Abstract
Despite multi-modality treatments, prognosis for advanced stage neuroblastoma (NB) remains challenging with residual long-term disabilities in survivors. Advanced stage NB is metastatic, which is a principal cause of cancer-related deaths. We presently document a primary role of MDA-9 in NB progression and define the molecular mechanisms by which MDA-9 promotes transformed phenotypes. NB cell lines and clinical samples display elevated MDA-9 expression and bioinformatic analysis supports an association between elevated MDA-9 and bone metastasis and poor prognosis. Genetic (shmda-9, mda-9 siRNA) or pharmacological (small molecule inhibitor of protein-protein interactions; PDZ1i) blockade of MDA-9 decreases NB migration, invasion and metastasis. Blocking mda-9 expression or disrupting MDA-9 partner protein interactions downregulates integrin α6 and β4, diminishing Src activity and suppressing Rho-Rac-Cdc42 activity. These signaling changes inhibit cofilin and matrix metalloproteinases reducing in vitro and in vivo NB cell migration. Overexpression of integrin α6 and β4 rescues the invasion phenotype and increases Src activity, supporting integrins as essential regulators of MDA-9-mediated NB migration and invasion. We identify MDA-9 as a key contributor to NB pathogenesis and show that genetic or pharmacological inhibition suppresses NB pathogenesis by an integrin-mediated Src-disruption pathway.
Keywords: neuroblastoma pathogenesis, MDA-9/Syntenin, metastasis, PDZ1i, integrins
Introduction
NB is one of the most common neuroectodermal pediatric solid tumors in infancy and early childhood. It can occur in tissues of the sympathetic nervous system, usually in the paraspinal ganglia or adrenal medulla, and it can present as a detectable mass in the chest, neck, pelvis, and abdomen [1]. Prognosis is highly variable and dependent on biological features of the tumor, which often displays a high rate of spontaneous regression. NB accounts for 6% of all pediatric cancers and in about 65% of cases metastasis has occurred at the time of diagnosis. The low risk group has a 5-year survival rate >95%, whereas 5-year survival in high risk patients is only approximately 40% [1]. Surgery alone or in combination with minimal therapeutic regimens can increase the survival rate in low risk patients, whereas in most cases metastatic disease is evident at the time of diagnosis and surgery is not an option. Rigorous multimodality therapies may improve the treatment of NB, but positive benefits are offset by toxicity [2]. In many stage III and IV NB patients, relapse occurs immediately after chemotherapy, and is associated with aggressive tumor behavior, resistance to chemotherapy, and organ metastasis. The primary cause of cancer-related deaths in most cancers involves metastasis. Preventing and reducing metastasis remains a major clinical challenge required to cure cancer and prevent recurrence [3, 4].
Colonization of metastatic cancer cells depends on cell adhesion to the extracellular matrix (ECM) and basement membrane. This complex process involves multiple cell signaling pathways that allow cancer cells to restructure the ECM, leading to cancer cell invasion, migration, and colonization at a new site [4, 5]. Cell invasion is mediated by both extracellular and intracellular factors and depends on specific, dynamic interactions of different cell surface receptors with the ECM [6]. Cell-cell interactions are also fundamental in cancer metastasis and tumor cell survival [6]. Extracellular adaptor proteins are major modifiers of ECM proteins, which modulate ECM–cell interactions affecting cancer cell migration and invasion [7]. One such adaptor protein, melanoma differentiation associated gene-9 protein (MDA-9) also known as Syntenin or Syndecan Binding Protein (SDCBP), was initially identified using subtraction hybridization in human melanoma cells induced to terminally differentiate [8, 9]. MDA-9/Syntenin is a unique scaffold protein containing two tandem PDZ domains, which are evolutionary conserved and influence a plethora of biological activities [7, 10]. PDZ domains are important and essential for diverse cell signaling processes regulating cell migration, invasion and metastasis [10]. Previous research from our group and others showed that MDA-9 affects cell migration, invasion, cytoskeletal reorganization, cell adhesion, protein trafficking and transcription factor activation [7, 10, 11]. MDA-9 also functions by forming complexes with multiple interacting partners such as Src, EGFR, IGF-R1, in various cancer contexts [7, 10, 12, 13].
As cancer cells migrate, invade and metastasize to new tissue sites they infiltrate and attach to the basal matrix of the target tissue [4, 14]. Tissue attachment is mediated by cell-surface receptors including integrins, which bind to components of the ECM. Integrins are crucial for invasion and migration, not only for physically binding cells to the matrix, but also for integrating (sending and receiving) molecular signals regulating these processes [15]. Integrins act as important rheostats of cell function through their capacity to mediate adhesion to ECMs, induce cytoskeletal rearrangements, and activate intracellular signaling pathways. Increases in αvβ3 integrin enhance IGF-IR-mediated NB cell migration and increase cell attachment to bone ECM components, thereby promoting metastasis [16].
The Rho family of small GTPases, Rho, Rac, and Cdc42, control organization of actin filaments. In this family of GTPases, Rho regulates the assembly of actin stress fibers and focal contacts by affecting downstream effectors, like Src. Inactivation of Rho-mediated stress fiber assembly also results in Src-mediated cytoskeleton disruption [17]. MDA-9-Src complexes are major promoters of anchorage-independent growth, motility and invasion of melanoma cells [18]. Macromolecular protein signaling complexes at the plasma membrane play important roles in regulating actin dynamics [12]. MDA-9 may also contribute to macromolecular protein signaling, which is required for cell migration by rearranging actin stress fibers [10, 12].
We presently scrutinized the role of MDA-9 in NB migration, invasion and metastasis and provide clarification of the molecular mechanism(s) involved in NB pathophysiology. MDA-9 is overexpressed in NB cells and tissues relative to normal cells/tissues and a correlation exists between enhanced MDA-9 expression and NB metastasis to bone and poor patient prognosis. Inhibition of MDA-9 genetically (shmda-9, mda-9 siRNA) or pharmacologically (PDZ1i) markedly impedes NB invasion, migration and metastasis both in vitro and in vivo. A functional link exists between MDA-9/Syntenin and the integrin-Src-Rho-Rac molecular pathways and disrupting these pathways suppresses migration, invasion and metastasis. These findings support the hypothesis that MDA-9 is a relevant molecular target that can be exploited to overcome NB progression and metastasis.
Results
Bioinformatic evidence for elevated mda-9 expression in stage IV NB displaying a metastatic phenotype and a negative correlation with prognosis
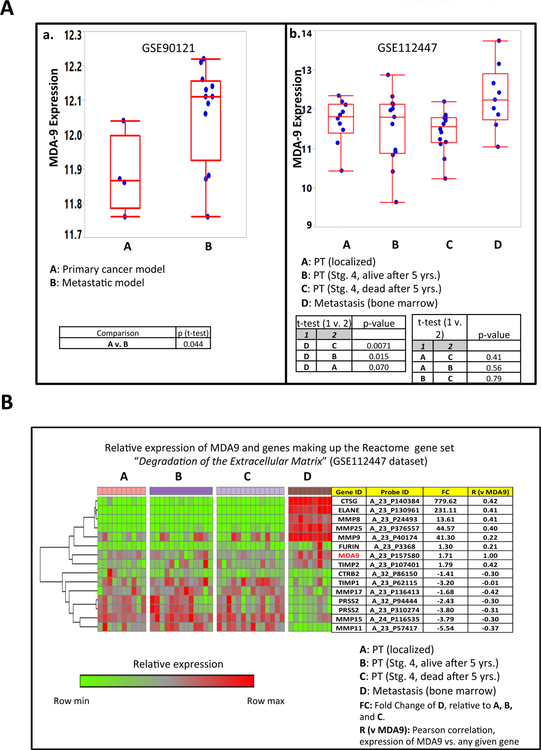
MDA-9 plays a prominent role in tumor progression and metastasis in many human cancers [3, 7, 10, 11, 12, 19–21] and a negative correlation between MDA-9 expression and poor prognosis has also been observed in specific cancers [7, 10, 12, 19–22]. Analysis (Fig. 1A.a) of a transcriptional dataset (GSE90121) from a mouse xenograft study [23] identified a subpopulation of metastatic NB cells with elevated MDA-9 expression vs. primary NB cells. Similarly, bone marrow metastatic samples from NB patients had higher MDA-9 levels vs. primary tumor samples classified as either localized, or Stage IV (irrespective of prognosis) (Fig. 1A.b). The transcriptional data (GSE112447) for the aforementioned patient samples have been partially reported in an earlier publication [24]. Further analysis of GSE112447, reveals a positive association between MDA-9 and specific genes coding for proteases, including matrix metalloproteinases MMP9, MMP8, and MMP25, which have direct roles in degradation of the extracellular matrix, an essential step in metastasis (Fig. 1B).
Figure 1. (A) Comparative expression of MDA-9 in two NB datasets:
GSE90121 (a), and GSE112447 (b). GSE90121 and GSE112447 were generated using Affymetrix Human Genome U133 Plus 2.0 Array and Agilent Whole Human Genome Microarray (4×44K) respectively. In a, the average expression of MDA-9 (as interrogated by the gene’s lone probe set) is higher in metastatic relative to primary NB mouse xenograft samples [23]. In b, the expression of MDA-9 is significantly higher in bone metastatic samples compared to primary tumors from children afflicted with NB [24]. The primary tumors were subdivided into 3 categories (localized, stage 4 with good prognosis, and stage 4 with poor prognosis). The generation of this figure (as well as the following (B) The relative expression of MDA-9 and genes comprising the Reactome pathway “Degradation of the Extracellular Matrix (ECM)” in 4 NB tissue subtypes (GSE112447). As the heatmap indicates, the expression levels of genes involved in the breakdown of ECM proteins and collagens (steps that are crucial to metastatic process) are relatively higher in bone marrow metastasis samples relative to primary tumors. These include the genes for the matrix metalloproteinases MMP9, MMP8, and MMP25; as well as the serine proteases ELANE and CTSG. The comparative gene expressions between metastasis and primary tumors are also summarized in the fold-change (FC) column. FC is calculated as follows: FC = 1 + (2x-2y)/ 2y, wherein X= average expression of samples belonging to D subset, Y= average expression of samples belonging to A, B, and C subsets. If FC < 1, it is further transformed to FC’ which is equal to −1/FC. As indicated, the transcript levels of the genes CTSG, ELANE, MMP8, MMP25, and TIMP2 exhibit significant positive correlation with that of MDA9. More detailed description of this particular Reactome pathway is found in: https://reactome.org/PathwayBrowser/#/R-HSA-1474228
Experimental confirmation of elevated MDA-9 expression in NB
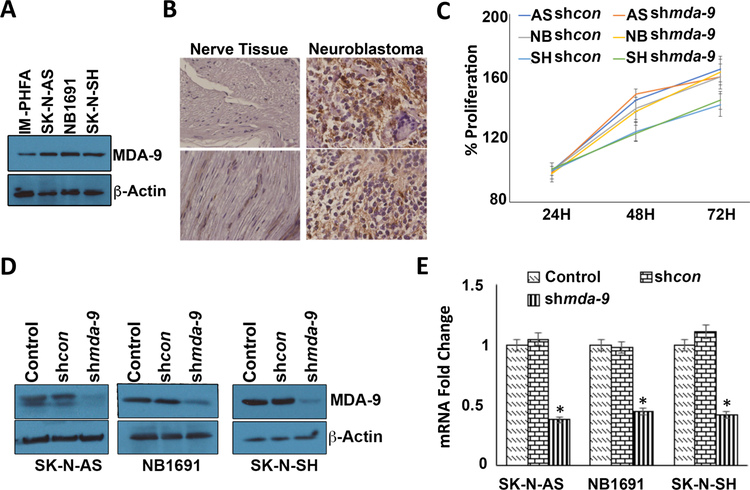
MDA-9 protein is elevated in multiple NB cell lines vs. IM-PHFA (immortal primary human fetal astrocyte cells) (Fig. 2A) and in about 80% of NB tumors in a commercial microarray of patient samples vs. adjacent peripheral nerve tissue (Fig. 2B; representative samples). A uniform pattern of MDA-9 expression was observed in tissue sections of a human tissue microarray containing peripheral nerve tissue and NB tumors (Fig. S1). Proliferation of NB cells was not significantly affected by downregulation of mda-9 using shmda-9 (Fig. 2C). Knockdown of mda-9 following infection with an adenovirus (Ad) expressing shmda-9 (Ad.shmda-9) or siRNA against mda-9 significantly inhibited MDA-9 expression on an mRNA and protein level in the three NB cell lines as compared to shcon virus-infected cells (Figs. 2D and E; S2A).
Figure 2: MDA-9 is upregulated in NB.
(A) IM-PHFA (immortalized primary human fetal astrocytes), SK-N-AS, NB1691 and SK-N-SH (NB cells) were grown for 24 hours in complete media and cell lysates were used for Western blotting analyses and probed for MDA-9 and β-actin, used as loading control. (B) Human NB tissue microarray was subjected to immunohistochemical analysis using MDA-9-specific antibody. (C) NB cells were infected with shcon or shmda-9 and at the indicated time points MTT assays were performed. (D) NB cells were treated for 48 hours as indicated and MDA-9 expression was determined by Western blotting analysis. (E) RT-PCR was performed for mda-9. Results are representative of three independent experiments presented in a graphical manner, Columns, mean of triplicate experiments. Bars, S.D.; *, P < 0.01 vs. control.
Inhibiting MDA-9 decreases NB cell migration
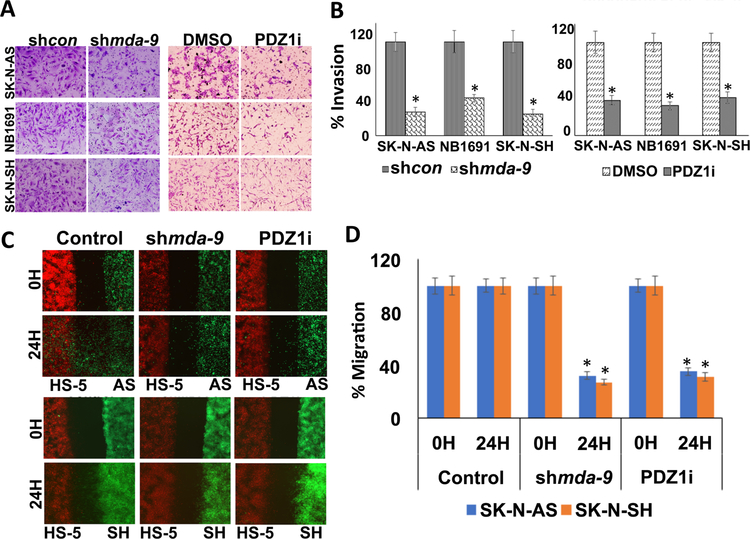
A two-dimensional spheroid assay [17] was used to investigate the effect of MDA-9 inhibition on NB cell migration. Blocking MDA-9 expression or activity using an Ad.shmda-9 or a PDZ1i small molecule inhibitor targeting the PDZ1 domain of MDA-9 [13], respectively, significantly blocked migration of cells from spheroids as compared with Ad.shcon–infected or DMSO-treated spheroids (Figs. 3A and B). A wound healing migration assay confirmed that MDA-9 knockdown or PDZ1i treatment reduced cell migration by ∼50% as compared to the control-infected or solvent-treated cells (Figs. 3C and D).
Figure 3: MDA-9 regulates NB cell migration.
NB cells were seeded onto low attachment plates and cultured until spheroids were formed [17]. (A) Spheroids were either infected with shcon or shmda-9 or (B) treated with DMSO or PDZ1i and cultured at 37°C for 48 hours. At the end of the migration assay, spheroids were fixed, stained and photographed (upper panel). Bottom panels, the distance of migration from the center of the spheroid was quantified using Image J software. (C) NB cells were seeded to near confluency and a straight wound was created and images were captured at 0 and 24 hours using a light microscope. (D) Cell migration was quantified and shown as a bar graph, Columns, mean of triplicate experiments. Bars, S.D.; *, P < 0.01 vs. control.
Blocking MDA-9 decreases NB cell invasion and migration towards bone marrow stromal cells
Matrigel-coated trans-well chambers were used to determine whether blocking MDA-9 suppresses NB invasion. NB cells were either infected with shcon or shmda-9 or treated with DMSO or PDZ1i for 48 hours, seeded onto Matrigel coated trans-well inserts and allowed to invade overnight. Ad.shmda-9 infection or treatment with PDZ1i significantly inhibited NB cell invasion as compared to shcon or DMSO controls. Quantification of this assay indicated that ~70% and ~58% fewer cells invaded in shmda-9–infected mda-9 or siRNA treated or PDZ1i-treated cells, respectively, vs. controls (Figs. 4A and B; S2B). A dose dependent inhibition of invasion was observed in PDZ1i-treated cells (Fig. S3). NB metastasis to bone marrow is an important prognostic factor and examining homing of cancer cells to the bone marrow is relevant [25]. Human bone marrow stromal cells (HS-5) and NB cells separated by a 500 μM space were placed on opposite sides of trans-well inserts. NB cells and HS-5 cells were pre-stained with either a green or red fluorescent dye and were untreated or treated with shmda-9 or PDZ1i for 48 hours. These cells were plated in different wells of the trans-well; once they attached the inserts were removed to provide a cell free gap and cancer cell migration was monitored using a fluorescence microscope. Decreased cancer cell migration towards HS-5 cells was evident when MDA-9 was inhibited genetically or its protein-protein interactions were blocked pharmacologically by PDZ1i (Figs. 4C and D; S4). These observations confirm that MDA-9 mediates NB cell migration and invasion, which can be blocked genetically or pharmacologically by targeting MDA-9.
Figure 4: MDA-9 regulates NB cell migration and invasion.
(A) NB cells were treated as indicated for 48 hours. Cells were then seeded onto Matrigel coated trans-well inserts and allowed to invade, and then fixed, stained and photographed. (B) Percentages of invading cells were quantified by counting 5 fields for each experimental condition. (C) NB cells were seeded into one well (Green) and HS-5 cells (Red) were seeded into another well of a trans-well insert. Once the cells attached, the culture-inserts were removed and incubated for 24 hours. Images were captured at 0 h and 24 hours. (D) Cell migration was quantified and shown as a bar graph, Columns, mean of triplicate experiments. Bars, S.D.; *, P < 0.01 versus control.
Molecular determinants of MDA-9 regulated NB cell invasion and migration
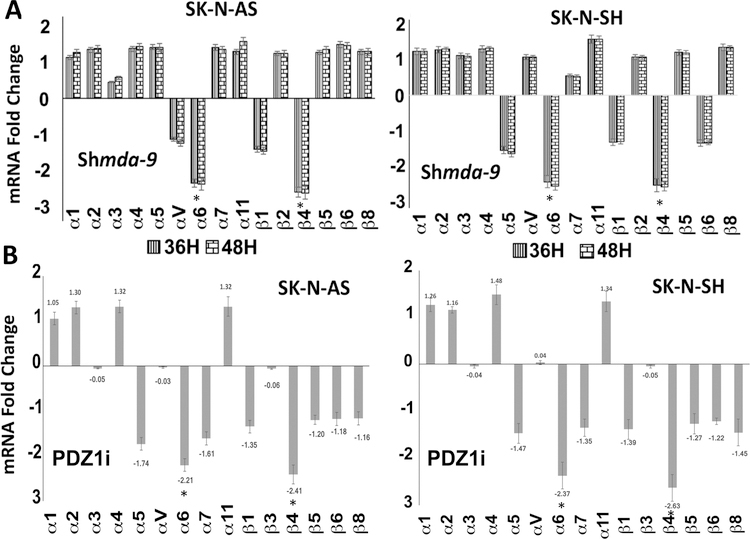
MDA-9 positively regulates polarized actin formation [7, 10, 12] by promoting actin cytoskeletal rearrangement through interactions with syndecans and regulation of epiboly movement [7, 10, 12]. Altered actin structures are observed in NB cells upon mda-9 inhibition (Fig. S5). Integrins are linked to actin cytoskeleton by clustering with multiple actin-associated partner proteins including α-actinin, vinculin, tensin and paxillin. Integrins directly mediate cell adhesion and also regulate intracellular signaling pathways that govern cytoskeletal organization [26]. Blocking MDA-9 using shmda-9 or siRNA treatment or PDZ1i altered integrin expression including a significant downregulation of integrin α6 and β4 (Figs. 5A, B and 6E; S6A). Several key migratory molecules changed when MDA-9 expression or function was inhibited using shmda-9 or PDZ1i, respectively. RECK inhibits the proteolytic activity of matrix metalloproteinase-9 (MMP-9) [27] and is elevated by shmda-9 or PDZ1i (Fig. 6A). This elevated level of RECK can affect MMP-9 directly and influence migration and invasion of NB cells. Blocking MDA-9 downregulated MMP-9, as determined by Western blotting and gelatin zymography (Figs. 6A and C). MMP-2 and MMP-9 are involved in matrix degradation and migration of cancer cells [28]. As observed with MMP-9 levels, MMP-2 levels were also inhibited when MDA-9 expression/function was inhibited (Figs. 6A; S6 and S7). Cofilin is expressed in eukaryotic cells and binds to Actin, thereby regulating rapid cycling of actin re-arrangement [29]. Interestingly, knockdown or inhibition of MDA-9 level or function, respectively, suppressed cofilin expression (Fig. 6A). EMT is an important process in the invasion and metastasis of tumor cells [30] and N-cadherin and vimentin play essential roles in EMT [31]. Treatment with either shmda-9 or PDZ1i decreased N-Cad and Vimentin levels (Fig. 6A). MDA-9 activates Src [18] and integrins facilitate Src-Fak interactions and cancer cell motility [18]. Active Src levels in shmda-9 or PDZ1i-treated NB cells decreased in shmda-9 or PDZ1i-treated NB cells (Figs. 6B; S6). These signaling molecules were also altered in a similar manner with siRNA targeting mda-9 (Fig. S6B). It is worth noting, that PDZ1i inhibits Src activity without direct binding to the PDZ1 domain of MDA-9 [18, 21]. Previous studies also showed that PDZ1i does not bind to the PDZ2 domain of MDA-9/Syntenin or to two closely related PDZ domains from Harmonin and X/11mint scaffold protein [13].
Figure 5: MDA-9 regulates integrin levels.
(A) NB cells were infected with shcon or shmda-9 or (B) treated with DMSO or PDZ1i and cultured at 37°C for 36 or 48 hours. RNA was isolated and converted to cDNAs. RT-PCR was performed for different integrins. GAPDH was used as a control. Results are representative of three independent experiments presented in a graphical manner, Columns, mean of triplicate experiments. Bars, S.E.; *, P < 0.01 vs. control.
Figure 6: MDA-9 regulates invasion and migration-related molecules in NB cells.
NB cells were treated as described in 4A and 4B and cell lysates were either incubated with GST fusion beads for 60 minutes or used directly for SDS-PAGE analysis. (A) Western blot analysis for MDA-9, active RhoA, active Rac1, active Cdc42, cofilin, MMP-2, MMP-9, RECK, N-Cadherin and Vimentin. β-actin was used as loading control. (B) Western blotting analysis for total and phospho Src. (C) MMP-9 and MMP-2 expression following shmda-9 or PDZ1i treatment for 48 hours. Gelatin zymography. (D) Western blotting analysis for total RhoA, Rac1, and Cdc42. (E) NB cells were either treated with shcon or shmda-9 or PDZ1i for 48 hours and cells were collected, fixed and stained for integrin α6 or β4. FACS analysis was performed to estimate levels of expression and data presented in a bar graph. Columns, mean of triplicate experiments. Bars, S.D.; *, P < 0.01 vs. control.
Integrins can facilitate Rho-, Rac- and Cdc42-mediated cell spreading in the absence of exogenous growth factors [32]. MDA-9 inhibition did not alter the total levels of these small GTPases, whereas active levels were downregulated as shown using GTP-Pulldown assays (Figs. 6A and D; S8). Taken together, these results suggest that suppressing MDA-9 or blocking its’ activity alters integrin α6 and β4 expression and affects Rho/Rac and Src activation in NB cells (Figs. 5 and 6; S9).
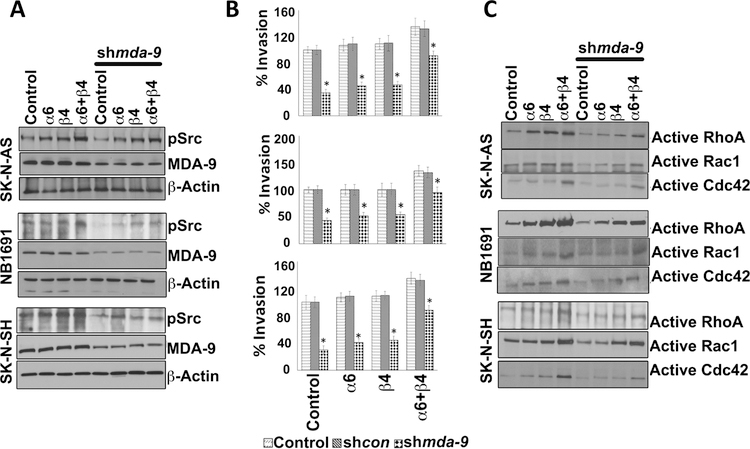
Integrin α6 and/or β4 over expression rescues MDA-9–mediated inhibition of NB cell invasion
α6/β4 integrins support motile behavior in single epidermal and A549 cancer cells [33, 34]. α6 and β4 integrin were expressed independently and in combination in NB cells to evaluate effects on MDA-9-mediated cell invasion and molecular signaling (Figs. 7; S9, S10, S11, and S12). Genetically modified cells received either shmda-9 or PDZ1i and cell migration and invasion were determined. Src activation plays a key role in MDA-9/Syntenin-mediated cell invasion and migration [7, 10, 12, 18]. Inhibition of pSrc by MDA-9 downregulation or PDZ1i treatment was partially rescued when α6 or β4 integrins were over-expressed and the level of rescue was elevated further when both integrins were overexpressed (Figs. 7A; S9, S10, and S12). Elevation in pSrc levels directly correlated with rescue in invasion inhibition in MDA-9-inhibited NB cells (Figs. 7A and B; S9). Integrin over-expression did not result in any change in MDA-9 levels suggesting integrins are functioning downstream of MDA-9 in NB cells (Figs. 7A; S9).
Figure 7: MDA-9 regulates NB invasion by modulating the integrin-Src-Rho pathway.
NB cells were transfected with integrin α6 or β4 alone or in combination together for 36 hours and treated with either shcon or shmda-9 for an additional 48 hours. Cell lysates were subjected to SDS-PAGE analysis. (A) Western blotting analysis for MDA-9 and pSrc. (B) Invasion assays performed as described [17]. (C) NB cells were treated as described in A for 48 hours and cell lysates were incubated with the GST fusion beads for 60 minutes. The beads were then washed three times, re-suspended in SDS loading buffer, and boiled for 10 minutes. SDS-PAGE and Western blotting analyses were performed for active RhoA, Rac1 and CDC42. Columns, mean of triplicate experiments. Bars, S.D.; *, P < 0.01 versus control.
Rho family proteins and the Src family kinase are implicated in MDA-9-induced invasion and migration mediated by integrins. We determined the relationship between integrins and Rho family GTPases in MDA-9-mediated signaling. Integrin overexpression resulted in rescue of activation of these GTPases in MDA-9-inhibited or PDZ1i treated cells (Figs. 7C; S11 and S12). Taken together, these results suggest that MDA-9 inhibition alters integrin α6 and β4, which in turn affects active-Src and the activation of small GTPases.
Blocking MDA-9 genetically or pharmacologically reduces tumor growth and inhibits metastasis in a NB metastatic animal model
Luciferase expressing NB1691 (NB1691-Luc) or shmda-9 pretreated NB1691-Luc cells were injected intravenously into SCID mice. For PDZ1i treatment, twenty-one days after injection of tumor cells, the animals were divided into vehicle control (5% DMSO in saline) or PDZ1i-treated groups. NB1691-Luc-injected animals became symptomatic within 6 weeks, and were sacrificed. The animals that received shmda-9 treated NB1691-Luc cells or animals treated with PDZ1i inhibitor lived longer than controls (Fig. 8A). Log-rank analysis revealed that a significant survival benefit was achieved in PDZ1i-treated (p<0.01), or shmda-9-treated group (p<0.001) as compared to the control group (Fig. 8A). The control animals developed tumors in their adrenal gland, liver, kidney, and spleen. In contrast, animals treated with PDZ1i or injected with shmda-9-treated cells displayed decreased tumor growth and had fewer metastatic lesions when imaged using an IVIS imager (Figs. 8B and C). These results suggest that inhibition of MDA-9 reduces metastasis and tumor burden in a xenograft model of NB.
Figure 8: Genetic or small molecule inhibition of MDA-9 suppresses NB metastasis.
NB xenografts created by intravenous injection of NB1691-Luc cells or shmda-9 treated NB1691-Luc cells were used for these experiments. For the PDZ1i treatment group, mice were injected with 30 mg/kg of PDZ1i or vehicle control intra-peritoneally after allowing tumors to develop for 21 days. (A) Log-rank analysis was performed on animal survival data and percent survival is graphically presented. Significant survival benefit was achieved in PDZ1i treated (p<0.001), or shmda-9 treated group (p<0.0001) as compared to control group. *p<0.001 vs Control; @ p<0.01 vs PDZ1i. (B) Mice were imaged using IVIS imager and representative images are shown after terminating the experiment. (C) Tumors were harvested, photographed at the terminal time points and representative images are shown. (D) Tumors were harvested, fixed in formalin, sectioned and immunohistochemistry determined MDA-9, CD-31 and pSrc expression. (E) Schematic diagram depicting the events resulted in decreased invasion and metastasis after MDA-9 inhibition in NB cells.
H&E analysis revealed a significant number of NB1691-Luc cells (Fig. 8D with arrows) had migrated from the core tumors in control mice. In contrast, few cells migrated and formed tumor lesions in mice injected with shmda-9-treated NB1691-Luc cells or mice treated with PDZ1i (Fig. 8D). To confirm MDA-9 inhibition in vivo, metastatic tumor sections from the liver were stained with a human MDA-9/Syntenin monoclonal antibody. Intense staining for MDA-9 was evident in control tumor sections as compared to tumors injected with shmda-9-treated cells, whereas MDA-9 levels in tumor sections from PDZ1i-treated groups were similar to controls. To confirm the in vitro molecular changes we analyzed the levels of Src activation in these tumor sections using a Src-Tyr416 specific antibody. Control tumor sections injected with NB1691-Luc cells showed high staining for pSrc as compared to tumors from animals treated with PDZ1i or shmda-9-treated tumors. MDA-9 also plays a critical role in tumor angiogenesis [35, 36]. Decreased CD-31 levels were evident in tumors from animals treated with PDZ1i or tumors established with shmda-9-treated cells. From the in vitro and animal experiments conducted in this study and previous experiments in the context of glioblastoma multiforme [13] and prostate cancer [37], it is evident that PDZ1i (at the doses used) is not toxic to normal or cancer cells in vitro and does not show overt signs of toxicity in vivo in animal models. Additionally, we checked Ki-67 (proliferation marker) and TUNEL staining (to detect cell death) (Fig. S13) in the tissue sections and observed decreased Ki-67 expression, but no evidence of cell death in shmda-9- or PDZ1i-treated tumor tissue. This confirms that there is no cytotoxicity in the tumors treated either with shmda-9 or PDZ1i in the context of NB. These results are consistent with the MDA-9-mediated inhibition of migration and tumor growth in other cancers [7, 10, 12, 13, 20, 37–39].
Discussion
NB arises from rapidly proliferating neuroblasts during fetal development and metastasis is the primary cause of death in these patients [40]. Interactions between extracellular matrix and tumor cells is a key component in metastasis, involving a multistep complex process comprising proliferation, attachment, migration, and invasion [4, 12, 41]. MDA-9 regulates multiple steps in tumor metastasis [10, 12]. In many cancers, such as melanomas, breast, prostate and gliomas, MDA-9 correlates with an aggressive, invasive and metastatic tumor phenotype [10–13, 21, 37, 38]. MDA-9-mediated regulation of tumor cell migration, invasion and metastasis depends on interacting partners or downstream molecules that are determinants of cell motility [7, 10]. Effects on cell growth, migration and tumor growth suggest that MDA-9 promotes cell-specific effects that also depend on regulation of ECM components [7, 10, 35–39]. The present study ascertained the role of MDA-9 inhibition (genetically and pharmacologically) in NB pathogenesis confirming that suppression of migration, invasion, and MMP-2 and MMP-9 activity corresponds with MDA-9 inhibition, without changing proliferation.
We determined the biochemical and molecular roles of MDA-9 in regulating NB cell migration, invasion and metastasis. Genetic suppression (shmda-9, siRNA) or pharmacological inhibition (PDZ1i) of MDA-9 inhibited tumor cell migration (spheroid migration assay) and wound healing migration and also impeded invasion. MDA-9 influences tumor cell migration in multiple cancers, where the regulated molecules appear cell-type specific [7, 10, 12, 21]. MDA-9 also plays a critical role in cancer stem cell survival and stemness, and anoikis-resistance [42–45]. Evidence is accumulating that MDA-9-targeting may have therapeutic applications as indicated by an ability to counteract invasion gains produced in glioma cells following radiation treatment [13, 45]. PDZ1i, a first-in-class PDZ small molecule inhibitor of MDA-9 which specifically targets the PDZ1 domain [13, 45], reduced the invasion phenotype and sensitized glioma to radiation therapy [13, 45]. Other studies in melanoma, and breast and prostate cancer suggest that silencing the expression of MDA-9 decreases metastasis [10, 12, 21, 38].
ECM components continually interact with epithelial cells by serving as ligands for cell receptors, including integrins. These interactions provoke changes that transmit signals regulating adhesion, migration and invasion [46]. Changes in the ECM can directly modulate integrins without modifying other molecules [46]. We show that MDA-9 inhibition or pathway disruption by blocking protein-protein interactions alters migration and invasion, which is accompanied by decreased integrin expression, mainly α6 and β4. In many cancers, Src signaling affects establishment of focal adhesions and the ECM [47]. In cancer metastasis, Src can directly interact with beta integrins. Integrin α6β4 mediates activation of PI3-K, which increases actin protrusions and may also stimulate other integrins [48]. In breast cancer, integrin α6β4 activates Src leading to downstream signaling events controlling migration and metastasis. MDA-9 downregulation reduces Src activation inhibiting the activity of small GTPases. In epithelial cells, integrin α6β4 affects signaling molecules involved in migration and invasion, especially PI3-K and Rho GTPases [48, 49]. Forced overexpression of integrins in shmda-9//PDZ1i-treated cells rescued Src activation resulting in increased activity of small GTPases, including Rho, Rac and Cdc42. This rescue activates biological activity as evidenced by increased invasion. These results show that MDA-9 inhibition or targeted disruption of protein-protein interactions modifies the ECM by directly affecting integrins. Overall, blocking MDA-9 downregulates integrin α6β4, inhibiting Src activation and reducing small GTPases, which in turn extinguishes invasion, migration and metastasis of NB cells (Fig. 8E). Moreover, considering the in vitro results indicating that MDA-9 inhibition reduces migration and invasion via integrin and Src leading to decreased activity of RhoA/Rac/Cdc42, we determined the effect of blocking MDA-9 on Src expression and angiogenesis in NB tumor cells in vivo (Fig. 8D). These studies demonstrated that disruption of MDA-9 genetically or pharmacologically suppressed Src and angiogenic activity in vivo.
In summary, genetic inhibition of endogenous MDA-9 expression or inhibition of MDA-9-partner protein interactions with PDZ1i decreased migration, invasion and metastasis of NB cells (Fig. 8E). Blocking MDA-9 resulted in integrin downregulation, which caused Src inhibition, leading to Rho, Rac, and Cdc42 inactivation. These pathway modifications inhibited cofilin and MMP’s culminating in decreased migration/invasion in vitro and in vivo. On the basis of these observations, it is evident that MDA-9 can directly regulate migration and metastasis in NB cells. Moreover, the current observations provide support for the application of genetic and pharmacological inhibitors of MDA-9 as potential reagents for the therapy of NB.
As a first generation drug, PDZ1i reveals remarkable inhibitory effects on invasion and metastasis in many cancer types with good pharmacological properties suggesting immense potential to become a viable anti-cancer drug. Before entering clinical trials, further modifications to enhance its properties may be required, which will become evident after detailed ADME and toxicology testing. Considering the initial positive activity toward a broad-spectrum of cancer cells and the absence of overt toxicity, developing PDZ1i as a potential drug for use in patients would appear to be a worthwhile endeavor.
Materials and Methods
Neuroblastoma and other cell lines
SK-N-AS and SK-N-SH (NB cell lines), and HS-5 (a human bone marrow stromal cell line immortalized by transduction with the human papilloma virus E6/E7 genes) cells were from the ATCC (Manassas, VA). The NB cell line NB1691 was provided by Dr. Houghton (Nationwide Children’s Hospital, Columbus, OH). NB1691 cell line was authenticated using the “CellCheck” service provided by the Research Animal Diagnostic Laboratory and compared with initial short tandem repeat profile generated by the collaborator (IDEXX BioResearch). Cells were cultured as described earlier [50]. Primary human fetal astrocytes were obtained from preterm abortions as previously described with institutional review board approval, and h-TERT–immortalized primary human fetal astrocytes (IM-PHFAs) were produced and cultured as described [13, 51]. The cumulative culture length of the cells was less than 6 months after recovery. Early passage cells were used for all experiments. All cell lines were frequently tested for mycoplasma contamination using a mycoplasma detection kit from Sigma.
Reagents and antibodies
Antibodies specific for Rho A, Rac, Cdc42, MMP-2, MMP-9, RECK, N-Cadherin, Vimentin, cofilin, Src and pSrc (Cell Signaling Technology Inc, Danvers, MA), β-actin and CD-31 (Abcam, Cambridge, MA), and MDA-9/Syntenin (Abnova) were used in this study. The other reagents were Transcriptor First Strand cDNA Synthesis Kit (Applied biosystems), MTT cell growth assay kit (Millipore Corporation, Billerica, MA), Neuroblastoma Tissue Microarray (US Biomax) and Matrigel Invasion chambers (BD Biosciences), siRNA against mda-9 (Ambion, Thermo Fisher).
mda-9 shRNA and PDZ1i treatment
The adenoviral shRNA for mda-9 (shmda-9) and scrambled vector (shcon) were constructed and amplified as described [19]. Virus infections were in serum-free media for 2 hours and then complete medium was added and cells were incubated for the indicated time periods. NB cells were treated with either DMSO or 50 μM PDZ1i for 48 hours.
Western blotting
Western blotting analysis was performed as described [17, 19]. SK-N-AS, SK-N-SH, or NB1691 cells were plated for 24 hours and treated with either shmda-9 or PDZ1i for 48 hours or treated with mda-9 siRNA for 72 hours. Cells were collected and Western blotting analysis was performed.
Cell proliferation assays
Cell growth was determined using a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as a measurement of mitochondrial metabolic activity as described [50]. Cells were treated with either shcon or shmda-9 and incubated at 37°C. After 0 to 72 hours, MTT reagent was added to the cells and they were incubated for 6 hours at 37°C. Absorbance of formazan was measured with a microplate reader at A550.
RT-PCR
NB cells were plated for 24 hours and treated with either shmda-9 or PDZ1i for 48 hours or treated with mda-9 siRNA for 72 hours. Total RNA was then extracted from these cells, cDNA was synthesized and real time PCR was performed as described [50].
GTPase activity assays
pGEX-Rac1, pGEX-Cdc42, and pGex-TRBD plasmids were expressed in Escherichia coli, and the fusion proteins GST-Rac1, GST-Cdc42, and GST-TRBD, respectively, were affinity purified. These purified glutathione agarose beads were incubated with either control (shcon), shmda-9 or PDZ1i treated lysates for 60 min. Agarose beads were then washed three times and eluted by incubating in loading buffer at 95 °C for 10 min. The samples were subjected to SDS-PAGE and immunoblotted with corresponding antibodies [17, 38].
Matrigel invasion assay
Matrigel invasion was determined as described [17, 19]. NB cells were plated for 24 hours and treated with either shmda-9 or PDZ1i for 48 hours or treated with mda-9 siRNA for 72 hours. The cells were re-plated on Matrigel-coated trans-well inserts and after overnight incubation invading cells were stained, photographed and counted. Five different fields per filter were analyzed, and all experiments were done in triplicate.
Wound healing migration
Wound healing migration assays were performed as described [17]. SK-N-AS, or SK-N-SH cells were treated as above and grown to full confluence to form a monolayer. A scratch was made as described [50], and the cells were allowed to migrate towards each other. Using a light microscope, we measured and quantified the distance the cells migrated over the indicated time periods. For HS-5 and NB cell studies, silicone inserts with a defined cell-free gap (500 μM) were used as described [17]. Migrated cell measurements were taken and the distance the cells migrated over the indicated time periods was quantified.
Plasmid transfections
NB cells were transfected with Integrin α6, β4 or both together or with mda-9 siRNA using FuGene HD transfection reagent according to the manufacturer’s protocol (Roche) [50].
Immunocytochemistry
A previously described protocol with minor changes was used for IHC analysis [50]. Briefly, the cells were cultured on 8-well chamber slides and fixed, permeabilized and blocked with 1% BSA (w/v) in PBS for 1 hour at 4°C. Cells were incubated overnight at 4°C with anti-integrin α6 and β4 antibody followed by fluorescence secondary antibody for 1 hour, and were washed and mounted with anti-fade mounting solution containing DAPI and analyzed with an inverted florescence microscope.
Animal studies and tissue section immunohistochemistry
For preparation of NB xenografts, NB1691-luc cells or shmda-9- or PDZ1i-treated NB1691-luc cells were re-suspended in serum free RPMI1640 media and 100 μL of this mixture containing 6 × 106 cells were injected in mice by intravenous tail vein injection [35–38]. These animals were imaged using IVIS imager to confirm the IV injections. Animals which contained luciferase positive cells (BLI) in the lungs were divided into three groups and used for experiments. For the PDZ1i treatment protocol, twenty-one days after injection of tumor cells, the animals were divided into vehicle control (5% DMSO in saline) or PDZ1i-treated groups (5 mice per group). Mice were euthanized when they lost >20% of body weight or had trouble ambulating, feeding, or grooming. The tumors were removed, fixed in 10% phosphate-buffered formaldehyde and used for IHC studies as described [13, 50].
Statistical analysis
All data are presented as mean ± SD of at least three independent experiments, each performed at least in triplicate. One-way ANOVA combined with the Tukey post hoc test of means was used for multiple comparisons. Statistical differences are presented at probability levels of P < 0.05.
Supplementary Material
Acknowledgements
We thank Dr. Xue-Ning Shen for outstanding technical assistance. We also thank Dr Martin A. Schwartz (Cardiovascular Research Center, Mellon Prostate Cancer Institute, Departments of Microbiology and Biomedical Engineering, University of Virginia, Charlottesville, VA) for providing pGEX-TRBD, Dr. Baroda S, for providing pGEX-Rac1, and pGEX-Cdc42 and we thank Dr. Filippo Giancotti for pRK5 alpha6, and beta4 plasmids. Services and products in support of the research project were also provided by the VCU Massey Cancer Center Cancer Mouse Model Shared Resource.
Grant support
The present study was supported in part by the National Foundation for Cancer Research (NFCR) (to P.B.F.), NCI Cancer Center Support Grant to VCU Massey Cancer Center (MCC) P30 CA016059 (to P.B.F. and D.S.), the VCU Institute of Molecular Medicine (VIMM) (P.B.F.) and the Genetics Enhancement Fund (P.B.F., S.K.D. and L.E.). Support was also provided by a Sponsored Research Agreement from InVaMet Therapeutics, Inc. (IVMT) (S.K.D.). P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research at the MCC.
Footnotes
Conflicts of Interest
P.B.F. is a founder of InVaMet Therapeutics, Inc. (IVMT). P.B.F., Virginia Commonwealth University and the Sanford Burnham Prebys Medical Discovery Institute own stock in IVMT. S.K.D. is the Principle Investigator of a SRA provided by InVaMet Therapeutics, Inc. to Virginia Commonwealth University. No other authors declare any potential conflicts with this research.
References
- 1.Shohet J, Foster J. Neuroblastoma. BMJ 2017;357:j1863. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi NK, McGuire TR, Coulter DW, Shukla A, McIntyre EM, Sharp JG, et al. Improved therapy for neuroblastoma using a combination approach: superior efficacy with vismodegib and topotecan. Oncotarget 2016;7:15215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarkar D, Boukerche H, Su Z-z, Fisher PB. mda-9/syntenin: more than just a simple adapter protein when it comes to cancer metastasis. Cancer Res 2008;68:3087–93. [DOI] [PubMed] [Google Scholar]
- 4.Welch DR, Fisher PB (Eds). Molecular and Cellular Basis of Metastasis: Road to Therapy. Adv. Cancer Res 2016;132:1–390.27613128 [Google Scholar]
- 5.Stivarou T, Patsavoudi E. Extracellular molecules involved in cancer cell invasion. Cancers (Basel) 2015;7:238–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminska K, Szczylik C, Bielecka ZF, Bartnik E, Porta C, Lian F, et al. The role of the cell-cell interactions in cancer progression. J Cell Mol Med 2015;19:283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kegelman TP, Das SK, Emdad L, Hu B, Menezes ME, Bhoopathi P, et al. Targeting tumor invasion: the roles of MDA-9/Syntenin. Expert Opin Ther Targets 2015; 19:97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JJ, Jiang H, Fisher PB. Characterization of a novel melanoma differentiation associated gene, mda-9, that is down-regulated during terminal cell differentiation. Mol Cell Different 1996; 4:317–33. [Google Scholar]
- 9.Lin JJ, Jiang H, Fisher PB. Melanoma differentiation associated gene-9 is a human gamma interferon responsive gene. Gene 1998;207:105–10. [DOI] [PubMed] [Google Scholar]
- 10.Das SK, Sarkar D, Emdad L, Fisher PB. MDA-9/Syntenin: an emerging global molecular target regulating cancer invasion and metastasis. Adv Cancer Res 2019; in press. [DOI] [PubMed]
- 11.Boukerche H, Su Z-z, Emdad L, Baril P, Balme B, Thomas L, et al. mda-9/Syntenin: a positive regulator of melanoma metastasis. Cancer Res 2005;65:10901–11. [DOI] [PubMed] [Google Scholar]
- 12.Das SK, Bhutia SK, Kegelman TP, Peachy L, Oyesanya RA, Dasgupta S, et al. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Bioscience 2012;17:1–15. [DOI] [PubMed] [Google Scholar]
- 13.Kegelman TP, Wu B, Das SK, Talukdar S, Beckta JM, Hu B, et al. Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proc Natl Acad Sci USA 2017;114:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan X Cancer metastases: challenges and opportunities. Acta Pharm Sin B 2015;5:402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer 2002;2:91–100. [DOI] [PubMed] [Google Scholar]
- 16.Meyer A, van Golen CM, Kim B, van Golen KL, Feldman EL. Integrin expression regulates neuroblastoma attachment and migration. Neoplasia 2004;6:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhoopathi P, Gondi CS, Gujrati M, Dinh DH, Lakka SS. SPARC mediates Src-induced disruption of actin cytoskeleton via inactivation of small GTPases Rho-Rac-Cdc42. Cell Signal 2011;23:1978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boukerche H, Su Z-z, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc Natl Acad Sci USA 2008;105:15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kegelman TP, Das SK, Hu B, Bacolod MD, Fuller CE, Menezes ME, et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol 2014;16:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dasgupta S, Menezes ME, Mukhopadhyay ND, Das SK, Shao C, Emdad L, et al. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res 2013;19:4621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das SK, Pradhan AK, Bhoopathi P, Talukdar S, Shen X-N, Sarker D, et al. The MDA-9/Syntenin/IGF-1R/STAT3 axis directs prostate cancer invasion. Cancer Res 2018;78:2852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacolod MD, Das SK, Sokhi UK, Bradley S, Fenstermacher DA, Pellecchia M, et al. Examination of epigenetic and other molecular factors associated with mda-9/Syntenin dysregulation in cancer through integrated analyses of public databases. Adv Cancer Res 2015;127:49–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seong BK, Fathers KE, Hallett R, Yung CK, Stein LD, Mouaaz S, et al. A metastatic mouse model identifies genes that regulate neuroblastoma metastasis. Cancer Res 2017;77:696–706. [DOI] [PubMed] [Google Scholar]
- 24.Morandi F, Scaruffi P, Gallo F, Stigliani S, Moretti S, Bonassi S, et al. Bone marrow-infiltrating human neuroblastoma cells express high levels of calprotectin and HLA-G proteins. PLoS One 2012;7:e29922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastogi P, Naseem S, Varma N, Das R, Ahluwalia J, Sachdeva MU, et al. Bone marrow involvement in neuroblastoma: a study of hemato-morphological features. Indian J Hematol Blood Transfus 2015;31:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol 2010;2:a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci U S A 1998;95:13221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP, et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017;17:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SH, Dominguez R. Regulation of actin cytoskeleton dynamics in cells. Mol Cells 2010;29:311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicol Res 2010;26:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res 2004;10:4125–33. [DOI] [PubMed] [Google Scholar]
- 32.Miao H, Li S, Hu YL, Yuan S, Zhao Y, Chen BP, et al. Differential regulation of Rho GTPases by beta1 and beta3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci 2002;115:2199–206. [DOI] [PubMed] [Google Scholar]
- 33.Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, et al. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci 2003;116:3543–56. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor KL, Chen M, Towers LN. Integrin alpha6beta4 cooperates with LPA signaling to stimulate Rac through AKAP-Lbc-mediated RhoA activation. Am J Physiol Cell Physiol 2012;302:C605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das SK, Bhutia SK, Azab B, Kegelman TP, Peachy L, Santhekadur PK, et al. MDA-9/Syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res 2013;73: 844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das SK, Guo C, Pradhan AK, Bhoopathi P, Talukdar S, Shen X-N, et al. Knockout of MDA-9/Syntenin (SDCBP) expression in the microenvironment dampens tumor-supporting inflammation and inhibits melanoma metastasis. Oncotarget 2016;7:46848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das SK, Kegelman TP, Pradhan AJ, Bhoopathi P, Talukdar S, Maji S, et al. Suppression of prostate cancer pathogenesis using MDA-9/Syntenin (SDCBP) PDZ1 small molecule inhibitor. Mol Cancer Ther 2019, in press. [DOI] [PubMed]
- 38.Menezes ME, Shen XN, Das SK, Emdad L, Sarkar D, Fisher PB. MDA-9/Syntenin (SDCBP) modulates small GTPases RhoA and Cdc42 via transforming growth factor beta1 to enhance epithelial-mesenchymal transition in breast cancer. Oncotarget 2016;7:80175–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das SK, Bhutia SK, Sokhi UK, Azab B, Su Z-z, Boukerche H, et al. Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res 2012;72:6217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer M, Ribeiro D, Arsenian-Henriksson M, Deller T, Rohrer H. Proliferation and survival of embryonic sympathetic neuroblasts by MYCN and activated ALK signaling. J Neurosci 2016;36:10425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melzer C, von der Ohe J, Hass R. Breast carcinoma: from initial tumor cell detachment to settlement at secondary sites. Biomed Res Int 2017;2017:8534371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talukdar S, Das SK, Pradhan AK, Emdad L, Shen XN, Windle JJ, et al. Novel function of MDA-9/Syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells. Oncotarget 2016;7:54102–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talukdar S, Pradhan AK, Bhoopathi P, Shen XN, August LA, Windle JJ, et al. Regulation of protective autophagy in anoikis-resistant glioma stem cells by SDCBP/MDA-9/Syntenin. Autophagy 2018;14:1845–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talukdar S, Pradhan AK, Bhoopathi P, Shen X-N, August LA, Windle JJ, et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc Natl Acad Sci U S A 2018;115:5768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das SK, Sarkar D, Cavenee WK, Emdad L, Fisher PB. Rethinking glioblastoma therapy: mda-9/syntenin targeted small molecule. ACS Chem Neurosci 2019, in press. [DOI] [PubMed]
- 46.Dominguez-Gimenez P, Brown NH, Martin-Bermudo MD. Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium. J Cell Sci 2007;120:1061–71. [DOI] [PubMed] [Google Scholar]
- 47.Bolos V, Gasent JM, Lopez-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther 2010;3:83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercurio AM, Rabinovitz I, Shaw LM. The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol 2001;13:541–5. [DOI] [PubMed] [Google Scholar]
- 49.Colburn ZT, Jones JCR. Complexes of α6β4 integrin and vimentin act as signaling hubs to regulate epithelial cell migration. J Cell Sci 2018;131(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhoopathi P, Lee N, Pradhan AK, Shen XN, Das SK, Sarkar D, et al. mda-7/IL-24 induces cell death in neuroblastoma through a novel mechanism involving AIF and ATM. Cancer Res 2016;76:3572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Z-Z, Kang D-c, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene 2002. 21:3592–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.