang Mai) | 0.8 | 1983 | 2012 | 111 | 6.9 | 180 | 17.6 |
| Australia/ New Zealand | |||||||
| Australia‡ | 8.6 | 1983 | 2012 | 865 | 5.4 | 2894 | 25.5 |
| New Zealand† | 2.2 | 1983 | 2012 | 249 | 6.1 | 799 | 28.3 |
| Central and South America | |||||||
| Colombia (Cali) | 1.2 | 1983 | 2012 | 146 | 6.6 | 273 | 24.9 |
| Costa Rica† | 2.2 | 1982 | 2011 | 160 | 4.9 | 229 | 15.5 |
| Ecuador (Quito) | 0.8 | 1985 | 2012 | 79 | 5.5 | 153 | 23.4 |
| North America | |||||||
| Canada‡ | 13.1 | 1983 | 2012 | 1726 | 6.8 | 5111 | 28.3 |
| United States‡: Black | 2.0 | 1978 | 2012 | 163 | 4.5 | 504 | 25.1 |
| United States‡: White | 10.8 | 1341 | 6.9 | 4744 | 32.0 | ||
| Eastern Europe | |||||||
| Belarus† | 5.1 | 1983 | 2012 | 1404 | 14.8 | 2775 | 37.2 |
| Slovakia† | 2.8 | 1971 | 2010 | 304 | 10.0 | 899 | 38.5 |
| Northern Europe | |||||||
| Denmark† | 2.8 | 1953 | 2012 | 311 | 6.1 | 1670 | 38.9 |
| Estonia† | 0.7 | 1983 | 2012 | 111 | 9.1 | 440 | 38.2 |
| Lithuania† | 1.7 | 1988 | 2012 | 436 | 13.7 | 1171 | 44.9 |
| Norway† | 2.4 | 1953 | 2012 | 255 | 5.8 | 1259 | 38.5 |
| United Kingdom (Scotland) | 2.7 | 1978 | 2012 | 336 | 6.6 | 1400 | 34.3 |
| Southern Europe | |||||||
| Croatia† | 2.3 | 1988 | 2012 | 437 | 10.9 | 1442 | 40.4 |
| Italy‡ | 1.5 | 1988 | 2012 | 190 | 7.2 | 645 | 30.9 |
| Slovenia† | 1.0 | 1983 | 2012 | 164 | 8.4 | 473 | 30.5 |
| Spain‡ | 3.0 | 1988 | 2010 | 241 | 6.7 | 648 | 27.1 |
| Western Europe | |||||||
| France‡ | 3.0 | 1983 | 2011 | 243 | 5.7 | 958 | 28.6 |
| Switzerland‡ | 1.0 | 1988 | 2012 | 79 | 4.2 | 400 | 29.7 |
Costa Rica (2008–2011); France (2008–2011); Japan (2008–2010); Slovakia (2008–2010); Spam (2008–2010)
National cancer registry
Australia - New South Wales, Tasmania, South Australia, Victoria, and Western Australia; Canada excludes Nunavut, Quebec and Yukon; France - Bas-Rhin, Calvados, Doubs, Haut-Rhin, Herault, Isere, and Somme; Italy - Modena, Parma, Ragusa, and Romagna; Japan - Miyagi, Nagasaki, and Osaka; Spain - Basque Country, Granada, Murcia, Navarra and Tarragona; Swi tzerland - Geneva, Neuchatel, St. Gall-Appenzell, and Vaud; United States - Georgia, Greater California, Idaho, Kentucky, Louisiana, Massachusetts, New York, Utah and Wisconsin (SEER-9)
Assessing the long-term trends among women aged 25–49 years, a clear declining trend were observed in several high-income countries, such as Australia, New Zealand, United States (White), Canada, Scotland, Norway, Denmark and France (Figure 2A). On the other hand, over the last 15 years most changes are statistically insignificant (Figure 3A & Table S1). Meanwhile, among women aged 50–74 years, marked declines in ovarian cancer incidence were observed in almost all countries, particularly in Northern and Western Europe, North America, as well as, in Australia and New Zealand (Figure 2B). The highest decrease in this region was seen in Denmark with an estimated annual percent change of −2.4% (95% CI=−3.9, −0.9) (Figure 3B & Table S1). Incidence trends in countries in economic transition were not significantly different over time in the last 15 years, although rising incidence rates were observed among post-menopausal Japanese and Indian women. As for the oldest age group, women aged 75+, incidence trends were generally stable (Figure S1 & S2).
Figure 2.
Age-standardized incidence rates (ASR, per 100,000 person-years, log scale) of ovarian cancer by age groups. Two age groups: (A) 25–49 years and (B) 50–74 years. Countries included: (Asia) CHN=China, IND=India, ISR=Israel, JPN=Japan, PHL=Philippines, THA=Thailand; (Oceania) AUS=Australia, NZL=New Zealand; (North America) CAN=Canada, USA=United States; (Central & South America) COL=Colombia, CRI=Costa Rica, ECU=Ecuador; (Eastern Europe) BLR=Belarus, CZE=Czech Republic, SVK=Slovakia; (Northern Europe) DNK=Denmark, EST=Estonia, LTU=Lithuania, NOR=Norway, SCO=Scotland; (Southern Europe) ESP=Spain, HRV=Croatia, ITA=Italy, SVN=Slovenia; (Western Europe) CHE=Switzerland, FRA=France.
Figure 3.
Estimated annual percent change (EAPC, %) of ovarian cancer between 1998 and 2012 by age groups. Costa Rica and France only until 2011; Japan, Slovakia, and Spain only until 2010. Statistically significant EAPC, 95% confidence interval not including zero, indicated by (*).
Birth cohort effects
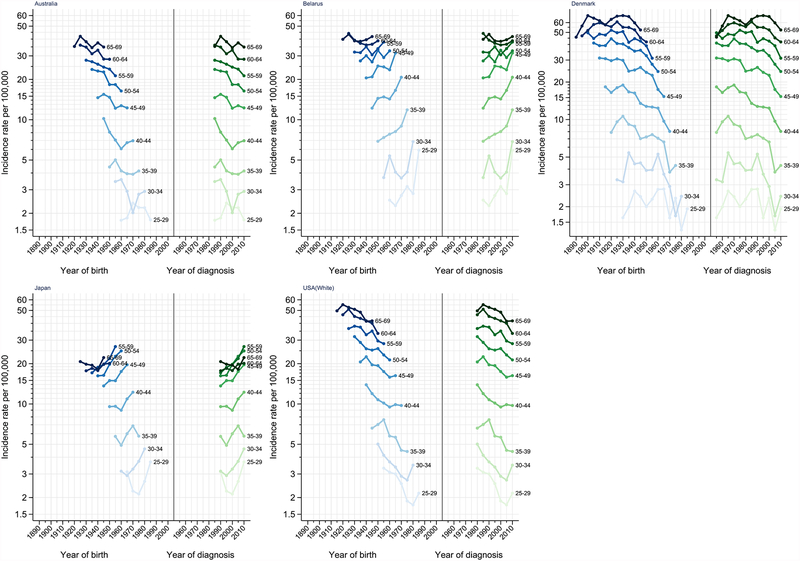
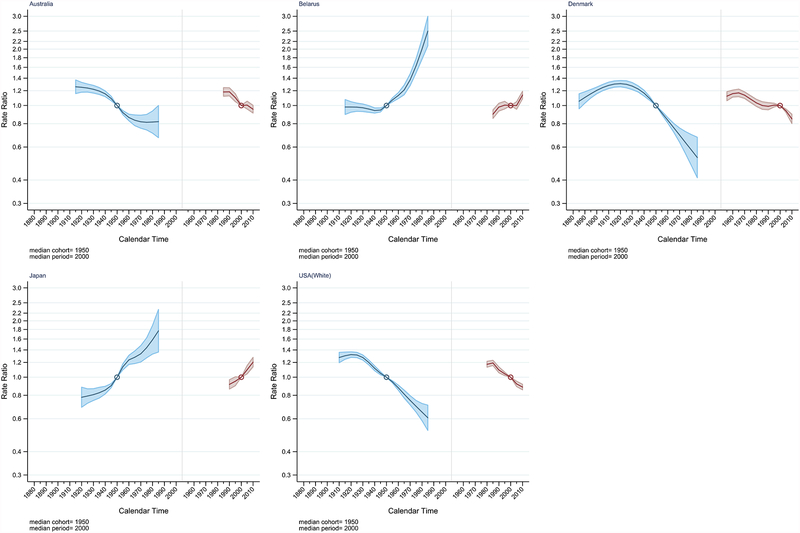
APC analysis was performed on five selected countries in the study: Australia, Belarus, Denmark, Japan, and United States (White). The full APC model was the best fitting model for all five countries (Table S2). Figure 4 illustrates the age-specific incidence rate of ovarian cancer by 5-year age group plotted against the year of birth (period effect) and years of diagnosis (cohort effect). The figure shows declining incidence rates of ovarian cancer for the majority of age groups among birth cohorts born after 1930 in Australia, Denmark and United States. Additionally, the United States and Denmark exhibited decreasing trend in IRR among birth cohorts born after 1930 that continued to the youngest cohort (IRR=0.61 [95% CI=0.52, 0.71] and IRR=0.53 [95% CI=0.41, 0.68] vs the 1950 birth cohort) (Figure 5 & Table S3). In comparison, the descending trend of IRR in Australia slowly levelled-off among the younger birth cohorts starting with cohorts born around 1965 (IRR=0.83, 95% CI=0.78, 0.89). Meanwhile, a consistent increase of IRR was observed across birth cohorts in Belarus born after 1950. An IRR of 2.50 (95% CI=2.09, 3.00) was observed in the youngest cohort born around 1985 compared to the reference birth cohort (1950). A steady rise of IRR across successive birth cohorts was also detected in Japan starting with the 1920 birth cohorts.
Figure 4.
Age-specific incidence rates for ovarian cancer by year of birth (cohort) and year of diagnosis (period). Australia includes New South Wales, Tasmania, Victoria, South Australia, and Western Australia. United States includes Georgia, Greater California, Idaho, Kentucky, Louisiana, Massachusetts, New York, Utah, and Wisconsin (SEER-9).
Figure 5.
Ovarian cancer incidence rate ratio for cohort (blue) and period (red) for selected countries. Australia includes New South Wales, Tasmania, Victoria, South Australia, and Western Australia. United States includes Georgia, Greater California, Idaho, Kentucky, Louisiana, Massachusetts, New York, Utah, and Wisconsin (SEER-9).
Period effect to the secular trend
Denmark and the United States also exhibited declining IRRs across periods of diagnosis after the year 2000 indicating possible period effect (Figure 4, 5 & Table S4). In contrast, IRRs in Japan has been steadily increasing across all periods of diagnosis, however, the non-linear period effect was not significant (Table S2). The IRR in Belarus was relatively stable until 2010 when an upsurge (IRR=1.14, 95% CI=1.09, 1.19 vs the 1998–2002 period) was observed.
Prevalence of selected risk and protective factors for ovarian cancer
Figure S3 illustrates the fertility rates by calendar year using data from World Bank Open Data (https://data.worldbank.org/), as well as the estimated prevalence of OC use in 2015 among married or in-union women aged 15–49 (based on data published by the United Nations, Population Division14) for selected countries plotted with their ovarian cancer ASRs. Similarly, Figure S4 shows the prevalence of obesity from 1988 to 2012 among women aged 20 and above using data from the NCD Risk Factor Collaboration (http://ncdrisc.org/).
Discussion
The current study provides a comprehensive overview of recent global burden and long-term trends in ovarian cancer incidence across 27 countries. Ovarian cancer occurs largely in post-menopausal women with incidence levels higher in European and North American countries relative to countries in Asia, Central and South America in the 50–74 years age group. For the past few decades, in most countries where rates were traditionally high, overall declines in incidence rates were observed particularly in post-menopausal women aged 50–74 years. Among the five countries analysed for APC, cohort and period effects were observed in Denmark and the United States. In contrast, large increases in ovarian cancer incidence rates were distinctly observed in Japan and Belarus, which were linked to generational changes over the birth cohorts.
Marked decreases in ovarian cancer were seen in Europe, North America, Australia and New Zealand have been reported in other studies and are driven by the changing incidence in post-menopausal women, potentially linked to the widespread use of OC5, 15, 16. A previous study observed a decrease in ovarian cancer incidence starting in cohorts born around the 1920s in Australia and the United States, which was suggested to be driven by the use of OC starting around the 1960s5. Moreover, several epidemiological studies showed that the use of OC is associated with a significant decrease in ovarian cancer with an estimated reduction of approximately 30–50% for OC use of at least five years, and the protective effect persisting at least 10–15 years since last use4, 17–19. Over the last decades decline in OC use and a slight increase in use of other methods of contraceptives14, 20, 21, as well as changes in OC formulations16, may impact future patterns and trends of ovarian cancer. Nonetheless, recent findings from a Danish prospective study showed reduced risks of ovarian cancer in women using any contemporary hormonal contraception, including various type of OC, patch, vaginal ring, and implants22 suggesting that the decrease in ovarian cancer may continue.
High-income countries in the current study generally exhibited declining incidence of ovarian cancer, except for Japan and Belarus where rates have markedly increased. A previous study has reported a steady increase of ovarian cancer in Niigata, Japan from 1983 to 200723. The observed trend in Japan may partly be explained by the low level of OC use among Japanese women. In 2015, the prevalence of OC use among married or in-union women aged 15–49 years in Japan was only1.1%, which is dramatically lower compared to other high-income countries such as the United States (16.0%) and France (39.5%)14. Another potential explanation is lower parity, for instance, studies have shown that a history of one or more full-term pregnancies decreases the risk of ovarian cancer in women, and with further reduction in risk for each additional pregnancy4, 17, 18. This might explain the increasing rates especially in the younger cohorts also in countries such as India and Thailand. We also observed that mucinous carcinoma, which is less closely linked to OC use19, was more commonly seen in Asia and South American countries (Figure S5 shows the distribution of histological groups in the current study for periods 1988–1992 and 2008–2012 by country). Adoption of western lifestyle in these countries may thus partly contribute to the increasing incidence24, 25. Hence, continued surveillance remains relevant to detect early changes in the incidence trends, particularly in the younger generations.
The current study also showed a continuous decrease of incidence in Israel. Israel has high frequency of BRCA1 and BRCA2 mutations, specifically among the Ashkenazi Jewish population with an estimated prevalence of 1 in 4026, 27. The cumulative cancer risk for ovarian cancer up to age 70 years is estimated to be approximately 40% for BRCA1 carriers and 18% for BRCA2 carriers27. High rates of risk reducing surgery for ovarian cancer in Israeli women28 with BRCA mutation may contribute to the decreasing trend observed in the country.
Other reproductive factors, such as age at menarche, age at menopause, tubal ligation, and use of menopausal hormone therapy4, 17, 29, 30, may also contribute to observed patterns in ovarian cancer incidence. Asides from reproductive risk factors, body mass index (BMI) has also been associated with ovarian cancer risk31, 32. Particularly, findings from a pooled study showed an association between BMI and risk of non-serous and low-grade serous ovarian cancer33. Thus, the rise of obesity may potentially contribute to the increase of incidence observed in the study, particularly in the younger generations, however further study is warranted.
The period effect observed in the study may be partially explained by changes in disease classifications and cancer registry practices, which impact the incidence rates of ovarian cancer for the entire population. In the study, a noticeable increase in incidence rates across all age groups was observed between the last two consecutive periods in Belarus, indicating a possible period effect perhaps due to changes in registration practices over time. On the other hand, decreases in incidence rates across women ages 25–64 years were observed in Denmark and United States between the period 2000 and 2005. This decline may likely be due to changes in the disease classification. The ICD-O 2nd edition (ICD-O-2) was published by the World Health Organization in 1990, and the most recent version, ICD-O 3rd edition (ICD-O-3), was published in 2000. In ICD-O-3 ovarian tumors with borderline malignancy or low malignant potential were no longer considered as malignant tumors. Generally, the incidence of borderline tumors ranged from 14–15% out of the total incidence of primary ovarian cancer neoplasms34. Thus, the observed period effects in Denmark and United States may be a reflection of the transition of the cancer registries to ICD-O-3 in early 2000.
Ovarian cancer mortality has also been persistently decreasing over time35, although the decline has been at a lower magnitude compared to the steep decrease in the incidence rates. The majority of women with ovarian cancer are diagnosed with advanced stage36, and there are no effective early detection or screening methods37, 38. Overall, ovarian cancer has low survival, and previous large population-based cancer survival studies have only shown slight improvements in ovarian cancer survival over the past decades39–41. Thus, decrease in mortality rates are likely due to the decreasing ovarian cancer incidence rather than improvements in survival.
The main strength of the study is the utilization of CI5 data. Only long established and reliable PBCRs with 25 consecutive years of data were included in the study to assess trends in incidence and to perform the APC analysis. The restrictive time inclusion, however, limits the number of countries represented in the study, and excluded most low- and middle-income countries, as well as limiting the number of PBCRs included in countries with sub-national cancer registries. Therefore, trends reported in this study from countries with sub-national cancer registries may not fully reflect the incidence trends experienced by these specific countries. In addition, women less than 25 years of age were excluded in the study due to very low number of cases leading to unstable ASRs. Ovarian cancer in this age group is uncommon, and are likely non-sporadic and linked to genetic factors. The study is also limited by the small number of cases in the younger age group resulting in wider IRR confidence intervals in the younger cohorts.
The study only had data for C56, malignant neoplasm of the ovary, while the more rare fallopian tubes and peritoneal primary carcinomas originating from the ovary were not accounted for in the current incidence estimate. Data from previous studies have suggested that many serous ovarian cancers likely originated from fallopian tubes42. Thus, the number of ovarian cancers coded as fallopian tubes may have increased recently, which may lead to the underestimation of the incidence in the most recent years of the study. Furthermore, previous studies showed that borderline ovarian cancer has been misclassified as carcinoma with misclassification ranging between 9–21%43, which may result in an overestimation of the true incidence in some registries. Finally, the data presented in our study lacks individual records on the risk and protective factors for ovarian cancer, therefore a firm conclusion regarding the true causes of the observed trends could not be drawn. Despite these limitations, the data used in this study were very high quality from a reliable database that allowed for valid comparisons across countries.
In conclusion, the current study provided an overview of ovarian cancer trends worldwide and examined the influence of birth cohort and period on secular trends. In general, a marked decline of ovarian cancer incidence among post-menopausal women was observed for over three decades in the majority of the countries in the study, which is likely due to the widespread use of OC. Meanwhile, less geographic and temporal variability was observed on the incidence trends of pre-menopausal women. Differences in incidence trends can partly be explained by variations in the prevalence of several reproductive risk and protective factors, as well as, changes in classification and cancer registry practices. Hence, on-going cancer surveillance plays an essential role in detecting early shifts in the incidence trends of ovarian cancer.
Supplementary Material
Figure S1. Age-standardized rates (per 100,000 person-years, log scale) of ovarian cancer among women 75+ years. Countries included: (Asia) CHN=China, IND=India, ISR=Israel, JPN=Japan, PHL=Philippines, THA=Thailand; (Oceania) AUS=Australia, NZL=New Zealand; (North America) CAN=Canada, USA=United States; (Central & South America) COL=Colombia, CRI=Costa Rica, ECU=Ecuador; (Eastern Europe) BLR=Belarus, CZE=Czech Republic, SVK=Slovakia; (Northern Europe) DNK=Denmark, EST=Estonia, LTU=Lithuania, NOR=Norway, SCO=Scotland; (Southern Europe) ESP=Spain, HRV=Croatia, ITA=Italy, SVN=Slovenia; (Western Europe) CHE=Switzerland, FRA=France.
Figure S2. Estimated annual percent change (EAPC, %) of ovarian cancer between 1998 and 2012 among women 75+ years. Costa Rica and France only until 2011; Japan, Slovakia, and Spain only until 2010. Statistically significant EAPC, 95% confidence interval not including zero, indicated by (*).
Figure S3. Ovarian cancer age-standardized incidence rates (per 100,000) plotted with fertility rates and estimated prevalence (%) of oral contraceptive use in 2015 for selected countries in the study. Note: Fertility rates data were derived from the World Bank Open Data (https://data.worldbank.org/). Estimated prevalence of oral contraceptive in 2015 were obtained from the report by UN Population Division14.
Figure S4. Ovarian cancer age-standardized incidence rates (per 100,000) plotted with prevalence of obesity (5) from 1988 to 2012 by country. Note: Data on the prevalence of obesity were derived from the NCD Risk Factor Collaboration network (http://ncdrisc.org/).
Figure S5. Distribution of ovarian cancer by histology groups and countries for periods 1988–1992 and 2008–2012. Note: Histological groups were adopted from the histological groupings provided in the volumes of Cancer Incidence in Five Contents that is based on the ICD-O system. Details on histology groups were not available for Belarus and Lithuania. ‘Other’ histology group includes sex cord-stromal tumor, germ cell tumor, and other specified morphology.
Novelty and Impact.
We provide an overview of international ovarian cancer patterns and trends in 27 countries using latest national estimates of incidence and recorded incidence from successive Volumes of Cancer Incidence in Five Continents. The influence of birth cohort and calendar period was examined in selected countries to better identify factors underlying the changing trends. We postulate on the reasons for the divergent trends in different populations, particularly the use of oral contraceptives and changing registration practice.
Financial support:
This work was supported by the International Agency for Research on Cancer. Dr. Trabert’s contribution was supported by the intramural research program of the U.S. National Cancer Institute.
Abbreviations:
- A
age
- Ad
age + drift
- AP
age + period
- AC
age + cohort
- APC
age-period-cohort
- ASR
age-standardized incidence rates
- BMI
body mass index
- BRCA1
breast cancer 1
- BRCA2
breast cancer 2
- CI
confidence interval
- CI5
Cancer Incidence in Five Continents
- df
degrees of freedom
- EAPC
estimated annual percent change
- ICD-O
International Classification of Disease for Oncology
- IRR
incidence rate ratio
- NCD
non-communicable disease
- NL
non-linear
- OC
oral contraceptive
- PBCR
population-based cancer registry
- SEER
Surveillance, Epidemiology, and End Result
- UN
United Nations
- AUS
Australia
- BLR
Belarus
- CAN
Canada
- CHE
Switzerland
- CHN
China
- COL
Colombia
- CRI
Costa Rica
- CZE
Czech Republic
- DNK
Denmark
- ECU
Ecuador
- ESP
Spain
- EST
Estonia
- FRA
France
- HRV
Croatia
- IND
India
- ISR
Israel
- ITA
Italy
- JPN
Japan
- LTU
Lithuania
- NOR
Norway
- NZL
New Zealand
- PHL
Philippines
- SCO
Scotland
- SVK
Slovakia
- SVN
Slovenia
- THA
Thailand
- USA (B/W)
United States (Black/White)
Footnotes
Disclosure Statement: None of the authors have any potential conflicts (financial, professional, or personal) related to the manuscript to disclose.
References
- 1.Cancer Today, vol. 2018: International Agency for Research on Cancer.
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136: E359–86. [DOI] [PubMed] [Google Scholar]
- 3.Coburn SB, Bray F, Sherman ME, Trabert B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer 2017;140: 2451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, Setiawan VW, Visvanathan K, Weiderpass E, Adami HO, Black A, Bernstein L, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016;34: 2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb PM, Green AC, Jordan SJ. Trends in hormone use and ovarian cancer incidence in US white and Australian women: implications for the future. Cancer Causes Control 2017;28: 365–70. [DOI] [PubMed] [Google Scholar]
- 6.Gatta G, Lasota MB, Verdecchia A. Survival of European women with gynaecological tumours, during the period 1978–1989. EUROCARE Working Group. Eur J Cancer 1998;34: 2218–25. [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, Pineros M, Steliarova-Foucher E, Swaminathan R, Antoni S, Soerjomataram I, Forman D. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer 2015;137: 2060–71. [DOI] [PubMed] [Google Scholar]
- 8.Doll Richard P P, Waterhouse John, Cancer Incidence in Five Continents: A Technical Report, 1966.
- 9.Segi M, Cancer Mortality for Selected Sites in 24 Countries (1950–57). Tohoku University of Medicine, 1960. [Google Scholar]
- 10.Holford TR. Analysing the temporal effects of age, period and cohort. StatMethods Med Res 1992;1: 317–37. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford MJ LP, Thompson JR. Age-period-cohort modeling. Stata Journal 2010;10:606–27. [Google Scholar]
- 12.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med 1987;6: 469–81. [DOI] [PubMed] [Google Scholar]
- 13.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med 1987;6: 449–67. [DOI] [PubMed] [Google Scholar]
- 14.Trends in Contraceptive Use Worldwide 2015. United Nations, Department of Economic and Social Affairs, Population Division, 2015.
- 15.Bray F, Loos AH, Tognazzo S, La Vecchia C. Ovarian cancer in Europe: Cross-sectional trends in incidence and mortality in 28 countries, 1953–2000. Int J Cancer 2005;113: 977–90. [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology Working Group Steering Committee OCACMotEWGSCiao, Doherty JA, Jensen A, Kelemen LE, Pearce CL, Poole E, Schildkraut JM, Terry KL, Tworoger SS, Webb PM, Wentzensen N. Current Gaps in Ovarian Cancer Epidemiology: The Need for New Population-Based Research. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol 1992;136: 1184–203. [DOI] [PubMed] [Google Scholar]
- 18.Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev 2005;14: 98–107. [PubMed] [Google Scholar]
- 19.Collaborative Group on Epidemiological Studies of Ovarian C, Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008;371: 303–14. [DOI] [PubMed] [Google Scholar]
- 20.Joshi R, Khadilkar S, Patel M. Global trends in use of long-acting reversible and permanent methods of contraception: Seeking a balance. Int J Gynaecol Obstet 2015;131 Suppl 1: S60–3. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco-Garrido P, Lopez de Andres A, Hernandez-Barrera V, Jimenez-Trujillo I, Esteban-Pena M, Perez-Farinos N, Jimenez-Garcia R. Trends in the use of oral contraceptives among adolescents and young women in Spain. ReprodHealth 2016;13: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iversen L, Fielding S, Lidegaard O, Morch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018;362: k3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yahata T, Banzai C, Tanaka K, Niigata Gynecological Cancer R. Histology-specific long-term trends in the incidence of ovarian cancer and borderline tumor in Japanese females: a population-based study from 1983 to 2007 in Niigata. J Obstet Gynaecol Res 2012;38: 645–50. [DOI] [PubMed] [Google Scholar]
- 24.Kato I, Tominaga S, Kuroishi T. Relationship between westernization of dietary habits and mortality from breast and ovarian cancers in Japan. Jpn J Cancer Res 1987;78: 349–57. [PubMed] [Google Scholar]
- 25.Khan MM, Khan A, Nojima M, Suzuki S, Fujino Y, Tokudome S, Tamakoshi K, Mori M, Tamakoshi A. Ovarian cancer mortality among women aged 40–79 years in relation to reproductive factors and body mass index: latest evidence from the Japan Collaborative Cohort study. J Gynecol Oncol 2013;24: 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, Beeri R, Gal M, Grinshpun-Cohen J, Djemal K, Mandell JB, Lee MK, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A 2014;111: 14205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 2010;12: 245–59. [DOI] [PubMed] [Google Scholar]
- 28.Laitman Y, Vaisman Y, Feldman D, Helpman L, Gitly M, Paluch Shimon S, Berger R, Cohen L, Narod SA, Friedman E. Rates of risk-reducing surgery in Israeli BRCA1 and BRCA2 mutation carriers. Clin Genet 2014;85: 68–71. [DOI] [PubMed] [Google Scholar]
- 29.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, Risch H, Wu AH, Webb PM, Moysich K, Doherty JA, Felberg A, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol 2013;42: 579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Liang Z, Liu X, Zhang Q, Li S. The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses. Int J Environ Res Public Health 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014;384: 755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer 2007;43: 690–709. [DOI] [PubMed] [Google Scholar]
- 33.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, Rossing MA, Terry KL, Wu AH, Risch HA, Yu H, Doherty JA, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer 2013;20: 251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skírnisdόttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer 2008;123: 1897–901. [DOI] [PubMed] [Google Scholar]
- 35.Malvezzi M, Carioli G, Rodriguez T, Negri E, La Vecchia C. Global trends and predictions in ovarian cancer mortality. Ann Oncol 2016;27: 2017–25. [DOI] [PubMed] [Google Scholar]
- 36.Chien J, Poole EM. Ovarian Cancer Prevention, Screening, and Early Detection: Report From the 11th Biennial Ovarian Cancer Research Symposium. Int J Gynecol Cancer 2017;27: S20–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs IJ, Menon U. Progress and challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics 2004;3: 355–66. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E, Cruickshank D, Crump DN, Davies SK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016;387: 945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, Bielska-Lasota M, Engholm G, et al. Cancer survival in Europe1999–2007 by country and age: results of EUROCARE−-5-a population-based study. Lancet Oncol 2014;15: 23–34. [DOI] [PubMed] [Google Scholar]
- 40.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, Bonaventure A, Valkov M, Johnson CJ, Esteve J, Ogunbiyi OJ, Azevedo ESG, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377: 127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klotz DM, Wimberger P. Cells of origin of ovarian cancer: ovarian surface epithelium or fallopian tube? Arch Gynecol Obstet 2017;296: 1055–62. [DOI] [PubMed] [Google Scholar]
- 43.Hannibal CG, Vang R, Junge J, Frederiksen K, Kjaerbye-Thygesen A, Andersen KK, Tabor A, Kurman RJ, Kjaer SK. A nationwide study of serous “borderline” ovarian tumors in Denmark 1978–2002: centralized pathology review and overall survival compared with the general population. Gynecol Oncol 2014;134: 267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Age-standardized rates (per 100,000 person-years, log scale) of ovarian cancer among women 75+ years. Countries included: (Asia) CHN=China, IND=India, ISR=Israel, JPN=Japan, PHL=Philippines, THA=Thailand; (Oceania) AUS=Australia, NZL=New Zealand; (North America) CAN=Canada, USA=United States; (Central & South America) COL=Colombia, CRI=Costa Rica, ECU=Ecuador; (Eastern Europe) BLR=Belarus, CZE=Czech Republic, SVK=Slovakia; (Northern Europe) DNK=Denmark, EST=Estonia, LTU=Lithuania, NOR=Norway, SCO=Scotland; (Southern Europe) ESP=Spain, HRV=Croatia, ITA=Italy, SVN=Slovenia; (Western Europe) CHE=Switzerland, FRA=France.
Figure S2. Estimated annual percent change (EAPC, %) of ovarian cancer between 1998 and 2012 among women 75+ years. Costa Rica and France only until 2011; Japan, Slovakia, and Spain only until 2010. Statistically significant EAPC, 95% confidence interval not including zero, indicated by (*).
Figure S3. Ovarian cancer age-standardized incidence rates (per 100,000) plotted with fertility rates and estimated prevalence (%) of oral contraceptive use in 2015 for selected countries in the study. Note: Fertility rates data were derived from the World Bank Open Data (https://data.worldbank.org/). Estimated prevalence of oral contraceptive in 2015 were obtained from the report by UN Population Division14.
Figure S4. Ovarian cancer age-standardized incidence rates (per 100,000) plotted with prevalence of obesity (5) from 1988 to 2012 by country. Note: Data on the prevalence of obesity were derived from the NCD Risk Factor Collaboration network (http://ncdrisc.org/).
Figure S5. Distribution of ovarian cancer by histology groups and countries for periods 1988–1992 and 2008–2012. Note: Histological groups were adopted from the histological groupings provided in the volumes of Cancer Incidence in Five Contents that is based on the ICD-O system. Details on histology groups were not available for Belarus and Lithuania. ‘Other’ histology group includes sex cord-stromal tumor, germ cell tumor, and other specified morphology.