Abstract
Captive elephant populations are not self-sustaining due to health concerns possibly related to obesity. Categorizing obesity relies on qualitative analyses like body condition scores (BCS). However, elephant indices have not been validated against measured body composition. The objective was to compare BCS systems to body composition determined by deuterium dilution in 28 zoo-kept Asian elephants. Elephants were weighed and given deuterated water orally (0.05 mL/kg). Blood was collected at ~0, 24, 120, 240, 360, and 480 h after dosing. Photographs were taken to score the elephant based on four BCS systems (BCSWemmer [0 to 11 scoring], BCSMorfeld [1 to 5 scoring], BCSFernando [0 to 10 scoring], BCSWijeyamohan [1 to 10 scoring]). Based on regression analysis, relative fat ranged from −305 kg to 515 kg, where negative values indicate less and positive values indicate more fat than expected for the elephant’s mass in this population. BCSFernando was associated with relative fat (P = 0.020, R2 = 0.194). Relative fat, adjusted for sex and age in the statistical model, was associated with BCSWemmer (P = 0.027, R2 = 0.389), BCSFernando (P=0.002, R2 = 0.502), and BCSWijeyamohan (P = 0.011, R2 = 0.426). Inclusion of zoo and familial relatedness resulted in all BCS systems associated with relative fat (P ≤ 0.015). Only BCSFernando predicted relative fat, unadjusted, suggesting it is the most capable system for practical use. Compared to absolute fat, relative fat may be more biologically relevant as greater fat relative to body mass is more likely to lead to health issues.
Keywords: body composition, BCS, zoo
Graphical Abstract
Introduction
Free-ranging elephant populations are rapidly declining, so captive breeding can be one means of insurance against extinction (Conde, Flesness, Colchero, Jones, & Scheuerlein, 2011; Hoffmann et al., 2010). However, most captive populations are not self-sustaining, and over the past decade, mortality rates have been greater than birth rates for elephants housed in North American zoos (Faust & Marti, 2011). This problem is in part due to logistics, as males are often not housed with reproductive females (Holt, Brown, & Comizzoli, 2014), but also to a high prevalence of reproductive and health issues, including arthritis, dystocia, and abnormal ovarian cycles (Brown, 2000; Clubb & Mason, 2002; Lewis, Shepherdson, Owens, & Keele, 2010), some of which may be related to excess fat.
In a recent survey of elephants housed in American Zoo and Aquarium (AZA) accredited zoos, 75% of female and 65% of male Asian elephants were characterized as being overweight or obese (Morfeld, Meehan, Hogan, & Brown, 2016). Similar findings have been shown in Asian elephants housed in European institutions (Schiffmann et al., 2018). However, assessing body condition is generally based on a qualitative body condition score (BCS), rather than quantified measures of total body fat. There are several BCS systems for Asian elephants, each involving a qualitative evaluation of key skeletal descriptors (e.g., ribs, pelvic bone, backbone) either by direct observation or via photographs (Morfeld et al., 2016; Wemmer et al., 2006). Based on the appearance of these anatomical regions, the elephant is given a numerical score, with lower numbers representing less and higher numbers representing more subcutaneous fat (Morfeld et al., 2016). Higher BCSs have been shown to be associated with increased ovarian acyclicity and changes in lipid, metabolic and adrenal status (Morfeld & Brown, 2014; Norkaew et al., 2018), as well as being a non-breeding female elephant, irrespective of species (Schiffmann et al., 2018). Although BCSs correlate positively with measures of subcutaneous fat (Morfeld, Lehnhardt, Alligood, Bolling, & Brown, 2014; Treiber, Reppert, & Ward, 2012), it has not been determined that BCSs accurately reflect the body composition of elephants.
The BCS technique was originally developed to assess the soft tissue (i.e., muscle and subcutaneous fat) of livestock to evaluate their nutritional and economic efficiency (Jefferies, 1961). However, BCSs are often extrapolated to infer a level of fatness, rather than soft tissue, which deviates from its original purpose with little quantitative supportive data. To date, elephant BCS protocols have been compared to serum triglycerides (Morfeld et al., 2016) and ultrasound measures (Morfeld et al., 2014), but neither are a measure of body composition. Although Morfeld (2016) found a positive relationship between BCSs and serum triglycerides, it is widely established that serum triglycerides are strongly influenced by diet (Liu et al., 2015; Mensink & Katan, 1992). In addition, triglycerides are not produced by adipocytes (fat cells). Ultrasound units are capable of estimating subcutaneous fat mass thickness (measured in millimeters not grams or kilograms), but in the elephant, they cannot account for visceral and ectopic fat, thereby not providing a measure of body composition. Further, the reliability of BCSs is contingent on the variation among and within the individuals scoring the elephant (Dugdale, Curtis, Milne, Harris, & Argo, 2011; Schiffmann, Clauss, Hoby, & Hatt, 2017). Therefore, prior to accepting BCSs as a valid means of estimating fatness in elephants, it is helpful to compare BCSs to a more direct measure of total body fat (Charette, Bigras-Poulin, & Martineau, 1996).
The best means to estimate total body fat mass (FM) in large species, such as the elephant, is by deuterium dilution. Deuterium dilution is a non-destructive technique that measures the animal’s total body water, which is then used to estimate fat free mass (FFM) (Wang, Pierson, & Heymsfield, 1992). Fat mass is calculated as the difference between body mass and FFM. Deuterium dilution has been validated in a range of animals, from bumblebees to the Atlantic walrus (Acquarone & Born, 2007; Wolf, Ellington, Davis, & Feltham, 1996), and we have previously shown the feasibility of using this method to measure body fat in African elephants (Chusyd et al., 2018). The primary objective of this study was to compare four commonly used BCS systems with FM estimated by deuterium dilution to determine which scoring system most accurately reflects FM in both female and male zoo Asian elephants.
Methods
Animals
This study was approved by the Institution Animal Care and Use Committee of the University of Alabama at Birmingham (UAB), the Smithsonian Conservation Biology Institute (SCBI), and participating zoos. A total of 28 elephants participated in the study (females: n=23 from seven zoos, mean age 31 ± 3.0 years, age range 8 – 56 years; males: n=5 from five zoos; mean age 21 ± 5.4 years; age range 8 – 34 years). Female elephants were not pregnant, but four had calves that ranged from 1.5 to 4.5 years of age. Male elephants were not in musth at the time of the study.
Body Composition
Body composition was assessed as previously described (Chusyd et al., 2018). In brief, using the institutions’ scales, elephants were weighed to the nearest 1 or 5 pounds. Zoo personnel collected venous blood from an ear or leg vein (~9 mL) to determine background isotope enrichment before administering deuterated water. The location of blood collection remained the same for subsequent samples by elephant. An oral dose of deuterium oxide (0.05 mL D2O/kg of body mass; 99.9% APE; DLM-4–1000, Cambridge Isotopes, Tewksbury, MA) was administered using bread (Publix®, Birmingham, AL) as the vehicle. Post deuterium administration, blood (~9 mL) was collected at regular intervals (~24, 120, 240, 360, and 480 h). Whole blood was centrifuged within 30 minutes to separate serum. Serum was aliquoted, and frozen at a minimum of −20 °C until shipped on dry ice overnight to UAB. Samples were kept in airtight containers in a frost-free −80 °C freezer until analysis.
Isotope ratio mass spectroscopy (Finningan Delta V Advantage, Thermo Fisher Scientific, USA) analysis was carried out by UAB’s Nutrition Obesity Research Center’s Metabolism Core with guidance and support from the Energetics Research Group at the University of Aberdeen, Aberdeen, Scotland (Chusyd et al., 2018). In brief, the 2H/1H delta value was converted to parts per million, and used to calculate FFM based on the mammalian hydration constant (0.73) (Speakman, 1997). Fat free mass was then subtracted from body mass to infer FM.
Body Condition Score (BCS)
The BCS system developed by Morfeld and colleagues (Morfeld et al., 2016), BCSMorfeld, is based on three anatomical regions (ribs, pelvic bone, and backbone) from various angles (e.g., lateral, rear-angle, and rear view) using a 1- to 5-point scoring system by whole numbers only. The BCS system developed by Wemmer and colleagues (Wemmer et al., 2006), BCSWemmer, is based on six anatomical regions (head, scapula, thoracic region, the area in front of the pelvic bone, backbone, pelvic bone) from various angles (e.g., lateral, rear, and elevated views) using a 0- to 11-point scoring system by 0.5 increments. The BCS system developed by Fernando and colleagues (Fernando, Janaka, Ekanayaka, Nishantha, & Pastorini, 2009), BCSFernando, compares the test elephant to five reference photographs preassigned a score of 1, 3, 5, 7, and 9 from a lateral view only. The test elephant is given one of the preassigned scores if it looks like the elephant in the reference photograph, or if it falls between the reference photographs, an even score is given, resulting in a scoring system from 0 to 10 by whole numbers only. The BCS system developed by Wijeyamohan and colleagues (Wijeyamohan, Treiber, Schmitt, & Santiapillai, 2015), BCSWijeyamohan, is based on reference photographs coupled with an explanatory key, scoring elephants from 1 to 10 by whole numbers only based on the lateral view of the elephant. For each index, lower scores imply less fat and higher scores imply more fat.
For each elephant, a set of photographs was taken by an observer around the elephant from every 45° angle along the horizontal plane (≥8 photographs per elephant beginning with a frontal view of the elephant), on the same day deuterated water was administered, to score body condition using each of the four BCS indices. To assess intra- and inter-assessor variability, three assessors scored each elephant three times, with a minimum of 1 week between scoring. Photographs were randomized prior to each scoring session. Scores were generated by the author (DEC) and two assessors who were trained by DEC. Intraclass correlations (ICC (2,1)) were done to evaluate intra- and inter-assessor variability. The first round of scoring showed the strongest intra-assessor reliability, ICC (2,1) = 0.655 – 0.831. Therefore, BCSs from the first round of scoring were averaged across assessors to determine the final BCSs for each elephant. There were no significant effects on the primary model outcomes when BCSs were used exclusively from one assessor’s scoring or from other rounds of scoring.
Statistical analyses
Statistical analyses for the primary models were performed using SAS v9.4 statistical software (SAS Institute, Cary, NC, USA), while secondary sensitivity analyses were performed using R (R Development Core Team, 2008). All statistical analyses were determined prior to examining the data unless otherwise stated. Although body composition was conducted on 30 elephants, two elephants (1 male, and 1 female) were excluded because they did not ingest their total deuterated water amount; thus, it was not possible to determine the exact amount ingested. Therefore, calculating body composition accurately was not possible. Therefore, all models included 28 elephants.
The primary models to address the main hypothesis were linear models regressing relative fat on each BCS system, with subsequent analyses including FM, FFM, and body mass. The BCS systems differed in the number of scores that can be given, thus it was predicted those systems with a more differentiated range would have higher R2 values in regard to quantitative measures. Sex and age were included in the primary model as covariates, followed by secondary sensitivity analyses including zoo. Linear mixed models regressed relative fat, FM, FFM, or body mass on each BCS system, with familial relationships treated as random effects. To address familial relationship, an R package called pedigreemm was used that allows for the correlations of genetic relationships by taking into account the variation within and between sire and dam (Vazquez, Bates, Rosa, Gianola, & Weigel, 2010). Out of 15 related pairs, 1 was a full sibling pair, 5 were half sibling pairs, and 9 were parent offspring pairs. In addition, 9 out of the 15 pairs resided at the same zoo. All model results included an Akaike Information Criterion (AIC) score to determine which model was the best fit based on the lowest score.
Relative FM was determined by the residual for each elephant when FM was regressed on body mass as done in other publications (Franco-Villoria et al., 2016; Goran et al., 1998; Secor & Nagy, 2003). Relative FM (i.e. the residual) is the amount of fat above or below their expected value after taking into account body size (i.e., body mass). As total FM and FFM typically increase with body size, this outcome variable is likely more biologically relevant than absolute FM. Shapiro-Wilk test was used to test normality for the residuals and was not significant (P=0.301).
Descriptive statistics were assessed for the total data set, and then by sex. Body fat percent was included as a descriptive statistic, but not as an outcome variable. Body fat percent is a ratio, and as such, may not be appropriate as an outcome variable in hypothesis testing. Specifically, to control for the denominator (i.e., body mass), the intercept of the regression of the numerator (i.e., fat mass) on the denominator needs to be zero (Allison, Paultre, Goran, Poehlman, & Heymsfield, 1995). Our data did not satisfy this requirement. Nonparametric testing was used when comparing by sex as the body composition data for males were not normally distributed. Significance was set at P < 0.05 (2-tailed).
Results
Descriptive statistics for the entire population and by sex are presented in Table 1. BCSs for each elephant by BCS system are presented in Table 2.
Table 1.
Sample characteristics of the study sample (Mean ± Standard Deviation).
| Total Sample N=28 |
Females N=23 |
Males N=5 |
|
|---|---|---|---|
| Age (years) | 29.14 ± 14.42 | 31.00 ± 14.44a | 20.60 ± 12.03b |
| Mass (kg) | 3506.25 ± 1055.86 | 3272.74 ± 712.45 a | 4580.40 ± 1725.61 b |
| Fat Free Mass (kg) | 3142.89 ± 899.71 | 2911.35 ± 578.11 a | 4208.00 ± 1378.19 b |
| Fat Mass (kg) | 363.39 ± 242.95 | 361.43 ± 221.12 | 372.40 ± 359.68 |
| Body Fat (%) | 9.91 ± 5.01 | 10.54 ± 5.07 | 7.01 ± 3.88 |
| BCSMorfeld (1–5) | 4.07 ± 0.77 | 3.96 ± 0.77 a | 4.60 ± 0.55 b |
| BCSWemmer (0–11) | 7.70 ± 1.63 | 7.57 ± 1.68 a | 8.30 ± 1.35 b |
| BCSFernando (0–10) | 7.00 ± 1.49 | 6.87 ± 1.55 a | 7.60 ± 1.14b |
| BCSWijeyamohan (1–10) | 6.54 ± 1.29 | 6.35 ± 1.23 a | 7.40 ± 1.34 b |
Different letters represent significant differences within the row. P < 0.05.
Table 2.
Body composition by deuterium dilution and BCSs for each elephant.
| ID | Sex | Age (years) |
Mass (kg) |
FFM (kg) |
FM (kg) |
BF% | BCSM (1–5) |
BCSW (0–11) |
BCSF (1–10) |
BCSWi (1–10) |
|---|---|---|---|---|---|---|---|---|---|---|
| 201 | F | 50 | 3343 | 3121 | 222 | 6.65 | 3 | 5.5 | 6 | 5 |
| 202 | F | 19 | 3699 | 2789 | 910 | 24.59 | 4 | 9.0 | 9 | 7 |
| 203 | F | 21 | 3483 | 2974 | 509 | 14.64 | 4 | 7.0 | 6 | 6 |
| 204 | F | 45 | 4345 | 3783 | 562 | 12.93 | 5 | 9.0 | 8 | 7 |
| 205 | F | 45 | 3611 | 3376 | 235 | 6.51 | 3 | 7.0 | 6 | 6 |
| 206 | F | 41 | 4819 | 4004 | 815 | 16.91 | 4 | 8.5 | 8 | 7 |
| 207 | F | 56 | 2854 | 2561 | 293 | 10.27 | 3 | 5.0 | 5 | 5 |
| 208 | F | 22 | 3313 | 3120 | 193 | 5.84 | 4 | 8.5 | 7 | 6 |
| 209 | F | 34 | 3733 | 3319 | 414 | 11.08 | 5 | 9.5 | 8 | 7 |
| 210 | F | 24 | 2089 | 1966 | 123 | 5.87 | 4 | 8.5 | 7 | 6 |
| 211 | M | 8 | 3198 | 3048 | 150 | 4.68 | 5 | 9.0 | 8 | 8 |
| 212 | F | 15 | 3520 | 3080 | 440 | 12.51 | 5 | 10.0 | 10 | 9 |
| 213 | F | 8 | 2538 | 2156 | 382 | 15.05 | 5 | 10.0 | 9 | 9 |
| 214 | F | 42 | 4216 | 3627 | 589 | 13.97 | 4 | 9.5 | 8 | 8 |
| 215 | M | 8 | 3128 | 2996 | 132 | 4.23 | 5 | 8.5 | 8 | 8 |
| 216 | M | 29 | 7382 | 6389 | 993 | 13.45 | 5 | 10.0 | 9 | 9 |
| 217 | F | 29 | 3484 | 3361 | 123 | 3.54 | 4 | 7.5 | 6 | 6 |
| 218 | F | 18 | 2762 | 2597 | 165 | 5.99 | 4 | 6.5 | 6 | 6 |
| 219 | F | 28 | 3526 | 2943 | 583 | 16.53 | 5 | 8.5 | 8 | 7 |
| 220 | M | 24 | 4740 | 4367 | 373 | 7.88 | 4 | 7.5 | 7 | 6 |
| 221 | F | 11 | 2064 | 1898 | 166 | 8.04 | 3 | 5.5 | 5 | 5 |
| 222 | F | 10 | 1823 | 1732 | 91 | 4.99 | 4 | 8.5 | 7 | 6 |
| 223 | F | 46 | 3062 | 2869 | 193 | 6.30 | 4 | 6.0 | 6 | 5 |
| 224 | F | 46 | 3329 | 2929 | 400 | 12.02 | 4 | 7.5 | 7 | 7 |
| 225 | F | 21 | 3300 | 3048 | 252 | 7.63 | 4 | 6.5 | 6 | 6 |
| 226 | F | 36 | 3146 | 2720 | 426 | 13.54 | 4 | 6.5 | 7 | 6 |
| 227 | F | 46 | 3214 | 2988 | 226 | 7.04 | 2 | 4.0 | 3 | 4 |
| 228 | M | 34 | 4454 | 4240 | 214 | 4.80 | 4 | 6.5 | 6 | 6 |
Sex: F=female, M=male; FFM: Fat free mass; FM: Fat mass; BF%: Body fat percent; BCSM: BCSMorfeld; BCSW: BCSWemmer; BCSF: BCSFernando; BCSWi: BCSWijeyamohan.
The primary models investigated the relationship between each BCS system and relative fat, with subsequent models investing absolute FM, FFM, and body mass (Table 3). Only BCSFernando was significantly associated with relative FM (Figure 1). BCSWemmer, BCSFernando, and BCSWijeyamohan significantly predicted absolute FM (Figure 2). BCSMorfeld was not significantly associated with either outcome.
Table 3.
Estimates for each BCS system in statistical models to predict body composition.
| Model | AIC | R2 | Estimate | SE | P |
|---|---|---|---|---|---|
| Rel. FM = BCSM | 371.64 | 0.03 | 36.03 | 43.19 | 0.412 |
| Rel. FM = BCSW | 369.16 | 0.11 | 34.63 | 19.43 | 0.087 |
| Rel. FM = BCSF | 366.41 | 0.19 | 50.29 | 20.22 | 0.020 |
| Rel. FM = BCSWi | 369.94 | 0.08 | 38.31 | 24.88 | 0.136 |
| FM = BCSM | 389.20 | 0.10 | 98.45 | 59.10 | 0.108 |
| FM = BCSW | 385.44 | 0.21 | 68.32 | 25.99 | 0.014 |
| FM = BCSF | 381.86 | 0.31 | 89.98 | 26.65 | 0.002 |
| FM = BCSWi | 384.58 | 0.23 | 91.05 | 32.32 | 0.009 |
| FFM = BCSM | 463.68 | 0.06 | 283.50 | 223.40 | 0.216 |
| FFM = BCSW | 463.56 | 0.06 | 137.80 | 104.90 | 0.200 |
| FFM = BCSF | 463.50 | 0.06 | 152.90 | 114.50 | 0.193 |
| FFM = BCSWi | 462.08 | 0.11 | 231.60 | 129.00 | 0.084 |
| Mass = BCSM | 472.08 | 0.08 | 382.00 | 259.60 | 0.153 |
| Mass = BCSW | 471.33 | 0.10 | 206.10 | 120.50 | 0.099 |
| Mass = BCSF | 470.82 | 0.12 | 242.90 | 130.50 | 0.074 |
| Mass = BCSWi | 469.59 | 0.16 | 322.70 | 147.50 | 0.038 |
Rel. FM: Relative fat mass; FM: fat mass; FFM: fat free mass; BCSM: BCSMorfeld; BCSW: BCSWemmer; BCSF: BCSFernando; BCSWi: BCSWijeyamohan.
Figure 1.
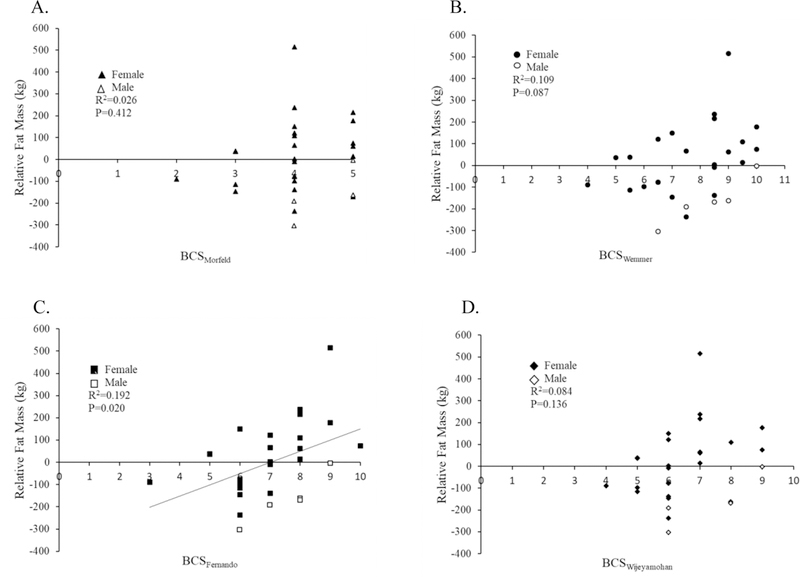
Distribution of Relative Fat Mass by the Morfeld BCS system (A), Wemmer BCS system (B), Fernando BCS system (C), and Wijeyamohan BCS system (D). Positive relative FM values imply the elephant has more fat than expected for their body mass, while negative relative FM values imply the elephant has less fat than expected for their body mass. Trendline indicates statistical significance.
Figure 2.
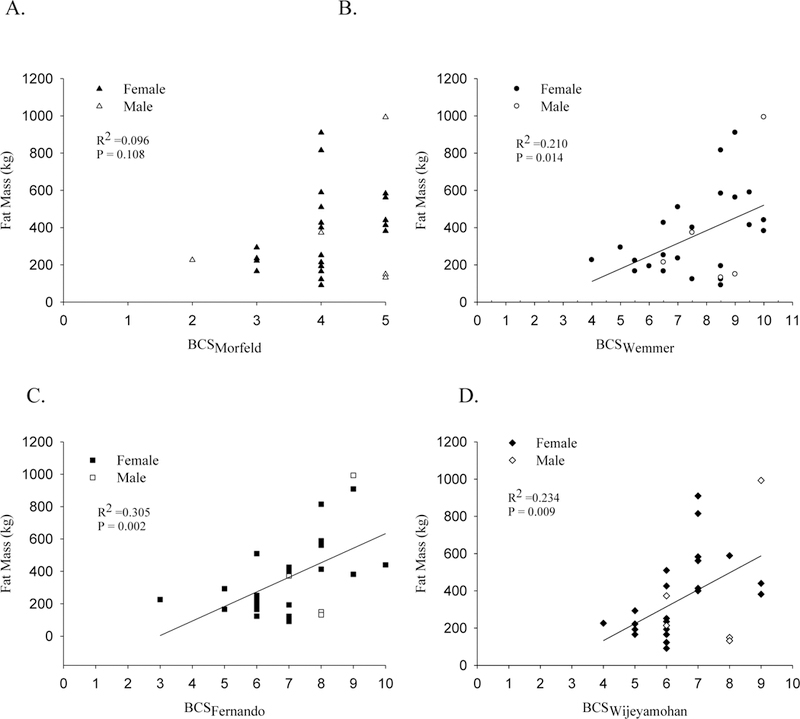
Distribution of absolute FM, unadjusted, by the Morfeld BCS system (A), Wemmer BCS system (B), Fernando BCS system (C), and Wijeyamohan BCS system (D). Trendline indicates statistical significance.
The primary models were then adjusted for sex and age (Table 4). BCSWemmer, BCSFernando, and BCSWijeyamohan were significantly associated with relative FM. All BCS systems were significantly associated with absolute FM, and body mass. BCSWemmer, BCSFernando, and BCSWijeyamohan were significantly associated with FFM, while BCSMorfeld almost reached significance. The models were also adjusted for sex only (Table S1) and age only (Table S2) to determine possible effects of just one covariate versus the other.
Table 4.
Estimates for each BCS system in statistical models to predict body composition, adjusted for sex and age.
| Model | AIC | R2 | Estimate | SE | P |
|---|---|---|---|---|---|
| Rel. FM = BCSM Sex Age | 365.34 | 0.33 | 73.00 | 43.31 | 0.105 |
| Rel. FM = BCSW Sex Age | 362.61 | 0.39 | 43.91 | 18.58 | 0.027 |
| Rel. FM = BCSF Sex Age | 356.87 | 0.50 | 62.93 | 17.93 | 0.002 |
| Rel. FM = BCSWi Sex Age | 360.85 | 0.43 | 63.37 | 23.12 | 0.011 |
| FM = BCSM Sex Age | 389.30 | 0.21 | 157.57 | 66.44 | 0.026 |
| FM = BCSW Sex Age | 383.30 | 0.37 | 95.79 | 26.88 | 0.002 |
| FM = BCSF Sex Age | 378.64 | 0.46 | 116.35 | 26.45 | <0.001 |
| FM = BCSWi Sex Age | 381.96 | 0.40 | 128.38 | 33.72 | <0.001 |
| FFM = BCSM Sex Age | 444.73 | 0.589 | 359.84 | 178.76 | 0.055 |
| FFM = BCSW Sex Age | 440.27 | 0.65 | 221.62 | 74.34 | 0.006 |
| FFM = BCSF Sex Age | 442.39 | 0.62 | 210.46 | 82.56 | 0.018 |
| FFM = BCSWi Sex Age | 441.34 | 0.63 | 269.42 | 97.35 | 0.011 |
| Mass = BCSM Sex Age | 458.08 | 0.52 | 517.42 | 226.90 | 0.032 |
| Mass = BCSW Sex Age | 452.34 | 0.61 | 317.42 | 92.21 | 0.002 |
| Mass = BCSF Sex Age | 453.33 | 0.59 | 326.83 | 100.38 | 0.003 |
| Mass = BCSWi Sex Age | 453.00 | 0.60 | 397.82 | 119.88 | 0.003 |
Rel. FM: Relative fat mass; FM: fat mass; FFM: fat free mass; BCSM: BCSMorfeld; BCSW: BCSWemmer; BCSF: BCSFernando; BCSWi: BCSWijeyamohan.
Results after accounting for zoo and familial relatedness in the model with age and sex are presented (Table 5). All BCS systems were associated with relative FM and absolute FM measures. Only BCSWemmer was significantly associated with FFM. BCSWemmer, BCSWijeyamohan and BCSFernando were significantly associated with body mass. The models were also adjusted for zoo only (Table S3) and familial relatedness only (Table S4) to determine possible effects of just one covariate versus the other.
Table 5.
Estimates for each BCS system in statistical models to predict body composition, adjusted for sex, age, zoo, and familial relatedness.
| Model | AIC | Estimate | SE | P |
|---|---|---|---|---|
| Rel. FM = BCSM Sex Age Zoo Pedigree | 271.98 | 111.98 | 45.97 | 0.015 |
| Rel. FM = BCSW Sex Age Zoo Pedigree | 265.29 | 86.42 | 19.45 | <0.001 |
| Rel. FM = BCSF Sex Age Zoo Pedigree | 265.48 | 81.32 | 18.38 | <0.001 |
| Rel. FM = BCSWi Sex Age Zoo Pedigree | 263.68 | 117.09 | 24.97 | <0.001 |
| FM = BCSM Sex Age Zoo Pedigree | 283.59 | 208.08 | 68.66 | 0.002 |
| FM = BCSW Sex Age Zoo Pedigree | 275.54 | 144.48 | 27.43 | <0.001 |
| FM = BCSF Sex Age Zoo Pedigree | 281.40 | 115.75 | 29.57 | <0.001 |
| FM = BCSWi Sex Age Zoo Pedigree | 277.72 | 193.53 | 42.28 | <0.001 |
| FFM = BCSM Sex Age Zoo Pedigree | 328.91 | 260.50 | 227.29 | 0.252 |
| FFM = BCSW Sex Age Zoo Pedigree | 327.87 | 221.85 | 110.66 | 0.045 |
| FFM = BCSF Sex Age Zoo Pedigree | 329.73 | 155.28 | 112.20 | 0.166 |
| FFM = BCSWi Sex Age Zoo Pedigree | 328.99 | 219.87 | 153.17 | 0.151 |
| Mass = BCSM Sex Age Zoo Pedigree | 336.26 | 457.56 | 292.17 | 0.117 |
| Mass = BCSW Sex Age Zoo Pedigree | 334.23 | 367.67 | 132.48 | 0.006 |
| Mass = BCSF Sex Age Zoo Pedigree | 335.88 | 312.45 | 134.28 | 0.020 |
| Mass = BCSWi Sex Age Zoo Pedigree | 335.81 | 397.54 | 200.63 | 0.048 |
Rel. FM: Relative fat mass; FM: fat mass; FFM: fat free mass; BCSM: BCSMorfeld; BCSW: BCSWemmer; BCSF: BCSFernando; BCSWi: BCSWijeyamohan; Pedigree: familial relatedness.
AIC score was included to determine the best fitting models on each outcome. For all BCS systems, and all outcomes, the best model adjusted for sex, age, zoo, and familial relatedness. For relative FM, BCSWemmer, BCSFernando, and BCSWijeyamohan models resulted in similar AIC scores, which were lower (i.e., better fit) than BCSMorfeld.
Intra-assessor reliability ranged for BCSMorfeld, BCSWemmer, BCSFernando, and BCSWijeyamohan, ICC (2,1) = 0.76–0.91, 0.83–0.97, 0.76–0.97, and 0.78–0.97, respectively. Inter-assessor reliability for BCSMorfeld, BCSWemmer, BCSFernando, and BCSWijeyamohan, ICC (2,1) = 0.58–0.74, 0.77–0.83, 0.60–0.82, and 0.59–0.66, respectively.
Discussion
To our knowledge, this is the first study to examine how different BCS systems correspond to measures of adiposity in elephants. BCSFernando was the most reliable system to predict relative FM, unadjusted. Relative fat refers to the amount of fat an individual has after body mass differences are accounted for, as larger individuals typically have more fat overall. In contrast, absolute fat refers to the total amount of fat the elephant has regardless of their size. Compared to absolute fat, relative fat may be more biologically relevant as greater fat mass relative to body mass is more likely to be linked to health issues associated with excess fat.
The use of deuterium dilution to quantify body composition/adiposity is a major strength of this study. Deuterium, a non-radioactive isotope of hydrogen, replaces hydrogen in water molecules, allowing the measurement of total body water (Pace & Rathbun, 1945). There is a relationship between total body water and FFM in mammals, termed the hydration constant, ultimately allowing for body composition quantification (Wang et al., 1999). Although assumptions were made (e.g., appropriate hydration constant used) and deuterium dilution has not been validated by total carcass analysis in Asian elephants, the method appears to be robust over time and species (Acquarone & Born, 2007; Burkholder & Thatcher, 1998; Cowan, Robinson, Greenhalgh, & McHattie, 1979; Dugdale et al., 2011; Farley & Robbins, 1994; Schloerb, Friis-Hansen, Edelman, Solomon, & Moore, 1950).
Relative FM is arguably the most important biological outcome when using a BCS system. Therefore, the primary linear regression model tested whether each BCS system could predict relative FM of the elephant. The Fernando system was the only system able to independently predict relative FM. A potential explanation for the Fernando system’s success may be related to the view of the elephant used for scoring. The Fernando scoring system, unlike the Morfeld and Wemmer systems, only relies on the lateral view of the elephant. Although the Wijeyamohan system relies only on the lateral view, differences in results may be due to the reference animals used and the simplicity of the Fernando system. The Fernando system uses five photographs aligned in one vertical column, progressing in body condition. The reference photographs used may have had greater consistency in subcutaneous fat changes with the increasing predefined BCS and allowed for the entire spectrum of scores to be visualized. Further, the lateral view may encompass the specific fat deposits that primarily expand during positive energy balance. To determine whether this was the case, we conducted a stepwise regression analysis to predict relative FM. The stepwise regression included six anatomical regions provided by the Wemmer system, in addition to the surface area of the elephant’s side region between the front and back legs. The six anatomical regions provided by the Wemmer system included the three anatomical regions used by the Morfeld system. Of the six anatomical regions used (temporal depression in the head, pronouncement of the scapula, visibility of the ribs, depression in front of the pelvic bone, visibility of the lumbar vertebrae viewed from behind the elephant, and visibility of the pelvic bone) and the surface area, the stepwise selection resulted in a model with only one explanatory variable, the ribs (AIC = 284.58). Subcutaneous fat over the ribs has not been measured via ultrasound due to practical limitations in locating the ribs reliably (Morfeld et al. 2014). The other anatomical regions relied upon may reflect anatomical changes associated with age rather than nutritional status as inferred by the wet/dry season. For example, Albl (1971) took a series of direct body measurements to investigate their relationship with subcutaneous fat and muscle mass in wild African elephants. Albl (1971) found that most of the direct measurements were indicative of age and not nutritional status as inferred based on the dry versus wet season. Of the relevant measurements, the temporal dent and the scapular depression showed a greater association with age. Further, in other species, older age is associated with increased muscle loss (Colman, McKiernan, Aiken, & Weindruch, 2005; Deschenes, 2004), particularly in females (Janssen, Heymsfield, Wang, & Ross, 2000). The clear pronouncement of certain anatomical regions may be related to preferential muscle loss. Collectively, these results suggest that in theory, scoring only the ribs may be required when interested in elephants’ relative fat stores.
When age and sex were accounted for in the primary model, the Fernando, Wemmer, and Wijeyamohan systems significantly predicted relative FM. Following exploratory analyses, it appeared that the relationship between relative FM and these systems was mediated through sex. Elephants exhibit sexual dimorphism, with males being much larger and heavier compared to their female counterparts, yet BCS systems have been generalized to either sex. The inclusion of both males and females in this study provided the opportunity to demonstrate that there may be inherent sex differences within BCS systems, albeit relying on a small sample size of males. The four included BCS systems assumed the criteria used to score the elephant are the same for males and females; however, this may not be appropriate, particularly when the scorer lacks extensive experience and may not be able to recognize subtle differences in developed musculature from fat deposits. Similar to other species (Wells, 2007), in this study sample, males overall have significantly greater FFM and relatively less FM compared to females. In addition, the majority of males tended to have less FM than expected (i.e., relative FM values for males fell below the regression line). This is because, in most mammalian species, relative to females, males have proportionately less fat (Ledger & Smith, 1964; Pitts & Bullard, 1968; Schoenemann, 2004; Wells, 2007). Therefore, it is feasible that a male could be scored a BCS =4 due to their greater FFM deposition obscuring bone structures, while a female could be scored a BCS =4 due to their greater FM obscuring bone structures. Both elephants receive the same score, but have overall different body compositions, which was recently independently posited by other investigators (Schiffmann et al., 2018).
The study population resided at eight different zoos and included 15 pairs of related individuals, the majority being a parent offspring pair (2 father offspring, 7 mother offspring), followed by half-sibling pairs. Therefore, zoo and family relatedness were included in the model to account for potentially correlated residuals attributed to the environment and genetic relatedness. BCSWemmer, BCSFernando, and BCSWijeyamohan models resulted in similar AIC scores, which were lower (i.e., better fit) than BCSMorfeld when predicting relative FM (Table 5). Therefore, visual BCS may be an appropriate tool for physical monitoring of zoo elephants when focusing on elephant health. Following exploratory analyses, it was determined that zoo accounted for much of the variability and helped isolate the effect of BCS on relative FM. Therefore, within a zoo, there may be a greater correlation between BCS and relative FM. Although from a statistical and research perspective it is important to know an elephant’s age, sex, familial relatedness, and housing institution when assigning a BCS, from a practical standpoint, a zookeeper cannot adjust for such factors when scoring their own elephants, thus they should focus on consistency when using a BCS system.
The Wemmer system consistently produced the highest ICC values, both in terms of intra- and inter-assessor reliability, while the Wijeyamohan system typically had the lowest. The stronger correlation between the Wemmer scores is likely attributed to the detailed and clear description of the anatomical regions, each of which is scored separately and then the scores added, which is supported by a similar composite BCS system used in black rhinoceros (Reuter & Adcock, 1998). In comparison, the Wijeyamohan method directly compares the focal elephant photograph to a series of reference photographs, with an accompanying description. However, the reference photographs for the Wijeyamohan system are placed on multiple pages throughout the publication, making comparisons more difficult. Our results contrast with recent findings from Schiffmann et al. (2017), which found the Wemmer system to have the highest inter-assessor variability, and Wijeyamohan to have some of the lowest inter-assessor variability. Differences in results may be attributed to Schiffmann et al. (2017) modifying the scoring systems, or to the differences in the background of the scorers. For example, Schiffmann et al. (2017) treated the Wijeyamohan system as a flow chart algorithm approach, providing more detail and direction in assigning scores. This suggests that the improved consistency in scoring is likely attributed to following the flow chart for this specific system, as in the present study, the Wijeyamohan system was used based on comparisons to example photographs only. Ultimately, descriptors must be clearly defined to allow assessors certainty of their interpretations (Teasdale & Jennett, 1974), and this ambiguity may have led to the lower ICC results. Scoring accuracy may also be contingent upon the pictures each BCS system uses as their examples. Although all four systems relied on example photographs in some capacity, the lighting, background, and amount of the photograph taken up by the elephant all varied. Lack of uniformity between and within each system may have contributed to how each elephant is scored. Ultimately, the homogeneity of the photographs may prove easier for the assessor to consistently score the elephant and future systems should consider the standardization of photographs. Lastly, a flexible scoring range seemed beneficial for the systems. For example, the Wemmer, Fernando, and Wijeyamohan BCS systems score the elephants on a minimum 10-point scale, while the Morfeld system only scores elephants on a 5-point scale. By having a smaller range of scores, the Morfeld system provides less differentiation and flexibility in assigning elephants to each score. Indeed, when examining the distribution of scores in this study, there was substantial overlap between elephants scored a 4 and those scored a 5, based on the inter-assessor data. Future inclusion of half scores in the Morfeld system should improve the differentiation between elephants.
In conclusion, this study suggests that BCS better explains relative FM than absolute FM, FFM, or body mass, with the Fernando system proving to be the most reliable system to use and a wider scoring range improving the overall predictability of body fat. The success of the Fernando system may be related to only using a photographic guide based on the lateral view of the elephant showing a clear progression in the loss of visibility of the ribs. In addition, by using multiple assessors to score each elephant multiple times, it was possible to examine which BCS system proved most consistent. The Wemmer system produces the most consistent scores. This is valuable information as BCS is a tool widely used by individuals of various backgrounds. Because only elephants under human care were used in this study, it is not known whether the results carry over to free-ranging populations. Nevertheless, this was the first step required in determining the validity of BCS systems for elephants. Further, when considering health implications and/or comparing individuals that vary in size, it is better to predict relative fat rather than absolute fat. Future development or refinement of current BCS systems should include only those measures (i.e., the ribs) that were significantly associated with relative FM as this could allow for clearer fat classifications. It is helpful to have a valid BCS system as BCSs are consistently used in Asian elephant husbandry, welfare, and research. Consistently using the same BCS system will allow keepers to recognize changes in elephants that may require intervention to improve overall wellness.
Supplementary Material
Acknowledgments
The authors thank Dr. Kenda Rigdon and Ms. Lindsay Pappas for scoring the elephants. Dr. Barbara Gower and Mrs. Cindy Zeng at the UAB Nutrition Obesity Research Center’s Metabolism Core for their assistance with mass spectroscopy. Dr. Paul Lin for assistance with statistical analyses. The authors graciously thank Columbus Zoo and Aquarium, Fort Worth Zoo, Little Rock Zoo, Oklahoma City Zoo, Oregon Zoo, Saint Louis Zoological Park, and Santa Barbara Zoo for their participation and hospitality. A big thank you to the zoos’ elephant keepers and elephants, as without them this study would not have happened. The Birmingham Zoo and Pat Flora and his elephant team were invaluable, helping improve the methods. This work was supported in part by the Smithsonian Institute, the Nutrition Obesity Research Center (P30DK056336), the Diabetes Research Center (P30DK079626), and the Nathan Shock Center on Aging (P30AG050886). DEC is supported by the National Institute of Aging (P30AG050886). The opinions expressed herein are those of the authors and not necessarily those of the NIH or any other organization.
References
- Acquarone M, & Born EW (2007). Estimation of water pool size, turnover rate and body composition of free-ranging Atlantic walruses (Odobenus rosmarus rosmarus) studied by isotope dilution. Journal of the Marine Biological Association of the United Kingdom, 87(1), 77–84. [Google Scholar]
- Albl P (1971). Studies on assessment of physical condition in African elephants. Biological Conservation, 3(2), 134–140. [Google Scholar]
- Allison D, Paultre F, Goran M, Poehlman E, & Heymsfield S (1995). Statistical considerations regarding the use of ratios to adjust data. International journal of obesity and related metabolic disorders: Journal of the International Association for the Study of Obesity, 19(9), 644–652. [PubMed] [Google Scholar]
- Brown JL (2000). Reproductive endocrine monitoring of elephants: an essential tool for assisting captive management. Zoo Biology, 19(5), 347–367. [Google Scholar]
- Burkholder WJ, & Thatcher CD (1998). Validation of predictive equations for use of deuterium oxide dilution to determine body composition of dogs. American Journal of Veterinary Research, 59(8), 927–937. [PubMed] [Google Scholar]
- Charette R, Bigras-Poulin M, & Martineau G-P (1996). Body condition evaluation in sows. Livestock Production Science, 46(2), 107–115. [Google Scholar]
- Chusyd DE, Brown JL, Hambly C, Johnson MS, Morfeld K, Patki A, . . . Nagy TR (2018). Adiposity and Reproductive Cycling Status in Zoo African Elephants. Obesity, 26(1), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clubb R, & Mason G (2002). A review of the welfare of zoo elephants in Europe: RSPCA. [Google Scholar]
- Colman RJ, McKiernan SH, Aiken JM, & Weindruch R (2005). Muscle mass loss in Rhesus monkeys: age of onset. Experimental Gerontology, 40(7), 573–581. [DOI] [PubMed] [Google Scholar]
- Conde DA, Flesness N, Colchero F, Jones OR, & Scheuerlein A (2011). An emerging role of zoos to conserve biodiversity. Science, 331(6023), 1390–1391. [DOI] [PubMed] [Google Scholar]
- Cowan R, Robinson J, Greenhalgh J, & McHattie I (1979). Body composition changes in lactating ewes estimated by serial slaughter and deuterium dilution. Animal Science, 29(1), 81–90. [Google Scholar]
- Deschenes MR (2004). Effects of aging on muscle fibre type and size. Sports Medicine, 34(12), 809–824. [DOI] [PubMed] [Google Scholar]
- Dugdale A, Curtis G, Milne E, Harris P, & Argo C (2011). Assessment of body fat in the pony: Part II. Validation of the deuterium oxide dilution technique for the measurement of body fat. Equine Veterinary Journal, 43(5), 562–570. [DOI] [PubMed] [Google Scholar]
- Farley SD, & Robbins CT (1994). Development of two methods to estimate body composition of bears. Canadian Journal of Zoology, 72(2), 220–226. [Google Scholar]
- Faust L, & Marti K (2011). Technical report on Zoo Risk modeling of the North American African elephant SSP population. Lincoln Park Zoo, Chicago. [Google Scholar]
- Franco-Villoria M, Wright CM, McColl JH, Sherriff A, Pearce MS, & team G. M. S. c. (2016). Assessment of adult body composition using bioelectrical impedance: comparison of researcher calculated to machine outputted values. BMJ Open, 6(1), e008922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Shewchuk R, Gower BA, Nagy TR, Carpenter WH, & Johnson RK (1998). Longitudinal changes in fatness in white children: no effect of childhood energy expenditure. The American Journal of Clinical Nutrition, 67(2), 309–316. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, . . . Cox NA (2010). The impact of conservation on the status of the world’s vertebrates. Science, 330(6010), 1503–1509. [DOI] [PubMed] [Google Scholar]
- Holt WV, Brown JL, & Comizzoli P (2014). Reproductive Sciences in Animal Conservation: Springer. [Google Scholar]
- Janssen I, Heymsfield SB, Wang Z, & Ross R (2000). Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal of Applied Physiology, 89(1), 81–88. [DOI] [PubMed] [Google Scholar]
- Jefferies B (1961). Body condition scoring and its use in management. Tasmanian Journal of Agriculture, 32, 19–21. [Google Scholar]
- Ledger H, & Smith N (1964). The carcass and body composition of the Uganda Kob. The Journal of Wildlife Management, 827–839. [Google Scholar]
- Lewis KD, Shepherdson DJ, Owens TM, & Keele M (2010). A survey of elephant husbandry and foot health in North American zoos. Zoo Biology, 29(2), 221–236. [DOI] [PubMed] [Google Scholar]
- Liu TW, Heden TD, Matthew Morris E, Fritsche KL, Vieira‐Potter VJ, & Thyfault JP (2015). High‐Fat Diet Alters Serum Fatty Acid Profiles in Obesity Prone Rats: Implications for InVitro Studies. Lipids, 50(10), 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink RP, & Katan MB (1992). Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arteriosclerosis and Thrombosis: A Journal of Vascular Biology, 12(8), 911–919. [DOI] [PubMed] [Google Scholar]
- Morfeld KA, & Brown JL (2014). Ovarian acyclicity in zoo African elephants (Loxodonta africana) is associated with high body condition scores and elevated serum insulin and leptin. Reproduction, Fertility and Development. [DOI] [PubMed] [Google Scholar]
- Morfeld KA, Lehnhardt J, Alligood C, Bolling J, & Brown JL (2014). Development of a body condition scoring index for female African elephants validated by ultrasound measurements of subcutaneous fat. PloS one, 9(4), e93802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeld KA, Meehan CL, Hogan JN, & Brown JL (2016). Assessment of body condition in African (Loxodonta africana) and Asian (Elephas maximus) elephants in North American zoos and management practices associated with high body condition scores. PloS one, 11(7), e0155146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkaew T, Brown JL, Bansiddhi P, Somgird C, Thitaram C, Punyapornwithaya V, . . . Khonmee J (2018). Body condition and adrenal glucocorticoid activity affects metabolic marker and lipid profiles in captive female elephants in Thailand. PloS one, 13(10), e0204965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N, & Rathbun EN (1945). Studies on body composition III. The body water and chemically combined nitrogen content in relation to fat content. Journal of Biological Chemistry, 158(3), 685–691. [Google Scholar]
- Pitts G, & Bullard T (1968). Some interspecific aspects of body composition in mammals Body composition in animals and man. Washington, DC: National Academy of Sciences, 45–70. [Google Scholar]
- Reuter H, & Adcock K (1998). Standardised body condition scoring system for black rhinoceros (Diceros bicornis). Pachyderm(26), 116–121. [Google Scholar]
- Schiffmann C, Clauss M, Fernando P, Pastorini J, Wandler P, Ertl N, . . . Hatt J-M (2018). Body condition scores of European zoo elephants (Elephas maximus and Loxodonta africana): Status quo and influencing factors. Journal of Zoo and Aquarium Research, 6(3), 91–103. [Google Scholar]
- Schiffmann C, Clauss M, Hoby S, & Hatt J-M (2017). Visual body condition scoring in zoo animals–composite, algorithm and overview approaches. Journal of Zoo and Aquarium Research, 5(1), 1–10. [Google Scholar]
- Schloerb PR, Friis-Hansen BJ, Edelman IS, Solomon A, & Moore FD (1950). The measurement of total body water in the human subject by deuterium oxide dilution: With a consideration of the dynamics of deuterium distribution. The Journal of Clinical Investigation, 29(10), 1296–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann PT (2004). Brain size scaling and body composition in mammals. Brain, Behavior and Evolution, 63(1), 47–60. [DOI] [PubMed] [Google Scholar]
- Secor SM, & Nagy TR (2003). Non-invasive measure of body composition of snakes using dual-energy x-ray absorptiometry. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 136(2), 379–389. [DOI] [PubMed] [Google Scholar]
- Teasdale G, & Jennett B (1974). Assessment of coma and impaired consciousness: a practical scale. The Lancet, 304(7872), 81–84. [DOI] [PubMed] [Google Scholar]
- Treiber K, Reppert N, & Ward A (2012). Transcutaneous rump ultrasound of Asian elephants (Elephas maximus): body fat, body condition and body weight. Paper presented at the The 7th Crissey Zoological Nutrition Symposium, Raleigh, NC. p. [Google Scholar]
- Vazquez A, Bates D, Rosa G, Gianola D, & Weigel K (2010). An R package for fitting generalized linear mixed models in animal breeding1. Journal of Animal Science, 88(2), 497–504. [DOI] [PubMed] [Google Scholar]
- Wang Z-M, Pierson R, & Heymsfield SB (1992). The five-level model: a new approach to organizing body-composition research. The American Journal of Clinical Nutrition, 56(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, & Heymsfield SB (1999). Hydration of fat-free body mass: review and critique of a classic body-composition constant. The American Journal of Clinical Nutrition, 69(5), 833–841. [DOI] [PubMed] [Google Scholar]
- Wells JC (2007). Sexual dimorphism of body composition. Best practice & research Clinical Endocrinology & Metabolism, 21(3), 415–430. [DOI] [PubMed] [Google Scholar]
- Wemmer C, Krishnamurthy V, Shrestha S, Hayek LA, Thant M, & Nanjappa KA (2006). Assessment of body condition in Asian elephants (Elephas maximus). Zoo Biology, 25(3), 187–200. doi: 10.1002/zoo.20099 [DOI] [Google Scholar]
- Wolf T, Ellington C, Davis S, & Feltham M (1996). Validation of the doubly labelled water technique for bumblebees Bombus terrestris (L.). Journal of Experimental Biology, 199(4), 959–972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


