Abstract
Objective:
The incidence of type 1 diabetes (T1D) is increasing, most notably in young children and in racial and ethnic minorities. Historically, screening for risk with T1D-associated antibodies has been limited to those with a family history, while up to 90% of newly diagnosed patients lack such a family history. To address the needs to screen diverse ethnic groups in the general population, we screened children for T1D-associated antibodies in the Denver, Colorado metro area at community health fairs.
Methods:
Children attending health fairs from 2015 to 2018 were offered free T1D screening by measuring the four prototypical T1D-associated antibodies. A finger stick capillary puncture was performed to collect blood spots on filter paper. Dried blood spots (DBSs) were eluted and antibodies were measured using fluid-phase radio-binding assays.
Results:
At 39 health fairs, children were educated on the signs and symptoms of diabetes, and screened for T1D-associated antibodies (n = 478), which represented 90% of those that attended. Median age was 9.0 years (range of 1–18) with diverse ethnic backgrounds: 37% Hispanic, 31% Caucasian, 20% African American, and 12% other. Nine children screened positive for antibodies, single n = 8 and multiple n = 1, and confirmation with serum samples showed excellent correlation to the measurements from DBSs for antibodies directed against GAD, IA-2, and ZnT8 (P < .01 for each).
Conclusions:
Screening for T1D risk at community health fairs using DBSs on filter paper is feasible and provides an avenue to screen children from ethnically diverse backgrounds.
Keywords: children, health fairs, islet autoantibodies, screening, type 1 diabetes
1 |. INTRODUCTION
Type 1 diabetes (T1D) is a common childhood chronic disease, with a prevalence of approximately 1 in 300 children developing the disease.1 The incidence of T1D is increasing in the United States population by 3%–5% per year and is especially evident in racial and ethnic minorities with Hispanic Americans experiencing the most dramatic increases.1,2 Often, children are not diagnosed before clinical symptoms of T1D are present. In Colorado, nearly half of children with new-onset T1D present with potentially life-threatening diabetic ketoacidosis (DKA), and other locations also report unacceptably high DKA rates.3,4 DKA is the major cause of morbidity and mortality in children with T1D as it can lead to cerebral edema and death. Additionally, studies indicate that DKA can have lifelong effects on the brain, such as impaired memory and cognition.5–8
T1D-associated antibodies, those directed against insulin (IAA), glutamic decarboxylase (GAD), islet antigen (IA-2), and zinc transporter 8 (ZnT8), are present in the peripheral blood years prior to clinical T1D onset. In children with multiple antibodies, the risk for developing T1D within 10 years is approximately 70% and their lifetime risk approaches 100%.9 Prospective birth cohort studies have shown that children receiving routine follow-up after screening positive for T1D-associated antibodies are less likely to present with DKA at T1D onset compared to children in the community.10–13 Importantly, screening for T1D allows children to have better long-term glycemic control and a reduced risk for complications, as children who present with DKA at T1D onset have higher hemoglobin A1c levels over time compared to children without DKA at diagnosis.14
Screening for T1D is currently recommended for family members of people with T1D by the American Diabetes Association.15 However, a family history of a first-degree relative with T1D is lacking in 88% to 90% of children. Because screening children for T1D-associated antibodies can reduce DKA and improve long-term outcomes when children are identified and followed over time, methods to screen ethnically diverse populations for T1D risk are needed. Historically, screening for diseases using dried blood spot (DBS) samples on filter paper has been successful and is routinely used in clinical practice (e.g, newborn screenings).16 Previously, we showed strong concordance between serum and DBS samples eluted off filter paper for measuring all four major T1D-associated antibodies.17 In this study, we screened children at community health fairs across the Denver, Colorado metro area for T1D risk by collecting DBS on filter paper to measure T1D-associated antibodies.
2 |. METHODS
2.1 |. Community health fairs
Screening for T1D-associated antibodies was offered to children aged 1 to 18 years attending community health fairs across the Denver, Colorado metro area between September 2015 and November 2018, which encompassed 39 fairs at 29 different locations. The fairs were sponsored by the 9Health Fair organization (www.9healthfair.org), which is one of the largest non-profit health, wellness, and preventive education programs in the United States.
2.2 |. Blood sampling and screening
Families were provided education about the types of diabetes, along with signs and symptoms. After obtaining consent and assent to screen for T1D risk, demographic and contact information was obtained. To collect samples for T1D antibody measurements at the screening visit (Figure 1), a capillary finger stick was performed and one hanging drop of blood was placed on each of the four 6 mm spots on Whatman #903 filter paper and allowed to completely dry; this represents approximately 120 μL of whole blood. All participants were notified by letter of their antibody results. Children who screened positive for one or more antibodies were invited to confirm the positive antibody results with a venipuncture for serum collection at the Barbara Davis Center for Diabetes. Hemoglobin A1c (HbA1c) and blood glucose were also measured at this confirmation visit. Those with confirmed positive antibodies were invited to enroll in a research study for semi-annual monitoring of T1D development. This study was approved by the Colorado Multiple Institutional Review Board.
FIGURE 1.
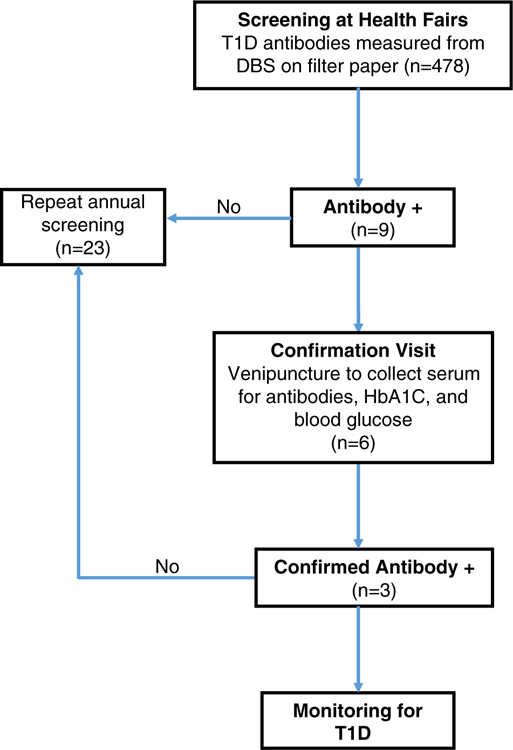
Flow diagram for screening children at community health fairs and confirmation of type 1 diabetes-associated antibodies
2.3 |. T1D-associated antibody measurements
DBSs were transported to the Michels’ laboratory at the Barbara Davis Center, and antibodies were eluted from DBS as previously described.17 Biochemical autoantibodies (IAA, GADA, IA-2A, and ZnT8A) were then measured from DBS eluents with fluid-phase radio-binding assays (RBA).18–20 Levels of autoantibodies are expressed as a DK unit (NIDDK unit) from a standard curve for GADA and IA-2A or an index for IAA and ZnT8A [index = (CPM sample-CPM negative control)/(CPM positive control-CPM negative control)] where CPM is counts per minute, with each antibody having its own positive standard for calculations. Serum samples obtained from confirmation visits were assayed in an identical manner. The same positive cut-off values were applied to both DBS and serum measurements with index 0.01 positive for insulin antibodies, 20 DK units for GADA, 5 DK units for IA-2A, and index 0.020 for ZnT8A. These cut-off values are based upon the 99th percentile from normative data measuring antibodies in non-diabetic individuals.
2.4 |. Statistical analyses
Linear regression was done using GraphPad Prism 8.0 software to compare antibody measurement from DBS on filter paper to those from a confirmation visit in which serum was obtained. A two-tailed P value of <.05 is considered significant.
3 |. RESULTS
Over the course of 3 years, 478 children were screened for T1D-associated antibodies at 39 separate community health fairs. This represents approximately 90% of children that attended these fairs. The ages of children screened ranged from 1 to 18 years with a median age of 9.0 and mean of 9.1 years (Figure 2A). Notably, many young children less than 5 years of age were screened (n = 107, 22.4%). 52% of the participants were female. The ethnic and racial distribution of children was diverse with the largest ethnicity being Hispanic at 37% of participants (Figure 2B). This corresponds to the sizeable Hispanic population within the state of Colorado. The vast majority of families did not report a first-degree relative with T1D (87.7%).
FIGURE 2.
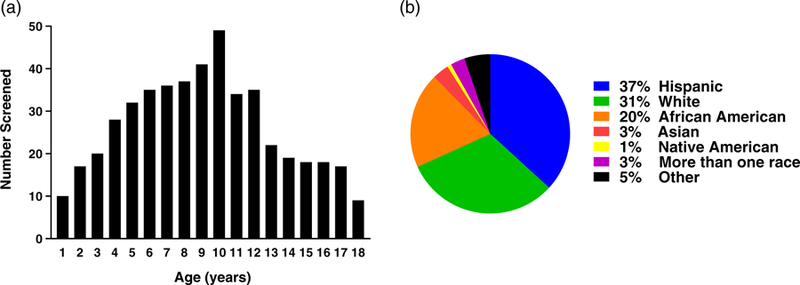
Demographic data of children screened at community health fairs. (A) Age and (B) racial distribution of the screened children
Of the children screened (n = 478), the vast majority of the samples collected as DBSs on filter paper were adequate to measure all four antibodies (98.7%), as samples were collected by trained volunteers at the health fairs. Only one sample was inadequate to measure any antibodies, and five samples had two or three antibodies measured. Nine children screened positive for T1D-associated antibodies with eight children having a single antibody (1.7%). Of those, five were positive for GADA and three for IAA. One child was positive for three antibodies (0.21%), which included GADA, IA-2A, and ZnT8A (Table 1). The racial distribution of those children that screened positive include: Hispanic (56%), African American (22%), Native American (11%), and Caucasian (11%). We found that 2.8% (5/176) of Hispanic children screened positive for T1D antibodies.
TABLE 1.
Type 1 diabetes-associated antibodies among those that screened positive
| Antibody | Screened+ (n = 9) | Confirmation visit (n = 6) | Confirmed+ (n = 3) |
|---|---|---|---|
| GADA | 6 | 4 | 3 |
| IA-2A | 1 | 1 | 1 |
| ZnT8A | 1 | 1 | 1 |
| IAA | 3 | 2 | 0 |
| Single+ | 8 | 5 | 2 |
| Multiple+ | 1 | 1 | 1 |
Abbreviations: GADA, glutamic decarboxylase antibodies; IA-2A, islet antigen antibodies; IAA, insulin autoantibodies; ZnT8A, zinc transporter 8 antibodies.
Six of the nine children that screened positive for at least one antibody completed a confirmation visit, which includes venipuncture to obtain serum for T1D-associated antibody measurements, HbA1c, and blood glucose level (Table 1). At this separate visit, two of three children who were positive for GADA alone at screening confirmed positive, and two children with low level IAA were negative. The child with multiple antibodies, GADA, IA-2A, and ZnT8, at screening was confirmed positive for all three antibodies. As depicted in Figure 3, there was a strong correlation between DBS sample measurements and serum for GADA (r2 = 0.99, P < .01), IA-2A (r2 = 0.99, P < .01), and ZnT8A (r2 = 1.0, P < .01), but less so for IAA (r2 = 0.04, P = n.s.). None of the children had blood glucose abnormalities (eg, hyperglycemia) at the confirmation visits, indicating that they were identified prior to clinical new-onset T1D.
FIGURE 3.
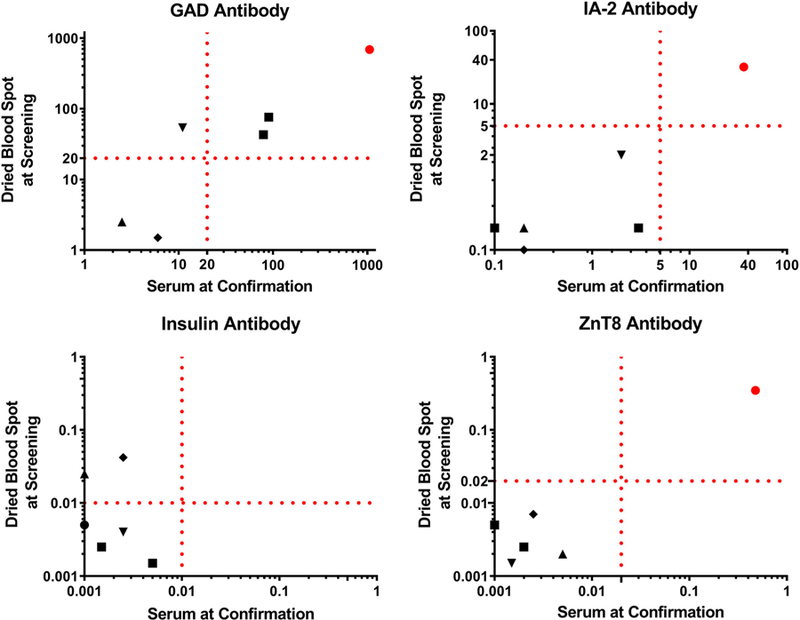
Comparison of type 1 diabetes-associated antibodies from children participating in a confirmation visit measured from serum and eluted dried blood spots at screening (n = 6). Dotted lines indicate positive thresholds for each antibody. Matching symbols are measurements from the same individual. The coefficient of determination (r2) is 0.99 for GADA, 0.99 for IA-2A, and 1.0 for ZnT8A, P < .0001; 0.04 for insulin, P = .72
4 |. DISCUSSION
Using an established community health fair network, we screened children for the four major T1D-associated antibodies by collecting samples as DBSs on filter paper. The samples were then transported to a reference laboratory able to perform sensitive and specific radio-immunoassays for each antibody. There is a strong need to screen children in the general population for T1D risk as many children present with life-threatening DKA,21 a family history is lacking in ~85% of those diagnosed with T1D, and the incidence of T1D is increasing. The large multicenter SEARCH for Diabetes in Youth Study indicates that T1D incidence has indeed increased from 2002 to 2012 with the largest increases in Hispanic children, 4.2% compared to 1.2% for non-Hispanic whites.2 In our study, we were able to screen a large number of Hispanic American children at community health fairs, and we found that 2.8% screened positive for T1D antibodies. This indicates it is feasible to identify those at risk for developing T1D in a Hispanic population.
There have been other screening efforts for T1D risk outside of individuals with a family history or those having a high genetic risk as determined by human leukocyte antigen (HLA) genes. In Bavaria, Germany the Fr1da study is a large screening effort to measure T1D-associated antibodies in children ages 2–5 years of age. Serum from a capillary finger stick is obtained at pediatric offices and used to measure GADA, IA-2A, and ZnT8A. The study has already screened over 25 000 children and their data indicates 0.4% of children have multiple T1D antibodies; the study plans to screen 100 000 children.22 In Denver, Colorado a pilot and feasibility study was conducted to measure T1D-associated antibodies and those for Celiac disease (gluten sensitivity) at local pediatrician offices. Samples from participating children (n = 200) were collected by venipuncture.23 The study showed feasibility for screening two autoimmune diseases; however, there was only a 26% acceptance rate by the families approached for screening. This is in contrast to our efforts screening children at community health fairs with a 90% acceptance rate in the same geographic region. One potential reason for this difference includes highly motivated families that attend health fairs, which is also apparent from 23 children that repeated screening in a subsequent year at a health fair location (Figure 1). Second, the ease of sample collection by a finger prick to collect blood spots on filter paper is less invasive than a blood draw via venipuncture.
We and others have shown a very good correlation between using DBS on filter paper and serum collected via venipuncture to measure T1D-associated antibodies.17,24,25 In this study, there was an excellent correlation for antibodies directed against GAD, IA-2, and ZnT8; the exception was insulin autoantibodies. Two children screened positive for low levels of insulin autoantibodies and at subsequent confirmation visits, up to 3 months later, antibodies were not detectable in serum. This indicates either the need for a higher cutoff detection level for insulin autoantibodies to avoid false positives or the fact that low level antibodies can relapse over time, both of which make insulin autoantibodies notoriously difficult to measure at low levels.26
In order for T1D screening to be widely adapted, it must be cost effective. Meehan et al recently evaluated the cost effectiveness of screening for T1D risk using a cost per case analysis.27 Their results indicate that the cost of screening to avoid DKA at diagnosis in children less than 5 years of age is not effective. The major cost associated with screening is the laboratory cost for measuring T1D-associated antibodies. Because collecting samples as a DBS eliminates the need for venipuncture and shipping expenses, this methodology lowers the cost per case and may be more favorable. An additional consideration is that early identification of T1D and reduction of DKA events has been shown to improve glycemic control over time. Improved glycemic control translates to reduced morbidity and mortality from diabetes, and therefore a potential lower cost over time.14 The cost effectiveness of screening children for T1D risk requires evaluation of both short-term (eg, DKA) and long-term diabetes outcomes.
In conclusion, screening children at community health fairs for T1D-associated antibodies by collecting DBS on filter paper is feasible and well accepted by families. We were able to screen ethnically diverse populations, which is significant as T1D incidence is increasing in children of minority racial and ethnic groups. Identifying subgroups of children at risk for T1D from various ethnic populations allows for future studies to understand the distinct factors and mechanisms that have resulted in an increased incidence of diabetes.
ACKNOWLEDGEMENTS
We would like to thank the 9Health Fair organization including Beth Brady and Karen Garcia for their help in coordinating the screening efforts. This work was supported by grant funding from the Juvenile Diabetes Research Foundation (2-SRA-2016–202-5-B) and the National Institute of Diabetes and Digestion and Kidney Disease (NIDDK) (K12 DK094712—Developing Pediatric Diabetes Investigators for the Future).
Funding information
Juvenile Diabetes Research Foundation International, Grant/Award Number: 2-SRA-2016–202-5-B; National Institute, Grant/Award Number: DK094712
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
REFERENCES
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017;376:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998–2012. JAMA 2015;313:1570–1572. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014;133:e938–e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron FJ, Scratch SE, Nadebaum C, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 2014; 37:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aye T, Mazaika PK, Mauras N, et al. Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care 2019;42: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin A, Northam EA, Werther GA, Cameron FJ. Risk factors for decline in IQ in youth with type 1 diabetes over the 12 years from diagnosis/-illness onset. Diabetes Care 2015;38:236–242. [DOI] [PubMed] [Google Scholar]
- 8.Semenkovich K, Bischoff A, Doty T, et al. Clinical presentation and memory function in youth with type 1 diabetes. Pediatr Diabetes 2016;17:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004;27:1399–1404. [DOI] [PubMed] [Google Scholar]
- 11.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 2012;13:308–313. [DOI] [PubMed] [Google Scholar]
- 12.Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34:2347–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: effect of prospective studies with new-born genetic screening and follow up of risk children. Pediatr Diabetes 2018;19:314–319. [DOI] [PubMed] [Google Scholar]
- 14.Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 2017;40:1249–1255. [DOI] [PubMed] [Google Scholar]
- 15.Chiang JL, Maahs DM, Garvey KC, et al. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care 2018;41(9):2026–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32:338–343. [PubMed] [Google Scholar]
- 17.Simmons KM, Alkanani AK, McDaniel KA, et al. Islet autoantibody measurements from dried blood spots on filter paper strongly correlate to serum levels. PLoS One 2016;11:e0166213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci USA 2000;97: 1701–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007;104:17040–17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duca LM, Reboussin BA, Pihoker C, et al. Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: the SEARCH for diabetes in youth study. Pediatr Diabetes 2019;20: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raab J, Haupt F, Scholz M, et al. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 2016;6: e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gesualdo PD, Bautista KA, Waugh KC, et al. Feasibility of screening for T1D and celiac disease in a pediatric clinic setting. Pediatr Diabetes 2016;17:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siraj ES, Rogers DG, Gupta MK, Reddy SS. A simple screening method for individuals at risk of developing type 1 diabetes: measurement of islet cell autoantibodies (GADA, IA-2A, and IAA) on dried capillary blood spots collected on filter paper. Horm Metab Res 2012;44:855–860. [DOI] [PubMed] [Google Scholar]
- 25.Bingley PJ, Rafkin LE, Matheson D, et al. Use of dried capillary blood sampling for islet autoantibody screening in relatives: a feasibility study. Diabetes Technol Ther 2015;17:867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlosser M, Mueller PW, Torn C, Bonifacio E, Bingley PJ, Participating L. Diabetes antibody standardization program: evaluation of assays for insulin autoantibodies. Diabetologia 2010;53:2611–2620. [DOI] [PubMed] [Google Scholar]
- 27.Meehan C, Fout B, Ashcraft J, Schatz DA, Haller MJ. Screening for T1D risk to reduce DKA is not economically viable. Pediatr Diabetes 2015;16:565–572. [DOI] [PubMed] [Google Scholar]


