Abstract
Objective:
Guidelines recommend critically ill children undergo continuous EEG monitoring (CEEG) for electrographic seizure (ES) identification and management. However, limited data exist on anti-seizure medication (ASM) safety for ES treatment in critically ill children.
Methods:
We performed a single-center prospective observational study of critically ill children undergoing CEEG. Clinical and EEG features and ASM utilization patterns were evaluated. We determined the incidence, types, and risk factors for adverse events associated with ASM administration.
Results:
472 consecutive critically ill children undergoing CEEG were enrolled. ES occurred in 131 children (28%). Clinicians administered ASM to 108 children with ES (82%). ES terminated after the initial ASM in 38% who received one ASM, after the second ASM in 35% patients who received two ASM, after the third ASM in 50% patients who received three ASM, and after the fourth ASM in 53% patients who received four ASM. Thirty patients (28%) received anesthetic infusions for ES management. Adverse events occurred in 18 patients (17%). Adverse effects were expected and resolved in all patients, and they were generally serious (15 patients) and definitely related (12 patients). Adverse events were rare in patients with acute symptomatic seizures requiring only 1–2 ASM for treatment but were more common in children with epilepsy, ictal-interictal continuum EEG patterns, or requiring more extensive ASM management.
Significance:
ES ceased after one ASM in only 38% of critically ill children but ceased after two ASM in 73% of critically ill children. Thus, ES management was often accomplished with readily available medications, but optimization of multi-step ES strategies might be beneficial. Adverse events were rare and manageable in children with acute symptomatic seizures requiring only 1–2 ASM for management. Future studies are needed to determine whether management of acute symptomatic ES improves neurobehavioral outcomes.
Keywords: Seizure, status epilepticus, anti-seizure medication, critical care, adverse events
INTRODUCTION
Electrographic seizures (ES) and electrographic status epilepticus (ESE) occur in 7–48% of critically ill children with acute encephalopathy undergoing continuous EEG monitoring (CEEG) in the pediatric intensive care unit (PICU).1–13 High ES exposure (i.e. ESE) in critically ill children is associated with unfavorable outcomes including worse global neurologic outcome, lower quality of life, worse adaptive behavior, and a higher risk for developing subsequent epilepsy.4; 7; 12–14 Given these data, clinicians rationalize that reduction in ES exposure could mitigate secondary brain injury and improve outcomes. As a result, rapid ES identification and aggressive management have become clinical goals. Guidelines and consensus statements recommend that encephalopathic or comatose critically ill children undergo CEEG for 24–48 hours to identify and rapidly manage ES.15; 16 Data indicate that the number of critically ill children undergoing CEEG at large institutions is increasing17 and that most physicians aim to terminate all or most ES using multi-drug regimens and even anesthetic infusions for refractory ES.18; 19
Despite these shifts in clinical practice which emphasize rapid ES identification and aggressive treatment, evidence-based ES management strategies are lacking. Anti-seizure medications (ASM) might produce adverse events, particularly when administered to critically ill children who often have multi-organ dysfunction, require intravenous medication administration, are being administered multi-drug regimens, and who often require ASM polypharmacy to achieve seizure cessation.19 Therefore, it is problematic that only limited data are available regarding the safety of ASM administration for ES treatment in critically ill children. In this prospective observational cohort study of critically ill children with acute encephalopathy who underwent CEEG, we aimed to: (1) determine ASM utilization patterns, (2) determine the incidence and types of adverse events associated with ASM administration, and (3) identify which children were at increased risk for experiencing adverse events due to ASM.
METHODS
This was a prospective observational study of consecutive critically ill children treated between April 2017 and July 2018 in the PICU of a quaternary care referral center. The study was approved by the Institutional Review Board with a consent waiver since the study was low-risk and data from consecutive children were required to avoid bias. The study is registered with clincialtrials.gov (). We applied the Strengthening the Reporting of Observational Studies in Epidemiology standards for reporting.20
Inclusion criteria were: (1) acute encephalopathy of any etiology, and (2) undergoing clinically indicated CEEG. There were three exclusion criteria. First, we excluded neonates (<30 days old) treated in the PICU since neonates have been the subject of other ASM investigations. Second, we excluded epilepsy surgery patients who received brief post-operative care in the PICU. Third, we excluded patients who were admitted after more than two days of care for refractory status epilepticus at a different institution since the data regarding early ASM utilization and adverse events were generally insufficient.
Patients with acute encephalopathy underwent clinically-indicated CEEG to screen for ES based on an institutional Critical Care CEEG Pathway21 derived from national guidelines and consensus statements.15; 22 The indications for CEEG included: (1) encephalopathy with a preceding clinically evident seizure; (2) encephalopathy without a preceding clinically evident seizure; and (3) encephalopathy with abnormal movements or vital sign fluctuations concerning for seizures. Video-EEG monitoring was performed using Natus Neuroworks equipment (Middleton, WI) with electrode placement according to the International 10–20 system. Patients were monitored for ≥24 hours to screen for ES, and patients with ES underwent CEEG for ≥24 hours after their last ES. EEG interpretation was performed by the Electroencephalography Service, and patients were managed by Critical Care Medicine and the Neurology Consultation Services. ASMs were selected based on ES severity and burden with guidance from institutional Critical Care EEG21 and Status Epilepticus23 pathways. The initial ASM could be a benzodiazepine or a non-benzodiazepine ASM depending on the severity and burden of the ES and concomitant medical problems.
Clinical and EEG data were collected prospectively using REDCap (Research Electronic Data Capture), a secure web-based application that provides validated data entry and audit trails. Clinical data included age, sex, acute neurologic disorders, prior neurodevelopmental status, medications, intubation status, CEEG indication, hospital and PICU admission and discharge dates, and ASMs used for acute seizure management. Acute seizures were categorized as: (1) epilepsy-related; (2) acute symptomatic structural (stroke, central nervous system inflammation or autoimmune disorder, traumatic brain injury, central nervous system infection, brain malformation, tumor/oncologic, and hypoxic-ischemic encephalopathy); and (3) acute symptomatic non-structural (sepsis, metabolic, pharmacologic sedation, toxin, paralytic administration). The acute seizure categories were selected based on the primary presenting problem/diagnosis given clinical information available at PICU admission. For example, a patient with epilepsy and a ventriculo-peritoneal shunt would be classified as acute symptomatic structural if a shunt malfunction were identified or epilepsy-related if no shunt problems were identified. EEG tracings were scored by the study pediatric electroencephalographers (FF and NSA) using standardized critical care EEG terminology24 for which most main variables have good reliability across elecroencephalographers.25; 26 EEG data included initiation and discontinuation date and time, EEG background features, and ES presence and timing. Consistent with prior studies8; 12 and proposed definitions,27 ES were defined as abnormal paroxysmal events that were different from the background, lasted >10 seconds, had a temporal-spatial evolution in morphology, frequency, and/or amplitude, and had a plausible electroencephalographic field. Benzodiazepine infusions used for seizure treatment were differentiated from those used for sedation by tracking whether infusion rates were increased around the time of ES identification and noted to be part of the ES management approach in the clinical records.
We assessed adverse events related to ASM administration within 12 hours of ES management by review of the clinical chart including physician and nursing documentation. We used a categorization system based on recommendations provided in Good Clinical Practice Guidelines and the National Cancer Institute’s Common Terminology statement.28; 29 Any medical condition that developed or worsened after ES management began was considered an adverse event. We assessed four adverse event categories: (1) in-hospital mortality; (2) cardiopulmonary problems [including subcategories of anaphylactic reactions, bradycardia (<5th percentile for age), hypotension (<5th percentile for age), hypoxemia (saturation <90%), and intubation requirement]; (3) organ dysfunction [including subcategories of allergic skin manifestations, acute kidney injury, hepatitis and acute liver injury, coagulopathy, and cytopenias]; and (4) hospital acquired infections (including subcategories of central line associated bloodstream infections, urinary tract infection, and ventilator associated pneumonia). For each adverse event, we described: (1) timing (during or after ES management); (2) relatedness (unrelated, unlikely, reasonable possibility, or definite); (3) seriousness (serious or not, as described below); (4) severity (grades 1–5, as described below); (5) expectedness (expected or not); (6) treatment/actions taken (none, seizure management modified, seizure management discontinued, or other management required); and (7) outcome (recovered/resolved, recovered/resolved with sequalae, recovering/resolving, not recovered/resolved, or fatal). Adverse events were considered serious if they led to death, were life threatening, prolonged hospitalization, or led to persistent significant incapacity. Adverse event severity was graded as Grade 1 (asymptomatic or mild symptoms; clinical or diagnostic observations only; no intervention indicated); Grade 2 (moderate; minimal, local or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living); Grade 3 (severe; or medically significant but not immediately life threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living); Grade 4 (life-threatening; urgent intervention indicated); or Grade 5 (death related to an adverse event).
To determine the study sample size, we calculated the number of subjects required to observe at least one subject with related adverse event for varying true adverse event rates. If 25 subjects were exposed to a medication, then the probability of observing at least one adverse event would be 0.72, 0.84, 0.93, and 0.98 if the true adverse event rate was 5%, 7%, 10%, and 15%, respectively.
All statistical analyses were performed using Stata 15.1 (College Station, TX). We report summary statistics as counts (and percentages) for categorical variables and medians [and interquartile ranges] for continuous variables. We analyzed nominal categorical predictive variables for seizures (dichotomous since present or absent) using the Pearson’s χ2 statistic or Fisher exact test, and Cochran-Armitage trend test for ordinal categorical predictors. We did not perform multi-variable analyses given the small number of subjects with adverse events.
RESULTS
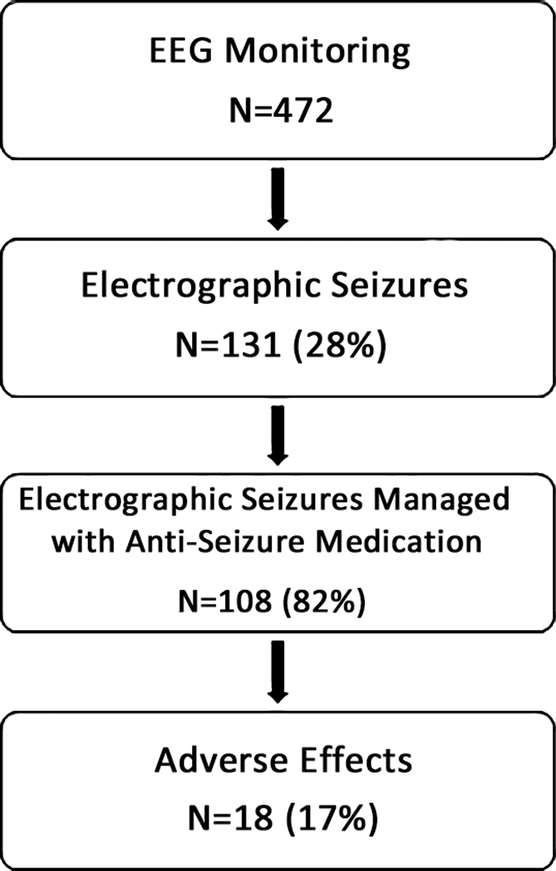
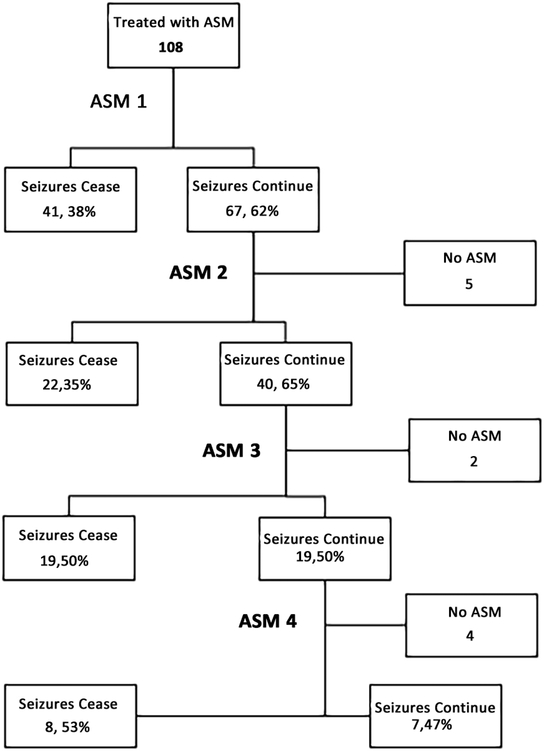
We enrolled 472 consecutive critically ill children who underwent clinically indicated CEEG. ES occurred in 131 children (28%) and ESE occurred in 27 children (6%). Twenty-eight children (6%) had EEG patterns along the ictal-interictal continuum (IIC), and most of these children (23 of 28, 82%) also had ES. Clinicians administered ASM to 108 patients (82%) (including 22 patients with IIC patterns and ES) and elected not to administer ASM for 23 patients (18%) (Figure 1). ES management involved one ASM in 47 patients (44%), two ASM in 24 patients (22%), three ASM in 22 patients (20%), and four or more ASM in 15 patients (14%). ES terminated after the initial ASM in 41 of 108 patients (38%), after the second ASM in 22 of 62 (35%) patients, after the third ASM in 19 of 38 (50%) patients, and after the fourth ASM in 8 of 15 (53%) (Figure 2). In some patients, ES persisted but no subsequent ASM was administered. For example, although only 41 subjects had ES terminate after the first ASM, 47 patients received only one ASM since clinicians elected not to escalate treatment for six patients. Thirty patients (28%) received anesthetic infusions targeting seizure suppression or burst-suppression for management of ES.
Figure 1.
Study subject flow diagram.
Figure 2.
Anti-seizure medication (ASM) flowchart.
We recorded the ASMs that were used in initial management of ES and the order in which they were administered. The most common ASM administered for ES identified during CEEG were: (1) first-line (levetiracetam in 39, lorazepam 31, phenobarbital in 12, and midazolam drip in 7); (2) second-line (levetiracetam in 22, phenobarbital in 15, fosphenytoin/phenytoin in 10, and lacosamide in 5); (3) third-line (midazolam infusion in 14, fosphenytoin/phenytoin in 7, levetiracetam in 6); and (4) fourth-line (midazolam infusion in 6). Combining first-line through third-line ASM treatments, the most commonly used ASM for ES management were levetiracetam in 67 patients, lorazepam in 31 patients, phenobarbital in 27 patients, midazolam infusion in 21 patients, fosphenytoin/phenytoin in 17 patients, and lacosamide in 5 patients. There were no differences in treatment efficacy between patients with epilepsy and acute symptomatic etiologies: (1) seizures ceased after the first ASM in 41% of patients with epilepsy versus 36% of patients with acute symptomatic etiologies (p=0.58); (2) seizures ceased after the second ASM in 28% of patients with epilepsy versus 41% of patients with acute symptomatic etiologies (p=0.31); (3) seizures ceased after the third ASM in 41% of patients with epilepsy versus 57% of patients with acute symptomatic etiologies (p=0.33); and (4) management included anesthetic infusions in 24% of patients with versus 28% of patients with acute symptomatic etiologies (p=0.67).
Adverse events occurred in 18 patients (17%) (Supplementary Table). Adverse events were definitely related to ASM administration in 12 patients and had a reasonable possibility of being related to ASM administration in 5 patients. Adverse events were serious in 15 patients, severe (Grade 4) in 15 patients. Adverse events were expected in all 18 patients and resolved in all 18 patients. The most common interventions involved initiation or increase in vasoactive medications (7 patients) and/or intubation (7 patients). Only five children experienced adverse events with ASM other than midazolam infusion. Phenobarbital was associated with hypotension in one patient and respiratory failure in one patient; fosphenytoin was associated with hypotension in one patient; lorazepam was associated with hypoxemia with respiratory failure in one patient; and lacosamide was associated with hypoxemia in one patient. Thirteen children who experienced adverse events were being administered midazolam infusions, and they were considered definitely related in 10 patients (77%). The most common adverse events with midazolam infusion were hypotension (10 patients) and respiratory failure (3 patients).
We identified several risk factors for adverse events (Table 1). Adverse events occurred more often in children with epilepsy (13/48, 27%) than children with acute symptomatic seizures from structural etiologies (4/42, 10%) or non-structural etiologies (1/18, 6%) (p=0.03). Several findings indicated that adverse events occurred more often in children who required extensive and more complex seizure management than those with shorter and simpler courses of treatment. Adverse events occurred more often in patients: (1) with ESE (11/26, 42%) than ES (7/82, 9%) (p<0.01); (2) in whom seizures persisted after a second ASM (persisted 12/40, 40%; terminated 1/22, 5%; p=0.02); (3) in whom management extended over more days (1 day 3/60, 5%; 2 days 4/24, 17%; 3 days 4/11, 36%; ≥4 days 7/13, 54%; p=<0.01); (4) in whom anesthetic infusions were required (required 12/30, 40%; not required 6/78, 8%; p=<0.01); and (5) in whom IIC EEG patterns were seen (IIC 8/22, 36%; no IIC 10/86, 12%; p<0.01).
Table 1.
Subjects with electroencephalographic seizures with and without adverse events.
| Variable | Total with ES (N=108) |
No Adverse events (N=90) |
Adverse events (N=18) |
p-value |
|---|---|---|---|---|
| Age (Days) | 2367 (IQR 280, 5040) | 2349 (IQR 290, 4995) | 2459 (IQR 225, 5188) | 0.86 |
| Sex | 1.0 | |||
| Male | 66 (61%) | 55 (61%) | 11 (61%) | |
| Female | 42 (39%) | 35 (39%) | 7 (39%) | |
| Race | 0.05 | |||
| Asian | 5 (4%) | 2 (2%) | 3 (17%) | |
| Black or African American | 41 (38%) | 35 (39%) | 6 (33%) | |
| White | 42 (39%) | 34 (38%) | 8 (44%) | |
| Ethnicity | 0.71 | |||
| Hispanic | 15 (14%) | 13 (14%) | 2 (11%) | |
| Non-Hispanic | 93 (86%) | 77 (86%) | 16 (89%) | |
| Sepsis | 0.24 | |||
| No | 98 (91%) | 83 (92%) | 15 (83%) | |
| Yes | 10 (9%) | 7 (8%) | 3 (17%) | |
| Acute Encephalopathy Category | 0.03 | |||
| Epilepsy | 48 (44%) | 35 (39%) | 13 (72%) | |
| Acute Structural | 42 (39%) | 38 (42%) | 4 (22%) | |
| Acute Non-Structural | 18 (17%) | 17 (19%) | 1 (6%) | |
| Mental Status at cEEG Onset | 0.74 | |||
| Not Comatose | 87 (81%) | 72 (80%) | 15 (83%) | |
| Comatose | 21 (19%) | 18 (20%) | 3 (17%) | |
| Clinically Evident Seizure(s) Prior to CEEG | 0.04 | |||
| No | 18 (17%) | 18 (20%) | 0 (0%) | |
| Yes | 90 (83%) | 72 (80%) | 18 (100%) | |
| Prior Epilepsy Diagnosis | 0.06 | |||
| No | 52 (48%) | 47 (52%) | 5 (28%) | |
| Yes | 56 (52%) | 43 (48%) | 13 (72%) | |
| ASM at cEEG initiation | 0.17 | |||
| No | 32 (30%) | 29 (33%) | 3 (17%) | |
| Yes | 74 (70%) | 59 (67%) | 15 (83%) | |
| Sedatives at cEEG initiation | 0.69 | |||
| No | 67 (62%) | 56 (62%) | 11 (61%) | |
| Intermittent boluses | 4 (4%) | 4 (4%) | 0 (0%) | |
| Continuous infusion | 37 (34%) | 30 (33%) | 7 (39%) | |
| Prior Developmental Delay or Intellectual Disability Diagnosis | 0.43 | |||
| No | 45 (42%) | 39 (43%) | 6 (33%) | |
| Yes | 63 (58%) | 51 (57%) | 12 (67%) | |
| EEG – Interictal Epileptiform Discharges | 0.09 | |||
| No | 22 (20%) | 21 (23%) | 1 (6%) | |
| Yes | 86 (80%) | 69 (77%) | 17 (94%) | |
| EEG – Ictal Interictal Continuum | <0.01 | |||
| No | 86 (80%) | 76 (84%) | 10 (56%) | |
| Yes | 22 (20%) | 14 (16%) | 8 (44%) | |
| EEG – Background (Asymmetry as Best) | 0.33 | |||
| Normal | 36 (33%) | 33 (37%) | 3 (17%) | |
| Slow-Disorganized | 62 (57%) | 50 (56%) | 12 (67%) | |
| Discontinuous or Burst Suppression | 7 (6%) | 5 (6%) | 2 (11%) | |
| Attenuated | 3 (3%) | 2 (2%) | 1 (6%) | |
| EEG – Background (Asymmetry as Worse) | 0.53 | |||
| Normal | 25 (23%) | 23 (26%) | 2 (11%) | |
| Slow-Disorganized | 67 (62%) | 55 (61%) | 12 (67%) | |
| Discontinuous or Burst Suppression | 12 (11%) | 9 (10%) | 3 (17%) | |
| Attenuated | 4 (4%) | 3 (3%) | 1 (6%) | |
| Seizure Exposure Category | <0.01 | |||
| Electrographic Seizure(s) | 82 (76%) | 75 (83%) | 7 (39%) | |
| Electrographic Status Epilepticus | 26 (24%) | 15 (17%) | 11 (61%) | |
| Status Epilepticus > 30 minutes | 0.001 | |||
| No | 99 (92%) | 86 (96%) | 13 (72%) | |
| Yes | 9 (8%) | 4 (4%) | 5 (28%) | |
| ES Terminate after Initial ASM | 0.12 | |||
| No | 66 (61%) | 52 (58%) | 14 (78%) | |
| Yes | 42 (39%) | 38 (42%) | 4 (22%) | |
| ES Terminate after Second ASM (N=62) | 0.02 | |||
| No | 40 (65%) | 28 (57%) | 12 (92%) | |
| Yes | 22 (35%) | 21 (43%) | 1 (8%) | |
| Management Extent | <0.01 | |||
| 1 day | 60 (56%) | 57 (63%) | 3 (17%) | |
| 2 days | 24 (22%) | 20 (22%) | 4 (22%) | |
| 3 days | 11 (10%) | 7 (8%) | 4 (22%) | |
| ≥4 days | 13 (12%) | 6 (7%) | 7 (39%) | |
| Anesthetic Infusions | <0.01 | |||
| No | 78 (72%) | 72 (80%) | 6 (33%) | |
| Yes | 30 (28%) | 18 (20%) | 12 (67%) |
ASM, anti-seizure medication; CEEG, continuous EEG monitoring; ES, electrographic seizure.
DISCUSSION
We evaluated ASM utilization patterns and adverse event incidence and risk factors among a large, consecutive, contemporary, and prospectively acquired cohort of critically ill children with acute encephalopathy undergoing CEEG. ES occurred in 28% of patients, consistent with other cohorts of critically ill children with acute encephalopathy.1–13 Further, 82% of patients with ES or ESE were treated with ASM, consistent with survey and prior observational data indicating that most physicians aim to terminate all or most ES using multi-drug regimens and even anesthetic infusions for treatment of refractory ES.18; 19 In our typical cohort of critically ill children with acute encephalopathy, we report four main findings.
First, only 39% of subjects had ES resolution after the initial ASM but an additional 35% of subjects had ES resolution after the second ASM, indicating 78% of children had seizure cessation after administration of 1–2 ASM. Overall, ES were managed with 1, 2, 3, or ≥4 ASM in 44%, 22%, 20%, and 14% of patients, respectively. Similarly, a study of critically ill adults found that ES persisted in about half of patients after initial treatment with lacosamide or phenytoin.30 Since most children had ES termination after 1–2 ASM, ES identification and management may be highly feasible in many patients. Conversely, about one-fifth of patients required four or more ASM, and 28% of patients required anesthetic infusions. Thus, it may be important to include multiple treatment steps in institutional management pathways and individualized seizure action plans for critically ill patients.
Second, adverse events occurred in 17% of children who received treatment for ES. The incidence of adverse events was similar to that occurring in critically ill adults undergoing treatment for non-convulsive seizures in which treatment-emergent adverse events occurred in 26% and 24% who received lacosamide and fosphenytoin respectively.30 Adverse events were more common in patients with a prior diagnosis of epilepsy, with higher ES exposure, with ES that persisted after administration of a second ASM, when treatment that extended over more days, when anesthetic infusions were needed, and with EEG findings classified along the IIC. In contrast, adverse effects were generally rare among children with acute symptomatic seizures (structural or non-structural etiologies) who required simpler treatment. Only two children without a prior diagnosis of epilepsy or ESE experienced adverse events. Recently, there has been much focus on identification and management of ES in patients with acute symptomatic seizure etiologies.22 Since a higher ES burden is associated with an increased risk for neurological decline, including among children with acute symptomatic seizure etiologies,7; 12; 13 ES exposure reduction could be a neuroprotective strategy. Our findings that ES management in children with acute symptomatic etiologies is often accomplished with 1–2 ASM with a low risk for adverse events suggest that ES management could be employed as a potential neuroprotective strategy with relatively low risk.31
Third, 82% of patients with IIC patterns also experienced ES. IIC patterns are often encountered in patients with acute brain injuries or in patients with epilepsy after status epilepticus and are associated with ES.1; 3 However, it is unclear whether these patterns cause secondary neuronal damage or whether they are merely symptomatic of acute brain injury. As such, there is uncertainty as to whether these patterns should be treated aggressively.32 Given that 44% of patients who underwent treatment for ES in the context of IIC patterns experienced adverse events, thoughtful consideration of the risks and benefits of treatment coupled with close monitoring may be warranted in these patients.
Fourth, 72% of patients who experienced adverse events were receiving midazolam infusions, and the most common adverse events were hypotension and respiratory failure. Midazolam is a common medication used to manage refractory status epilepticus,15; 16 and both of these adverse effects are known to be associated with midazolam administration.33; 34 The adverse event categorization system defined hypotension as blood pressure below the normal expected for an individual and respiratory failure as impaired gas exchange by the respiratory system resulting in hypoxemia or desaturation on pulse oximetry.29 Furthermore, the categorization system classified these Grade 4 severity, indicating life-threatening consequences requiring urgent intervention with intubation or ventilatory support.29 However, the adverse events resolved in all patients and are considered highly treatable with standard management by pediatric intensivists.
Fifth, levetiracetam was the most commonly administered first-line ASM. In contrast to some ASM, levetiracetam can be readily accessed in PICU medication distribution machines and prepared by nurses, can be administered rapidly as a bolus, and is often considered safe and with minimal drug-drug interactions. Furthermore, guidelines on status epilepticus management designate levetiracetam as an appropriate first-line ASM,15; 16 and recent clinical trials indicate levetiracetam is effective as a second-line therapy for pediatric convulsive status epilepticus.35; 36
The clinical impact of ES identification and treatment remains unclear. Accumulating evidence indicates that high ES exposure is associated with unfavorable short- and long-term neurobehavioral outcomes independent of acute encephalopathy etiology and severity of critical illness. Children with acute encephalopathy and ESE (and sometimes also ES) have a higher mortality, worse functional outcome scores on discharge, worse functional outcome at follow-up, and a higher risk developing subsequent epilepsy.4; 5; 7; 12–14; 37 Presumably, more rapid ES identification results in earlier treatment and reduction of seizure burden. Lower ES exposure could potentially minimize secondary brain injury and improve neurobehavioral outcomes. Therefore, recent guidelines by the Neurocritical Care Society recommend that status epilepticus, including ESE, be treated aggressively with the goal of terminating ES.15 There is evidence from both animal models and human clinical data that ASM may have higher efficacy when administered earlier during a seizure. Animal models indicate that ASM treatment before GABA receptors have internalized is associated with higher ASM responsiveness.38 Concordantly, studies in humans demonstrate higher efficacy when ASM are administered earlier during convulsive status epilepticus management,39 leading to an emphasis on timely administration of first- and second-line ASMs.15; 16 Similarly, among critically ill children with ES, implementation of a pathway optimizing ES management led to a reduction in the median time between ES onset and treatment initiation and also to a higher percentage of patients with ES cessation after the first-line ASM.40 Together, these findings suggest that patients might benefit from optimized and rapid ES management strategies that increase the likelihood of ES termination with initial ASM administration, thereby reducing the need for more complex therapeutic regimens associated with a higher risk for ASM-related adverse events.
This study has several key strengths. First, this was a prospective cohort study which is the optimal study design for estimating the incidence of and risk factors for adverse events. Second, this was a pragmatic observational study driven by need for clinical decision-making in ES management in the absence of optimal clinical trial data. Third, since all data were collected during the acute period and we acquired data from consecutive patients, the study avoided limitations related to loss to follow-up, subject withdrawal, or incomplete data sets. Fourth, adverse event assessment was performed by clinicians knowledgeable in ES management in critically ill children. Fifth, adverse event assessment and categorization were performed using standardized and recommended systems.28; 29 Although the National Cancer Institute’s Common Terminology Criteria for Adverse Events29 was developed for oncology research, it provided applicable standardized terminology for assessing drug-related adverse events.
The study also has several important limitations. First, we only assessed acute adverse events and did not assess longer-term neurobehavioral or patient-centered outcomes. Thus, we cannot determine whether any aspects of acute ES management might impact long-term outcomes. Second, the small number of patients who experienced adverse events precluded multi-variate analyses regarding risk factors for adverse events. A larger multi-center study focused on the cohort of patients that are most likely to experience adverse events might provide additional information. Finally, although adverse events resolved in all patients, the study was conducted in a quaternary care PICU with in-hospital pediatric intensivists. Thus, there were presumably well-developed mechanisms in place for rapid identification and management of adverse events. Some adverse events involving respiratory depression and need for vasoactive medication may have been more problematic in less resource-rich environments.
Conclusions
Few data are available regarding the safety and efficacy of ASM administration for ES in critically ill children, thereby limiting clinicians’ abilities to make evidence-based assessments of treatment risk versus benefit. A better understanding of the risks for adverse events among critically ill children with ES will help determine which patients, ES types, and ES burdens may benefit from treatment. Adverse events were rare among patients with acute symptomatic seizures due to acute symptomatic structural or non-structural etiologies requiring simpler ASM treatment regimens. Thus, approaches to rapidly identify and manage ES may be relatively safe in those children. If these approaches reduce secondary brain injury caused by higher ES exposure, then they could be a neuroprotective strategy. Further work is warranted to better establish evidence-based optimal ES management approaches and evaluate their impact on neurobehavioral outcomes for critically ill children.
Supplementary Material
KEY POINTS.
Electrographic seizures terminated after 1–2 anti-seizure medications in about 2/3 of critically ill children with acute encephalopathy.
Adverse events were rare in patients with acute symptomatic seizures requiring management with 1–2 anti-seizure medications.
Adverse events were more common in children with epilepsy, ictal-interictal continuum EEG patterns, or requiring more extensive ASM management.
Acknowledgements:
Dr. Abend is funded by NIH K02NS096058.
Footnotes
Ethical Publication Statement
We confirm that we have read the journal’s position on issues involvd in ethical publication and affirm that this report is consistent with these guidelines.
Disclosure
France W. Fung has no conflicts of interest.
Marin Jacobwitz has no conflicts of interest.
Lisa Vala has no conflicts of interest.
Darshana Parikh has no conflicts of interest.
Maureen Donnelly has no conflicts of interest.
Rui Xiao has no conflicts of interest.
Alexis A. Topjian has no conflicts of interest.
Nicholas S. Abend has received support from Demos Publishing (royalties).
REFERENCES
- 1.Jette N, Claassen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006;63:1750–1755. [DOI] [PubMed] [Google Scholar]
- 2.Shahwan A, Bailey C, Shekerdemian L, et al. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia 2010;51:1198–1204. [DOI] [PubMed] [Google Scholar]
- 3.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia 2011;52:1130–1136. [DOI] [PubMed] [Google Scholar]
- 4.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med 2012;38:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology 2013;81:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber JM, Zelleke T, Gaillard WD, et al. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care 2012;17:31–38. [DOI] [PubMed] [Google Scholar]
- 7.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014;137:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology 2011;76:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia 2011;52:1973–1978. [DOI] [PubMed] [Google Scholar]
- 10.Vlachy J, Jo M, Li Q, et al. Risk Factors for Seizures Among Young Children Monitored With Continuous Electroencephalography in Intensive Care Unit: A Retrospective Study. Front Pediatr 2018;6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sansevere AJ, Duncan ED, Libenson MH, et al. Continuous EEG in Pediatric Critical Care: Yield and Efficiency of Seizure Detection. J Clin Neurophysiol 2017;34:421–426. [DOI] [PubMed] [Google Scholar]
- 12.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Crit Care Med 2013;41:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014;82:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav 2015;49:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 16.Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG monitoring: current resources and practice in the United States and Canada. J Clin Neurophysiol 2013;30:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abend NS, Dlugos DJ, Hahn CD, et al. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care 2010;12:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abend NS, Sanchez SM, Berg RA, et al. Treatment of electrographic seizures and status epilepticus in critically ill children: A single center experience. Seizure 2013;22:467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 21.Children’s Hospital of Philadelphia, Critical Care Pathway for EEG Monitoring, 2019. Available at: http://www.chop.edu/clinical-pathway/critical-care-pathway-eeg-monitoring-clinical-pathways. Accessed July 20, 2019.
- 22.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol 2015;32:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Children’s Hospital of Philadelphia, Pathway for Evaluation/Treatment of Status Epilepticus, 2019. Available at: https://www.chop.edu/clinical-pathway/status-epilepticus-clinical-pathway. Accessed July 20, 2019.
- 24.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 25.Abend NS, Gutierrez-Colina A, Zhao H, et al. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol 2011;28:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mani R, Arif H, Hirsch LJ, et al. Interrater reliability of ICU EEG research terminology. J Clin Neurophysiol 2012;29:203–212. [DOI] [PubMed] [Google Scholar]
- 27.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013;54 Suppl 6:28–29. [DOI] [PubMed] [Google Scholar]
- 28.Food and Drug Administration. Guidance for Clinical Investigators, Sponsors, and IRBs: Adverse Event Reporting to IRBs — Improving Human Subject Protection, 2009. Available at: https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126572.pdf. Accessed March 1, 2019.
- 29.National Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE) (version 5.0), 2017. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Accessed March 1, 2019.
- 30.Husain AM, Lee JW, Kolls BJ, et al. Randomized trial of lacosamide versus fosphenytoin for nonconvulsive seizures. Ann Neurol 2018;83:1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abend NS, Topjian AA, Williams S. How much does it cost to identify a critically ill child experiencing electrographic seizures? J Clin Neurophysiol 2015;32:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claassen J How I Treat Patients with EEG Patterns on the Ictal-Interictal Continuum in the Neuro ICU. Neurocrit Care 2009;11:437–444. [DOI] [PubMed] [Google Scholar]
- 33.Singhi S, Murthy A, Singhi P, et al. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol 2002;17:106–110. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi K, Osawa M, Aihara M, et al. Efficacy of intravenous midazolam for status epilepticus in childhood. Pediatr Neurol 2007;36:366–372. [DOI] [PubMed] [Google Scholar]
- 35.Dalziel SR, Borland ML, Furyk J, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet 2019;393:2135–2145. [DOI] [PubMed] [Google Scholar]
- 36.Lyttle MD, Rainford NEA, Gamble C, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 2019;393:2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia 2008;49:615–625. [DOI] [PubMed] [Google Scholar]
- 38.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci 2008;28:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chin RF, Neville BG, Peckham C, et al. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol 2008;7:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams RP, Banwell B, Berg RA, et al. Impact of an ICU EEG monitoring pathway on timeliness of therapeutic intervention and electrographic seizure termination. Epilepsia 2016;57:786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.