Abstract
Research on stress and disease has often afforded an important role to emotion, typically conceptualized in broad categories (e.g., negative emotions), viewed as playing a causal role (e.g., anger contributing to pathophysiology of cardiovascular disease), and measured using self-report inventories. In this paper, I argue for the value of evaluating specific emotions, considering bidirectional causal influences, and assessing actual emotional responding when considering the role that emotions play in the stress-disease relationship. In terms of specificity, specific emotions (e.g., anger, sadness, embarrassment) can be linked with particular health outcomes (e.g., cardiovascular disease, musculoskeletal disease). In terms of bidirectionality, the influences of emotions on disease as well as the influences of disease on emotional functioning can be considered. In terms of assessing actual emotional responding, emotions can be studied in vivo under controlled conditions that allow behavioral, physiological, and subjective responses to be measured during different kinds of emotional functioning (e.g., responding to emotional stimuli, interacting with relationship partners, down-regulating emotional responses). With these considerations in mind, I review early theories and empirical studies in psychosomatic medicine that considered the role of specific emotions and emotion-related behaviors. Studies from our laboratory are presented that illustrate: (a) differences in patterns of autonomic nervous system responding associated with specific emotions; (b) relationships between specific emotions and particular health outcomes in the context of social relationships; (c) age as a moderator of the relationship between specific emotions and well-being; (d) bidirectional influences (emotions influencing disease and disease influencing emotional functioning); and (e) impact of changes in emotional functioning in individuals with neurodegenerative diseases on the health of familial caregivers.
Keywords: emotion, stress, health and disease, autonomic nervous system, specificity, dementia
This paper is based on an invited talk I gave at the October 2017 midyear meeting of the American Psychosomatic Society, which was devoted to the theme “Emotions in social relationships: Implications for health and disease”. As with the original talk, my overarching goal is to consider the role that specific emotions play in the stress-disease relationship (in this paper, the term stress primarily refers to the exposure to stressors that can result in threat or harm to the individual).To accomplish this goal, I discuss: (a) the history of specific emotions in psychosomatic research; (b) the notion that specific emotions have different patterns of associated physiological responding; (c) evidence that specific emotions and emotional behaviors that arise in social relationships are associated with particular health problems; (d) changes in the relationship between specific emotions and health that occur with aging; (e) influences of neurodegenerative disease on patients’ emotional functioning; and (f) influences of changes in emotional functioning in patients on the health of familial caregivers.
This paper draws heavily on the methods and perspectives of contemporary affective science. Although emotions have often been studied using self-report inventories, new approaches enable studying emotions in vivo under conditions that allow behavioral, physiological, and subjective responses to be measured precisely during particular kinds of emotional functioning (e.g., responding to emotional stimuli, interacting with relationship partners, down-regulating emotional responses). This approach is well-suited for considering the role that specific emotions play in the stress-disease relationship. Consistent with the mandate of the original talk, in this paper I present both old and new ideas, speculate on implications and future directions, and provide a number of illustrative research examples.
Stress and disease: A brief history
Fifty years ago, in a landmark study, Rahe [1] presented a classic “black box” finding. In a sample of approximately 2,500 U.S. sailors, the number of life events requiring adjustments in accustomed patterns that the sailors had encountered during a six month period prior to shipping out [measured with a self-report inventory; 2] was associated with the amount of illness they experienced during the ensuing six months (measured by examining medical records). Sailors in the high-risk group (i.e., top 30% of total life events) had significantly more first illnesses during the first two months of the cruise compared to those in the low-risk group (i.e., lowest 30% of total life events). These differences were most dramatic in the first month of the cruise during which approximately 8% of the high-risk sailors had their first illness compared to 5% of the low-risk sailors. In a subsequent review of similar studies [3], the correlations typically found between life stress and future disease (usually measured over 6–12 month periods) were significant but small (average r=.12).
In the period since the initial empirical studies [e.g., 4] there has been increasing refinement in our understanding of the intermediate stages linking life stress to the onset of disease. Consistent with changes in the scientific zeitgeist, Rahe’s “lens” model [3], with its inclusion of the psychodynamic concept of “psychological defenses” was supplanted by cognitive models emphasizing appraisal and coping [e.g., 5] and ultimately by models [e.g., 6, 7] that afford a prominent role to emotions.
In the stage model proposed by Cohen and colleagues [Figure 1, redrawn from 6], environmental events are appraised in terms of their stressfulness, leading to negative emotional responses. These negative emotions activate behavioral (health decisions and behaviors) and physiological pathways (sympathetic adrenal medullary and hypothalamic pituitary adrenal responding) that create disease-related physiological changes that promote disease onset and progression.
Figure 1.
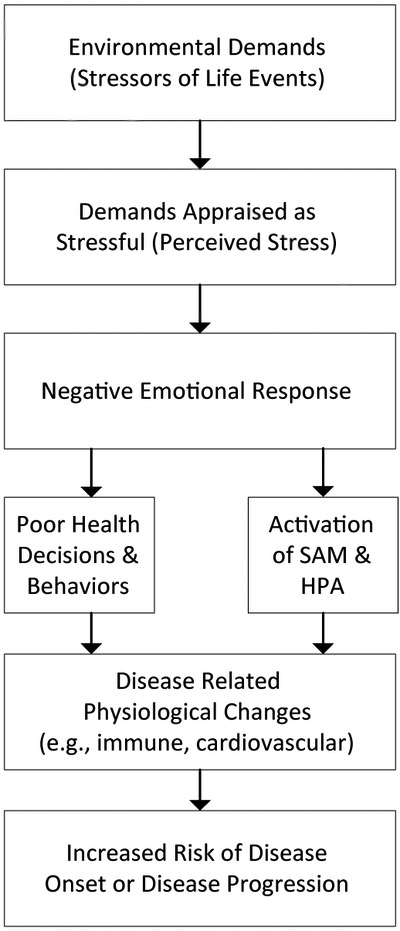
Stage model of stress and disease
Figure 6.
Caregivers’ depression scores on CESD (N=107)
The role of emotions in this model is consistent with evolutionary/functionalist views [e.g., 8] that envision emotions as activating biological and behavioral systems rapidly in ways that are most likely to enable the organism to deal optimally with a small set of species-typical challenges and opportunities. Inherent in these evolutionary/functionalist views is the notion that emotions are not all the same, but rather differ in evoking conditions and patterns of attendant behavioral and biological responses. Thus, rather than associating all kinds of negative emotions with broad behavioral adaptations such as “fight-flight” [9], it may be useful to parse fight and flight, distinguishing among conditions involving threats to life and limb (which are most likely to elicit the emotion of fear and behavioral adaptations of “flight” or “freeze”) and those that involve frustration of progress toward goals (which are most likely to elicit the emotion of anger and the behavioral adaption of “fight”). To these we would add conditions that create other prototypical challenges and opportunities. For example, loss of an attachment figure might elicit the emotion of sadness and behavioral adaptations involving reaching out for comfort and support; encountering contamination might elicit the emotion of disgust and activate withdrawal and expulsive behaviors.
Introducing these more specific negative emotions and their different associated action patterns [10, 11] sets the stage for the possibility that behaviors (e.g., facial expressions, postural changes) and physiological responses (e.g., patterns of autonomic nervous system [ANS] and endocrine activity) are different for specific emotions. Thus, the facial expressions that signal anger and the ANS and somatic changes that prepare the organism to defend its turf are likely to be quite different from the facial expressions that signal disgust and the ANS and somatic changes that prepare the organism for rapid withdrawal from sources of contamination.
Although I expect the stage model can readily incorporate these kinds of elaborations around more specific negative emotions and the possibility that they are accompanied by different patterns of behavioral and physiological activation, it is important to realize that environmental challenges and opportunities can also produce positive emotions as well as self-conscious emotions (which can have positive or negative valence).Thus, for example, dealing successfully with a threat or challenge can produce the positive emotion of joy. Meeting or exceeding social expectations can produce the positive self-conscious emotion of pride; failing to meet these expectations can lead to the negative self-conscious emotion of shame. Although at one time all positive emotions were thought to share a common facial expression [i.e., the smile; 12] and most were thought to calm the ANS rather than activating it [13], more recent research indicates that positive and self-conscious emotions have much more diverse patterns of associated behavioral [14] and physiological activation [15]. This greater specificity among positive and self-conscious emotions is likely to have important consequences for disease-related physiological changes and for theoretical models that link emotions and health [16].
Before considering how a more differentiated view of emotion can be applied to research on stress and disease, it is important to note that not all affective scientists embrace the functionalist/evolutionary and discrete emotions perspectives described above. Contemporary affective science encompasses several quite different theoretical models [for alternative social constructivist and dimensional views see 17, 18, 19] as well as disparate views concerning the evidence supporting and disconfirming physiological and behavioral differences among specific emotions [e.g., 17, 20, 21].
Specific emotions and health
Early studies
Although the notion that different emotions have different patterns of associated behavior and physiology is often traced back to the 19th century [22, 23], its application to individual differences in health arguably began almost a century later with Alexander’s [24] assertion that different diseases have their own associated emotional conflict. Alexander’s model stood in stark contrast to Selye’s [25] highly influential and very non-specific model that envisioned a generalized physiological response to stress that, when activated chronically, eroded diverse biological systems and created vulnerability to a range of diseases.
Although Alexander did conduct empirical studies to test his ideas--for example, using films to elicit emotional states [reported in 24]--more rigorous empirical tests of the role of specific emotions and related states (e.g., emotion-related attitudes) awaited the emergence of improved methods of psychophysiological measurement. These new methods enabled researchers to monitor and characterize the activity of multiple physiological systems during different emotional states [e.g., fear versus anger in 26]. Prominent in this era was the work of Graham and colleagues, who viewed diseases as being associated with different “attitudes” [27]. For example, based on extensive patient interviews, they concluded that individuals with hives typically felt that they were being “unfairly treated” whereas those with hypertension typically felt that they had to be “on guard”. In a classic experimental study [28], these specific attitudes were suggested to healthy individuals under hypnosis and their psychophysiological responses were measured. When the hives attitude was suggested, participants showed increases in hand temperature (consistent with hives pathophysiology); when the hypertension attitude was suggested, participants showed increases in diastolic blood pressure (consistent with hypertension pathophysiology). Although studies by Graham and colleagues focused on specific attitudes rather than specific emotions, it would not be too large a leap to assume that being “unfairly treated” (hives) was associated with the emotion of anger whereas being “on guard” (hypertension) was more likely to be associated with the emotion of fear.
The notion of links between specific emotions and disease was also found in research on the Type A personality and cardiovascular disease [29]. Although early studies focused on a broad set of behaviors, later studies identified hostility as being most important [30]. Hostility is generally considered to be an emotion-related personality trait that is closely linked to habitual expression (or inhibited expression) of the emotion of anger.
Autonomic specificity
A fundamental notion in most theories that link specific emotions with particular diseases is that different emotions produce different patterns of physiological responding. There was a spate of experimental studies of “autonomic specificity” during the 1950s and 60s [e.g., 26, 31, 32] and a revival of interest beginning in the 1980s [e.g., 33, 34, 35]. In the best of these studies: (a) laboratory procedures are used that produce reasonably intense instances of particular emotions; (b) within-subject designs are used so that differences between multiple emotions can be compared in the same individuals; (c) emotional responses are verified in some way to ensure that “apples” are in fact being compared with “oranges” (rather than comparing unspecified varieties of emotional “fruit salad”); and (d) physiological responses relevant to the emotions of interest are measured and precisely linked in time with the occurrence of emotion [36-39]. Under these more stringent experimental conditions (which are unfortunately met by few studies), reliable autonomic differences among emotions are most likely to be found, belying the notion that all emotions (or all negative emotions) are physiologically the same [21, 36, 37, 39].
Another common notion is that physiological differences among emotions “blur” as emotions become more intense. According to this view, a state of “undifferentiated physiological arousal” [9, 40] is common to all highly intense emotions. This notion can be hard to evaluate in the laboratory. For both methodological and ethical reasons, emotions studied in the laboratory typically occur at mild to moderate levels of intensity (e.g., heart rate changes from pre-trial baselines in the range of 5–10 beats per minute). Even when larger responses are evoked [e.g., to sudden loud noises; 41] the physiological changes may be relatively short-lived. In real life, emotions often occur at much higher levels of intensity and attendant physiological changes are commensurately larger and longer-lasting.
High intensity emotions: A case study
As part of our research program, we have periodically conducted case and group studies of individuals with specialized training and life experiences that might affect emotional functioning [e.g., highly skilled meditators, dancers, and actors; 35, 42, 43]. One such individual, studied in collaboration with my colleague Paul Ekman, was a Bay Area therapist who had developed a method for helping clients access blocked emotions. At our initial interview, she described her ability to produce highly intense emotional states on demand. At the time we had been primarily eliciting emotion using relived memories and directed facial actions [35], both of which produce emotions of only mild to moderate intensity. We invited her to come to the laboratory so that we could observe her method for producing emotions. She agreed and we conducted two laboratory sessions studying emotions elicited using her method and using our own eliciting methods (her emotional responses to our methods were unremarkable, quite consistent with those we had seen in others). Throughout these sessions we videotaped her facial expressions, recorded multiple ANS measures on a second-by-second basis, and queried her about her subjective emotion experiences.
I cannot provide rich detail or insights about the method she used to produce emotional states, but she described them as accessing the emotions directly rather than via a typical indirect pathway (e.g., recalling and reliving memories, posing expressions, imagining situations). As we observed her producing a range of discrete emotions, we saw facial expressions that were highly prototypical for these emotions [12], even when examined in slow motion using the Facial Action Coding System [44]. Moreover, the facial muscle contractions were extremely intense and dynamically appropriate and coherent, not having the kind of static artificiality and temporal discoordination often seen when participants are asked to pose emotional expressions (e.g., telling someone to “look angry”). These highly intense facial muscle contractions were also accompanied by large ANS perturbations. For example, when she produced anger (Figure 2), her heart rate increased initially by over 60 beats per minute and then rose an additional 10 beats per minute (around second 80) after she asked if we wanted her to allow the anger to “move toward rage”. At this point her heart rate was around 160 beats per minute and we thought it wise not to go further. Needless to say, we had not seen emotions at this level of physiological intensity in any of our prior studies. Importantly, based on our collective experience, the emotions she produced appeared to be quite “real”, despite their being created in a somewhat unusual way.
Figure 2.
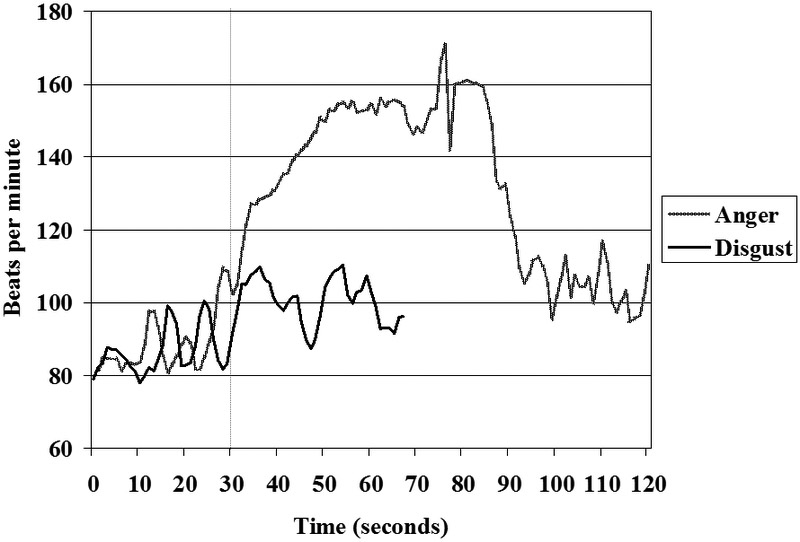
High intensity emotions
Comparing the heart rate changes that occurred when she produced anger versus disgust (Figure 2) is quite informative. When accessing both emotions (the emotions start at second 30), she produced high intensity, dynamically-coordinated prototypical facial expressions and large increases in the pace (respiration cycles lasting approximately 5 seconds) and depth of respiration. These elevations in somatic and respiratory activity would normally be expected to produce large increases in heart rate [45, 46]. However, the two emotions differed markedly in their associated magnitude and patterns of heart rate change. As noted earlier, in anger, her heart rate increased by over 75 beats per minute. Moreover, the typical rise and fall of heart rate that follows the inspiratory and expiratory phases of respiration [i.e., vagally-mediated respiratory sinus arrhythmia; 47] essentially disappeared (see the “flattening” of heart rate during anger between seconds 30–80 compared to the higher variability before [seconds 10–30] and after [seconds 110–120] in Figure 2). In contrast, the heart rate during disgust increased much less markedly and the patterns of respiratory sinus arrhythmia were quite apparent (e.g., see the clear rise and fall of disgust heart rate approximately every five seconds between seconds 40–70 in Figure 2). Although we cannot know with certainty what patterns of neural activation produced these particular heart rate responses, they are consistent with a release of vagal restraint during anger and an assertion of vagal influence during disgust. Given the magnitude and rapid onset of heart rate increase during anger, it is likely that there was sympathetic activation as well.
Clearly, these two episodes of emotion in one specially-selected individual do not constitute a reliable finding. Nonetheless, similar differences in mean heart rate response during disgust relative to anger (and other negative emotions) are among the most consistent differences found in the autonomic specificity literature [21, 48]. For example, Figure 3 illustrates heart rate changes associated with different voluntarily-produced emotional facial expressions [35]. Even with this somewhat unusual and relatively mild form of emotion elicitation, a simular pattern of larger heart rate increases in anger compared to disgust can be seen.
Figure 3.
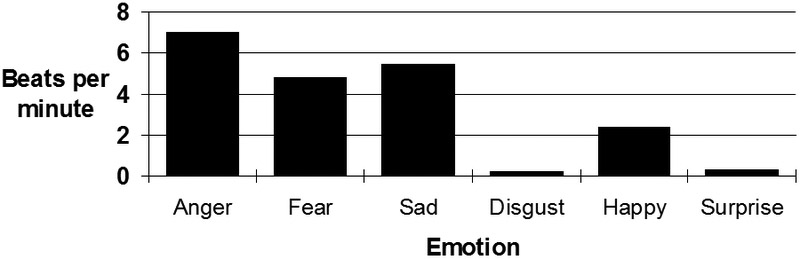
Heart rate change during directed facial actions
Emotion regulation
Although emotional reactivity (i.e., generating emotion in response to challenges and opportunities) has been the primary focus of research linking emotion with health, affective scientists have become increasingly concerned with emotion regulation (i.e., adjusting emotional responses to meet situational demands and personal goals). Emotion regulation research has focused less on the regulation of specific emotions (e.g., comparing regulation of fear with regulation of anger) and more on different kinds of regulation. For example, many studies have contrasted down-regulation of emotion by reappraisal (changing the way the eliciting situation is construed) with down-regulation by suppression (e.g., reducing the expressive signs of emotion). These strategies of emotion regulation differ both in their intended target (thoughts versus expressive responses) and their temporal location in the emotion-eliciting sequence (reappraisal occurring early often before the emotion is fully formed and suppression occurring later when the emotion is being expressed). Importantly, reappraisal and suppression have quite different physiological concomitants in both the autonomic and central nervous system [49-52]. In general, suppression is seen as less “healthy” (i.e., requiring additional physiological resources to constrain the powerful expressive forces) than reappraisal (which requires less physiological activation). For example, in our research, we have found the cardiovascular response (e.g., increase in heart rate) associated with suppressing an emotion to be approximately twice as large as the response associated with simply expressing that emotion [41, 53]. Despite the greater metabolic costs associated with suppression; it would be unwise to conclude that suppression is always the unhealthy form of regulation and that reappraisal is always the healthy form [e.g., findings that the association between suppression and health can be moderated by culture; 54].
Specific emotions: Specific health problems
A recent study from our research group [55] ilustrates the potential for considering specific emotions that occur in social relationships in health research. In this study, we utilized data from a 20-year longitudinal study of marriage [56, 57]. The study began in 1989 with the recruitment of a sample of 156 middle-aged (40–50 years old, married at least 15 years) and older (60–70 years old; married at least 35 years) couples. The study had a number of features that made it particularly useful for addressing the emotion-health relationship: (a) it was a laboratory-based observational study of actual marital interactions rather than a survey of attitudes and beliefs; (b) the sample was recruited to be representative of the local community (e.g., in ethnicity and socioeconomic status); (c) couples were observed in the laboratory repeatedly over the course of the study (every five years); and (d) participants were moving into ages where their vulnerability to significant health problems increased.
Using a well-established procedure [58], couples came to our laboratory and engaged in three unrehearsed 15-minute conversations about different relationship topics (recent events, area of conflict, enjoyable activities). Conversations were videotaped and rated by trained coders using the Specific Affect Coding System [SPAFF; 59], which considers facial expression, tone of voice, posture, and speech content to identify the occurrence of specific emotions (e.g., anger) and emotion-related behaviors (e.g., stonewalling, defensiveness). For this study, we focused on three negative emotions (anger, fear, sadness) and one emotion-related behavior (stonewalling: stiff frozen face, clenched jaw, rigid neck muscles) that were expressed by participants during the conflict discussion in 1989. Health (focusing on cardiovascular, musculoskeletal, and respiratory symptoms) was measured using the Cornell Medical Index [CMI; 60] in 1989, 1995, 2001, and 2008.
Results indicated that emotions and emotional behaviors expressed in 1989 predicted increasing health problems over the ensuing 20 years. Importantly, there was considerable specificity in the findings: (a) anger predicted increasing cardiovascular symptoms and stonewalling predicted increasing musculoskeletal symptoms, but not vice versa; (b) neither fear nor sadness predicted cardiovascular or musculoskeletal symptoms; (c) none of the emotional behaviors predicted respiratory symptoms. Two striking aspects of the findings were that they only emerged over time and were strongest for husbands. Illustrating the emergence over time, in Figure 4 there were no differences in cardiovascular symptoms in 1989/90 between participants who expressed high, moderate, or low levels of anger in their conflict discussions. However, by 1995/96, the group expressing high levels of anger was clearly showing more cardiovascular symptoms, and these symptoms continued to increase most rapidly in this group over the final two measurement periods. Overall, the risk odds ratio of anger behavior predicting future cardiovascular symptoms was 1.5.
Figure 4.
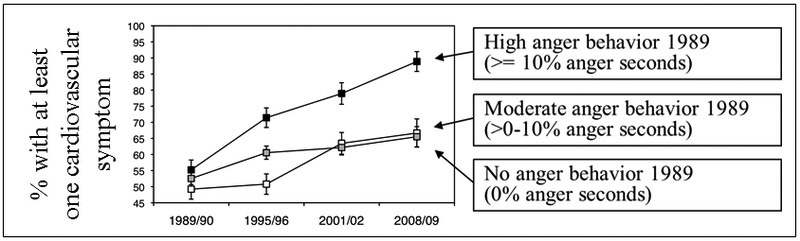
Cardiovascular symptoms and anger behavior over 20 years
This finding of relationships between particular emotions and emotional behaviors and particular health outcomes is illustrative of the promise of this approach. Of course, pending replication and extension, we cannot know how robust and generalizable these findings will be. However, the linking of anger, an emotion thought to produce widespread activation of the cardiovascular system [21, 35, 61], with cardiovascular symptoms, and stonewalling, an emotional behavior defined by high levels of muscle tension [59], with musculoskeletal symptoms has a great deal of face validity. Studies of emotion and health typically do not observe specific emotional behaviors in interpersonal situations, do not study older samples, and are either cross-sectional or, if longitudinal, span shorter periods of time. Studies using these more typical research designs might fail to detect the kinds of relationships between specific emotions and emotional behaviors and particular health symptoms that emerged over time in our research.
Specific emotions and health: Moderation by age
Just as the early studies of stress and illness studied young sailors in the prime of life, it is common for contemporary laboratory research to be conducted with younger college students. Studies in which the dependent measures are related to physiological and emotional reactivity can be highly informative in younger participants, but these populations may be less well suited for studies where the dependent measure is disease occurrence (due to low base rates of illness in younger samples). Fortunately, in the realms of health and well-being, a number of population-based studies have been conducted that include participants at many different ages and, in some of these, subsamples have participated in laboratory studies of emotional functioning [e.g., 62]. Just as health risk and disease incidence clearly change with age, the relationships between emotions, health, and well-being may also change. Simply stated, it is reasonable to expect that the optimal “emotional palette” for maintaining health and well-being for a person entering adulthood differs from that best suited for a person in the last decade of life.
We explored this issue in a study of emotional correlates of well-being in three different age groups [63]. This was a cross-sectional laboratory study (participants were in their 20s, 40s, or 60s) with participants recruited to be representative of the local community. Participants viewed an excerpt from the film “Stranger Than Paradise” and rated how intensely they experienced specific emotions (e.g., anger, fear, disgust, sadness). In the excerpt, two men struggle to maintain an extremely empty, boring, and labored conversation. In many ways, this excerpt is an “emotional Rorschach”, with the viewer needing to project emotional meaning on to fairly ambiguous stimulus material. Participants also completed a standard measure of well-being [64]. Findings revealed a striking moderation by age. For older participants, the more sadness that was reported in response to the film, the greater their reported well-being. For middle-aged subjects, the more anger that was reported, the greater their reported well-being. In thinking about these findings, we speculated that anger might be particularly functional in middle-age as people compete for resources, defend past gains, and engage in the give and take of the workplace. In contrast, sadness might be particularly functional in old age, helping people deal with the inevitable losses encountered in that stage of life and also to signal the need for connection with others [65]. The notion that life’s challenges and opportunities change with age is a central tenet of many theories of life-span development [66-69]. Because of this, different emotions, with their capacity to address different challenges and opportunities, may become maximally adaptive at different ages. Given the strong connections between well-being and health [70, 71], it is reasonable to expect that the connections between particular emotions and health may change in similar ways.
Influences of disease on emotion
Thus far, our discussion of the relationship between emotion and disease has focused on the influences of emotion on disease, a theme that is found throughout the history of psychosomatic medicine. Although there have been elegant experimental [e.g., 72] and naturalistic longitudinal [e.g., 73] studies showing that stress can increase susceptibility to illness and slow the pace of healing, most studies in this literature using human participants have been correlational in nature. Thus, when associations are found between emotion and disease, we cannot know whether emotions influence disease, disease influences emotion, or influences are bidirectional. There have been studies that experimentally induced illness or altered inflammation and examined the effects on emotional and/or behavioral functioning [e.g., 74, 75-78]. Albeit and understandably rare, such studies underscore the role that disease processes play in influencing emotional functioning [e.g., the role of inflammation in the etiology and treatment of depression; 79].
Neurodegenerative disease: A patient model for studying influences of disease on emotional functioning
Studies of emotional functioning in individuals with particular diseases provide a “natural experiment” for exploring the influence of disease on emotion. Of course, data from these studies come with certain caveats (e.g., the research designs are not truly experimental). These inherent limitations notwithstanding, the quality of data derived from patient studies can be improved by contrasting emotional functioning across multiple patient groups with different diseases, including healthy controls, controlling for the more general effects of “illness”, using longitudinal designs (which allow some disambiguation of temporal sequences of causal influence), and incorporating more precise assessments of patients’ actual emotional functioning.
Neurodegenerative disease
In recent years we have been conducting research on late-life dementia and other neurodegenerative diseases, focusing on the ways that these diseases influence emotional functioning. In this work, we typically compare the effects of different diseases (carefully diagnosed and characterized), include healthy controls, control for disease severity, and measure patients’ actual emotional functioning in the laboratory. This research is aided by laboratory procedures we have developed [80] to study specific emotions; consider particular emotional processes (including reactivity--generating emotions, regulation--altering emotional responding, and recognition--identifying emotions in others); and assess physiological, expressive, and subjective aspects of emotional responding.
Because neurodegenerative diseases are becoming increasingly common with the aging population, we have been able to go beyond case studies and small-N studies to conduct research with sufficiently large samples of patients to obtain reasonable statistical power [e.g., 144 patients with dementia and 45 healthy controls in 81]. To date, most of our work has been cross-sectional, comparing emotional functioning in different patient groups at a particular moment in time. However, we are moving to more longitudinal designs in which disease progression and emotional functioning will be assessed repeatedly over time.
Of particular interest in our research has been frontotemporal dementia (FTD), a progressive neurodegenerative disease that targets frontal and temporal brain regions critical for emotional functioning including the amygdala, insula, temporal pole, and frontal lobes. Reflecting the affected anatomy, initial symptoms in FTD include declines in ability to generate, regulate, and recognize emotion with relative preservation of many aspects of cognitive functioning such as memory [82, 83]. Neurodegeneration in FTD often targets ANS control centers in the brain [84, 85]. Given the important role the ANS plays in numerous aspects of emotion [39], this can further compromise emotional functioning in individuals with FTD.
Alzheimer’s disease (AD) has a very different pattern of neurodegeneration and behavioral deficits than those seen in FTD. AD targets more posterior brain regions (e.g., hippocampus, entorhinal cortex, posterior cingulate). Reflecting this anatomy, initial symptoms in AD include declines in memory and spatial ability, with relative preservation of emotional functioning [e.g., 86, 87, 88].
Clearly, with progressive neurodegenerative diseases, if deficits are found in emotional functioning, the notion that the disease causes changes in emotional functioning becomes more compelling than the notion that changes in emotional functioning cause the disease.
Emotional reactivity: Specific emotions
Emotional reactivity involves the actions of widely distributed brain regions [83]. Thus, it is unlikely that there would be unique and non-overlapping regional anatomies associated with specific emotions. Nonetheless, specific emotions may differ in terms of how much they require the involvement of different brain structures. Although FTD has widespread effects on emotional functioning, we have nonetheless found it useful to examine its effects on specific emotions, especially in the early stages of the disease when emotional deficits may be less widespread.
Embarrassment and self-conscious emotions.
In one line of research we have examined embarrassment, a “self-conscious” emotion that alerts us to instances in which we have violated social norms and need to take corrective actions [89]. Clinical observations suggest that individuals with FTD often engage in socially-inappropriate behaviors that are quite distressing for friends and families but do not seem to bother the person with FTD (e.g., inappropriate touching of others). To determine if there are deficits in the capacity to generate embarrassment in individuals with FTD, we [90, 91] used a “karaoke” laboratory task in which participants were shown a video recording of their singing “My Girl”. Neurologically healthy participants find this task to be quite embarrassing and typically show facial expressions that signal embarrassment and amusement and attendant increases in ANS arousal. Figure 5 depicts the results of a study comparing the responses to this task in individuals with FTD (N=26) and age-matched healthy controls (N=16). In both the amount of positive emotional facial expressions (primarily amusement) shown in the left panel and the skin conductance response shown in the right panel, individuals with FTD were dramatically less reactive than controls. Lowered physiological reactivity in FTD was also seen in measures of cardiovascular (i.e., heart rate) and respiratory (i.e., respiration depth) responding [90].
Figure 5.
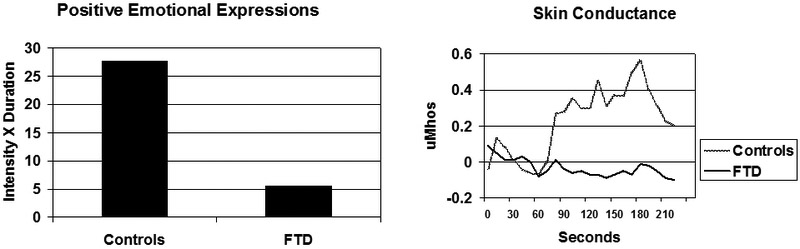
Facial and electrodermal responding during karaoke task
In another study of embarrassment in FTD, we [91] used an unexpected acoustic startle (a sudden loud burst of white noise) stimulus. In healthy individuals this stimulus produces a two-stage response. The initial response is a reflexive defensive response and the secondary response is an emotional reaction that unfolds as the person becomes aware of their initial response [92]. Using this startle stimulus, during the secondary response we found deficits in embarrassment behavior in FTD patients compared to healthy controls, similar to our findings using the Karaoke task [90]. In contrast, during the initial defensive stage, FTD patients did not differ from healthy controls in their physiological or behavioral response. This suggests some specificity of the deficit in the realm of the secondary self-conscious emotional response.
In a follow-up study using structural imaging to quantify neurodegeneration, we [93] established some anatomical specificity for deficits in self-conscious emotional responding during the karaoke task. In this study we found that greater degeneration in the right pregenual anterior cingulate cortex (a region involved in processing the social milieu) was associated with lower levels of embarrassment responding (facial expressions and physiology) in the karaoke task. This association remained significant even after statistically controlling for the association of degeneration in this region with sadness responding when viewing a sad film clip [93].
Disgust.
In another line of research, we have documented marked deficits in the ability of individuals with FTD to generate disgust responses to laboratory stimuli (disgusting films) [94]. As was the case with the embarrassment research reviewed in the previous section, these laboratory findings are quite consistent with clinical observations that individuals with FTD often engage in behaviors and activities that others find quite disgusting (e.g., eating unusual things). This suggests that individuals with FTD may not experience disgust (which would normally cause them to withdraw from these things rather than approaching and consuming them).
As with the embarrassment findings, the disgust findings also show some specificity in the relationships between patterns of neurodegeneration and deficits in emotional functioning. In a sample of individuals with a range of neurodegenerative diseases (N=84) who viewed disgusting and sad films, we [95] found that lower levels of disgust responding was associated with greater degeneration in anterior insular regions [which are thought to be critical for processing visceral information; 96]. The findings revealed specificity both in terms of emotions (insular volumes were not associated with sadness responding) and anatomy (disgust responding was not associated with degeneration in other regions including putamen, pallidum, and caudate).
Emotion regulation and recognition
To understand the impact of disease on emotional functioning more broadly, it is important to examine particular kinds of emotion regulation and emotion recognition. As noted earlier, emotional deficits become increasingly pervasive as neurodegeneration progresses. Nonetheless, in the earlier stages of disease, when patients can still participate in laboratory sessions, we have found evidence for some specificity in the influence of disease on both emotion regulation and emotion recognition.
In terms of emotion regulation, we have found that, compared to healthy controls, individuals with FTD and AD both have difficulties suppressing observable emotional responses when instructed to down-regulate emotional responding to an aversive audio stimulus. However, when told exactly when the stimulus will occur but not given explicit instructions about regulation, FTD patients show much more profound deficits in down-regulation than both AD patients and healthy controls [88]. Thus, it appears that FTD patients can still follow instructions to down-regulate (suggesting their ability to down-regulate is still intact) but fail to do so when they have to evaluate the situational demands and initiate down-regulation on their own (suggesting problems in evaluating contextual factors and using this information to motivate appropriate behavior).
In terms of emotion recognition, we have found that individuals with FTD have greater difficulty identifying positive, negative, and self-conscious emotions displayed by characters in brief films compared to individuals with AD and healthy controls. However, deficits in emotion recognition in FTD patients are particularly pronounced in their ability to recognize self-conscious emotions such as embarrassment, pride, and shame [87].
Implications for the influence of other diseases on emotion
These examples of disease influencing emotional functioning in relatively specific ways are all drawn from our studies of individuals with neurodegenerative disease. Because neurodegenerative diseases produce different patterns of injury to emotion-critical brain circuitry, they often provide plausible explanations for the different patterns of associated change in emotional functioning. Other diseases certainly can have strong associations with changes in emotional functioning [e.g., depression and heart disease; 97, 98]. Based on our findings with individuals with neurodegenerative diseases, future research with other diseases should investigate links with specific emotions (e.g., heart disease and anger) and particular emotional processes (e.g., considering associations with deficits in emotional reactivity, regulation, and recognition) and give consider the relative strength of bidirectional influences. Moreover, for this kind of research to be most effective, it should include studies that go beyond self-report inventories and carefully measure actual emotional functioning.
Influences of Emotion on the Health of Others
Emotions are deeply embedded in the social fabric of our lives [99] providing the threads that bind us to others and the forces that drive us asunder [8]. Nowhere is the role of emotion more profound than in marriage and other intimate relationships [100]. Our research with individuals with neurodegenerative disease caused us to become increasingly interested in the plight of their spousal and other familial caregivers. This has led to a number of studies in which we examine the impact that deficits in specific aspects of emotional functioning in the person with dementia have on the health of their caregivers.
Dementia and dementia caregiving: A growing public health challenge
AD, FTD, and other forms of dementia constitute a major public health challenge worldwide. There are currently approximately 5.5 million cases of AD and 60,000 cases of FTD in the US and approximately 16 million family members and friends who are providing 18 billion hours of unpaid care for people with dementia [101, 102]. Because dementia rates increase with age [e.g., 44% of individuals between the ages of 75–84 have AD; 103] the worldwide “graying” of the population will make the challenges of dementia and dementia caregiving even greater in the future. In 2006 there were 700 million people worldwide over the age of 60; by 2050 this number will triple to 2.1 billion, and in the US, 11.4% of the population will be older than 75 [104].The staggering number of people already living with dementia; the prospects for dramatic increases in the number of future cases; the enormous associated economic, social, and personal costs; and the lack of effective preventative and curative treatments have made dementia one of the most critical public health challenges of our time.
Adverse effects on the health of caregivers
Although dementia has devastating effects on the person with the disease, caregivers often suffer “collateral damage”. The elevated psychiatric and physical morbidity associated with caregiving is well-established. In terms of mental health, caregivers have up to four-fold increases in rates of depression, three-fold increases in seeking treatment for anxiety, greater use of psychotropic medications, and greater suicidal ideation compared to non-caregiving adults of similar age [105-113]. These elevations in psychiatric disorders are all the more striking given that, among people over the age of 65, the prevalence of these mental health disorders normally stabilizes or decreases [114].
In terms of physical health, dementia caregivers have greater physical morbidity [115, 116]; lower self-rated health [117]; greater heath care utilization [118]; greater decline in cellular immune functioning [73]; greater heart rate reactivity to stress [119]; higher rates of dementia [120]; and shorter lives [121, 122].
Individual differences in caregiver health
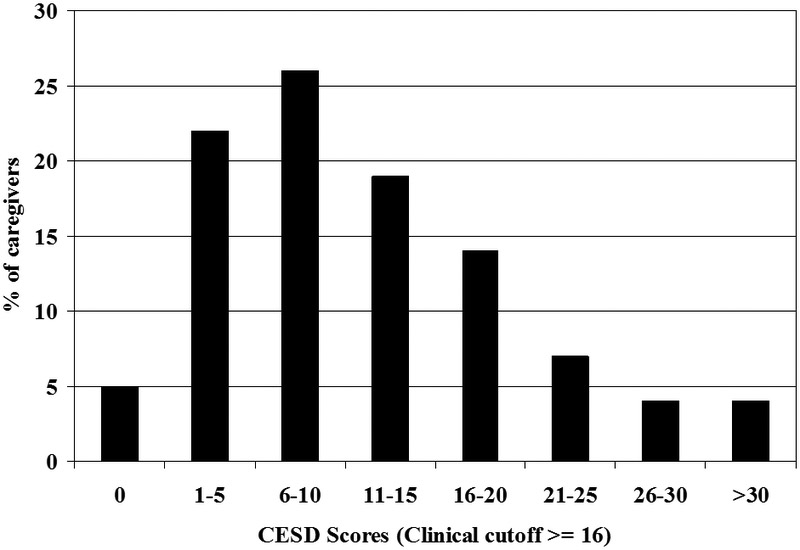
Although challenging to all, some caregivers remain relatively unimpaired, whereas others spiral downward in a trajectory of declining mental health, physical health, and well-being. These individual differences are illustrated in Figure 6, which portrays depression scores [CESD; 123] in 107 consecutive familial caregivers studied in our Berkeley laboratory. The variation in depressive symptoms is striking (e.g., 27% scored above the typical clinical cutoff score of 16, but 5% reported no symptoms). Existing research indicates the promise of identifying risk factors in the external environment, in individuals with dementia, in caregivers, and in the patient-caregiver relationship that account for these individual differences [122, 124-128].
The role of specific emotions
Emotional changes in individuals with dementia underlie many of the problematic behaviors and psychological symptoms [e.g., aggression, agitation; 125] that create high levels of caregiver burden. Consequently, our initial studies of caregiver health focused on emotional deficits in individuals with dementia.
In one line of work, we have focused on atypical emotional responding in individuals with dementia. A person viewing a film in which a child’s father dies will typically report high levels of sadness and low levels of other emotions [e.g., disgust or amusement; 129]. In individuals with dementia, report of these other non-target emotions is elevated [81]. In a laboratory study of emotional reactivity (N=178), we [126] found that greater experience of non-target emotions by individuals with dementia in response to films that elicited either amusement or sadness was associated with greater mental illness in their caregivers.
In another line of work, we have examined the emotions that individual with dementia express during interactions with their spousal caregivers. Facial expressions provide conspecifics with emotional information that is critical for maintaining social relationships [99]. Using the Facial Action Coding System [44], we [124] analyzed the smiles expressed by individuals with dementia and their spousal caregivers (N=57 dyads) during a 10-minute naturalistic discussion of an area of marital conflict. Smiles were classified as genuine enjoyment (i.e., “Duchenne smiles”) or non-enjoyment smiles using well-established morphological criteria [130]. Findings revealed that low levels of genuine enjoyment smiles in individuals with dementia were associated with greater physical and mental illness in caregivers. This relationship was not found for non-enjoyment smiles in individuals with dementia or for either type of smile in caregivers.
Thus, it appears that changes in quite specific aspects of emotional responding in individuals with dementia (e.g., not producing focused emotional responses, lack of genuine smiling) assessed in the laboratory can provide important clues that help explain individual differences among caregivers in their susceptibility to the adverse health effects of caregiving. Although these initial studies have focused on fairly broad measures of mental and physical health, we expect that relationships between specific emotions and particular health problems will emerge as our samples become larger and our studies become more longitudinal. Importantly, this kind of research has the potential for revealing early indicators that identify caregivers who are at heightened risk for later health problems and for illuminating mechanisms that could become targets for focused interventions aimed at protecting caregivers’ health.
Conclusion
Emotions play a critical role in explaining the well-established connections between stress exposure and disease outcomes. They provide a plausible pathway through which reactions to life’s challenges and opportunities can activate biological systems in ways that increase (or decrease) vulnerability to disease. Although not present in earlier models, emotions assume more prominent roles in newer theoretical models in psychosomatic medicine [e.g., 6]. Despite this increasing prominence, emotions are often considered only in broad classes (e.g., negative emotions) rather than in terms of specific emotional states (e.g., anger, disgust) and emotional processes (e.g., different forms of emotion regulation). In this article, I have argued for a more careful consideration of the role that specific emotions and emotional processes play in health and disease. Moreover, using examples from our own research, I have tried to underscore the importance of considering bidirectional causal influences (specific aspects of emotional functioning causing particular diseases as well vice versa) and the impact of changes in one person’s emotional functioning on the health of intimate others (e.g., association between emotional deficits in individuals with dementia and health problems in their familial caregivers). Designing research in ways that allows specific emotions to be linked with particular health outcomes will require additional effort in: (a) selecting and developing emotion-eliciting stimuli; (b) measuring and verifying emotional states; (c) conducting longitudinal research spanning longer periods of time; (d) studying populations at heightened risk for disease; (e) assessing particular health problems; and (f) measuring physiological, expressive, and subjective aspects of actual emotional responding. The payoff for these additional efforts lies in their potential for providing a deeper and more precise understanding of the emotional pathways connecting exposure to stress and disease processes.
Acknowledgments
This article is based on a talk presented at the October 2017 midyear meeting of the American Psychosomatic Society (“Emotions in social relationships: Implications for health and disease”). The research was supported by NIA Grants AG019724, AG041762, and AG059458. The author has no conflicts of interest to report.
Abbreviations:
- ANS
autonomic nervous system
- AD
Alzheimer’s disease
- FTD
frontotemporal dementia
References
- 1.Rahe RH, Life-change measurement as a predictor of illness. Proc R Soc Med, 1968. 61(11 Part 1): p. 1124–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes TH and Rahe RH, The social readjustment rating scale. Journal of Psychosomatic Research, 1967. 11: p. 213–218. [DOI] [PubMed] [Google Scholar]
- 3.Rahe RH and Arthur RJ, Life change and illness studies: past history and future directions. J Human Stress, 1978. 4(1): p. 3–15. [DOI] [PubMed] [Google Scholar]
- 4.Rahe RH, Meyer M, Smith M, Kjaer G, and Holmes TH, Social stress and illness onset. Journal of Psychosomatic Research, 1964. 8(1): p. 35–44. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus RS and Folkman S, Stress appraisal and coping. 1984, New York: Springer. [Google Scholar]
- 6.Cohen S, Gianaros PJ, and Manuck SB, A stage model of stress and disease. Perspectives on Psychological Science, 2016. 11(4): p. 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, Kessler RC, and Gordon LU, Strategies for measuring stress in studies of psychiatric and physical disorders, in Cohen Sheldon ∣ Kessler Ronald C ∣ Gordon Lynn Underwood (ed.), Measuring stress: a guide for health and social scientists. 1995. p. (1995), p pp 3. [Google Scholar]
- 8.Levenson RW, Human emotion: A functional view. The nature of emotion: Fundamental questions, 1994: p. 123–126. [Google Scholar]
- 9.Cannon WB, The wisdom of the body. 1932, New York,: W.W. Norton & Company; xv p., 1 *., 19–312 p. [Google Scholar]
- 10.Frijda NH, The emotions. 1986, Cambridge: Cambridge University Press. [Google Scholar]
- 11.Frijda NH, Kuipers P, and Schure E.t., Relations among emotion, appraisal, and emotional action readiness. Journal of Personality and Social Psychology, 1989. 57(2): p. 212. [Google Scholar]
- 12.Ekman P and Friesen WV, Unmasking the face: A guide to recognizing emotions from facial clues. 1975, Englewood Cliffs, New Jersey: Prentice-Hall. [Google Scholar]
- 13.Fredrickson BL and Levenson RW, Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion, 1998. 12(2): p. 191–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracy JL and Robins RW, The nonverbal expression of pride: evidence for cross-cultural recognition. Journal of Personality and Social Psychology, 2008. 94(3): p. 516–30. [DOI] [PubMed] [Google Scholar]
- 15.Shiota MN, Campos B, Oveis C, Hertenstein MJ, Simon-Thomas E, and Keltner D, Beyond Happiness: Building a Science of Discrete Positive Emotions. The American Psychologist, 2017. 72(7): p. 617. [DOI] [PubMed] [Google Scholar]
- 16.Pressman SD and Cohen S, Does positive affect influence health? Psychol Bull, 2005. 131(6): p. 925–971. [DOI] [PubMed] [Google Scholar]
- 17.Russell JA, Is there universal recognition of emotion from facial expressions? A review of the cross-cultural studies. Psychological Bulletin, 1994. 115(1): p. 102–141. [DOI] [PubMed] [Google Scholar]
- 18.Barrett LF, Are emotions natural kinds? Perspectives on Psychological Science, 2006. 1(1): p. 28–58. [DOI] [PubMed] [Google Scholar]
- 19.Barrett LF, How emotions are made : the secret life of the brain. 2017, Boston: Houghton Mifflin Harcourt; xv, 425 pages. [Google Scholar]
- 20.Friedman BH and Kreibig SD, The biopsychology of emotion: Current theoretical, empirical, and methodological perspectives. Biological Psychology, 2010. 84(3): p. 381–382. [DOI] [PubMed] [Google Scholar]
- 21.Kreibig SD, Autonomic nervous system activity in emotion: A review. Biological Psychology, 2010. 84(3): p. 394–421. [DOI] [PubMed] [Google Scholar]
- 22.Darwin C, The expression of the emotions in man and animals. 1872, London: Murray. [Google Scholar]
- 23.James W, What is an emotion? Mind, 1884. 9: p. 188–205. [Google Scholar]
- 24.Alexander F, Psychosomatic medicine, its principles and applications. 1950, New York: Norton. [Google Scholar]
- 25.Selye H, The stress of life. 1966, New York: McGraw-Hill. [Google Scholar]
- 26.Ax AF, The physiological differentiation between fear and anger in humans. Psychosomatic Medicine, 1953. 15(5): p. 433–442. [DOI] [PubMed] [Google Scholar]
- 27.Grace WJ and Graham DT, Relationship of specific attitudes and emotions to certain bodily diseases. Psychosomatic Medicine, 1952. 14: p. 243–251. [DOI] [PubMed] [Google Scholar]
- 28.Graham DT, Stern JA, and Winokur G, Experimental investigation of the specificity of attitude hypothesis in psychosomatic disease. Psychosomatic Medicine, 1958. 20: p. 446–457. [DOI] [PubMed] [Google Scholar]
- 29.Friedman M and Rosenman RH, Association of specific overt behavior pattern with blood and cardiovascular findings; blood cholesterol level, blood clotting time, incidence of arcus senilis, and clinical coronary artery disease. J Am Med Assoc, 1959. 169(12): p. 1286–96. [DOI] [PubMed] [Google Scholar]
- 30.Williams RB Jr., Haney TL, Lee KL, Kong Y, Blumenthal JA, and Whalen RE, Type A behavior, hostility, and coronary atherosclerosis. Psychosomatic Medicine, 1980. 42: p. 539–549. [DOI] [PubMed] [Google Scholar]
- 31.Funkenstein DH, King SH, and Drolette M, The direction of anger during a laboratory stress-inducing situation. Psychosomatic Medicine, 1954. 16: p. 404–413. [DOI] [PubMed] [Google Scholar]
- 32.Sternbach RA, Assessing differential autonomic patterns in emotions. Journal of Psychosomatic Research, 1962. 6(2): p. 87–91. [DOI] [PubMed] [Google Scholar]
- 33.Levenson RW, Ekman P, Heider K, and Friesen WV, Emotion and autonomic nervous system activity in the Minangkabau of West Sumatra. Journal of Personality & Social Psychology, 1992. 62(6): p. 972–988. [DOI] [PubMed] [Google Scholar]
- 34.Levenson RW, Ekman P, and Friesen WV, Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology, 1990. 27(4): p. 363–384. [DOI] [PubMed] [Google Scholar]
- 35.Ekman P, Levenson RW, and Friesen WV, Autonomic nervous system activity distinguishes among emotions. Science, 1983. 221(4616): p. 1208–1210. [DOI] [PubMed] [Google Scholar]
- 36.Levenson RW, The autonomic nervous system and emotion. Emotion Review, 2014. 6(2): p. 100–112. [Google Scholar]
- 37.Levenson RW, Autonomic specificity and emotion, in Handbook of affective sciences, Davidson RJ, Scherer KR, and Goldsmith HH, Editors. 2003, Oxford University Press: New York: p. 212–224. [Google Scholar]
- 38.Levenson RW, Emotion and the autonomic nervous system: A prospectus for research on autonomic specificity. Social psychophysiology and emotion: Theory and clinical applications, 1988: p. 17–42. [Google Scholar]
- 39.Levenson RW, Lwi SJ, Brown CL, Ford BQ, Otero MC, and Verstaen A, Emotion, in Handbook of psychophysiology (4th edition), Cacioppo JT, Tassinary LG, and Berntson GG, Editors. 2017, Cambridge University Press: New York: p. 444–464. [Google Scholar]
- 40.Cannon WB, The James-Lange theory of emotions: A critical examination and an alternative theory. American Journal of Psychology, 1927. 39: p. 106–124. [PubMed] [Google Scholar]
- 41.Hagemann T, Levenson RW, and Gross JJ, Expressive suppression during an acoustic startle. Psychophysiology, 2006. 43(1): p. 104–112. [DOI] [PubMed] [Google Scholar]
- 42.Levenson RW, Ekman P, and Ricard M, Meditation and the startle response: A case study. Emotion, 2012. 12(3): p. 650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sze JA, Gyurak A, Yuan JW, and Levenson RW, Coherence between emotional experience and physiology: Does body awareness training have an impact? Emotion, 2010. 10(6): p. 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ekman P and Friesen WV, Facial action coding system. 1978, Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 45.Obrist PA, Webb RA, Sutterer JR, and Howard JL, The cardiac-somatic relationship: Some reformulations. Psychophysiology, 1970. 6(5): p. 569–587. [DOI] [PubMed] [Google Scholar]
- 46.Levenson RW, Cardiac-respiratory-somatic relationships and feedback effects in a multiple session heart rate control experiment. Psychophysiology, 1979. 16(4): p. 367–373. [DOI] [PubMed] [Google Scholar]
- 47.Grossman P and Taylor EW, Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 2007. 74(2): p. 263–285. [DOI] [PubMed] [Google Scholar]
- 48.Levenson RW, Autonomic nervous system differences among emotions. Psychological Science, 1992. 3(1): p. 23–27. [Google Scholar]
- 49.Gross JJ and Levenson RW, Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology, 1997. 106(1): p. 95–103. [DOI] [PubMed] [Google Scholar]
- 50.Gross JJ, Antecedent-and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 1998. 74(1): p. 224–237. [DOI] [PubMed] [Google Scholar]
- 51.Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JD, and Gross JJ, Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci, 2009. 20(11): p. 1322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldin PR, McRae K, Ramel W, and Gross JJ, The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry, 2008. 63(6): p. 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross JJ and Levenson RW, Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology, 1993. 64(6): p. 970–986. [DOI] [PubMed] [Google Scholar]
- 54.Soto JA, Perez CR, Kim Y-H, Lee EA, and Minnick MR, Is expressive suppression always associated with poorer psychological functioning? A cross-cultural comparison between European Americans and Hong Kong Chinese. Emotion, 2011. 11(6): p. 1450–1455. [DOI] [PubMed] [Google Scholar]
- 55.Haase CM, Holley SR, Bloch L, Verstaen A, and Levenson RW, Interpersonal emotional behaviors and physical health: A 20-year longitudinal study of long-term married couples. Emotion, 2016. 16(7): p. 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levenson RW, Carstensen LL, and Gottman JM, Influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriages. Journal of Personality & Social Psychology, 1994. 67(1): p. 56–68. [DOI] [PubMed] [Google Scholar]
- 57.Levenson RW, Carstensen LL, and Gottman JM, Long-term marriage: Age, gender, and satisfaction. Psychology and Aging, 1993. 8(2): p. 301–313. [DOI] [PubMed] [Google Scholar]
- 58.Levenson RW and Gottman JM, Marital interaction: physiological linkage and affective exchange. Journal of Personality and Social Psychology, 1983. 45(3): p. 587–97. [DOI] [PubMed] [Google Scholar]
- 59.Coan JA and Gottman JM, The Specific Affect Coding System (SPAFF). 2007, US: Oxford University Press. [Google Scholar]
- 60.Brodman K, Erdmann AJ Jr., and Wolff HG, Cornell medical index, health questionnaire: Manual. 1974, New York: Cornell University Medical College. [Google Scholar]
- 61.Kreibig SD, Emotion, motivation, and cardiovascular response. 2012, US: American Psychological Association. [Google Scholar]
- 62.Brim OG, Ryff CD, and Kessler RC, The MIDUS National Survey: An Overview, in Brim Orville Gilbert; Ryff Carol D.; Kessler Ronald C. (2004). How healthy are we?: A national study of well-being at midlife. The John D. and Catherine T. MacArthur foundation series on mental health and development. Studies on successful midlife development. (pp. 1–34). Chicago, IL, US: University of Chicago Press; ix, 687 pp. 2004, University of Chicago Press: Chicago, IL. [Google Scholar]
- 63.Haase CM, Seider BH, Shiota MN, and Levenson RW, Anger and sadness in response to an emotionally neutral film: Evidence for age-specific associations with well-being. Psychology and Aging, 2012. 27(2): p. 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diener E, Emmons RA, Larsen RJ, and Griffin S, The Satisfaction With Life Scale. Journal of Personality Assessment, 1985. 49(1): p. 71–75. [DOI] [PubMed] [Google Scholar]
- 65.Lwi SJ, Haase CM, Shiota MN, Newton SL, and Levenson RW, Responding to the emotions of others: Age differences in facial expressions and age-specific associations with relational connectedness. Emotion, in press. [DOI] [PubMed] [Google Scholar]
- 66.Carstensen LL, Isaacowitz DM, and Charles ST, Taking time seriously: A theory of socioemotional selectivity. American Psychologist, 1999. 54: p. 165–181. [DOI] [PubMed] [Google Scholar]
- 67.Heckhausen J, Wrosch C, and Schulz R, A motivational theory of lifespan development. Psychological Review, 2010. 117: p. 32–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wrosch C and Heckhausen J, Peceived control of life regrets: Good for young and bad for old adults. Psychology and Aging, 2002. 17(2): p. 340–350. [PubMed] [Google Scholar]
- 69.Erikson EH, Identify and the life cycle: Selected papers. 1959, Oxford, U.K: International University Press. [Google Scholar]
- 70.Ryff CD, Eudaimonic Well-Being, Inequality, and Health: Recent Findings and Future Directions. International Review of Economics, 2017. 64(2): p. 159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diener E and Chan MY, Happy people live longer: Subjective well‐being contributes to health and longevity. Applied Psychology: Health and Well-Being, 2011. 3(1): p. 1–43. [DOI] [PubMed] [Google Scholar]
- 72.Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, and Manuck SB, Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosomatic Medicine, 2002. 64(2): p. 302–310. [DOI] [PubMed] [Google Scholar]
- 73.Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, and Glaser R, Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosomatic Medicine, 1991. 53(4): p. 345–362. [DOI] [PubMed] [Google Scholar]
- 74.Smith AP, Tyrrell DA, Al-Nakib W, Coyle KB, Donovan CB, Higgins PG, and Willman JS, The effects of experimentally induced respiratory virus infections on performance. Psychol Med, 1988. 18(1): p. 65–71. [DOI] [PubMed] [Google Scholar]
- 75.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, and Irwin MR, Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biological Psychiatry, 2010. 68(8): p. 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, and Pollmächer T, Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry, 2001. 58(5): p. 445–452. [DOI] [PubMed] [Google Scholar]
- 77.Prather AA, Rabinovitz M, Pollock BG, and Lotrich FE, Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain, behavior, and immunity, 2009. 23(8): p. 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raison CL, Demetrashvili M, Capuron L, and Miller AH, Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs, 2005. 19(2): p. 105–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller AH and Raison CL, The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews. Immunology, 2016. 16(1): p. 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levenson RW, Emotion elicitation with neurological patients, in The handbook of emotion elicitation and assessment, Coan JA and Allen JJB, Editors. 2007, Oxford University Press: New York: p. 158–168. [Google Scholar]
- 81.Chen K, Lwi SJ, Hua AY, Haase CM, Miller BL, and Levenson RW, Increased subjective experience of non-target emotions in patients with frontotemporal dementia and Alzheimer’s disease. Current Opinion in Behavioral Sciences, 2017. 15: p. 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levenson RW and Miller BM, Loss of cells--loss of self. Frontotemporal lobar degeneration and human emotion. Current Directions in Psychological Science, 2007. 15(6): p. 289–294. [Google Scholar]
- 83.Rosen HJ and Levenson RW, The emotional brain: Combining insights from patients and basic science. Neurocase, 2009. 15(3): p. 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sturm VE, Brown JA, Hua AY, Lwi SJ, Zhou J, Kurt F, Eickhoff SB, Rosen HJ, Kamer JH, Miller BL, Levenson RW, and Seeley WW, Network architecture underlying basal autonomic outflow: Evidence from frontotemporal dementia. Journal of Neuroscience, 2018. 38(42): p. 8943–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY, Crawford R, Stables L, Kramer JH, Rankin K, Levenson RW, Rosen HJ, Miller BL, and Seeley WW, Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc Natl Acad Sci U S A, 2016. 113(17): p. E2430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavenu I, Pasquier F, Lebert F, Petit H, and Van der Linden M, Perception of emotion in frontotemporal dementia and Alzheimer disease. Alzheimer Dis Assoc Disord, 1999. 13(2): p. 96–101. [DOI] [PubMed] [Google Scholar]
- 87.Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, and Levenson RW, Emotion recognition in frontotemporal dementia and Alzheimer’s disease: A new film-based assessment. Emotion, 2015. 15(4): p. 416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goodkind MS, Gyurak A, McCarthy M, Miller BL, and Levenson RW, Emotion regulation deficits in frontotemporal lobar degeneration and Alzheimer’s disease. Psychology and Aging, 2010. 25(1): p. 30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keltner D and Anderson C, Saving face for Darwin: The functions and uses of embarrassment. Current Directions in Psychological Science, 2000. 9(6): p. 187–192. [Google Scholar]
- 90.Sturm VE, Ascher EA, Miller BL, and Levenson RW, Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion, 2008. 8(6): p. 861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sturm VE, Rosen HJ, Allison S, Miller BL, and Levenson RW, Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain: A Journal of Neurology, 2006. 129(9): p. 2508–2516. [DOI] [PubMed] [Google Scholar]
- 92.Ekman P, Friesen WV, and Simons RC, Is the startle reaction an emotion? Journal of Personality and Social Psychology, 1985. 49: p. 1416–1426. [DOI] [PubMed] [Google Scholar]
- 93.Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, and Levenson RW, Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci, 2013. 8(4): p. 468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eckart JA, Sturm VE, Miller BL, and Levenson RW, Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia, 2012. 50(5): p. 786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Verstaen A, Eckart JA, Muhtadie L, Otero MC, Sturm VE, Haase CM, Miller BL, and Levenson RW, Insular atrophy and diminished disgust reactivity. Emotion, 2016. 16(6): p. 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Craig AD, How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience, 2009. 10(1): p. 59–70. [DOI] [PubMed] [Google Scholar]
- 97.Barth J, Schumacher M, and Herrmann-Lingen C, Depression as a Risk Factor for Mortality in Patients with Coronary Heart Disease: A Meta-analysis. Psychosomatic Medicine, 2004. 66(6): p. 802–813. [DOI] [PubMed] [Google Scholar]
- 98.Rugulies R, Depression as a predictor for coronary heart disease: A review and meta-analysis. American Journal of Preventive Medicine, 2002. 23(1): p. 51–61. [DOI] [PubMed] [Google Scholar]
- 99.Keltner D and Kring AM, Emotion, social function, and psychopathology. Review of General Psychology, 1998. 2(3): p. 320–342. [Google Scholar]
- 100.Gottman JM and Levenson RW, Assessing the role of emotion in marriage. Behavioral Assessment, 1986. 8(1): p. 31–48. [Google Scholar]
- 101.Alzheimer’s Association, 2017 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 2017. 13(4): p. 325–373. [Google Scholar]
- 102.Galvin JE, Howard DH, Denny SS, Dickinson S, and Tatton N, The social and economic burden of frontotemporal degeneration. Neurology, 2017. 89(20): p. 2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hebert LE, Weuve J, Scherr PA, and Evans DA, Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 2013. 80(19): p. 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawas CH and Brookmeyer R, Aging and the public health effects of dementia. The New England Journal of Medicine, 2001. 344(15): p. 1160–1. [DOI] [PubMed] [Google Scholar]
- 105.Coope B, Ballard C, Saad K, Patel A, Bentham P, Bannister C, Graham C, and Wilcock G, The prevalence of depression in the carers of dementia sufferers. International Journal of Geriatric Psychiatry, 1995. 10(3): p. 237–242. [Google Scholar]
- 106.Cuijpers P, Depressive disorders in caregivers of dementia patients: A systematic review. Aging & Mental Health, 2005. 9(4): p. 325–330. [DOI] [PubMed] [Google Scholar]
- 107.Brodaty H and Donkin M, Family caregivers of people with dementia. Dialogues Clin Neurosci, 2009. 11(2): p. 217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joling KJ, van Hout HPJ, Schellevis FG, van der Horst HE, Scheltens P, Knol DL, and van Marwijk HWJ, Incidence of depression and anxiety in the spouses of patients with dementia: A naturalistic cohort study of recorded morbidity with a 6-year follow-up. The American Journal of Geriatric Psychiatry, 2010. 18(2): p. 146–153. [DOI] [PubMed] [Google Scholar]
- 109.Kolanowski AM, Fick D, Waller JL, and Shea D, Spouses of persons with dementia: Their healthcare problems, utilization, and costs. Research in Nursing & Health, 2004. 27(5): p. 296–306. [DOI] [PubMed] [Google Scholar]
- 110.Grafstrom M, Fratiglioni L, Sandman PO, and Winblad B, Health and social consequences for relatives of demented and non-demented elderly. A population-based study. J Clin Epidemiol, 1992. 45(8): p. 861–70. [DOI] [PubMed] [Google Scholar]
- 111.Baumgarten M, Hanley JA, Infante-Rivard C, Battista RN, Becker R, and Gauthier S, Health of family members caring for elderly persons with dementia. A longitudinal study. Ann Intern Med, 1994. 120(2): p. 126–32. [DOI] [PubMed] [Google Scholar]
- 112.Dura JR, Stukenberg KW, and Kiecolt-Glaser JK, Anxiety and depressive disorders in adult children caring for demented parents. Psychol Aging, 1991. 6(3): p. 467–73. [DOI] [PubMed] [Google Scholar]
- 113.O’Dwyer ST, Moyle W, Zimmer-Gembeck M, and De Leo D, Suicidal ideation in family carers of people with dementia: a pilot study. Int J Geriatr Psychiatry, 2013. 28(11): p. 1182–8. [DOI] [PubMed] [Google Scholar]
- 114.Blazer D, Geriatric psychiatry, in Hales Robert E.; Yudofsky Stuart C.; Talbott John A. (1994). The American Psychiatric Press textbook of psychiatry (2nd ed.). (pp. 1405–1421). Washington, DC, US: American Psychiatric Association; xxiii, 1694 pp. 1994, American Psychiatric Association: Washington, DC. [Google Scholar]
- 115.Dassel KB and Carr DC, Does dementia caregiving accelerate frailty? Findings from the health and retirement study. Gerontologist, 2016. 56(3): p. 444–50. [DOI] [PubMed] [Google Scholar]
- 116.Vitaliano PP, Zhang J, and Scanlan JM, Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological bulletin, 2003. 129(6): p. 946–972. [DOI] [PubMed] [Google Scholar]
- 117.Baumgarten M, Battista RN, Infante-Rivard C, Hanley JA, Becker R, and Gauthier S, The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemiol, 1992. 45(1): p. 61–70. [DOI] [PubMed] [Google Scholar]
- 118.Moritz DJ, Kasl SV, and Ostfeld AM, The health impact of living with a cognitively impaired elderly spouse: Blood pressure, self-rated health, and health behaviors. Journal of Aging and Health, 1992. 4(2): p. 244–267. [Google Scholar]
- 119.Uchino BN, Kiecolt-Glaser JK, and Cacioppo JT, Age-related changes in cardiovascular response as a function of a chronic stressor and social support. J Pers Soc Psychol, 1992. 63(5): p. 839–46. [DOI] [PubMed] [Google Scholar]
- 120.Norton MC, Smith KR, Østbye T, Tschanz JT, Corcoran C, Schwartz S, Piercy KW, Rabins PV, Steffens DC, Skoog I, Breitner JCS, and Welsh-Bohmer KA, Greater risk of dementia when spouse has dementia? The Cache County Study. Journal of the American Geriatrics Society, 2010. 58(5): p. 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schulz R and Beach SR, Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA : the journal of the American Medical Association, 1999. 282(23): p. 2215–9. [DOI] [PubMed] [Google Scholar]
- 122.Schulz R, O’Brien AT, Bookwala J, and Fleissner K, Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. The Gerontologist, 1995. 35(6): p. 771–791. [DOI] [PubMed] [Google Scholar]
- 123.Radloff LS, The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1977. 1: p. 385–401. [Google Scholar]
- 124.Lwi SJ, Casey JJ, Verstaen A, Connelly DE, Merrilees J, and Levenson RW, More genuine smiles by patients during marital interactions are associated with better caregiver mental health. Journal of Gerontology: Psychological Sciences, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ornstein K and Gaugler JE, The problem with “problem behaviors”: A systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient–caregiver dyad. International Psychogeriatrics, 2012. 24(10): p. 1536–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen KH, Wells JL, Otero MC, Lwi SJ, Haase CM, and Levenson RW, Greater experience of negative non-target emotions by patients with neurodegenerative diseases is related to lower emotional well-being in caregivers. Dementia and Geriatric Cognitive Disorders, 2017. 44(5–6): p. 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Otero MC and Levenson RW, Lower visual avoidance in dementia patients is associated with greater psychological distress in caregivers. Dementia and Geriatric Cognitive Disorders, 2017. 43(5–6): p. 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brown CL, Lwi SJ, Goodkind MS, Rankin KP, Merrilees J, Miller BL, and Levenson RW, Empathic accuracy deficits in patients with neurodegenerative disease: Association with caregiver depression. American Journal of Geriatric Psychiatry, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gross JJ and Levenson RW, Emotion elicitation using films. Cognition & Emotion, 1995. 9(1): p. 87–108. [Google Scholar]
- 130.Ekman P and Friesen WV, Felt, false and miserable smiles. Journal of Nonverbal Behavior, 1982. 6: p. 238–252. [Google Scholar]


