Abstract
1) Predators and pathogens are fundamental components of ecological communities that have the potential to influence each other via their interactions with victims and to initiate density- and trait-mediated effects, including trophic cascades. Despite this, experimental tests of the healthy herds hypothesis, wherein predators influence pathogen transmission, are rare. Moreover, no studies have separated effects mediated by density vs. traits. Using a semi-natural mesocosm experiment, we investigated the interactive effects of predatory dragonfly larvae (caged or lethal [free-ranging]) and a viral pathogen, ranavirus, on larval amphibians (gray treefrogs and northern leopard frogs).
2) We determined the influence of predators on ranavirus transmission and the relative importance of density- and trait-mediated effects on observed patterns. Lethal predators reduced ranavirus infection prevalence by 57–83% compared to no-predator and caged-predator treatments. The healthy-herds effect was more strongly associated with reductions in tadpole density than behavioral responses to predators.
3) We also assessed whether ranavirus altered the responses of tadpoles to predators. In the absence of virus, tadpoles reduced activity levels and developed deeper tails in the presence of predators. However, there was no evidence that virus presence or infection altered responses to predators.
4) Finally, we compared the magnitude of trophic cascades initiated by individual and combined natural enemies. Lethal predators initiated a trophic cascade by reducing tadpole density, but caged predators and ranavirus did not. The absence of a virus-induced trophic cascade is ostensibly the consequence of limited virus-induced mortality and the ability of infected individuals to continue interacting within the community.
5) Our results provide support for the healthy herds hypothesis in amphibian communities. We uniquely demonstrate that density-mediated effects of predators outweigh trait-mediated effects in driving this pattern. Moreover, this study was one of the first to directly compare trophic cascades caused by predators and pathogens. Our results underscore the importance of examining the interactions between predators and pathogens in ecology.
Keywords: disease ecology, food web, higher order interaction, natural enemy ecology, non-consumptive effect, parasite
Graphical Abstract
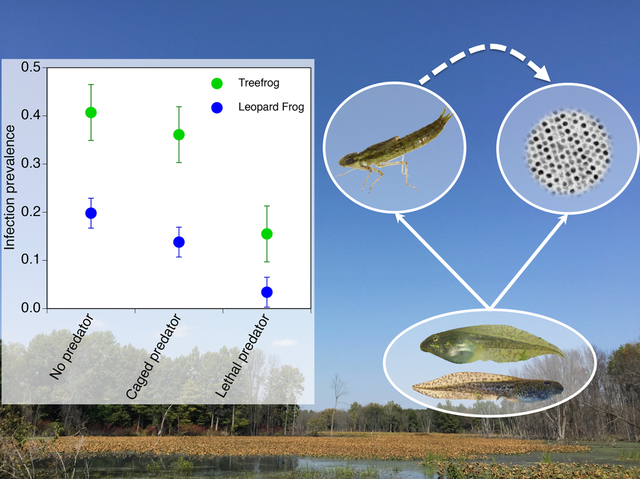
The authors provide empirical support for the healthy herds hypothesis, which has largely been explored using theory. Moreover, they uniquely assessed the relative importance of trait- vs. density-mediated effects in driving the pattern. Lastly, they directly compared trophic cascades initiated by predators and pathogens in the same food web.
Introduction
The influence of predators and pathogens (e.g., natural enemies) on their resources (i.e. prey and hosts) and associated communities has received considerable attention for decades (Abrams & Ginzburg, 2000; Hudson et al., 2002). Natural enemies can alter the structure and function of communities via direct and indirect interactions with their resources, and may also result in additive or synergistic effects on community dynamics through their interactions with one another (Werner & Peacor, 2003; Keesing, Holt & Ostfeld, 2006). Because predators and pathogens frequently co-occur in communities, there is a need to determine whether and how the simultaneous presence of multiple natural enemies influences food web dynamics to inform ecological theory and advance the field of natural enemy ecology.
Predators have the potential to positively or negatively influence disease dynamics through their effects on prey density and traits (e.g., phenotypic plasticity). For instance, a reduction in host density via predation can lower pathogen transmission by reducing contact rates between competent hosts (Lafferty, 2004; Ostfeld & Holt, 2004). Moreover, some predators selectively remove infected hosts from a system because afflicted individuals generally exhibit pathogen-induced morbidity that increases vulnerability to predation (Lefcort & Eiger, 1993; Packer et al., 2003; Joly & Messier, 2004; Johnson et al., 2006). These observations form the foundation of the healthy herds hypothesis, which posits that predators can reduce pathogen transmission and disease risk within communities (Packer et al., 2003). Beyond consumptive effects, predators also alter the traits of their prey in many systems (Lima, 1998; Werner & Peacor, 2003). Although such defensive strategies can reduce the risk of predation, they can also increase or decrease infection risk (Decaestecker, De Meester & Ebert, 2002; Duffy et al., 2011; Orlofske et al., 2012; Koprivnikar & Urichuk, 2017). While both density- and trait-mediated effects of predators on host-pathogen interactions have been explored, the relative contributions of these effects to disease dynamics are rarely examined (Raffel, Martin & Rohr, 2008; Hawlena, Abramsky & Bouskila, 2010). Moreover, theoretical models of the healthy herds effect have largely focused on density-mediated effects of predators (Bertram et al., 2013). Because the magnitude and direction of trait-mediated effects of predators may interact with density-mediated effects, research addressing these effects is critical to predict the influence of predators on disease dynamics and inform conservation and management strategies.
Similar to predators, pathogens can affect rates of predation by inducing changes in traits and reducing host densities. The ability of trophically-transmitted parasites to manipulate host behavior to increase predation rates, and transmission to a definitive host, is well-documented (Cezilly, Gregoire & Bertin, 2000). Conversely, predators might avoid infected prey, particularly if the pathogen is infectious to them (Meyling & Pell, 2006). Although behavioral changes are often induced in order to maximize pathogen fitness, some are the byproduct of infection (Adelman & Martin, 2009). Sickness behaviors such as lethargy and decreased foraging can have positive or negative effects on predation rates (Hoverman & Searle, 2016). Additionally, infection has the potential to alter the expression of inducible defenses due to resource investment trade-offs. In terms of density effects, theory suggests that pathogens regulate population oscillations associated with predator-prey relationships (Grenfell, 1992; Ives & Murray, 1997), with the extreme occurring when pathogen-induced crashes in prey populations concomitantly decrease predator populations that rely on the host (Calvete, 2006). Thus, pathogen-mediated mortality can alter predator-prey dynamics (Hatcher, Dick & Dunn, 2006). The diversity of potential effects of pathogens on predator-prey dynamics underscores both the challenge and importance of examining interactions among these natural enemies.
Natural enemies can cause trophic cascades by reducing species density (i.e. density-mediated indirect effect, DMIE) or altering species traits (i.e. trait-mediated indirect effect, TMIE). Both pathogens and predators can initiate trophic cascades (Werner & Peacor, 2003; Preisser, Bolnick & Benard, 2005; Ripple et al., 2016; Buck & Ripple, 2017), yet comparisons of these cascades in the same food web have received limited attention (Raffel, Martin & Rohr, 2008). A key difference between pathogen- and predator-induced trophic cascades may be in how quickly the cascade occurs. In predator-prey systems, consumed prey are immediately removed from the community, which can initiate a DMIE. Additionally, prey typically respond rapidly (e.g., seconds, hours) to the presence of predators by altering behavior (e.g., habitat use, activity level), which can contribute to the strength of trophic cascades. In contrast, we might expect pathogen-induced trophic cascades to develop more gradually due to the constraints of transmission and disease progression. Moreover, infections are not always lethal to the host; infected individuals can therefore continue to interact within their communities and contribute to food web dynamics. However, like predator-prey systems, there is evidence that hosts can detect the presence of pathogens or infected hosts (Buck, Weinstein & Young, 2018; Stephenson, Perkins & Cable, 2018; Weinstein, Buck & Young, 2018). To date, the magnitude of pre-encounter trait shifts in pathogen-host systems is largely unexplored relative to predator-prey systems. Given the potential for high costs associated with trait changes (e.g., altered foraging, physiological changes, immune system priming) and that most pathogens are not highly lethal to hosts, the magnitude of pre-encounter trait changes induced by pathogens could be smaller compared to predators. Collectively, these factors are expected to increase the time between initial introduction of the pathogen in the system and effects on host behavior and/or density that would be needed to initiate trophic cascades.
Amphibians provide an excellent system for studying natural enemy ecology because they commonly encounter concurrent predation and disease threats as larvae in aquatic communities. In particular, larval dragonflies (Anax spp.) and viral pathogens in the genus Ranavirus are widespread and common enemies in North American wetlands (Van Buskirk, 1988; Brunner et al., 2015). Moreover, there is a rich literature addressing the effects of these natural enemies on tadpoles. Larval dragonflies are generalist predators that can consume several tadpoles per hour (Relyea, 2001b). Tadpoles exhibit decreased activity and morphological changes (e.g., smaller bodies, deeper tails) in response to predator cues that function to reduce predation rates (Van Buskirk, 2002). At the community level, DMIEs and TMIEs of predators on primary producers have been documented in this system (Werner & Peacor, 2006). Ranaviruses are hemorrhagic viral pathogens of ectothermic vertebrates that have caused epizootics in numerous amphibian species across the globe (Duffus et al., 2015). Ranaviruses spread between individuals via direct contact, contaminated water, fomites, or necrophagy (Brunner, Schock & Collins, 2007). Individuals may become lethargic upon infection and mortality can occur 7–10 d post-infection (Hoverman et al., 2011). Importantly, the effects of ranaviruses on communities and food webs are unknown (Brunner et al., 2015).
We examined the interactive effects of predators and ranavirus on a tadpole assemblage using a semi-natural mesocosm experiment. Our first objective was to determine whether predators influence ranavirus transmission within the tadpole assemblage. We included caged and free-ranging (lethal) predator treatments to assess whether effects are mediated by changes in tadpole behavior or changes in tadpole density. Given that tadpoles generally reduce activity levels in the presence of predators and the role of direct contacts in ranavirus transmission (Relyea, 2001a; Brunner, Schock & Collins, 2007), we predicted that the presence of caged predators would decrease ranavirus prevalence in the assemblage. With lethal predators, we expected the combination of reduced tadpole activity and lower host densities to further reduce ranavirus transmission. Our second objective was to determine whether ranavirus infection alters the responses of tadpoles to predators. Because infection is likely to alter the allocation of energy and resources by the host (Lochmiller & Deerenberg, 2000), we predicted that the magnitude of inducible defenses would be reduced for exposed and infected individuals. Finally, we sought to quantify the strength of trophic cascades initiated by natural enemies by measuring primary productivity. As described above, we expected predators to initiate stronger trophic cascades than pathogens because of their immediate effects on tadpole mortality and potentially stronger effects on tadpole behavior.
Materials and methods
Focal species
Our amphibian assemblage included northern leopard frogs (Rana pipiens) and gray treefrogs (Hyla versicolor), which were collected from the Purdue Wildlife Area (PWA) in West Lafayette, Indiana, USA. We collected 8 partial leopard frog egg masses and 25 treefrog breeding pairs on 9 March and 9–10 May 2016, respectively. We housed leopard frog egg masses in separate, outdoor 200-L culture tanks and checked their health and development daily. For treefrogs, we collected and placed each breeding pair into a 15-L tub containing 8 L of UV-irradiated, filtered well water to oviposit. We maintained eggs in the tubs until hatching and then transferred them to culture tanks. We also included wood frogs (Rana sylvatica) to serve as the initial source of ranavirus infection in the experiment. We collected 10 partial wood frog egg masses on 9 March 2016 from a forested wetland in Nashville, Indiana, USA, and housed them in culture tanks. Once tadpoles were free-swimming, they were fed ad libitum with either Tetramin (for early stage treefrogs; Tetra, Virginia, USA) or rabbit chow (Purina, Missouri, USA) until used in the experiment.
Experimental setup
We conducted an outdoor mesocosm experiment at the PWA from May–June 2016. The experimental design was a factorial combination of two ranavirus treatments (present or absent) crossed with three predator treatments (absent, caged, or lethal). The six treatments were replicated 10 times for a total of 60 experimental units. Our experimental units were 1,200-L cattle tanks (Rubbermaid, Georgia, USA), filled with 500 L of well water on 2 and 3 May, and covered with 70% shade cloth lids. We arranged the tanks into a 5×12 grid and randomly assigned two replicates of each treatment to each of the five blocks. To each tank, we added 150 g of dry oak leaves (Quercus spp.) for refuge and 30 g of rabbit chow as an initial nutrient source. We also added 1 L of water from nearby ponds to inoculate the tanks with phytoplankton and periphyton and added 180 mL of water containing concentrated zooplankton. We sorted and removed all potential tadpole and zooplankton predators by hand and by straining through a 1 mm sieve. We added two clay tiles (10 × 10 cm) facing south against the inside of each tank to monitor periphyton growth during the experiment. We allowed the algal and zooplankton communities to establish for 3 wk prior to the start of the experiment. On 1 June, we added 30 leopard frogs (79.4 ± 5.6 mg; median Gosner stage 25, range 25–26) and 30 treefrogs (34.1 ± 1.7 mg; median Gosner stage 26, range 25–27) to each tank. In the laboratory, we set aside a sample of 30 individuals for each species to monitor mortality due to handling; all individuals survived for 24 hr.
For the virus treatments, we added previously-infected or -uninfected wood frog tadpoles to the experimental units. This approach simulates natural routes of ranavirus transmission (e.g., direct contact, necrophagy, shed virions in the water) and has proven successful in previous experiments (Wuerthner et al. 2017). We began by setting up 15-L tubs under a 12:12 day:night cycle at 21°C (n = 8 tubs) in the laboratory. The tubs were filled with 4 L of UV-irradiated, filtered well water 24 hr prior to introducing wood frogs to allow the water to equilibrate. To each tub, we then added 45 wood frog tadpoles (156.5 ± 9.4 mg; median Gosner stage 31, range 28–36). We used a ranavirus strain isolated from an infected green frog found at the PWA (Pochini and Hoverman 2017). We cultured the virus on fathead minnow cells and Eagle’s minimum essential media containing 5% fetal bovine serum (MEM) to a titer of 1.3 × 106 PFU mL−1. On 31 May, we inoculated four tubs with 3.076 mL of the ranavirus isolate to achieve a final concentration 103 PFU mL−1. The remaining tubs received 3.076 mL of sterile MEM and served as our controls. After 24 hours, we added 3 L of water to the tubs to bring the volume to 7 L and the tadpoles were maintained in the laboratory for 3 d before being released into the experimental units. On 3 June (day 1 of experiment), we pooled together all individuals that were exposed to virus in one tub and those not exposed to virus into a separate tub. We then randomly selected five infected individuals for addition to each virus tank and five uninfected individuals for addition to the no-virus tanks. In the laboratory, we set aside a sample of 20 individuals per exposure treatment to monitor mortality due to handling; all individuals survived for 24 hr. We also tested the 20 individuals exposed to ranavirus to determine infection status (described below); all individuals tested positive.
Our focal predators were dragonfly larvae (Anax spp.), which were collected from nearby permanent ponds at PWA. Individuals were housed in 1-L containers filled with 500 mL of water and fed treefrog tadpoles until the start of the experiment. To each tank, we added a single predator cage constructed from 7.5-cm diameter polyethylene corrugated drainage pipe with 10-cm squares of window screen secured on each end with rubber bands. Predator cages allow chemical cues released by predators (kairomones) and prey items (alarm cues) to permeate throughout the tank (Schoeppner & Relyea, 2009). We placed a 2.5-cm cube of polystyrene foam into each cage to provide buoyancy. On 1 June, we placed all the predators into their cages, fed them ~800 mg of total tadpole biomass (~400 mg of treefrog and ~400 mg of leopard frog), and placed the cages into the appropriate treatment tanks (i.e., lethal and caged predator treatments). The lethal predators were initially caged to allow tadpoles to acclimate to their presence prior to release. We also placed empty cages into the no-predator treatment tanks. For the lethal predator treatment, the predator was released from the cage after 2 d (day 1 of experiment). For the caged-predator treatment, we fed each predator three times per week as described above. During the experiment, we only replaced one caged predator because it was not eating. To equalize disturbance among treatments, we briefly lifted and replaced all empty cages in replicates of the lethal- and no-predator treatments.
On day 10, we destructively sampled half of the experimental units (1 replicate per treatment per block) because mortality rates due to ranavirus infection typically increase 10 d post-exposure (Hoverman et al., 2011). Thus, we selected this time point to capture infection prevalence prior to substantial ranavirus-associated mortality in the experiment. We removed all tadpoles from each tank, euthanized them using MS-222, and preserved them in 70% ethanol. Additionally, we removed one clay tile from each tank to quantify periphyton biomass at this timepoint. The tiles were scrubbed using a toothbrush inside plastic bags with 200 mL UV-irradiated, filtered water to remove the attached periphyton. The water containing the suspended periphyton was then vacuum pumped through a dried, pre-weighed 90 mm GF/C Whatman filter. We dried the filters in a drying oven for 24 hours at 80°C and then weighed the filter to determine periphyton dry weight on each tile. On day 20, the remaining experimental units were taken down following the same protocol. In previous experiments, 20 d was sufficient to observe predator effects on tadpole traits (e.g., mass development, morphology) and algal biomass (Peacor & Werner, 1997; Relyea, 2002; Relyea, 2003). Additionally, this time frame was selected to ensure that lethal predators did not consume all tadpoles in the mesocosms (Relyea & Hoverman, 2008).
Tadpole behavior
We observed tadpole behavior on days 5 and 12 (at ~11:00 h) of the experiment using scan sampling (Relyea & Hoverman, 2003). Observers walked around each tank while recording the number of visible tadpoles and the proportion of those tadpoles that were actively swimming or foraging. Within each observation period, we conducted 10 observations of each tank with up to five different observers. Because we were unable to reliably differentiate between species in the tanks, our estimates of tadpole behavior were pooled across species. We only observed the tanks that were destructively sampled on day 10 during behavioral observations on day 5 while we only observed the tanks that were sampled on day 20 during behavioral observations on day 12.
Tadpole morphology and ranavirus infection determination
We identified individuals to species to determine survival during the experiment. Additionally, we recorded the mass, developmental stage (Gosner, 1960), body length, and tail depth of each individual from the samples collected on day 20. We obtained body length and tail depth using ImageJ on photographs that included a 150-millimeter ruler for scale. To determine infection status and load, we dissected all individuals from the virus treatments and two randomly-selected individuals of each species from the no-virus treatments. We removed a section of the liver for DNA extractions using DNeasy Blood and Tissue Kits (Qiagen, Hilden, Germany). We changed gloves, and cleaned and soaked all instruments, tools, and surfaces with a 10% bleach solution between samples to prevent cross-contamination. We used a NanoDrop 2000c (Thermo Fisher Scientific, Waltham, Massachusetts, USA) to both confirm and quantify genomic DNA presence in the extracted sample. We stored eluted DNA and dissected tissue samples at −80°C.
We used quantitative polymerase chain reaction (qPCR) to determine infection status and viral loads (Wuerthner, Hua & Hoverman, 2017). For each reaction, we added 6.25 μL of SsoAdvancedTM Universal Probes Supermix (Bio-Rad Laboratories, California, USA), 0.1125 μL of forward and reverse primers, 0.0313 μL of fluorescent probe, 3.49 μL of reverse osmosis water, and 2.5 μL of template. We used a CFX Connect- (Bio-Rad Laboratories, California, USA) to conduct qPCR. We ran each plate with four standards containing known concentrations of the target sequence and a negative control containing reverse osmosis water as template. We calculated the number of copies of ranavirus DNA (viral copies μL−1) for each individual and then divided by the total DNA present in that sample (ng DNA μL−1) to obtain viral load (viral copies ng−1 DNA). For each species within a tank, we calculated infection prevalence as the number of infected individuals out of the total number of survivors. Additionally, we calculated the mean viral load of just the infected individuals of each species in a mesocosm for our viral load response variable.
Statistical analyses
Our response variables were infection prevalence, viral load, survival, activity, individual-level trait values (stage, mass, body length, and tail depth), and periphyton biomass. We used analysis of variance (ANOVA) to examine the effects of takedown day, predator treatment, and the interaction on infection prevalence and viral load. The no-virus treatments were excluded from the analyses because no infections were detected. We also used ANOVA tests to examine the additive and interactive influence of takedown day, predator treatment, and virus exposure on activity, survival, and periphyton biomass. Therefore, we included terms for takedown day, predator and virus treatments, and all possible interactions among the three terms in ANOVA tests. Following ANOVA tests, we conducted pairwise comparisons using Tukey’s HSD (Zar, 1999). We used univariate linear mixed-effects models in R package ‘nlme’ to investigate the influence of predator treatment, virus exposure, and their interactions, on mass, stage, body length, and tail depth (individual-level traits). We conducted separate analyses per species because we anticipated large differences between morphological traits of tadpoles between both species (Zuur et al., 2009). For body length and tail depth, we accounted for mass and included it as a fixed effect in our models because it was strongly correlated (P < 0.001, ρ> 0.72) with the traits, tested with Pearson’s correlation coefficient (Zar, 1999; Zuur et al., 2009). We nested observations within tanks that individuals were sampled from, and included it as a random effect, to account for dependence among individuals from the same tanks (Zuur et al., 2009). We tested for interactions between predator and virus treatments on individual-level trait values by comparing additive (predator + virus) and interactive models (predator + virus + predator*virus) with Akaike Information Criterion (AIC; Burnham & Anderson, 2004). We selected the reduced additive models unless AIC of interactive models was ≥ 4 AIC units fewer, and interactive terms were statistically significant (P < 0.05; Anderson & Burnham, 2002). We also used univariate linear mixed-effects models to investigate the influence of infection status (infected or not) on body mass, stage, length, and tail depth within the virus treatments. This allowed us to more directly compare the influence of infection status on predator-induced responses. We accounted for mass, and nested observations within tanks, as described above. We log-transformed individual-level variables (mass, body length, and tail depth) to meet statistical assumptions of normality. We conducted analyses in SPSS v24 (IBM Corp., 2016) or Program R v3.4.1 (R Core Team, 2016).
Results
Infection prevalence and viral load
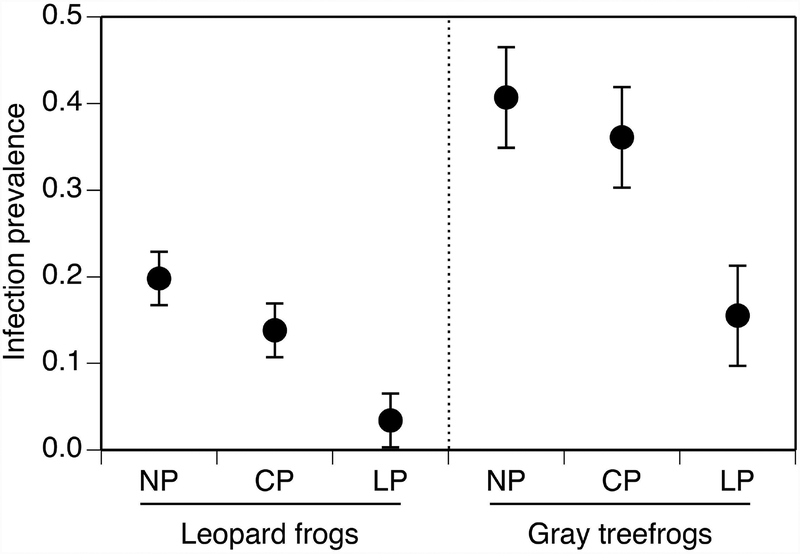
For both leopard frogs and treefrogs, infection prevalence was affected by predators (F2,24 ≥ 6.9, P ≤ 0.004) but not takedown day (F1,24 ≤ 0.3, P ≥ 0.57) or the interaction (F2,48 ≤ 1.7, P ≥ 0.204; Fig. 1). Similar responses to the predator treatments were observed for both species; infection prevalence was 57–83% lower in the lethal-predator treatment compared to the no-predator and caged-predator treatment (P ≤ 0.006). There was no difference in infection prevalence between the no-predator and caged-predator treatment (P ≥ 0.328). Infection prevalence was 60% lower in leopard frogs compared to treefrogs, pooled across all treatments. There was no effect of predators (F1,16 ≤ 1.7, P ≥ 0.314), takedown day (F1,16 ≤ 4.3, P ≥ 0.053), or their interaction (F1,16 ≤ 0.2, P ≥ 0.657) on viral load.
Figure 1.
Ranavirus infection prevalence of northern leopard frog and gray treefrog tadpoles across predator treatments within the virus treatment. Data (least-squares means ± 1 SE) are averaged across day 10 and 20. The no-virus treatment was excluded from the figure because no infections were detected. Predator treatments are: no-predator (NP), caged predator (CP), and lethal predator (LP).
Tadpole survival
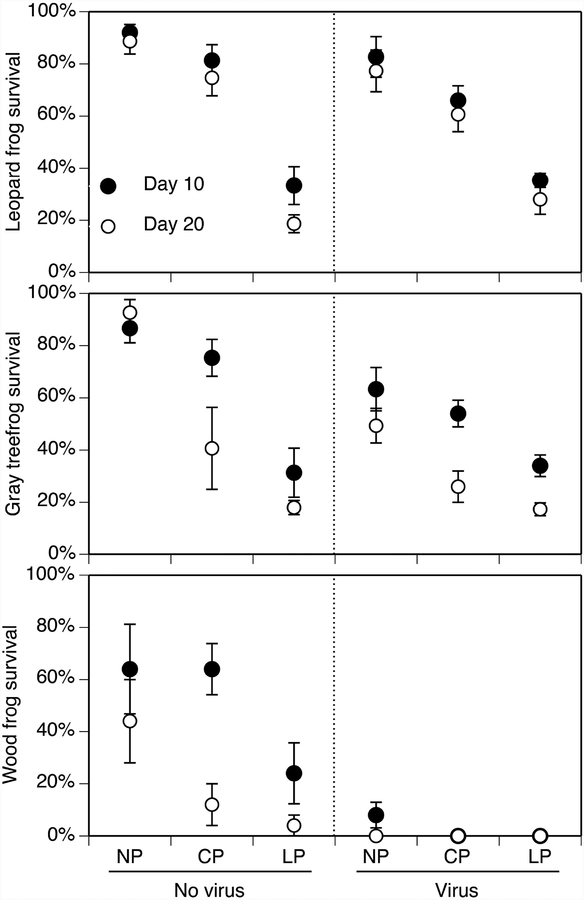
Leopard frog survival was influenced by day, predators, and the predator-by-virus interaction (Table 1, Figure 2). In the absence of predators and ranavirus, leopard frog survival was high (89–92%) on both takedown days. However, survival was 11% lower on day 20 compared to day 10 (P = 0.043). Virus effects within the caged-predator treatment but not the no-predator or lethal-predator treatment drove the predator-by-virus interaction. Within the caged-predator treatment, survival was 19% lower in the virus treatment than the no-virus treatment (P = 0.017). Within each of the virus treatments, predator effects showed similar patterns. Survival was 59–66% lower in lethal-predator treatment compared to the caged-predator and no-predator treatments (P < 0.001). Additionally, survival was 17% lower in the caged-predator treatment compared to the no-predator treatment (P < 0.001).
Table 1:
Results of ANOVAs examining the effects of predator and virus treatments on northern leopard frog (R. pipiens) and gray treefrog (H. versicolor) survival at the two takedown points (days 10 and 20).
| Leopard frogs | Gray treefrogs | ||||
|---|---|---|---|---|---|
| df | F | P | F | P | |
| Day | 1 | 4.3 | 0.043 | 15.7 | < 0.001 |
| Predator | 2 | 97.3 | < 0.001 | 42.5 | < 0.001 |
| Virus | 1 | 3.5 | 0.066 | 15.7 | < 0.001 |
| Day* Predator | 2 | 0.3 | 0.712 | 3.5 | 0.038 |
| Day*Virus | 1 | 0.1 | 0.747 | 0.4 | 0.515 |
| Predator*Virus | 2 | 3.3 | 0.047 | 5.5 | 0.007 |
| Day*Predator*Virus | 2 | 0.2 | 0.853 | 0.8 | 0.437 |
| Error | 48 | ||||
Figure 2.
The effects of predator treatment and virus exposure on the survival of northern leopard frog, gray treefrog, and wood frog tadpoles on day 10 (closed circle) and 20 (open circle). Predator treatments are as described in Fig. 1. Data are means ± 1 SE.
Treefrog survival was influenced by the predator and virus treatment, and the predator-by-virus and day-by-predator interactions (Table 1, Figure 2). The day-by-predator interaction was largely influenced by the caged-predator treatment wherein survival in the caged-predator treatment was similar to the no-predator treatment, but higher than the lethal-predator treatment on day 10. However, this pattern was reversed on day 20. The predator-by-virus interaction was driven by virus effects within the no-predator and caged-predator treatments (P ≤ 0.018), but not in the lethal-predator treatment (P = 0.892). In the former two treatments, survival was 29% lower in the virus treatment compared to the no-virus treatment. Within each of the virus treatments, predator effects showed similar patterns. Survival was 49 – 65% lower in lethal-predator treatment compared to the caged-predator and no-predator treatments (P < 0.001). Additionally, survival was 17% lower in the caged-predator treatment compared to the no-predator treatment (P < 0.001).
Because wood frogs were largely eliminated from the virus treatment, our analysis examined predator effects on wood frogs within the no-virus treatment. Wood frog survival was affected by predators (F2,24 = 5.6, P = 0.01) and takedown day (F1,24 = 9.8, P = 0.005) but not the interaction (F2,48 = 1.2, P = 0.323). Survival was 61% lower on day 20 compared to day 10. Survival in the lethal-predator treatment was 61% lower compared to the no-predator treatment (P = 0.003). There was no difference in survival between the no-predator and caged-predator treatment (P = 0.057).
Tadpole behavior
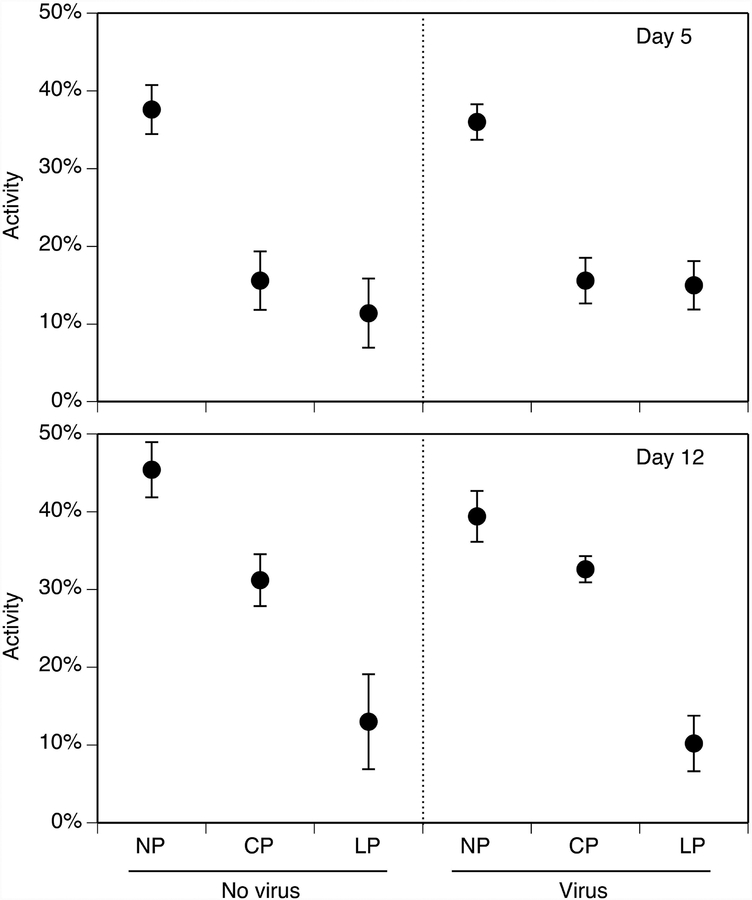
We examined treatment effects within each observation day because we found significant effects of observation day (F1,48 = 10.1, P = 0.003) and the day-by-predator interaction (F2,48 = 5.9, P = 0.005) on tadpole activity. On both observation days, tadpole activity was influenced by predators (F2,24 ≥ 30.3, P ≤ 0.001) but not virus (F1,24 ≤ 0.6, P ≥ 0.447) or the virus-by-predator interaction (F2,24 ≤ 0.4, P ≥ 0.624; Fig. 3). On day 5, tadpoles were 57–64% less active in the caged- and lethal-predator treatments compared to the no-predator treatment (P ≤ 0.001). However, there was no difference in behavior between the caged- and lethal-predator treatments (P ≥ 0.391). On day 12, tadpoles were 25–73% less active in the caged- and lethal-predator treatments compared to the no-predator treatment (P ≤ 0.012). Additionally, tadpoles were 64% less active in the lethal-predator treatment compared to the caged-predator treatment (P ≤ 0.001).
Figure 3.
The effects of predator treatment and virus exposure on the percent activity of the observed tadpoles on day 5 (A) and day 12 (B) of the experiment. Predator treatments are as described in Fig. 1. Data are means ± 1 SE.
Tadpole individual-level traits
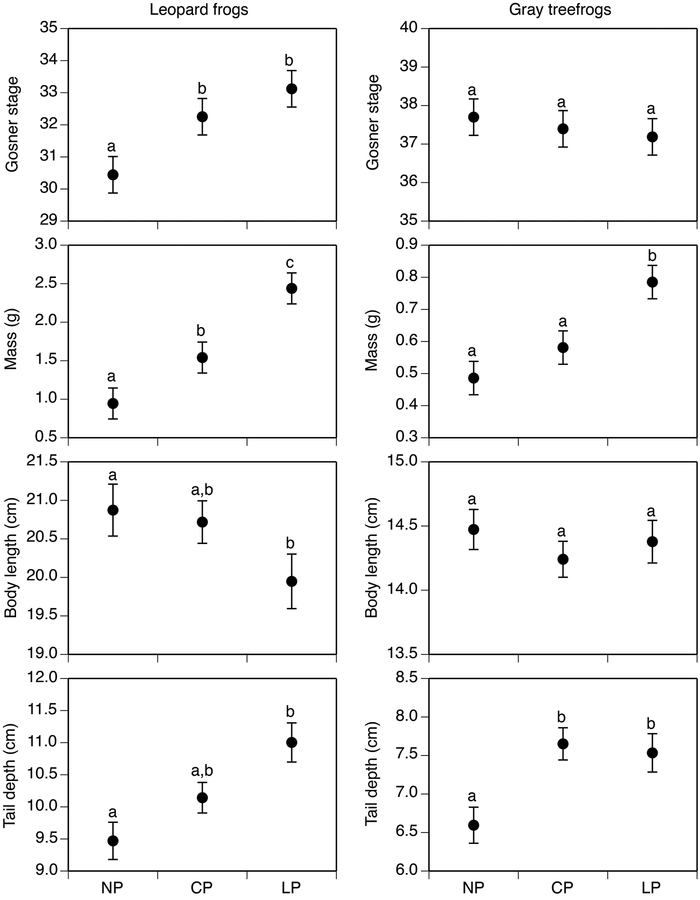
We did not detect interactions between predator and virus treatments influencing any individual-level traits and present results from additive models (see Table S1). Individual-level traits of tadpoles were influenced by exposure to predators (P ≤ 0.037), but not by exposure to virus (P > 0.09; Table 2; Fig. 4). Leopard frog tadpoles in the lethal-predator treatment had 9% higher stage, 158% greater mass, 4% shorter bodies, and 16% deeper tails (P ≤ 0.037) than tadpoles in the no-predator treatment. Similarly, treefrog tadpoles in the lethal-predator treatment had 63% greater mass and 14% deeper tails than tadpoles in the no-predator treatment (P = 0.001). Leopard frog tadpoles in the caged-predator treatment had 6% greater stage and 56% greater mass (P = 0.025) than tadpoles in the no-predator treatment. Treefrog tadpoles in the caged-predator treatment had 16% deeper tails compared to tadpoles in the no-predator treatment (P = 0.015)
Table 2.
Summary statistics for univariate linear mixed effects models investigating the influence of predator and virus treatments on the individual-level traits of northern leopard frogs (R. pipiens) and gray treefrog tadpoles (H. versicolor) on day 20. The reference level for all models is the control (no-virus and no-predator) treatment.
| Leopard frogs | Gray treefrogs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Treatment | ß | SE | df | t | P | ß | SE | df | t | P |
| Stage | Virus | 0.07 | 0.63 | 27 | 0.10 | 0.918 | 0.34 | 0.59 | 27 | 0.57 | 0.571 |
| Caged predator | 1.81 | 0.73 | 27 | 2.49 | 0.019 | −0.17 | 0.71 | 27 | −0.24 | 0.813 | |
| Lethal predator | 2.65 | 0.79 | 27 | 3.35 | 0.001 | −0.45 | 0.72 | 27 | −0.63 | 0.534 | |
| Mass | Virus | 0.13 | 0.16 | 27 | 0.83 | 0.413 | 0.02 | 0.09 | 27 | 0.23 | 0.818 |
| Caged predator | 0.44 | 0.19 | 27 | 2.37 | 0.025 | 0.17 | 0.11 | 27 | 1.60 | 0.121 | |
| Lethal predator | 0.87 | 0.18 | 27 | 4.72 | < 0.001 | 0.46 | 0.11 | 27 | 4.21 | < 0.001 | |
| Body length | Virus | 0.01 | 0.01 | 27 | 0.57 | 0.576 | 0.00 | 0.01 | 27 | 0.29 | 0.772 |
| Caged predator | −0.02 | 0.01 | 27 | −2.00 | 0.056 | −0.02 | 0.01 | 27 | −2.62 | 0.015 | |
| Lethal predator | −0.03 | 0.01 | 27 | −2.10 | 0.037 | 0.00 | 0.01 | 27 | 0.21 | 0.832 | |
| Tail depth | Virus | −0.02 | 0.03 | 27 | −0.73 | 0.474 | 0.05 | 0.03 | 27 | 1.75 | 0.092 |
| Caged predator | 0.06 | 0.03 | 27 | 1.89 | 0.070 | 0.11 | 0.03 | 27 | 3.32 | 0.003 | |
| Lethal predator | 0.16 | 0.04 | 27 | 4.35 | < 0.001 | 0.14 | 0.04 | 27 | 3.66 | 0.001 | |
Figure 4.
Individual-level traits of northern leopard frog (left panels) and gray treefrog (right panels) tadpoles on day 20 in the predator treatments. Data (least-squares means ± 1 SE) are averaged across virus treatments. Body length and tail depth represent mass-adjusted values. Predator treatments are as described in Fig. 1. Treatments sharing lower case letters are not significantly different from each other based on Tukey’s HSD test (P > 0.05).
We also examined the influence of infection status on individual-level traits. Mass of treefrog tadpoles was influenced by ranavirus infection (Table 3). Treefrog tadpoles that were infected with ranavirus had 23% lower mass than those that were not infected (P < 0.001). We did not detect any other relationships between infection and individual-level traits for either species (P > 0.360).
Table 3.
Summary statistics for univariate linear mixed effects models investigating the influence of infection on individual-level traits of northern leopard frog (R. pipiens) and gray treefrog (H. versicolor) tadpoles on day 20. The reference level for models represents tadpoles that were not infected with ranavirus.
| Species | Trait | ß | SE | df | t | P |
|---|---|---|---|---|---|---|
| Leopard frog | Stage | −0.02 | 0.58 | 227 | −0.04 | 0.969 |
| Mass | −0.08 | 0.08 | 227 | −0.92 | 0.360 | |
| Body length | 0.01 | 0.01 | 226 | 0.86 | 0.389 | |
| Tail depth | 0.05 | 0.03 | 226 | 1.54 | 0.126 | |
| Gray Treefrog | Stage | −0.60 | 0.44 | 100 | −1.38 | 0.172 |
| Mass | −0.27 | 0.05 | 100 | −5.06 | < 0.001 | |
| Body length | 0.01 | 0.01 | 99 | 0.51 | 0.613 | |
| Tail depth | 0.00 | 0.01 | 99 | −0.34 | 0.736 |
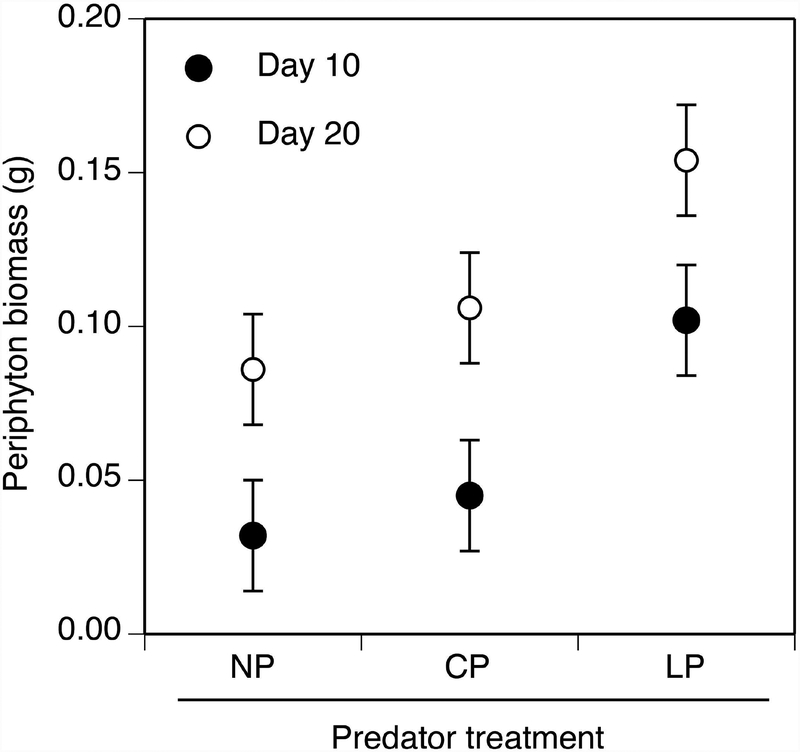
Periphyton biomass
Periphyton biomass was influenced by takedown day (F1,48 = 24.4, P < 0.001) and predator treatment (F2,48 = 9.2, P < 0.001), but not by virus treatment or interactions among explanatory variables (F ≤ 1.4, P ≥ 0.262). Periphyton biomass increased 93% between day 10 and 20. Periphyton biomass was 117 and 68% higher in the lethal-predator treatment than in the no-predator and caged-predator treatments, respectively (P ≤ 0.005). However, there was no difference in periphyton biomass between the no-predator and caged-predator treatment (P = 0.234).
Discussion
Using a semi-natural mesocosm experiment, we found that larval dragonflies reduced the prevalence of ranavirus in a larval amphibian assemblage. This effect appeared to be mediated by reductions in tadpole density rather than behavioral changes. However, we did not find any evidence that virus presence or infection altered the responses of tadpoles to predators. At the community level, predators caused trophic cascades (e.g., increased periphyton growth) via their negative effects on tadpole density. However, virus exposure did not contribute to trophic cascades. The field of ecology has increasingly focused on improving our understanding of how shared natural enemies of victim species influence population- and community-level dynamics (Sih, Englund & Wooster, 1998). Given the effect predators had on infection, our results underscore the importance of examining these interactions.
The healthy herds hypothesis posits that predators can reduce pathogen prevalence and transmission rates by removing infected individuals from a community and/or reducing host densities (Packer et al., 2003). Our results suggest that larval dragonflies keep tadpole ‘herds’ healthy by reducing ranavirus transmission, as we observed a 57 to 83% reduction in infection prevalence with lethal predators. The infection patterns were consistent across both sample dates suggesting that transmission dynamics were established relatively early in the experiment and maintained throughout. Moreover, our transmitting species, wood frogs, were largely eliminated by the day 10 sampling event across all the virus treatments, which suggests that most of the transmission occurred early in the experiment. However, given that ranaviruses can be transmitted through necrophagy (Gray, Miller & Hoverman, 2009), it is possible that carcasses served as a source of exposure beyond day 10. Notably, infection prevalence in leopard frogs dropped from 20% without predators to 4% with lethal predators, suggesting that predators can nearly eliminate infection risk in this species. Theoretical models have demonstrated that a given natural enemy may be excluded from a system if victim density is reduced below a specific threshold by a second natural enemy (Anderson & May, 1986), though additional research is necessary to determine these thresholds in our system. Given that numerous systems – including ours – contain multiple victim species, it will be critical for future theoretical work to explore variation in traits within communities, such as the relative susceptibilities of species to different natural enemies. For example, a more diverse community including species that are highly susceptible to infection might maintain the pathogen in a system, as well as increase the probability of spillover to less susceptible species.
A predator-associated reduction in pathogen prevalence can be mediated by a reduction in host density, changes in host traits, or the selective consumption of infected individuals (Packer et al., 2003). Our study was only designed to examine the potential effects of changes in host density and host traits. Of these two mechanisms, it appears that host density was the main driver of reduced infection prevalence. Early in the experiment, when most of the virus transmission appeared to occur, we observed the same trends in both survival and infection prevalence among predator treatments. More specifically, survival and infection prevalence were relatively high in no-predator and caged-predator treatments, but both were low in lethal-predator treatments. At the same time, tadpole activity was reduced in both caged- and lethal-predator treatments, suggesting that activity played a minor role in altering virus transmission. These patterns broke down later in the experiment because of increased gray treefrog mortality and increased activity levels in caged-predator treatments. Although we did not examine selective predation, it may have also contributed to our results. Tadpoles infected with ranavirus often display erratic behavior in the early stages of infection that could make them more easily detectable by predators (Gray, Miller & Hoverman, 2009). Although theoretical models have shown that both selective and non-selective predation can contribute to healthy herds, effects are most pronounced when infected individuals are selectively removed (Packer et al., 2003). Future experiments examining whether amphibian predators selectively remove infected prey will be valuable for informing models that explore dynamics in nature.
In response to predators, many prey species express inducible defenses that reduce encounter rates with predators or the probability of capture once detected (Tollrian & Harvell, 1999). In our experiment, tadpoles responded to both caged and lethal predators by reducing activity levels and developing deeper tails. In the lethal-predator treatment, this could be the result of natural selection in addition to phenotypic plasticity. Regardless, these findings are consistent with previous studies examining tadpole responses to predators (Relyea, 2001a; Relyea & Hoverman, 2003). We also observed that the behavioral response to caged predators was weaker on day 12 compared to day 5. These results are consistent with previous research demonstrating that tadpoles invest less in behavioral responses once morphological responses (e.g., deeper tails) are formed (Relyea, 2003). Given that induced traits are energetically costly to produce and maintain (McCollum & Van Buskirk, 1996), we expected virus exposure and infection to interfere with their expression. However, we did not find evidence that virus presence or infection altered the formation of inducible defenses. Although ranavirus did not compromise inducible defenses, it did reduce growth of treefrog tadpoles. Virus infection could therefore be altering resource allocation or host metabolism, as has been documented in other systems (Lochmiller & Deerenberg, 2000).
Natural enemies are capable of initiating trophic cascades within communities through their effects on host densities and traits. However, comparisons of these cascades initiated by predators and pathogens in the same food web have received limited attention. In our study, only lethal predators caused a trophic cascade, a trend that appeared to be largely driven by tadpole density. This is consistent with other work that has observed predator-initiated trophic cascades (Werner & Peacor, 2006). Presumably as a result of greater periphyton biomass and per-capita resource levels, surviving tadpoles were larger and more developed with lethal predators. These findings may have contributed to the healthy herd effect that we observed. More specifically, individuals in the lethal-predator treatment may have been in better body condition and more capable of resisting or clearing ranavirus infections. We did not observe a virus-associated trophic cascade, a result that can be attributed to the weak effect of ranavirus on tadpole survival. We saw a more immediate influence of dragonflies on tadpole density, as survival in lethal predator tanks decreased considerably compared to other treatments by day 10. Individuals exposed to virus continue to interact within their communities as disease progresses. Moreover, infection does not always result in mortality. In contrast, predation immediately removes individuals from the community. Because of the relatively short duration of our study, future studies focused on the trophic effects of ranavirus infection should be conducted over longer time frames (>1 month) to allow sufficient time for the propagation of cascades.
The healthy herds hypothesis is largely based on theoretical work (Packer et al., 2003), though it has been documented in some systems (Lafferty, 2004; Duffy et al., 2005; Hawlena, Abramsky & Bouskila, 2010). Our results provide empirical support for the healthy herds effect and underscore the importance of examining the interactions between predators and pathogens. The ability of larval dragonflies to influence ranavirus dynamics in our system suggests that they could play an important role in altering disease dynamics in nature. However, future work that examines the relationship between larval dragonfly abundance and infection prevalence in natural systems is needed. Additionally, larval amphibians have a diversity of predators that vary in predation mode, foraging rate, and risk level (Relyea, 2001b). Given these differences among tadpole predators, we would expect variation in whether particular predators can initiate the healthy herds effect and at what magnitude. Such comparative studies are lacking in the literature but are needed to broaden the knowledge base in natural enemy ecology. Notably, although predators dramatically reduced infection prevalence in the assemblage, they also significantly reduced tadpole survival. Thus, there are tradeoffs associated with the interactive effects of multiple natural enemies that influence population-level dynamics. Future work in the field of natural enemy ecology should seek to examine the complexities associated with the presence of multiple natural enemies in communities.
Supplementary Material
Figure 5.
Periphyton biomass on day 10 (closed circle) and 20 (open circle) in the predator treatments. Data (least-squares means ± 1 SE) are averaged across virus treatments for each predator treatment. Predator treatments are as described in Fig. 1.
Acknowledgements
We thank M. Sepúlveda, W. Hopkins, and members of the Hoverman Lab for their comments on earlier drafts of the manuscript. We thank several anonymous reviewers for their helpful comments on the manuscript. We collected all eggs under Indiana Department of Natural Resources permit #16–015 issued to JH. All methods were approved by the Purdue University IACUC (protocol 1302000823). This research was supported by funding from the National Institutes of Health, Ecology, and Evolution of Infectious Diseases Program grant (R01GM109499).
Footnotes
Data Accessibility
Data associated with this paper are deposited in the Purdue University Research Repository https://doi.org/10.4231/BVZE-3057 (Hoverman & Gallagher, 2019).
Literature Cited
- Abrams PA & Ginzburg LR (2000) The nature of predation: prey dependent, ratio dependent or neither? Trends in Ecology & Evolution, 15, 337–341. [DOI] [PubMed] [Google Scholar]
- Adelman JS & Martin LB (2009) Vertebrate sickness behaviors: Adaptive and integrated neuroendocrine immune responses. Integrative and Comparative Biology, 49, 202–214. [DOI] [PubMed] [Google Scholar]
- Anderson DR & Burnham KP (2002) Avoiding pitfalls when using information-theoretic methods. Journal of Wildlife Management, 66, 912–918. [Google Scholar]
- Anderson RM & May RM (1986) The invasion, persistence and spread of infectious-diseases within animal and plant-communities. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 314, 533–570. [DOI] [PubMed] [Google Scholar]
- Bertram CR, Pinkowski M, Hall SR, Duffy MA & Caceres CE (2013) Trait-mediated indirect effects, predators, and disease: test of a size-based model. Oecologia, 173, 1023–1032. [DOI] [PubMed] [Google Scholar]
- Brunner J, Storfer A, Gray MJ & Hoverman JT (2015) Ranavirus ecology and evolution: From epidemiology to extinction Ranaviruses: Lethal pathogens of ectothermic vertebrates (eds Gray MJ & Chinchar GD), pp. 71–104. Springer, New York, U.S.A. [Google Scholar]
- Brunner JL, Schock DM & Collins JP (2007) Transmission dynamics of the amphibian ranavirus Ambystoma tigrinum virus. Diseases of Aquatic Organisms, 77, 87–95. [DOI] [PubMed] [Google Scholar]
- Buck JC & Ripple WJ (2017) Infectious Agents Trigger Trophic Cascades. Trends in Ecology & Evolution, 32, 681–694. [DOI] [PubMed] [Google Scholar]
- Buck JC, Weinstein SB & Young HS (2018) Ecological and evolutionary consequences of parasite avoidance. Trends in Ecology & Evolution, 33, 619–632. [DOI] [PubMed] [Google Scholar]
- Burnham KP & Anderson DR (2004) Multimodel inference - understanding AIC and BIC in model selection. Sociological Methods & Research, 33, 261–304. [Google Scholar]
- Calvete C (2006) Modeling the effect of population dynamics on the impact of rabbit hemorrhagic disease. Conservation Biology, 20, 1232–1241. [DOI] [PubMed] [Google Scholar]
- Cezilly F, Gregoire A & Bertin A (2000) Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology, 120, 625–630. [DOI] [PubMed] [Google Scholar]
- Decaestecker E, De Meester L & Ebert D (2002) In deep trouble: Habitat selection constrained by multiple enemies in zooplankton. Proceedings of the National Academy of Sciences of the United States of America, 99, 5481–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffus ALJ, Waltzek TB, Stöhr AC, Allender MC, Gotesman M, Whittington RJ, … Marschang R (2015) Distribution and Host Range of Ranaviruses Ranaviruses: Lethal pathogens of ectothermic vertebrates (eds Gray MJ & Chinchar GD), pp. 9–57. Springer, New York, U.S.A. [Google Scholar]
- Duffy MA, Hall SR, Tessier AJ & Huebner M (2005) Selective predators and their parasitized prey: Are epidemics in zooplankton under top-down control? Limnology and Oceanography, 50, 412–420. [Google Scholar]
- Duffy MA, Housley JM, Penczykowski RM, Caceres CE & Hall SR (2011) Unhealthy herds: indirect effects of predators enhance two drivers of disease spread. Functional Ecology, 25, 945–953. [Google Scholar]
- Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes and identification. Herpetologica, 16, 183–190. [Google Scholar]
- Gray MJ, Miller DL & Hoverman JT (2009) Ecology and pathology of amphibian ranaviruses. Diseases of Aquatic Organisms, 87, 243–266. [DOI] [PubMed] [Google Scholar]
- Grenfell BT (1992) Parasitism and the dynamics of ungulate grazing systems. American Naturalist, 139, 907–929. [Google Scholar]
- Hatcher MJ, Dick JTA & Dunn AM (2006) How parasites affect interactions between competitors and predators. Ecology Letters, 9, 1253–1271. [DOI] [PubMed] [Google Scholar]
- Hawlena D, Abramsky Z & Bouskila A (2010) Bird predation alters infestation of desert lizards by parasitic mites. Oikos, 119, 730–736. [Google Scholar]
- Hoverman JT & Gallagher SJ (2019) Healthy but smaller herds: Predators reduce pathogen transmission in an amphibian assemblage. Purdue University Research Repository, 10.4231/BVZE-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoverman JT, Gray MJ, Haislip NA & Miller DL (2011) Phylogeny, life history, and ecology contribute to differences in amphibian susceptibility to ranaviruses. EcoHealth, 8, 301–319. [DOI] [PubMed] [Google Scholar]
- Hoverman JT & Searle CL (2016) Behavioural influences on disease risk: implications for conservation and management. Animal Behaviour, 120, 263–271. [Google Scholar]
- Hudson PJ, Rizzoli A, Grenfell BT, Hesterbeek H & Dobson AP (2002) Ecology of wildlife diseases. Oxford University Press, Oxford. [Google Scholar]
- Corp IBM. (2016) IMB SPSS Statistics for Windows, Version 24.0. IBM Corp., Armonk, NY. [Google Scholar]
- Ives AR & Murray DL (1997) Can sublethal parasitism destabilize predator-prey population dynamics? A model of snowshoe hares, predators and parasites. Journal of Animal Ecology, 66, 265–278. [Google Scholar]
- Johnson PTJ, Stanton DE, Preu ER, Forshay KJ & Carpenter SR (2006) Dining on disease: How interactions between infection and environment affect predation risk. Ecology, 87, 1973–1980. [DOI] [PubMed] [Google Scholar]
- Joly DO & Messier F (2004) The distribution of Echinococcus granulosus in moose: evidence for parasite-induced vulnerability to predation by wolves? Oecologia, 140, 586–590. [DOI] [PubMed] [Google Scholar]
- Keesing F, Holt RD & Ostfeld RS (2006) Effects of species diversity on disease risk. Ecology Letters, 9, 485–498. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J & Urichuk TMY (2017) Time-lagged effect of predators on tadpole behaviour and parasite infection. Biology Letters, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD (2004) Fishing for lobsters indirectly increases epidemics in sea urchins. Ecological Applications, 14, 1566–1573. [Google Scholar]
- Lefcort H & Eiger SM (1993) Antipredatory behavior of feverish tadpoles - Implications for pathogen transmission. Behaviour, 126, 13–27. [Google Scholar]
- Lima SL (1998) Stress and decision making under the risk of predation: Recent developments from behavioral, reproductive, and ecological perspectives. Stress and Behavior, 27, 215–290. [Google Scholar]
- Lochmiller RL & Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos, 88, 87–98. [Google Scholar]
- McCollum SA & Van Buskirk J (1996) Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution, 50, 583–593. [DOI] [PubMed] [Google Scholar]
- Meyling NV & Pell JK (2006) Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecological Entomology, 31, 162–171. [Google Scholar]
- Orlofske SA, Jadin RC, Preston DL & Johnson PTJ (2012) Parasite transmission in complex communities: Predators and alternative hosts alter pathogenic infections in amphibians. Ecology, 93, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS & Holt RD (2004) Are predators good for your health? Evaluating evidence for top-down regulation of zoonotic disease reservoirs. Frontiers in Ecology and the Environment, 2, 13–20. [Google Scholar]
- Packer C, Holt RD, Hudson PJ, Lafferty KD & Dobson AP (2003) Keeping the herds healthy and alert: implications of predator control for infectious disease. Ecology Letters, 6, 797–802. [Google Scholar]
- Peacor SD & Werner EE (1997) Trait-mediated indirect interactions in a simple aquatic food web. Ecology, 78, 1146–1156. [Google Scholar]
- Preisser EL, Bolnick DI & Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology, 86, 501–509. [Google Scholar]
- R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Raffel TR, Martin LB & Rohr JR (2008) Parasites as predators: unifying natural enemy ecology. Trends in Ecology & Evolution, 23, 610–618. [DOI] [PubMed] [Google Scholar]
- Relyea RA (2001a) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology, 82, 523–540. [Google Scholar]
- Relyea RA (2001b) The relationship between predation risk and antipredator responses in larval anurans. Ecology, 82, 541–554. [Google Scholar]
- Relyea RA (2002) The many faces of predation: how induction, selection, and thinning combine to alter prey phenotypes. Ecology, 83, 1953–1964. [Google Scholar]
- Relyea RA (2003) Predators come and predators go: the reversibility of predator-induced traits. Ecology, 84, 1840–1848. [Google Scholar]
- Relyea RA & Hoverman JT (2003) The impact of larval predators and competitors on the morphology and fitness of juvenile treefrogs. Oecologia, 134, 596–604. [DOI] [PubMed] [Google Scholar]
- Relyea RA & Hoverman JT (2008) Interactive effects of predators and a pesticide on aquatic communities. Oikos, 117, 1647–1658. [Google Scholar]
- Ripple WJ, Estes JA, Schmitz OJ, Constant V, Kaylor MJ, Lenz A, … Wolf C (2016) What is a Trophic Cascade? Trends in Ecology & Evolution, 31, 842–849. [DOI] [PubMed] [Google Scholar]
- Schoeppner NM & Relyea RA (2009) Interpreting the smells of predation: how alarm cues and kairomones induce different prey defences. Functional Ecology, 23, 1114–1121. [Google Scholar]
- Sih A, Englund G & Wooster D (1998) Emergent impacts of multiple predators on prey. Trends in Ecology & Evolution, 13, 350–355. [DOI] [PubMed] [Google Scholar]
- Stephenson JF, Perkins SE & Cable J (2018) Transmission risk predicts avoidance of infected conspecifics in Trinidadian guppies. Journal of Animal Ecology, 87, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Tollrian R & Harvell D (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, New Jersey, U.S.A. [Google Scholar]
- Van Buskirk J (1988) Interactive effects of dragonfly predation in expermental pond communities. Ecology, 69, 857–867. [Google Scholar]
- Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. American Naturalist, 160, 87–102. [DOI] [PubMed] [Google Scholar]
- Weinstein SB, Buck JC & Young HS (2018) A landscape of disgust. Science, 359, 1213–1214. [DOI] [PubMed] [Google Scholar]
- Werner EE & Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology, 84, 1083–1100. [Google Scholar]
- Werner EE & Peacor SD (2006) Lethal and nonlethal predator effects on an herbivore guild mediated by system productivity. Ecology, 87, 347–361. [DOI] [PubMed] [Google Scholar]
- Wuerthner VP, Hua J & Hoverman JT (2017) The benefits of coinfection: trematodes alter disease outcomes associated with virus infection. Journal of Animal Ecology, 86, 921–931. [DOI] [PubMed] [Google Scholar]
- Zar JH (1999) Biostatistical analysis. Pearson Education India. [Google Scholar]
- Zuur AF, Leno EN, Wlaker N, Saveliev AA & Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.